Synthesis of Silver Nanoparticles Using Extracts from Different Parts of the Paullinia cupana Kunth Plant: Characterization and In Vitro Antimicrobial Activity
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profile of Aqueous Extracts Characterized by UHPLC-HRMS/MS
2.2. Quantification of Total Phenol Content (TPC) and Antioxidant Capacity of Aqueous Extracts of Paullinia cupana Leaves and Flowers
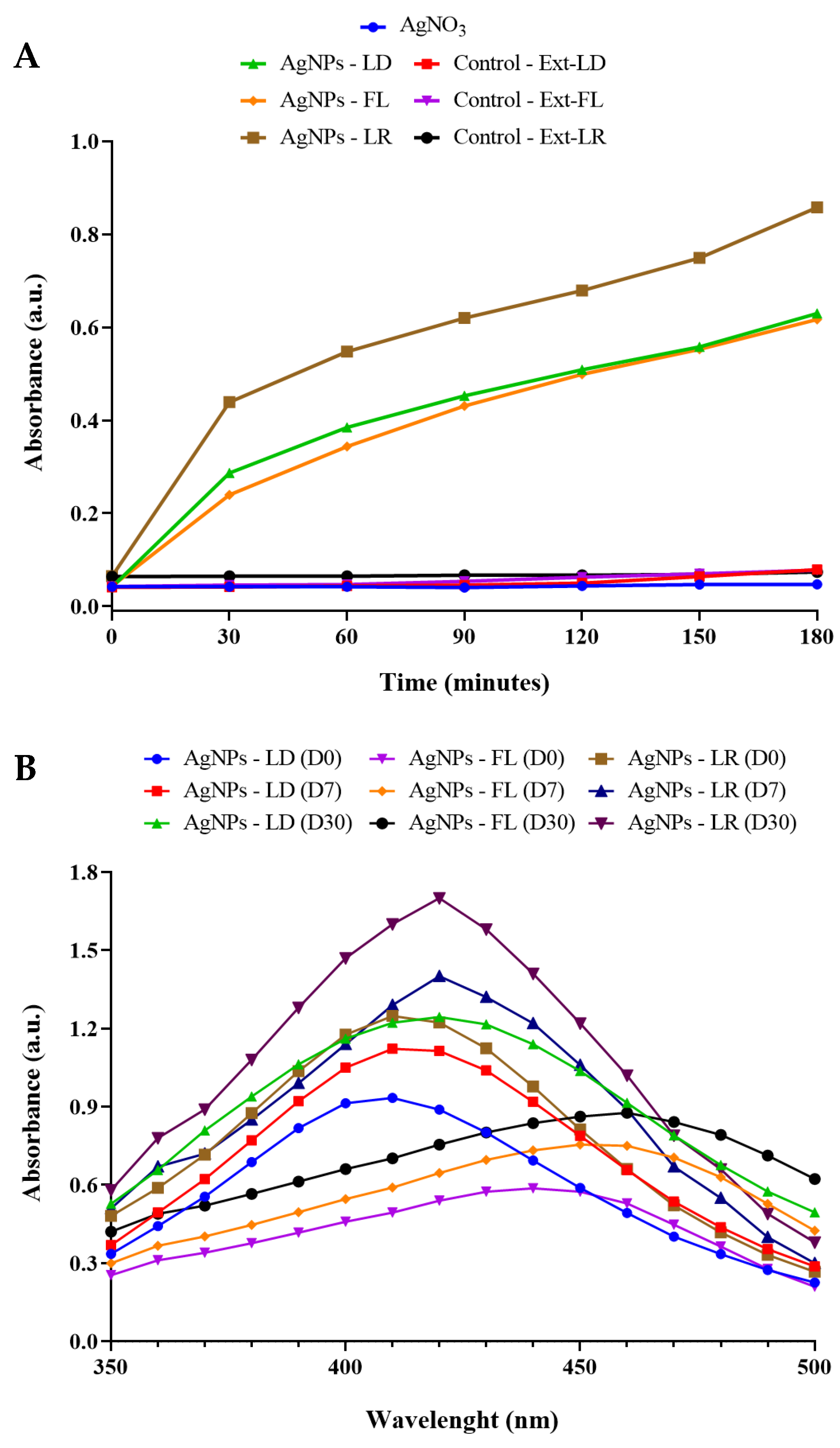
2.3. Visual Aspects and UV/Vis Spectral Analysis
2.4. Evaluation of Colloidal Stability by DLS and Zeta Potential
2.5. Nanoparticle Tracking Analysis (NTA)
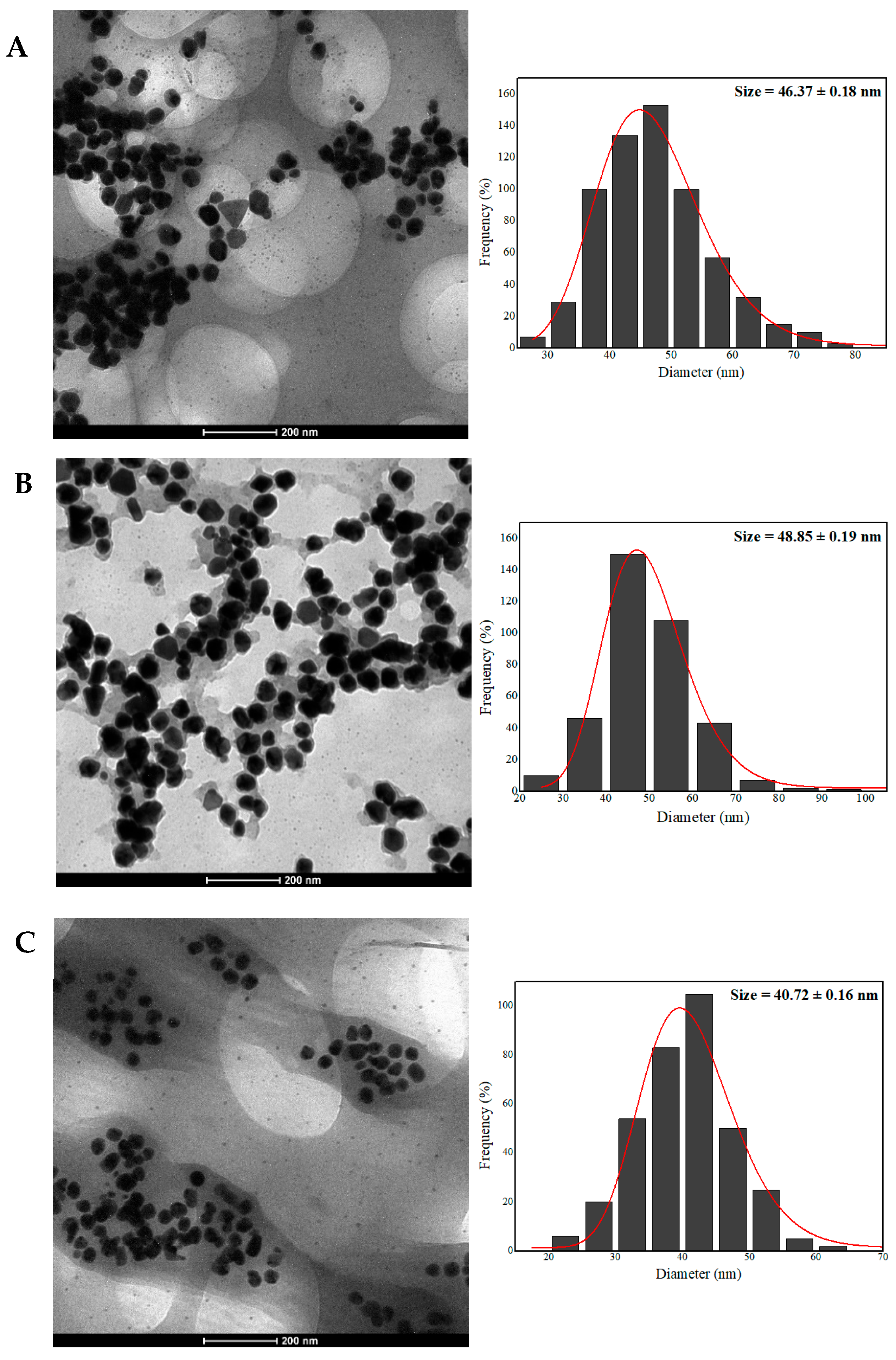
2.6. Transmission Electron Microscopy (TEM) and Energy-Dispersive X-ray Spectroscopy (EDX)
2.7. Evaluation of Antimicrobial Activity
3. Material and Methods
3.1. Chemicals and Reagents
3.2. Collection of Plant Material and Preparation of Aqueous Extracts of Paullinia cupana Leaves and Flowers
3.3. Phytochemical Characterization and Antioxidant Potential of Paullinia cupana Plant Extracts
3.3.1. UHPLC-HRMS/MS
3.3.2. Quantification of Total Phenol Content (TPC)
3.3.3. DPPH
3.3.4. ABTS
3.4. Green Synthesis of AgNPs Using Paullinia cupana Extracts
3.5. UV/Vis Spectrophotometric Analysis
3.6. Dynamic Light Scattering (DLS) and Electrophoretic Mobility (Zeta Potential) Analysis
3.7. Nanoparticle Tracking Analysis (NTA)
3.8. Transmission Electron Microscopy (TEM)
3.9. Energy-Dispersive X-rays (EDX)
3.10. Antimicrobial Activity
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, J.W. History of the medical use of silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef]
- Raj, S.; Singh, H.; Trivedi, R.; Soni, V. Biogenic synthesis of AgNPs employing Terminalia arjuna leaf extract and its efficacy towards catalytic degradation of organic dyes. Sci. Rep. 2020, 10, 9616. [Google Scholar] [CrossRef]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of stable Au, Ag and Au–Ag alloy nanoparticles using J. Sambac leaves extract, and their enhanced antimicrobial activity in presence of organic antimicrobials. Spectrochim. Acta Part A 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Vadakkan, K.; Rumjit, N.P.; Ngangbam, A.K.; Vijayanand, S.; Nedumpillil, N.K. Novel advancements in the sustainable green synthesis approach of silver nanoparticles (AgNPs) for antibacterial therapeutic applications. Coord. Chem. Rev. 2024, 499, 215528. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmad, S.; Ali, S.; Esa, M.; Khan, A.; Yan, H. Recent Advancements and Unexplored Biomedical Applications of Green Synthesized Ag and Au Nanoparticles: A Review. Int. J. Nanomed. 2024, 19, 3187–3215. [Google Scholar] [CrossRef]
- Terenteva, E.A.; Apyari, V.V.; Dmitrienko, S.G.; Zolotov, Y.A. Formation of plasmonic silver nanoparticles by flavonoid reduction: A comparative study and application for determination of these substances. Spectrochim. Acta Part A 2015, 151, 89–95. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Ahmed, K.B.R.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. Vitr. 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Bharath, L.V. Mechanism of plant-mediated synthesis of silver nanoparticles—A review on biomolecules involved, characterisation and antibacterial activity. Chem.-Biol. Interact. 2017, 273, 219–227. [Google Scholar] [CrossRef]
- Basile, A.; Rigano, D.; Conte, B.; Bruno, M.; Rosselli, S.; Sorbo, S. Antibacterial and antifungal activities of acetonic extract from Paullinia cupana Mart. seeds. Nat. Prod. Res. 2013, 27, 2084–2090. [Google Scholar] [CrossRef]
- Aguiar, B.A.A.; Bueno, F.G.; Panizzon, G.; Silva, D.B.D.; Athaydes, B.R.; Gonçalves, R.D.C.R.; Kitagawa, R.R.; Marques, L.L.M.; Antonelli-Ushirobira, T.M.; Paula, M.N.; et al. Chemical analysis of the semipurified extract of Paullinia cupana and evaluation of in vitro inhibitory effects against Helicobacter pylori. Nat. Prod. Res. 2020, 34, 2332–2335. [Google Scholar] [CrossRef]
- Dalonso, N.; De Oliveira Petkowicz, C.L. Guarana powder polysaccharides: Characterisation and evaluation of the antioxidant activity of a pectic fraction. Food Chem. 2012, 134, 1804–1812. [Google Scholar] [CrossRef]
- Santana, A.L.; Macedo, G.A. Health and technological aspects of methylxanthines and polyphenols from guarana: A review. J. Funct. Foods 2018, 47, 457–468. [Google Scholar] [CrossRef]
- Schimpl, F.C.; Kiyota, E.; Mayer, J.L.S.; de Carvalho Gonçalves, J.F.; da Silva, J.F.; Mazzafera, P. Molecular and biochemical characterization of caffeine synthase and purine alkaloid concentration in guarana fruit. Phytochemistry 2014, 105, 25–36. [Google Scholar] [CrossRef]
- Ushirobira, T.M.A.; Yamaguti, E.; Uemura, L.M.; Nakamura, C.V.; Dias Filho, B.P.; Mello, J.P. Chemical and microbiological study of extract from seeds of guaraná (Paullinia cupana var. sorbilis). Acta Farm. Bonaer. 2007, 26, 5–9. [Google Scholar]
- Carvalho, L.V.D.N.; Cordeiro, M.F.; Sampaio, M.C.P.D.; de Mello, G.S.V.; da Costa, V.D.C.M.; Marques, L.L.M.; Klein, T.; De Mello, J.C.P.; Cavalcanti, I.M.F.; Pitta, I.R.; et al. Evaluation of antibacterial, antineoplastic, and immunomodulatory activity of Paullinia cupana seeds crude extract and ethyl-acetate fraction. Evid. Based Complement. Alternat. Med. 2016, 2016, 1203274. [Google Scholar] [CrossRef]
- De Oliveira Salles, R.C.; Muniz, M.P.; Nunomura, R.D.C.S.; Nunomura, S.M. Geographical origin of guarana seeds from untargeted UHPLC-MS and chemometrics analysis. Food Chem. 2022, 371, 131068. [Google Scholar] [CrossRef]
- De Oliveira, A.L.L.; Muniz, M.P.; Silva, F.M.A.; Nascimento, A.H.; Santos-Barnett, T.C.; Gomes, F.B.; Nunomura, S.M.; Krug, C. Chemical composition of guarana flowers and nectar and their ecological significance. Biochem. Syst. Ecol. 2024, 112, 104769. [Google Scholar] [CrossRef]
- Gröning, R.; Breitkreutz, J.; Baroth, V.; Müller, R.S. Nanoparticles in plant extracts--factors which influence the formation of nanoparticles in black tea infusions. Pharmazie 2001, 56, 790–792. [Google Scholar]
- Bose, D.; Chatterjee, S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl. Nanosci. 2016, 6, 895–901. [Google Scholar] [CrossRef]
- Osonga, F.J.; Kariuki, V.M.; Yazgan, I.; Jimenez, A.; Luther, D.; Schulte, J.; Sadik, O.A. Synthesis and antibacterial characterization of sustainable nanosilver using naturally-derived macromolecules. Sci Total Environ. 2016, 563, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Karan, T.; Gonulalan, Z.; Erenler, R.; Kolemen, U.; Eminagaoglu, O. Green synthesis of silver nanoparticles using Sambucus ebulus leaves extract: Characterization, quantitative analysis of bioactive molecules, antioxidant and antibacterial activities. J. Mol. Struct. 2024, 1296, 136836. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Talat, M.; Singh, D.P.; Srivastava, O.N. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J. Nanopart. Res. 2010, 12, 1667–1675. [Google Scholar] [CrossRef]
- Das, J.; Velusamy, P. Antibacterial effects of biosynthesized silver nanoparticles using aqueous leaf extract of Rosmarinus officinalis L. Mater. Res. Bull. 2013, 48, 4531–4537. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Catalytically and biologically active silver nanoparticles synthesized using essential oil. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 132, 743–750. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural characterization of flavonoid glycoconjugates and their derivatives with mass spectrometric techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-mediated green synthesis of nanostructures: Mechanisms, characterization, and applications. Interface Sci. Technol. 2019, 28, 199–322. [Google Scholar] [CrossRef]
- Ovais, M.; Hoque, M.Z.; Khalil, A.T.; Ayaz, M.; Ahmad, I. Mechanisms underlying the anticancer applications of biosynthesized nanoparticles. Biog. Nanopart. Cancer Theranost. 2021, 229–248. [Google Scholar] [CrossRef]
- Majhenič, L.; Škerget, M.; Knez, Ž. Antioxidant and antimicrobial activity of guarana seed extracts. Food Chem. 2007, 104, 1258–1268. [Google Scholar] [CrossRef]
- Silva, M.P.; Thomazini, M.; Holkem, A.T.; Pinho, L.S.; Genovese, M.I.; Fávaro-Trindade, C.S. Production and characterization of solid lipid microparticles loaded with guarana (Paullinia cupana) seed extract. Food Res. Int. 2019, 123, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.; Silva, L.M.A.; Alves Filho, E.G.; Canuto, K.M.; de Brito, E.S.; de Jesus, R. 1H quantitative nuclear magnetic resonance and principal component analysis as tool for discrimination of guarana seeds from different geographic regions of Brazil. In Proceedings of the XIII International Conference on the Applications of Magnetic Resonance in Food Science, Karlsruhe, Germany, 7–10 June 2016; Volume 6, pp. 21–25. [Google Scholar]
- Da Silva, G.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Brito, E.S.; Nascimento, M.M.; Zocolo, G.J.; Coutinho, J.P.; de Jesus, R.M. Chemical profiling of guarana seeds (Paullinia cupana) from different geographical origins using UPLC-QTOF-MS combined with chemometrics. Food Res. Int. 2017, 102, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.P.; Palma, J.; Simeon, N.C.; Jin, X.; Liu, X.; Ngabire, D.; Kim, N.H.; Tarte, N.H.; Kim, G.D. Sasa borealis leaf extract-mediated green synthesis of silver–silver chloride nanoparticles and their antibacterial and anticancer activities. New J. Chem. 2017, 41, 1363–1371. [Google Scholar] [CrossRef]
- Manosalva, N.; Tortella, G.; Cristina Diez, M.; Schalchli, H.; Seabra, A.B.; Durán, N.; Rubilar, O. Green synthesis of silver nanoparticles: Effect of synthesis reaction parameters on antimicrobial activity. World J. Microbiol. Biotechnol. 2019, 35, 88. [Google Scholar] [CrossRef]
- Mock, J.J.; Barbic, M.; Smith, D.R.; Schultz, D.A.; Schultz, S. Localized surface plasmon resonance effects by naturally occurring Chinese yam particles. J. Chem. Phys. 2002, 116, 6755. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Xu, T.; Zheng, H.; Yu, M.; Li, G.; Xu, J.; Wu, J. Probing bianisotropic biomolecules via a surface plasmon resonance sensor. Opt. Express 2018, 26, 28277–28287. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.M.; Polez, V.L.P.; Sousa, M.H.; Silva, L.P. Modulating physical, chemical, and biological properties of silver nanoparticles obtained by green synthesis using different parts of the tree Handroanthus heptaphyllus (Vell.) Mattos. Colloids Interface Sci. Commun. 2020, 34, 100224. [Google Scholar] [CrossRef]
- Zhang, N.A.; Sun, J.; Yin, L.; Liu, J.; Chen, C. Silver nanoparticles: From in vitro green synthesis to in vivo biological effects in plants. Adv. Agrochem. 2023, 2, 313–323. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Rajkumari, K.; Biswas, A.; Adhikari, P.P.; Lalfakzuala, R.; Rokhum, L. Green synthesis of silver nanoparticles using Nostoc linckia and its antimicrobial activity: A novel biological approach. Bionanoscience 2018, 8, 624–631. [Google Scholar] [CrossRef]
- Lakshmi, P.; Kumar, G.A. Nanosuspension technology: A review. Int. J. Pharm. Pharm. Sci. 2010, 2, 35–40. [Google Scholar]
- ASTM. Standard Test Method for Oil and Grease and Petroleum Hydrocarbons in Water; American Society for Testing and Materials: West Conshohocken, PA, USA, 1985; pp. 3921–3985. [Google Scholar]
- Oliveira, G.Z.S.; Lopes, C.A.P.; Sousa, M.H.; Silva, L.P. Synthesis of silver nanoparticles using aqueous extracts of Pterodon emarginatus leaves collected in the summer and winter seasons. Int. Nano Lett. 2019, 9, 109–117. [Google Scholar] [CrossRef]
- Ahmad, B.; Chang, L.; Satti, U.Q.; Rehman, S.U.; Arshad, H.; Mustafa, G.; Shaukat, U.; Wang, F.; Tong, C. Phyto-synthesis, characterization, and in vitro antibacterial activity of silver nanoparticles using various plant extracts. Bioengineering 2022, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.K.O.; Vasconcelos, A.A.; Kobayashi, R.K.T.; Nakazato, G.; de Campos Braga, H.; Taube, P.S. Green synthesis: Characterization and biological activity of silver nanoparticles using aqueous extracts of plants from the Arecaceae family. Acta Sci. Technol. 2021, 43, e52011. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Kuskoski, E.M.; Rios, J.J.; Martín Bueno, J.; Fett, R.; Troncoso González, A.M.; García Asuero, A. Capillary Gas Chromatography-Mass Spectromety (CGC-MS) Analysis and Antioxidant Activities of Phenolic and Components of Guarana and Derivatives. Open Med. Chem. J. 2012, 6. [Google Scholar]
- Sarini, G.; Bopaiah, A.K. Phytochemical screening of the leaf and flower extracts of five Ipomoea species collected from in and around Bangalore. Int. J. Pharm. Bio Sci. 2016, 7, 71–73. [Google Scholar] [CrossRef]
- Shitan, N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016, 80, 1283–1293. [Google Scholar] [CrossRef]
- Gavhane, A.J.; Padmanabhan, P.; Kamble, S.P.; Jangle, S.N. Synthesis of silver nanoparticles using extract of Neem leaf and Triphala and evaluation of their antimicrobial activities. Int. J. Pharm. Bio Sci. 2012, 3, 88–100. [Google Scholar]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 2016, 62, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Prabula, S.S.; Hentry, C.; Rose, B.L.; Parvathiraja, C.; Mani, A.; Wabaidur, S.M.; Eldesoky, G.E.; Islam, M.A. Synthesis of silver nanoparticles by using Cassia auriculata flower extract and their photocatalytic behavior. Chem. Eng. Technol. 2022, 45, 1919–1925. [Google Scholar] [CrossRef]
- Shirzadi-Ahodashti, M.; Hashemi, Z.; Mortazavi, Y.; Khormali, K.; Mortazavi-Derazkola, S.; Ebrahimzadeh, M.A. Discovery of high antibacterial and catalytic activities against multi-drug resistant clinical bacteria and hazardous pollutants by biosynthesized of silver nanoparticles using Stachys inflata extract (AgNPs@ SI). Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126383. [Google Scholar] [CrossRef]
- Din, S.M.; Malek, N.A.N.N.; Shamsuddin, M.; Matmin, J.; Hadi, A.A.; Asraf, M.H. Antibacterial silver nanoparticles using different organs of Ficus deltoidea Jack var. kunstleri (King) Corner. Biocatal. Agric. Biotechnol. 2022, 44, 102473. [Google Scholar] [CrossRef]
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277–4290. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, S. Green synthesis of silver nanoparticles using extracts of Ananas comosus. Green Sustain. Chem. 2012, 2, 141–147. [Google Scholar] [CrossRef]
- Sadeghi, B.; Gholamhoseinpoor, F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 134, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile green synthesis of zinc oxide nanoparticles with potential synergistic activity with common antifungal agents against multidrug-resistant candidal strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- Rizwana, H.; Aljowaie, R.M.; Al Otibi, F.; Alwahibi, M.S.; Alharbi, S.A.; Al Asmari, S.A.; Aldosari, N.S.; Aldehaish, H.A. Antimicrobial and antioxidant potential of the silver nanoparticles synthesized using aqueous extracts of coconut meat (Cocos nucifera L.). Sci. Rep. 2023, 13, 16270. [Google Scholar] [CrossRef]
- Pathak, M.; Pathak, P.; Khalilullah, H.; Grishina, M.; Potemkin, V.; Kumar, V.; Majee, R.; Ramteke, P.W.; Abdellattif, M.H.; Shahbaaz, M.; et al. Green synthesis of silver nanoformulation of Scindapsus officinalis as potent anticancer and predicted anticovid alternative: Exploration via experimental and computational methods. Biocatal. Agric. Biotechnol. 2021, 35, 102072. [Google Scholar] [CrossRef]
- Hazman, Ö.; Khamidov, G.; Yilmaz, M.A.; Bozkurt, M.F.; Kargioğlu, M.; Savrik, M.; Tukhtaev, D.; Erol, I. Green synthesis of Ag nanoparticles from Verbascum insulare Boiss. and Heldr.: Evaluation of antimicrobial, anticancer, antioxidant properties and photocatalytic degradation of MB. J. Photochem. Photobiol. A 2024, 453, 115601. [Google Scholar] [CrossRef]
- Hijazi, B.U.; Faraj, M.; Mhanna, R.; El-Dakdouki, M.H. Biosynthesis of silver nanoparticles as a reliable alternative for the catalytic degradation of organic dyes and antibacterial applications. Curr. Res. Green Sustain. Chem. 2024, 8, 100408. [Google Scholar] [CrossRef]
- Katta, V.K.M.; Dubey, R.S. Green synthesis of silver nanoparticles using Tagetes erecta plant and investigation of their structural, optical, chemical and morphological properties. Mater. Today Proc. 2021, 45, 794–798. [Google Scholar] [CrossRef]
- Raguvaran, K.; Kalpana, M.; Ragavendran, C.; Manimegalai, T.; Kamaraj, C.; Maheswaran, R. Facile synthesis and surface characterization of silver metal nanoparticles using Acorus calamus and its applications. Inorg. Chem. Commun. 2024, 161, 112095. [Google Scholar] [CrossRef]
- Sabzevar, A.H.; Aflakian, F.; Hashemitabar, G. Characterization and evaluation of antibacterial, antioxidant and cytotoxic activities of green synthesized silver nanoparticles using Haloxylon persicum. J. Mol. Struct. 2024, 1304, 137615. [Google Scholar] [CrossRef]
- Shashiraj, K.N.; Nayaka, S.; Kumar, R.S.; Kantli, G.B.; Basavarajappa, D.S.; Gunagambhire, P.V.; Almansour, A.I.; Perumal, K. Rotheca serrata flower bud extract mediated bio-friendly preparation of silver nanoparticles: Their characterizations, anticancer, and apoptosis inducing ability against pancreatic ductal adenocarcinoma cell line. Processes 2023, 11, 893. [Google Scholar] [CrossRef]
- Suriyakala, G.; Sathiyaraj, S.; Devanesan, S.; AlSalhi, M.S.; Rajasekar, A.; Maruthamuthu, M.K.; Babujanarthanam, R. Phytosynthesis of silver nanoparticles from Jatropha integerrima Jacq. flower extract and their possible applications as antibacterial and antioxidant agent. Saudi J. Biol. Sci. 2022, 29, 680–688. [Google Scholar] [CrossRef]
- Hashemitabar, G.; Aflakian, F.; Sabzevar, A.H. Assessment of antibacterial, antioxidant, and anticancer effects of biosynthesized silver nanoparticles using Teucrium polium extract. J. Mol. Struct. 2023, 1291, 136076. [Google Scholar] [CrossRef]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Green synthesis and characterization of gold and silver nanoparticles using Mussaenda glabrata leaf extract and their environmental applications to dye degradation. Environ. Sci. Pollut. Res. 2017, 24, 17347–17357. [Google Scholar] [CrossRef]
- Jan, H.; Zaman, G.; Usman, H.; Ansir, R.; Drouet, S.; Gigliolo-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Biogenically proficient synthesis and characterization of silver nanoparticles (Ag-NPs) employing aqueous extract of Aquilegia pubiflora along with their in vitro antimicrobial, anti-cancer and other biological applications. J Mater. Res. Technol. 2021, 15, 950–968. [Google Scholar] [CrossRef]
- Essghaier, B.; Toukabri, N.; Dridi, R.; Hannachi, H.; Limam, I.; Mottola, F.; Mokni, M.; Zid, M.F.; Rocoo, L.; Abdelkarim, M. First report of the biosynthesis and characterization of silver nanoparticles using Scabiosa atropurpurea subsp. maritima fruit extracts and their antioxidant, antimicrobial and cytotoxic properties. Nanomaterials 2022, 12, 1585. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef]
- Chauhan, N.; Tyagi, A.K.; Kumar, P.; Malik, A. Antibacterial potential of Jatropha curcas synthesized silver nanoparticles against food borne pathogens. Front. Microbiol. 2016, 7, 188541. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The antibacterial mechanism of silver nanoparticles and its application in dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Jian, Y.; Chen, X.; Ahmed, T.; Shang, Q.; Zhang, S.; Ma, Z.; Yin, Y. Toxicity and action mechanisms of silver nanoparticles against the mycotoxin-producing fungus Fusarium graminearum. J. Adv. Res. 2022, 38, 1–12. [Google Scholar] [CrossRef]
- Reis, G.F.; Thaler, J.; Lustosa, B.P.R.; Geraldo, M.R.; Silveira, E.S.; Meis, J.F.; Panagio, L.A.; Junio, A.G.B.; Vicente, V.A. Nanotechnology on agent identification, diseases diagnosis and therapeutic approaches. Biotechnol. Res. Innov. 2022, 5, e2021006. [Google Scholar] [CrossRef]
- Pietrzak, K.; Glińska, S.; Gapińska, M.; Ruman, T.; Nowak, A.; Aydin, E.; Gutarowska, B. Silver nanoparticles: A mechanism of action on moulds. Metallomics 2016, 8, 1294–1302. [Google Scholar] [CrossRef]
- Bocate, K.P.; Reis, G.F.; de Souza, P.C.; Junior, A.G.O.; Durán, N.; Nakazato, G.; Furlaneto, M.C.; De Almeida, R.S.; Panagio, L.A. Antifungal activity of silver nanoparticles and simvastatin against toxigenic species of Aspergillus. Int. J. Food Microbiol. 2019, 291, 79–86. [Google Scholar] [CrossRef]
- Netala, V.R.; Kotakadi, V.S.; Nagam, V.; Bobbu, P.; Ghosh, S.B.; Tartte, V. First report of biomimetic synthesis of silver nanoparticles using aqueous callus extract of Centella asiatica and their antimicrobial activity. Appl. Nanosci. 2015, 5, 801–807. [Google Scholar] [CrossRef]
- Quinteros, M.A.; Aristizábal, V.C.; Dalmasso, P.R.; Paraje, M.G.; Páez, P.L. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol. Vitr. 2016, 36, 216–223. [Google Scholar] [CrossRef]
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The potential of silver nanoparticles for antiviral and antibacterial applications: A mechanism of action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef]
- Shen, T.; Wang, Q.; Li, C.; Zhou, B.; Li, Y.; Liu, Y. Transcriptome sequencing analysis reveals silver nanoparticles antifungal molecular mechanism of the soil fungi Fusarium solani species complex. J. Hazard. Mater. 2020, 388, 122063. [Google Scholar] [CrossRef]
- Basile, A.; Ferrara, L.; Del Pezzo, M.; Mele, G.; Sorbo, S.; Bassi, P.; Montesano, D. Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J. Ethnopharmacol. 2005, 102, 32–36. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.D.A. Antioxidant and antimicrobial properties of ethanolic extracts of guarana, boldo, rosemary and cinnamon. Braz. J. Food Technol. 2017, 20, e2016024. [Google Scholar] [CrossRef]
- Lima, A.K.O.; Silveira, A.P.; Silva, R.C.; Machado, Y.A.A.; Araujo, A.R.; Araujo, S.S.M.; Vieira, I.R.S.; Araujo, J.L.; Santos, L.C.; Rodrigues, K.A.F.; et al. Phytosynthesis of silver nanoparticles using guarana (Paullinia cupana Kunth) leaf extract employing different routes: Characterization and investigation of in vitro bioactivities. Biomass Convers. Biorefin. 2024, 1–17. [Google Scholar] [CrossRef]
- Pires, J.; Torres, P.B.; Santos, D.Y.A.C.; Chow, F. Ensaio em Microplaca de Substâncias Redutoras pelo Método do Folin-Ciocalteu para Extratos de Algas; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2017; pp. 1–5. [Google Scholar]
- Pires, J.; Torres, P.B.; Santos, D.Y.A.C.; Chow, F. Ensaio em Microplaca do Potencial Antioxidante Através do Método de Sequestro do Radical Livre DPPH para Extratos de Algas; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2017; Volume 12, pp. 1–6. [Google Scholar]
- Torres, P.B.; Pires, J.S.; Santos, D.Y.A.C.; Chow, F. Ensaio do Potencial Antioxidante de Extratos de Algas Através do Sequestro do ABTS•+ em Microplaca; Instituto de Biociências, Universidade de São Paulo: São Paulo, Brazil, 2017; pp. 1–4. [Google Scholar]
- Ribeiro, T.D.C.; Sábio, R.M.; Luiz, M.T.; Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.A.; Planeta, C.S.; Chorilli, M. Curcumin-loaded mesoporous silica nanoparticles dispersed in thermo-responsive hydrogel as potential Alzheimer disease therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef]
- CLSI Standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; p. 112.
- CLSI Document M27-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2002.
- CLSI Document M38-A2; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.

| Ext-LD | |||||
|---|---|---|---|---|---|
| Peak | Retention Time (min) | m/z [M+H]+ | m/z [M+H]− | Molecular Formula | Compound |
| 3 | 1.000 | 176.0919 | – | C7H13NO4 | Calystegin B2 |
| 4 | 1.084 | 148.0976 | – | C6H13NO3 | Fagomine |
| 7 | 1.562 | 130.0862 | – | C6H11NO2 | Pipecolic acid |
| 7 | 1.243 | – | 191.0566 | C7H12O6 | Quinic acid |
| 9 | 8.332 | 181.0725 | – | C7H8N4O2 | Theobromine |
| 11 | 14.262 | – | 449.1110 | C21H22O11 | Astilbin |
| 12 | 14.933 | – | 447.0950 | C21H20O11 | Quercitrin |
| 14 | 15.752 | – | 431.0997 | C21H20O10 | Afzelin |
| Ext-FL | |||||
| 4 | 1.030 | 176.0918 | – | C7H13NO4 | Calystegin B2 |
| 4 | 1.218 | – | 191.0567 | C7H12O6 | Quinic acid |
| 7 | 1.261 | 138.0546 | – | C7H7NO2 | Trigonelin |
| 8 | 11.490 | – | 289.0722 | C15H14O6 | Mikanolide |
| 13 | 8.138 | 181.0724 | – | C7H8N4O2 | Theobromine |
| 13 | 14.823 | – | 447.0945 | C21H20O11 | Quercitrin |
| 15 | 10.535 | 195.0880 | – | C8H10N4O2 | Caffeine |
| 17 | 11.786 | 291.0867 | – | C15H14O6 | Luteoforol |
| 19 | 12.098 | 865.2003 | – | C45H36O18 | Cinnamtannin D1 |
| 25 | 14.609 | 551.1049 | – | C24H22O15 | Quercetin 3-O-malonylglucoside |
| Ext-LR | |||||
| 3 | 0.979 | – | 174.0768 | C7H13NO4 | Calystegin B2 |
| 6 | 0.953 | 176.0918 | – | C7H13NO4 | Calystegin B2 |
| 7 | 1.124 | 148.0973 | – | C6H13NO3 | Fagomine |
| 9 | 9.510 | – | 225.0760 | C11H14O5 | Genipin |
| 11 | 10.559 | – | 577.1339 | – | Procyanidin |
| 12 | 11.134 | – | 289.0705 | C15H14O6 | Mikanolide |
| Extract | TPC | DPPH | ABTS |
|---|---|---|---|
| Ext-LD | 437.5 ± 0.093 | 40.57 ± 0.038 | 19.24 ± 0.003 |
| Ext-FL | 646.8 ± 0.165 | 40.37 ± 0.008 | 19.26 ± 0.002 |
| Ext-LR | 728.4 ± 0.087 | 41.72 ± 0.023 | 19.24 ± 0.002 |
| Time/Storage | AgNPs-LD | ||
|---|---|---|---|
| HD (nm) | PdI | ZP (mV) | |
| D0 | 79.29 ± 17.40 | 0.303 ± 0.057 | −28.7 ± 0.80 |
| D1—RT | 69.70 ± 9.10 | 0.262 ± 0.065 | −30.8 ± 2.20 |
| D1—REF | 79.78 ± 15.40 | 0.285 ± 0.089 | −35.2 ± 1.20 |
| D7—RT | 61.98 ± 8.04 | 0.358 ± 0.091 | −18.4 ± 5.35 × |
| D7—REF | 66.61 ± 1.67 | 0.406 ± 0.022 | −18.4 ± 2.26 × |
| D30—RT | 77.29 ± 2.64 | 0.441 ± 0.028 | −39.1 ± 0.32 × |
| D30—REF | 74.28 ± 4.24 | 0.479 ± 0.062 | −36.6 ± 1.14 × |
| Time/Storage | AgNPs-FL | ||
| HD (nm) | PdI | ZP (mV) | |
| D0 | 78.87 ± 4.10 | 0.311 ± 0.075 | −33.8 ± 0.50 |
| D1—RT | 70.74 ± 2.70 | 0.324 ± 0.069 | −26.6 ± 1.90 * |
| D1—REF | 73.85 ± 4.40 | 0.310 ± 0.078 | −36.0 ± 2.40 |
| D7—RT | 63.66 ± 1.46 * | 0.280 ± 0.012 | −39.7 ± 3.72 |
| D7—REF | 68.54 ± 1.74 * | 0.335 ± 0.039 | −37.9 ± 1.40 |
| D30—RT | 66.60 ± 0.97 * | 0.277 ± 0.008 | − 39.4 ± 1.42 |
| D30—REF | 70.25 ± 5.03 | 0.392 ± 0.091 | −38.2 ± 2.73 |
| Time/Storage | AgNPs-LR | ||
| HD (nm) | PdI | ZP (mV) | |
| D0 | 86.76 ± 9.90 | 0.417 ± 0.036 | −38.5 ± 1.40 |
| D1—RT | 78.63 ± 6.00 | 0.347 ± 0.028 | −34.5 ± 0.40 |
| D1—REF | 85.20 ± 10.20 | 0.311 ± 0.032 ϕ | −35.2 ± 0.30 |
| D7—RT | 71.69 ± 2.89 | 0.322 ± 0.041 ϕ | −36.7 ± 3.11 |
| D7—REF | 77.55 ± 0.99 | 0.374 ± 0.017 | −37.8 ± 0.89 |
| D30—RT | 101.6 ± 19.07 | 0.299 ± 0.044 ϕ | −36.0 ± 1.06 |
| D30—REF | 81.52 ± 1.99 | 0.350 ± 0.025 | −16.8 ± 2.34 ϕ |
| Samples | Diameter (nm) | Concentration (Particles/mL) |
|---|---|---|
| AgNPs-LD | 68.9 ± 0.7 | 1.56 × 108 |
| AgNPs-FL | 61.4 ± 1.0 | 1.68 × 1011 |
| AgNPs-LR | 78.4 ± 2.6 | 1.43 × 1010 |
| Samples | Microorganisms | |||||
|---|---|---|---|---|---|---|
| A. fumigatus | C. albicans | E. coli | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| AgNPs-LD | 2.12 | – | 2.12 | – | 2.65 | 2.65 |
| AgNPs-FL | 2.12 | – | 4.25 | – | 5.3 | 5.3 |
| AgNPs-LR | 2.6 | – | 5.3 | – | 10.6 | 10.6 |
| AgNO3 | 1.3 | – | 1.3 | – | 10.6 | 21.25 |
| F. oxysporum | P. chrysogenum | S. aureus | ||||
| MIC | MBC | MIC | MBC | MIC | MBC | |
| AgNPs-LD | 4.25 | – | 2.12 | – | 10.6 | 21.25 |
| AgNPs-FL | 4.25 | – | 2.12 | – | 10.6 | 21.25 |
| AgNPs-LR | 21.25 | – | 2.6 | – | 10.6 | 10.6 |
| AgNO3 | 1.3 | – | 2.6 | – | 10.6 | 10.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, A.K.O.; Souza, L.M.d.S.; Reis, G.F.; Junior, A.G.T.; Araújo, V.H.S.; Santos, L.C.d.; Silva, V.R.P.d.; Chorilli, M.; Braga, H.d.C.; Tada, D.B.; et al. Synthesis of Silver Nanoparticles Using Extracts from Different Parts of the Paullinia cupana Kunth Plant: Characterization and In Vitro Antimicrobial Activity. Pharmaceuticals 2024, 17, 869. https://doi.org/10.3390/ph17070869
Lima AKO, Souza LMdS, Reis GF, Junior AGT, Araújo VHS, Santos LCd, Silva VRPd, Chorilli M, Braga HdC, Tada DB, et al. Synthesis of Silver Nanoparticles Using Extracts from Different Parts of the Paullinia cupana Kunth Plant: Characterization and In Vitro Antimicrobial Activity. Pharmaceuticals. 2024; 17(7):869. https://doi.org/10.3390/ph17070869
Chicago/Turabian StyleLima, Alan Kelbis Oliveira, Lucas Marcelino dos Santos Souza, Guilherme Fonseca Reis, Alberto Gomes Tavares Junior, Victor Hugo Sousa Araújo, Lucas Carvalho dos Santos, Vitória Regina Pereira da Silva, Marlus Chorilli, Hugo de Campos Braga, Dayane Batista Tada, and et al. 2024. "Synthesis of Silver Nanoparticles Using Extracts from Different Parts of the Paullinia cupana Kunth Plant: Characterization and In Vitro Antimicrobial Activity" Pharmaceuticals 17, no. 7: 869. https://doi.org/10.3390/ph17070869
APA StyleLima, A. K. O., Souza, L. M. d. S., Reis, G. F., Junior, A. G. T., Araújo, V. H. S., Santos, L. C. d., Silva, V. R. P. d., Chorilli, M., Braga, H. d. C., Tada, D. B., Ribeiro, J. A. d. A., Rodrigues, C. M., Nakazato, G., Muehlmann, L. A., & Garcia, M. P. (2024). Synthesis of Silver Nanoparticles Using Extracts from Different Parts of the Paullinia cupana Kunth Plant: Characterization and In Vitro Antimicrobial Activity. Pharmaceuticals, 17(7), 869. https://doi.org/10.3390/ph17070869









