Electrospun Gelatin Scaffolds with Incorporated Antibiotics for Skin Wound Healing
Abstract
1. Introduction
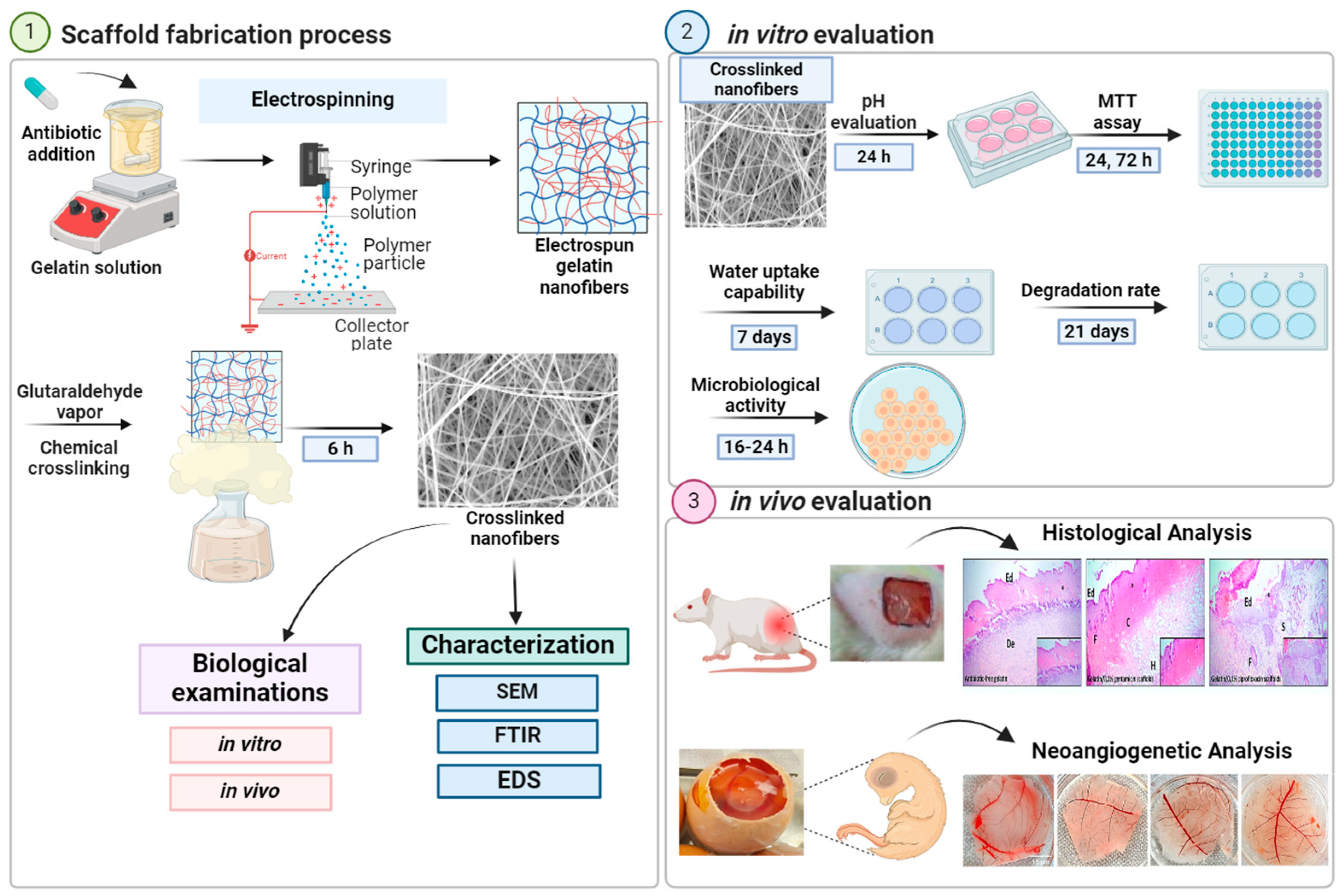
2. Results
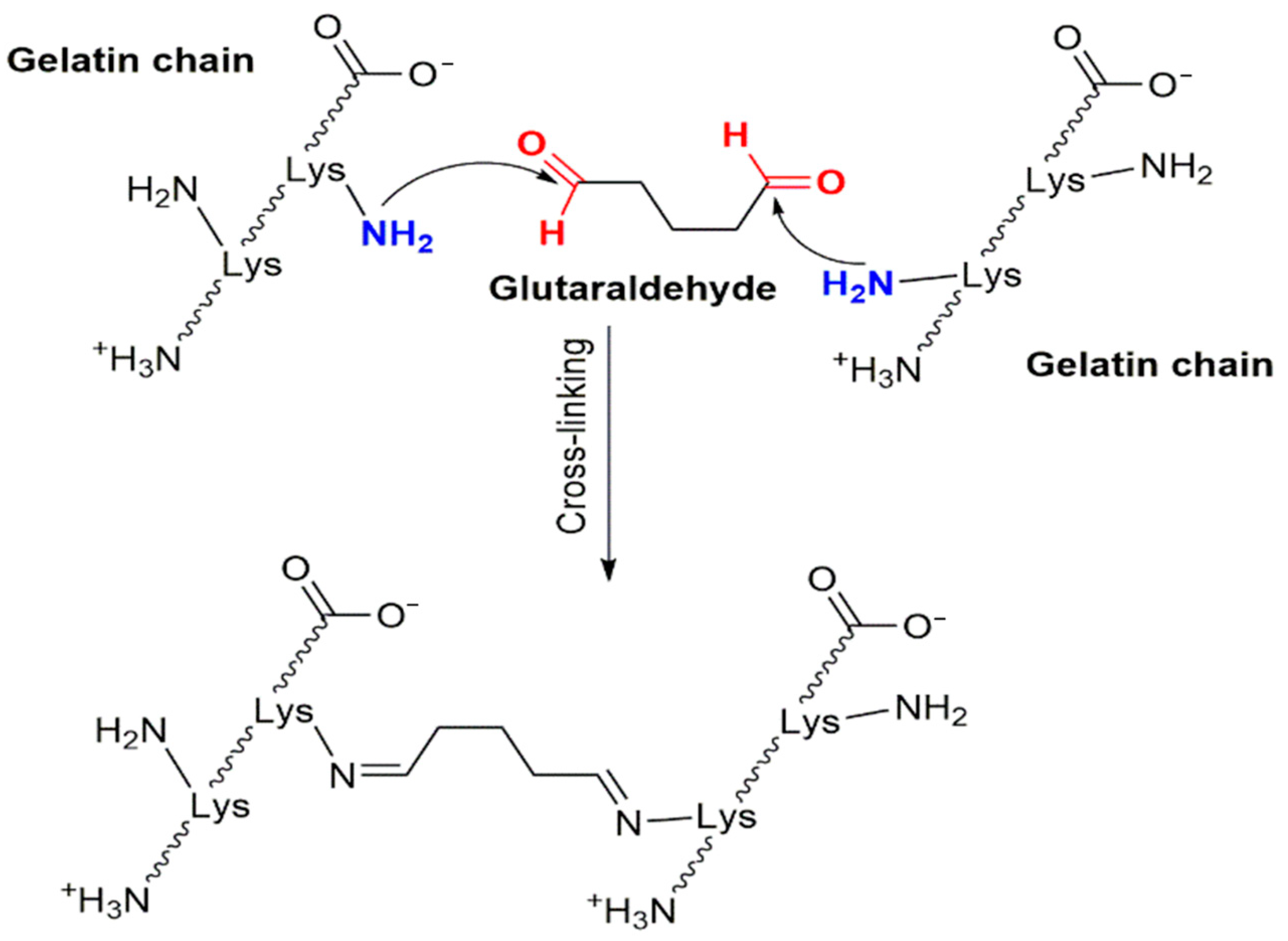
2.1. Crosslinking
2.2. The Effect of the Acidity Content and pH Value of Nanofibers in a Physiological Environment
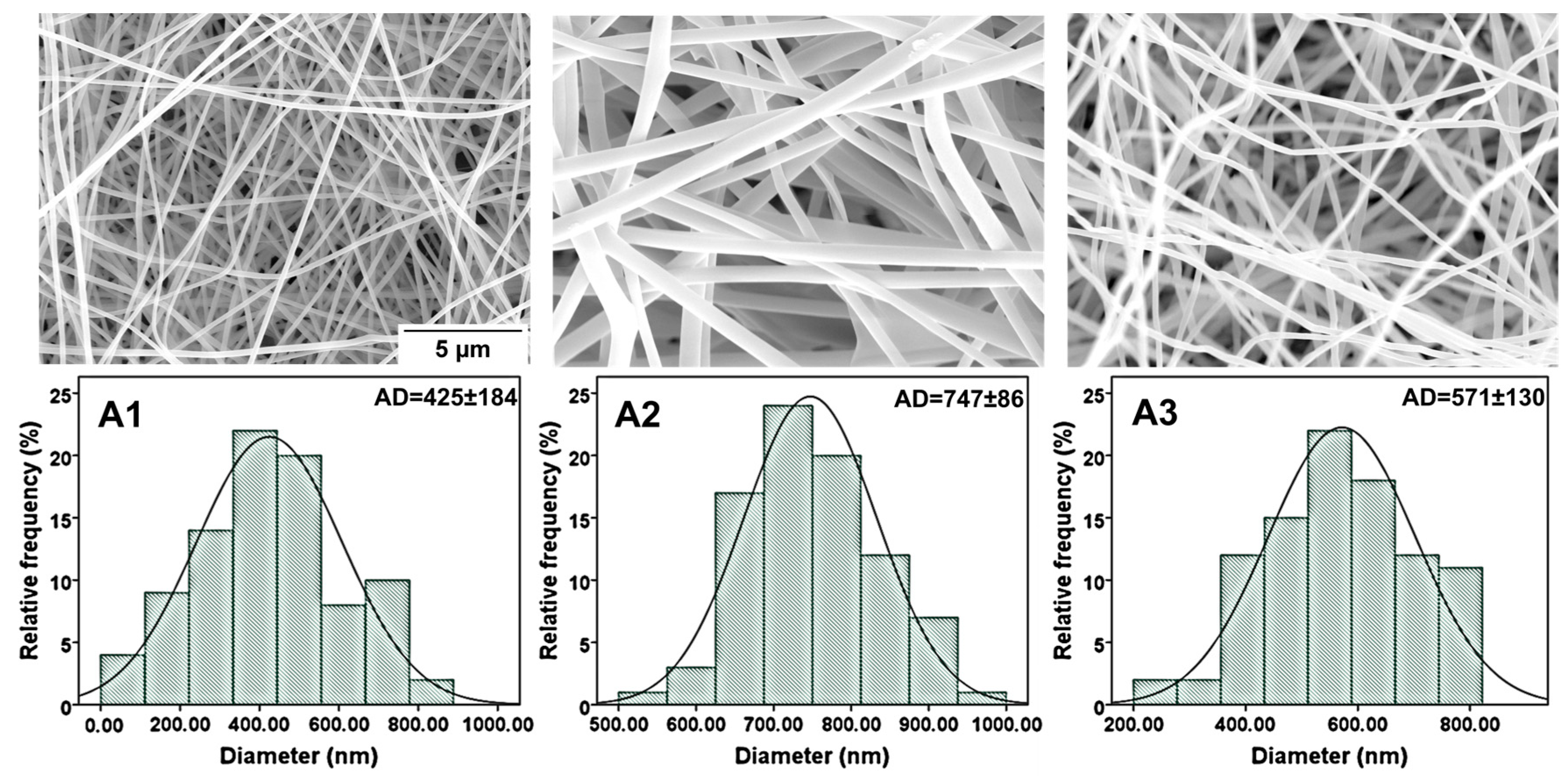
2.3. Morphology Analyses of Nanofibers
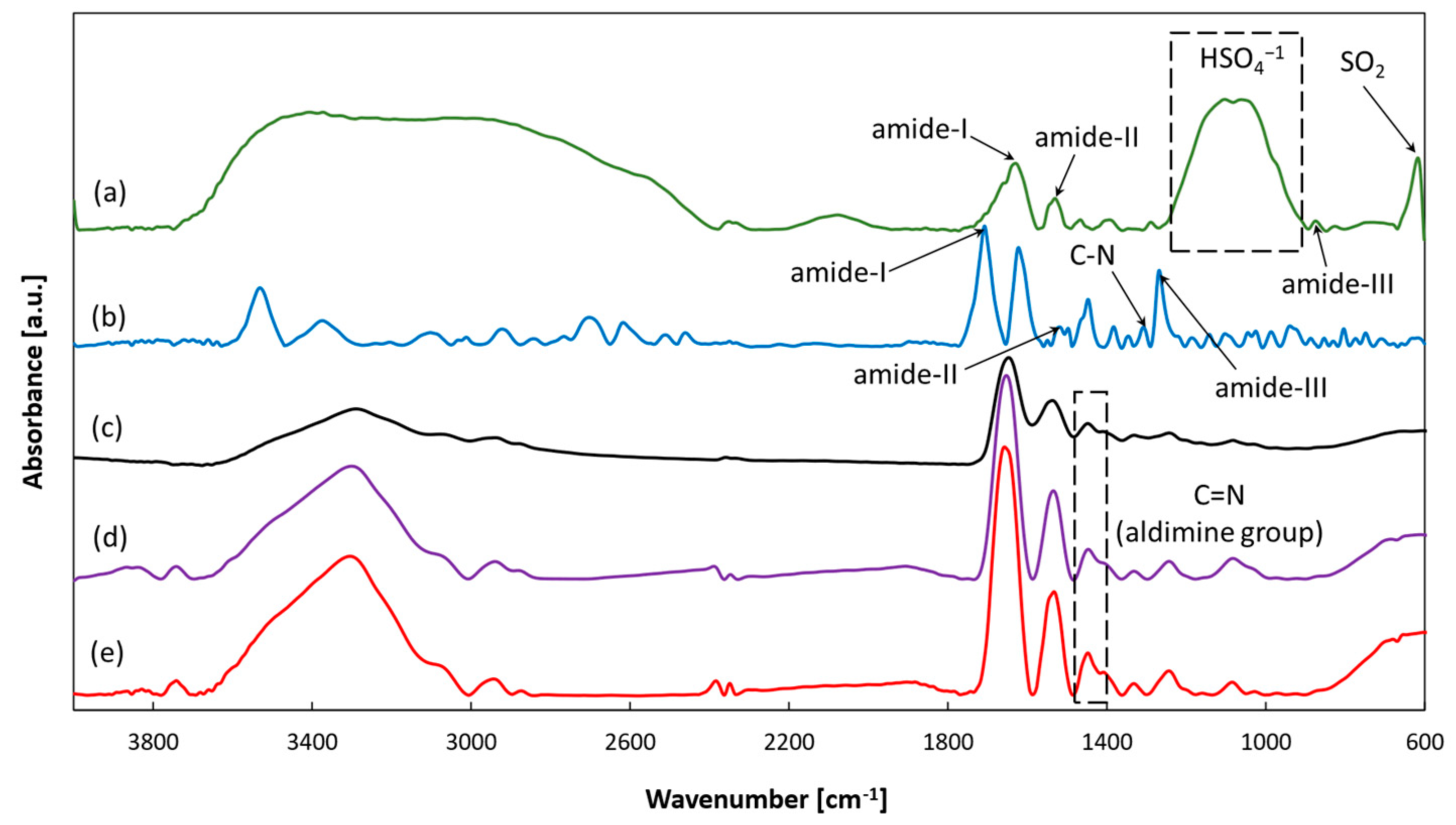
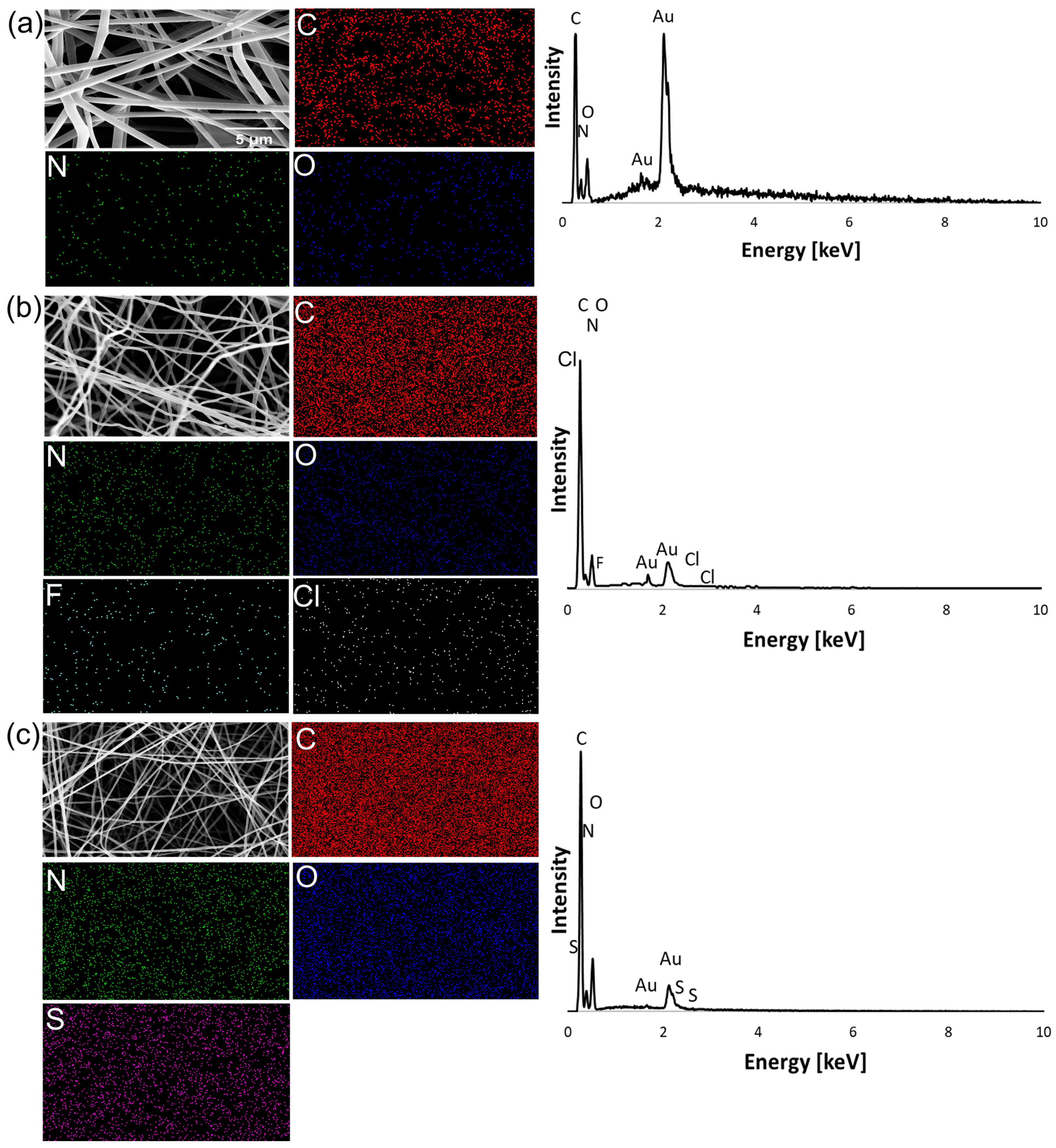
2.3.1. FTIR and EDS Analysis
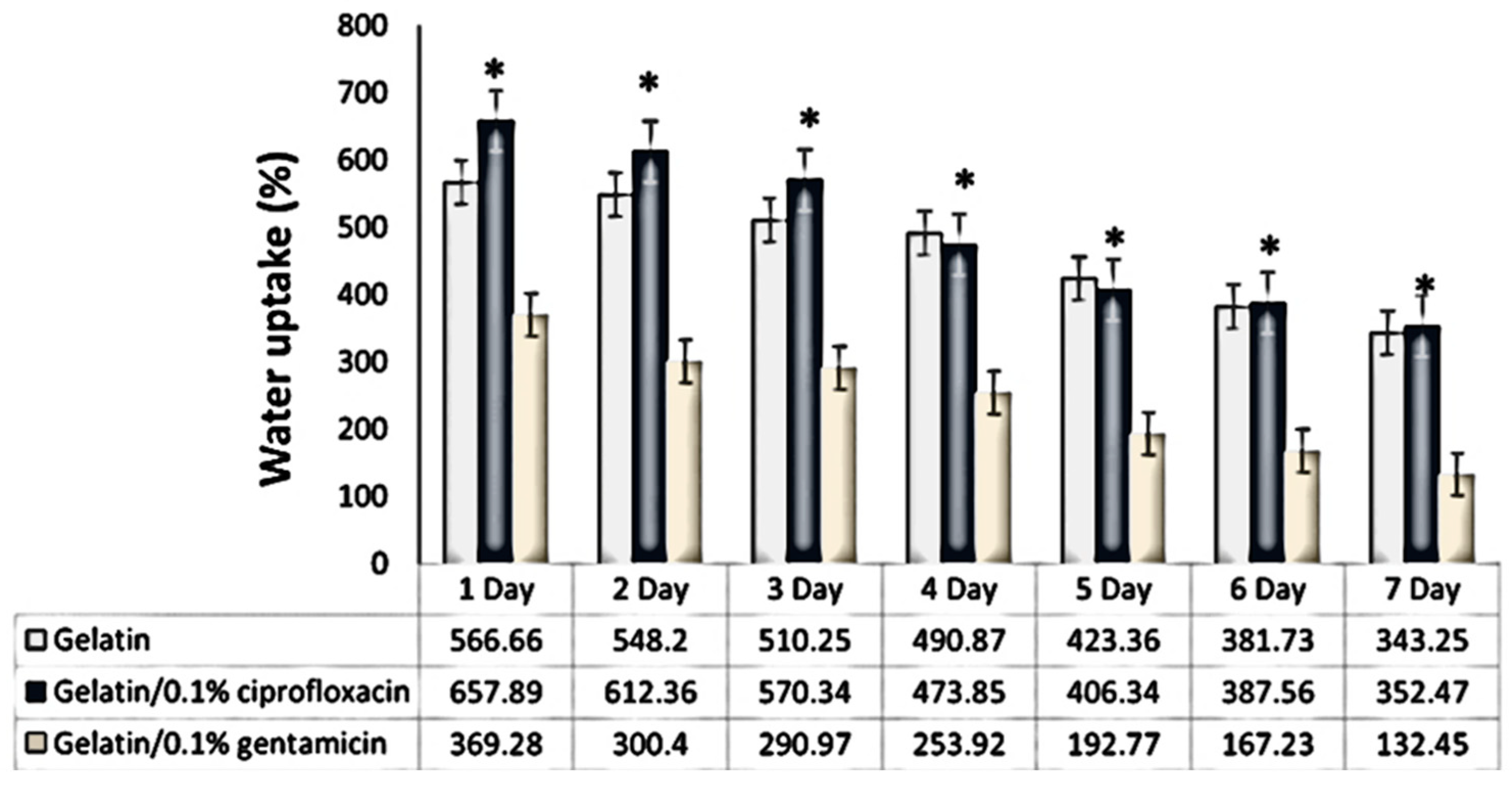
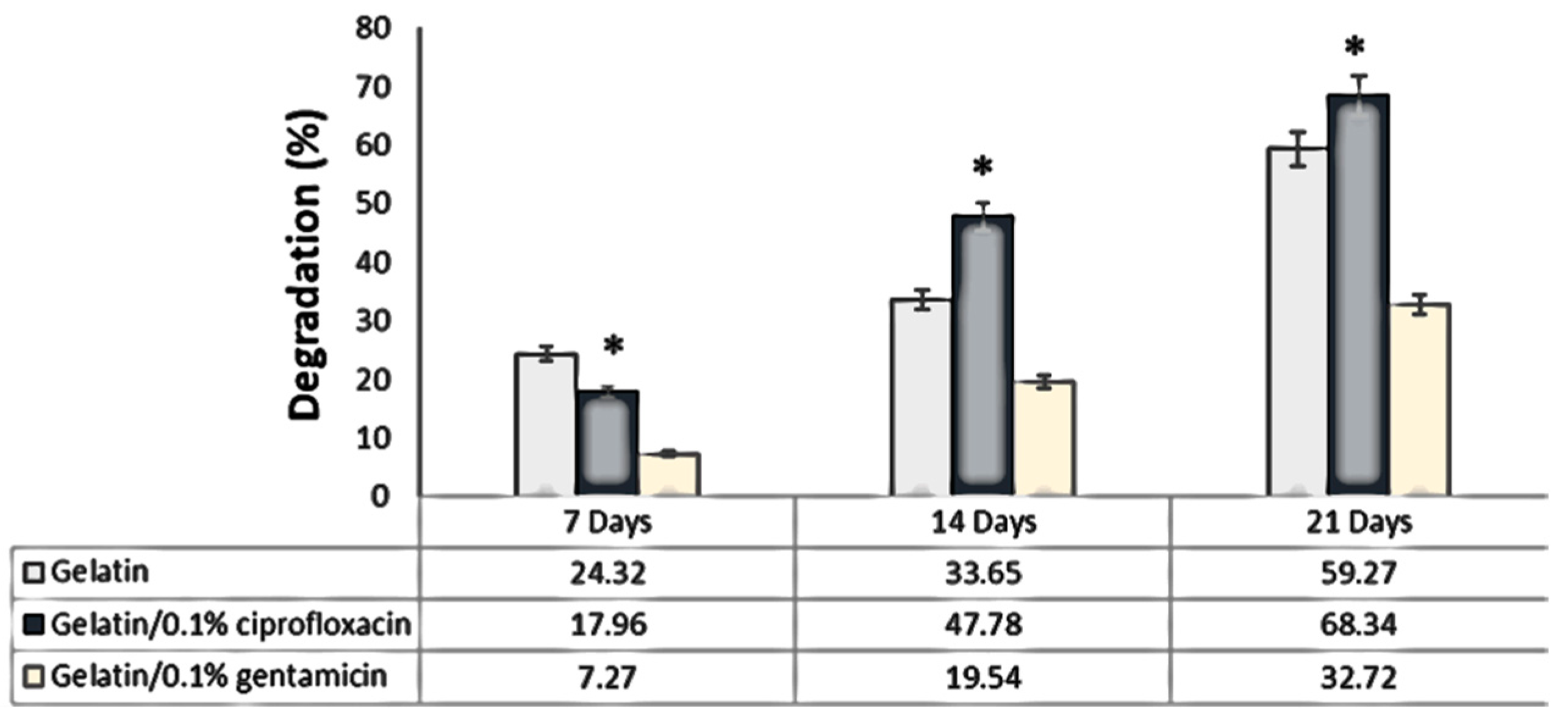
2.3.2. Test of Absorption and Degradation
Absorption
Degradation
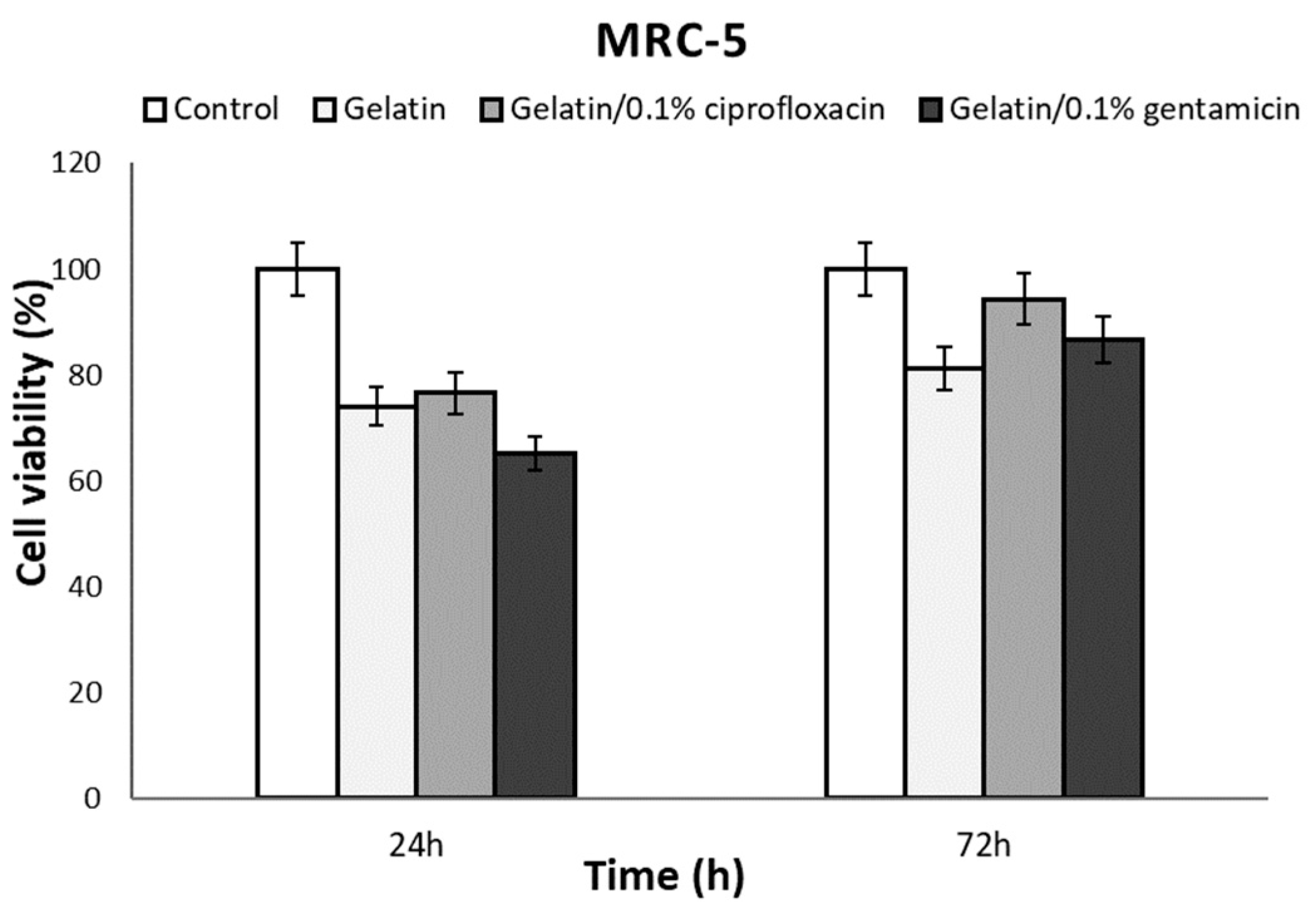
2.3.3. In Vitro Cytotoxicity Assay
2.3.4. Analysis of the Antibacterial Activity of Gelatin Material Impregnated with Antibiotics
2.3.5. In Vivo Application
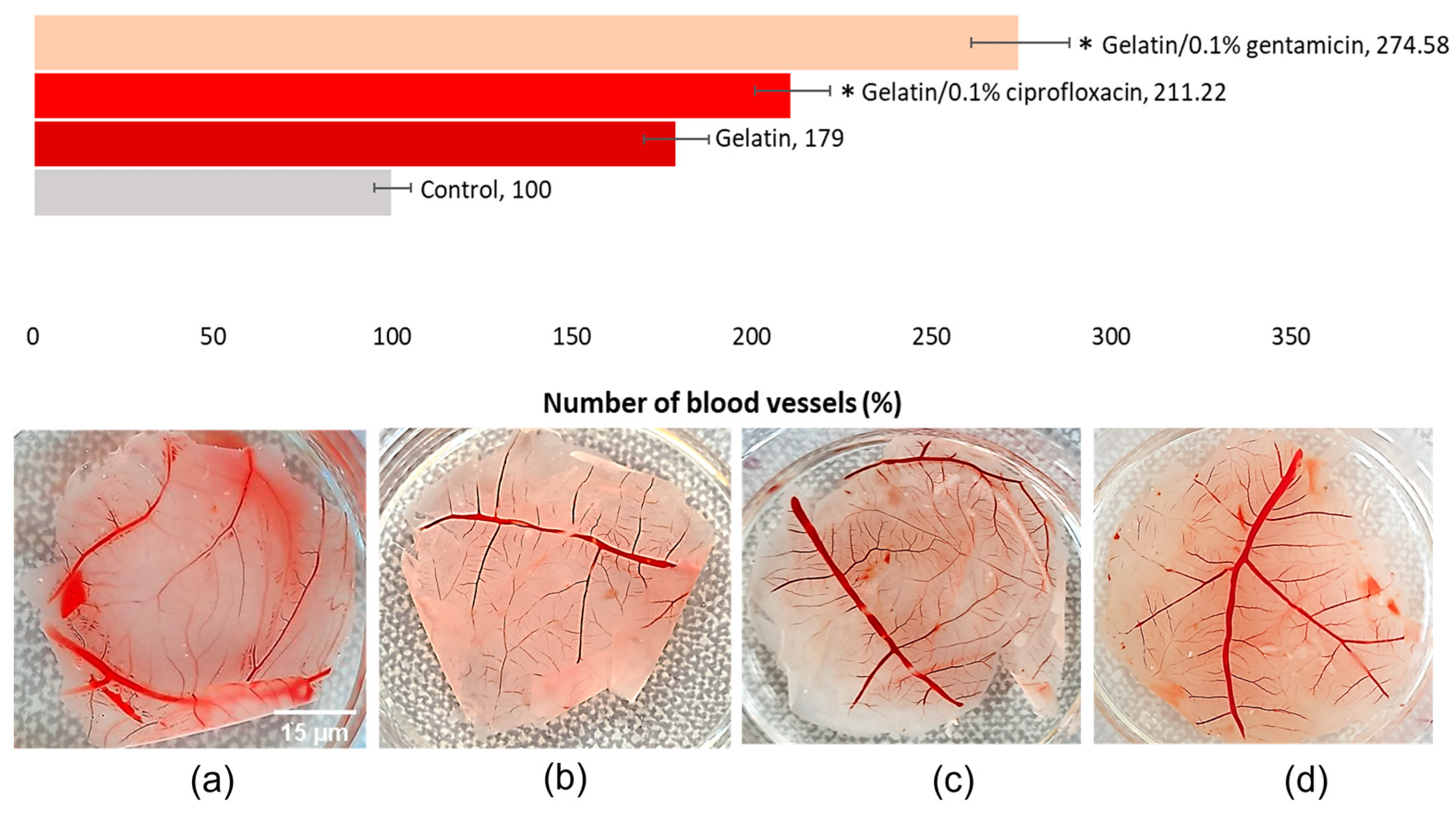
Neoangiogenesis Evaluation—CAM Assay Approach
2.3.6. In Vivo Wound Healing
3. Discussion
4. Experimental
4.1. Materials and Methods
4.2. Fabrication of Gelatin Nanofibers
4.3. Crosslinking of Nanofibers
4.3.1. pH Evaluation
4.3.2. Characterization and Morphology of Nanofibrous Mats
4.3.3. SEM and EDS Analysis
4.3.4. FTIR Spectroscopy
4.3.5. Cytotoxicity Assay
4.3.6. Test of Absorption and Degradation
4.3.7. Antibacterial Activity
Preparation of Bacterial Suspensions and Tested Materials
Disk-Diffusion Susceptibility Test
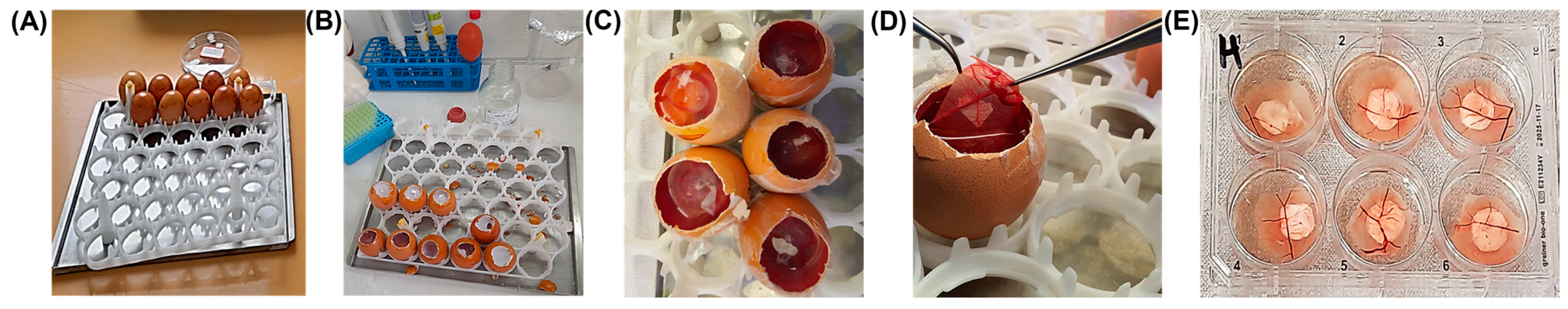
4.3.8. Chick Embryo Chorioallantoic Membrane (CAM) Assay
4.3.9. In Vivo Wound Healing
- Negative control—healthy skin from the dorsal middle back of rats;
- Positive control group—rats were subjected to burn wounds without any further treatment, serving as the control for the experiment;
- Antibiotic-free gelatin—burn wounds on rats were treated with antibiotic-free gelatin;
- Gelatin/0.1% ciprofloxacin scaffolds—burn wounds on rats were treated with scaffolds containing gelatin/0.1% ciprofloxacin;
- Gelatin/0.1% gentamicin scaffolds—burn wounds on rats were treated with scaffolds containing gelatin/0.1% gentamicin.
Experimental Model of Burn Wounds
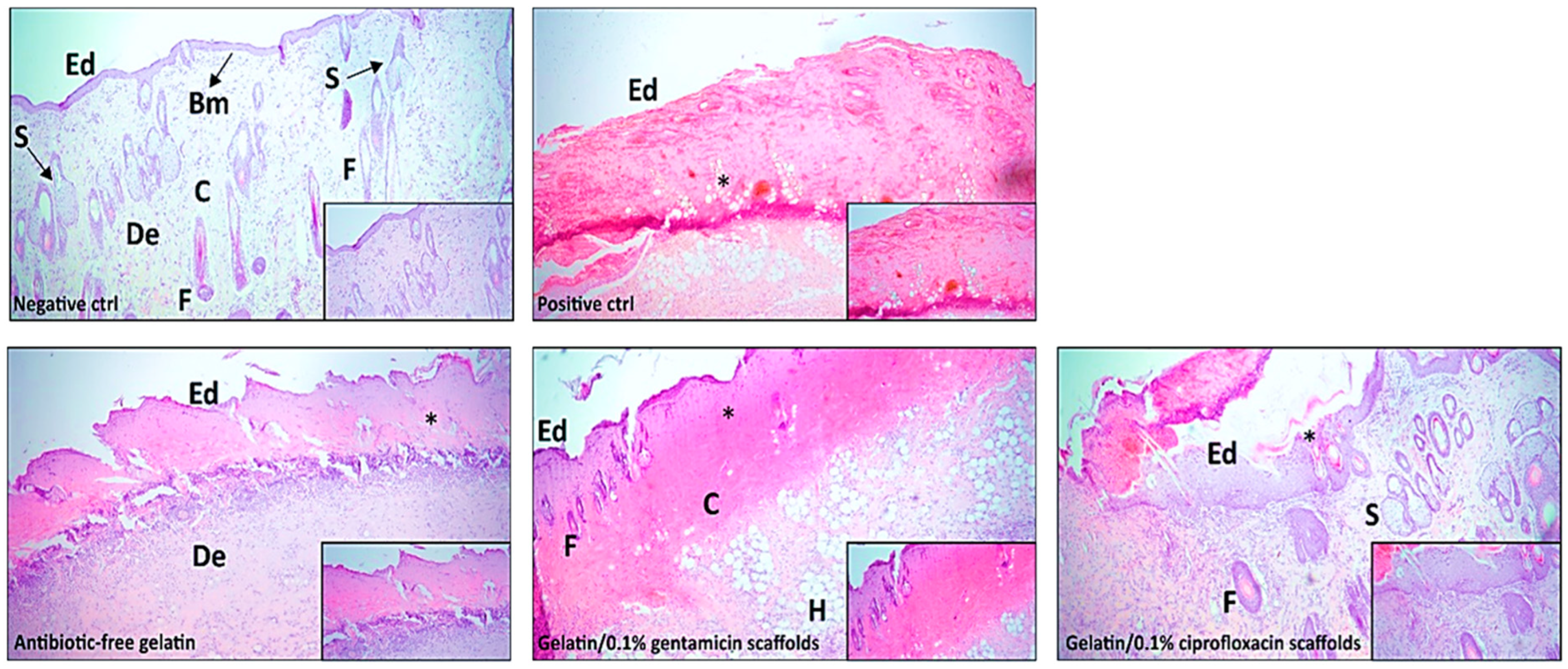
Histopathological Analysis
4.3.10. Ethics Approval
4.3.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Advancement of Wound Care from Grafts to Bioengineered Smart Skin Substitutes. Prog. Biomater. 2014, 3, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, B.A.; Krishnaswamy, M.; Xu, H.; Hoque, M.E. Electrospinning of Biomedical Nanofibers/Nanomembranes: Effects of Process Parameters. Polymers 2022, 14, 3719. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic Natural Biomaterials for Tissue Engineering and Regenerative Medicine: New Biosynthesis Methods, Recent Advances, and Emerging Applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Wu, S.; Chen, S.; Ma, J.; Zhou, F. Electrospun Chitosan Nanofibers for Wound Healing Application. Eng. Regen. 2021, 2, 82–90. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and Their Applications in Tissue Engineering. Int. J. Nanomed. 2006, 1, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.; Phan, T.; Lim, I.; Zhang, Y.; Bay, B.; Ramakrishna, S.; Lim, C. Evaluation of Electrospun PCL/Gelatin Nanofibrous Scaffold for Wound Healing and Layered Dermal Reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Sabzi, M.; Pirmoradian, A.; Behshad, Y.; Sharifi, F.; Alipour, S.; Jiang, L. Development of Antibiotic Releasing Electrospun Nanofibrous Mats Based on Gelatin. Mater Chem Horiz. 2023, 2, 185–193. [Google Scholar] [CrossRef]
- Mozaffari, A.; Parvinzadeh, M. Effect of Voltage and Distance in Electrospinning of Gelatin. Iran. J. Text. Nano-Bio Modif. 2022, 1, 32–38. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transla Med. 2019, 4, 96–115. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on Developments in Synthetic, Analytical, and Medicinal Aspects. J. Enzym. Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lu, L.; Liang, Y.; Cheng, B. Fabrication of Centrifugally Spun Prepared Poly(Lactic Acid)/Gelatin/Ciprofloxacin Nanofibers for Antimicrobial Wound Dressing. RSC Adv. 2019, 9, 35328–35335. [Google Scholar] [CrossRef] [PubMed]
- Thy, M.; Timsit, J.-F.; De Montmollin, E. Aminoglycosides for the Treatment of Severe Infection Due to Resistant Gram-Negative Pathogens. Antibiotics 2023, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- López-Calderón, H.D.; Avilés-Arnaut, H.; Galán-Wong, L.J.; Almaguer-Cantú, V.; Laguna-Camacho, J.R.; Calderón-Ramón, C.; Escalante-Martínez, J.E.; Arévalo-Niño, K. Electrospun Polyvinylpyrrolidone-Gelatin and Cellulose Acetate Bi-Layer Scaffold Loaded with Gentamicin as Possible Wound Dressing. Polymers 2020, 12, 2311. [Google Scholar] [CrossRef] [PubMed]
- Ehrmann, A. Non-Toxic Crosslinking of Electrospun Gelatin Nanofibers for Tissue Engineering and Biomedicine—A Review. Polymers 2021, 13, 1973. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ma, M.; Xu, H.; Zhang, B.; Cao, X.; Guo, Y. Gelatin Nanofibers Prepared by Spiral-Electrospinning and Cross-Linked by Vapor and Liquid-Phase Glutaraldehyde. Mater. Lett. 2015, 140, 1–4. [Google Scholar] [CrossRef]
- Chen, H.; Jao, W.; Yang, M. Characterization of Gelatin Nanofibers Electrospun Using Ethanol/Formic Acid/Water as a Solvent. Polym. Adv. Techs 2009, 20, 98–103. [Google Scholar] [CrossRef]
- Pal, A.; Kumar Bajpai, A.; Bajpai, J. Study on Facile Designing, Swelling Properties and Structural Relationship of Gelatin Nanoparticles. J. Macromol. Sci. Part A 2019, 56, 206–214. [Google Scholar] [CrossRef]
- Lin, J.; Pan, D.; Sun, Y.; Ou, C.; Wang, Y.; Cao, J. The Modification of Gelatin Films: Based on Various Cross-linking Mechanism of Glutaraldehyde at Acidic and Alkaline Conditions. Food Sci. Nutr. 2019, 7, 4140–4146. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, H.; Pandey, A.; Ramteke, P. Fabrication and Assessment of Gentamicin Loaded Electrospun Nanofibrous Scaffolds as a Quick Wound Healing Dressing Material. Curr. Nanosci. 2015, 11, 222–228. [Google Scholar] [CrossRef]
- Silva, D.M.; Vyas, H.K.N.; Sanderson-Smith, M.L.; Sencadas, V. Development and Optimization of Ciprofloxacin-Loaded Gelatin Microparticles by Single-Step Spray-Drying Technique. Powder Technol. 2018, 330, 201–209. [Google Scholar] [CrossRef]
- Lin, M.; Liu, Y.; Gao, J.; Wang, D.; Xia, D.; Liang, C.; Li, N.; Xu, R. Synergistic Effect of Co-Delivering Ciprofloxacin and Tetracycline Hydrochloride for Promoted Wound Healing by Utilizing Coaxial PCL/Gelatin Nanofiber Membrane. Int. J. Mol. Sci. 2022, 23, 1895. [Google Scholar] [CrossRef] [PubMed]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and Thermal Properties of Gelatin Films at Different Degrees of Glutaraldehyde Crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef] [PubMed]
- EUCAST—European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. Available online: https://www.eucast.org (accessed on 8 February 2024.).
- Salimi, F.; Mohammadipanah, F. Nanomaterials Versus The Microbial Compounds With Wound Healing Property. Front. Nanotechnol. 2021, 2, 584489. [Google Scholar] [CrossRef]
- Anjum, S.; Rahman, F.; Pandey, P.; Arya, D.K.; Alam, M.; Rajinikanth, P.S.; Ao, Q. Electrospun Biomimetic Nanofibrous Scaffolds: A Promising Prospect for Bone Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 9206. [Google Scholar] [CrossRef]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The Use of Natural Polymers in Tissue Engineering: A Focus on Electrospun Extracellular Matrix Analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Lazaridou, M.; Bikiaris, D.N.; Lamprou, D.A. 3D Bioprinted Chitosan-Based Hydrogel Scaffolds in Tissue Engineering and Localised Drug Delivery. Pharmaceutics 2022, 14, 1978. [Google Scholar] [CrossRef]
- Lazaridou, M.; Moroni, S.; Klonos, P.; Kyritsis, A.; Bikiaris, D.N.; Lamprou, D.A. 3D-Printed Hydrogels Based on Amphiphilic Chitosan Derivative Loaded with Levofloxacin for Wound Healing Applications. Int. J. Polym. Mater. Polym. Biomater. 2024, 1–18. [Google Scholar] [CrossRef]
- Silva, R.S.G.; Bandeira, S.F.; Pinto, L.A.A. Characteristics and Chemical Composition of Skins Gelatin from Cobia (Rachycentron Canadum). LWT—Food Sci. Technol. 2014, 57, 580–585. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L. Cross-Linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef]
- Chen, W.; Gao, Z.; He, M.; Dou, Y.; Yin, G.; Ding, J. Vapor-Phase Glutaraldehyde Crosslinked Waste Protein-Based Nanofiber Nonwovens as an Environmentally Friendly Wound Dressing. React. Funct. Polym. 2022, 172, 105203. [Google Scholar] [CrossRef]
- Banafati Zadeh, F.; Zamanian, A. Glutaraldehyde: Introducing Optimum Condition for Cross-Linking the Chitosan/Gelatin Scaffolds for Bone Tissue Engineering. Int. J. Eng. 2022, 35, 1967–1980. [Google Scholar] [CrossRef]
- Aoki, H.; Miyoshi, H.; Yamagata, Y. Electrospinning of Gelatin Nanofiber Scaffolds with Mild Neutral Cosolvents for Use in Tissue Engineering. Polym. J. 2015, 47, 267–277. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning Based on Benign Solvents: Current Definitions, Implications and Strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Chi, H.Y.; Chang, N.Y.; Li, C.; Chan, V.; Hsieh, J.H.; Tsai, Y.-H.; Lin, T. Fabrication of Gelatin Nanofibers by Electrospinning—Mixture of Gelatin and Polyvinyl Alcohol. Polymers 2022, 14, 2610. [Google Scholar] [CrossRef] [PubMed]
- Abdulkadhim, M.K.; Habeeb, S.A. Electro Spun Uniform Nanofiber from Gelatin: Chitosan at Low Concentration. Mater. Today Proc. 2023, 87, 299–306. [Google Scholar] [CrossRef]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanás, J.; Carrillo, F. Electrospinning of Gelatin Fibers Using Solutions with Low Acetic Acid Concentration: Effect of Solvent Composition on Both Diameter of Electrospun Fibers and Cytotoxicity. J. Appl. Polym. Sci. 2015, 132, app.42115. [Google Scholar] [CrossRef]
- Binulal, N.S.; Natarajan, A.; Menon, D.; Bhaskaran, V.K.; Mony, U.; Nair, S.V. PCL–Gelatin Composite Nanofibers Electrospun Using Diluted Acetic Acid–Ethyl Acetate Solvent System for Stem Cell-Based Bone Tissue Engineering. J. Biomater. Sci. Polym. Ed. 2014, 25, 325–340. [Google Scholar] [CrossRef]
- Jalaja, K.; James, N.R. Electrospun Gelatin Nanofibers: A Facile Cross-Linking Approach Using Oxidized Sucrose. Int. J. Biol. Macromol. 2015, 73, 270–278. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Ponzini, S.; De Stefano, P.; Ortoleva, G.; Vignati, L.; Draghi, L. Cross-Linking Optimization for Electrospun Gelatin: Challenge of Preserving Fiber Topography. Polymers 2020, 12, 2472. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Venugopal, J.; Huang, Z.-M.; Lim, C.T.; Ramakrishna, S. Crosslinking of the Electrospun Gelatin Nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Deng, L.; Taxipalati, M.; Zhang, A.; Que, F.; Wei, H.; Feng, F.; Zhang, H. Electrospun Chitosan/Poly(Ethylene Oxide)/Lauric Arginate Nanofibrous Film with Enhanced Antimicrobial Activity. J. Agric. Food Chem. 2018, 66, 6219–6226. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, G.; Bonde, G.V.; Mittal, P.; Khan, G.; Pandey, V.K.; Bakade, B.V.; Mishra, B. Biomimetic PCL-Gelatin Based Nanofibers Loaded with Ciprofloxacin Hydrochloride and Quercetin: A Potential Antibacterial and Anti-Oxidant Dressing Material for Accelerated Healing of a Full Thickness Wound. Int. J. Pharm. 2019, 567, 118480. [Google Scholar] [CrossRef] [PubMed]
- Cesur, S.; Ilhan, E.; Tut, T.A.; Kaya, E.; Dalbayrak, B.; Bosgelmez-Tinaz, G.; Arısan, E.D.; Gunduz, O.; Kijeńska-Gawrońska, E. Design of Cinnamaldehyde- and Gentamicin-Loaded Double-Layer Corneal Nanofiber Patches with Antibiofilm and Antimicrobial Effects. ACS Omega 2023, 8, 28109–28121. [Google Scholar] [CrossRef] [PubMed]
- Okutan, N.; Terzi, P.; Altay, F. Affecting Parameters on Electrospinning Process and Characterization of Electrospun Gelatin Nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Baykara, D.; Pilavci, E.; Cesur, S.; Ilhan, E.; Ulag, S.; Sengor, M.; Kijeńska-Gawrońska, E.; Gunduz, O. Controlled Release of Gentamicin from Electrospun Poly(Vinyl Alcohol)/Gelatin Nanofibers: The Effect of Crosslinking Time Using Glutaraldehyde Vapor. ChemistrySelect 2023, 8, e202203681. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Hu, F.; Rausch-Fan, X.; Steinberg, T.; Lan, Z.; Zhang, X. The Effect of Modifying the Nanostructure of Gelatin Fiber Scaffolds on Early Angiogenesis in Vitro and in Vivo. Biomed. Mater. 2022, 17, 015010. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, S.; Amiri, S.; Bahrami, S.H. PCL-Based Nanofibers Loaded with Ciprofloxacin/Cyclodextrin Containers. J. Text. Inst. 2018, 109, 1044–1053. [Google Scholar] [CrossRef]
- Cui, C.; Sun, S.; Li, X.; Chen, S.; Wu, S.; Zhou, F.; Ma, J. Optimizing the Chitosan-PCL Based Membranes with Random/Aligned Fiber Structure for Controlled Ciprofloxacin Delivery and Wound Healing. Int. J. Biol. Macromol. 2022, 205, 500–510. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Z.; Du, Y.; Kennedy, J.F. Controlled Release of Ciprofloxacin Hydrochloride from Chitosan/Polyethylene Glycol Blend Films. Carbohydr. Polym. 2007, 69, 336–343. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2021, 10, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Morsi, Y.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X. In Vitro Evaluation of Electrospun Gelatin–Glutaraldehyde Nanofibers. Front. Mater. Sci. 2016, 10, 90–100. [Google Scholar] [CrossRef]
- Liang, Y.; Liang, Y.; Zhang, H.; Guo, B. Antibacterial Biomaterials for Skin Wound Dressing. Asian J. Pharm. Sci. 2022, 17, 353–384. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A. Drug Delivery of Gelatin Nanoparticles as a Biodegradable Polymer for the Treatment of Infectious Diseases: Perspectives and Challenges. Polymers 2023, 15, 4327. [Google Scholar] [CrossRef] [PubMed]
- Thairin, T.; Wutticharoenmongkol, P. Ciprofloxacin-Loaded Alginate/Poly (Vinyl Alcohol)/Gelatin Electrospun Nanofiber Mats as Antibacterial Wound Dressings. J. Ind. Text. 2022, 51, 1296S–1322S. [Google Scholar] [CrossRef]
- Dreesmann, L.; Ahlers, M.; Schlosshauer, B. The Pro-Angiogenic Characteristics of a Cross-Linked Gelatin Matrix. Biomaterials 2007, 28, 5536–5543. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Hashemikia, S.; Farhangpazhouh, F.; Parsa, M.; Hasan, M.; Hassanzadeh, A.; Hamidi, M. Fabrication of Ciprofloxacin-Loaded Chitosan/Polyethylene Oxide/Silica Nanofibers for Wound Dressing Application: In Vitro and in Vivo Evaluations. Int. J. Pharm. 2021, 597, 120313. [Google Scholar] [CrossRef]
- Li, H.; Williams, G.R.; Wu, J.; Lv, Y.; Sun, X.; Wu, H.; Zhu, L.-M. Thermosensitive Nanofibers Loaded with Ciprofloxacin as Antibacterial Wound Dressing Materials. Int. J. Pharm. 2017, 517, 135–147. [Google Scholar] [CrossRef]
- Andrews, J.M. BSAC Standardized Disc Susceptibility Testing Method (Version 4). J. Antimicrob. Chemother. 2005, 56, 60–76. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Liang, Y.; Zhang, T.; Ma, P.X.; Guo, B. Antibacterial Adhesive Injectable Hydrogels with Rapid Self-Healing, Extensibility and Compressibility as Wound Dressing for Joints Skin Wound Healing. Biomater 2018, 183, 185–199. [Google Scholar] [CrossRef] [PubMed]
| Tested Bacteria/ Tested Material | Staphylococcus aureus ATCC 25923 | Pseudomonas aeruginosa ATCC 27853 |
|---|---|---|
| Antibiotic-free gelatin scaffold | 3.67 ± 0.47 * | 2.00 ± 1.41 |
| Gelatin/0.1% gentamicin scaffold | 21.67 ± 1.25 | 22.33 ± 1.70 |
| Filter paper/gentamicin 1 mg/mL | 20.00 ± 2.16 | 14.33 ± 3.40 |
| Gelatin/0.1% ciprofloxacin scaffold | 28.00 ± 3.74 | 18.33 ± 4.11 |
| Filter paper/ciprofloxacin 1 mg/mL | 27.33 ± 1.25 | 25.33 ± 0.47 |
| Solution Parameters | Electrospinning Conditions | ||||||
|---|---|---|---|---|---|---|---|
| Gelatin Concentration | AcA/DMSO (v/v%) | Voltage (kV) | Flow Rate (mL/h) | Nozzle Size (gauge) | Collector and Needle Distance (cm) | Temperature and Humidity Conditions | Average Diameter (nm) |
| 23% | 70% AcA: 9% DMSO (93:7) | 15 | 0.15 | 18 | 15 | 28 °C, 45% | 376 ± 122 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virijević, K.; Živanović, M.; Pavić, J.; Dragačević, L.; Ljujić, B.; Miletić Kovačević, M.; Papić, M.; Živanović, S.; Milenković, S.; Radojević, I.; et al. Electrospun Gelatin Scaffolds with Incorporated Antibiotics for Skin Wound Healing. Pharmaceuticals 2024, 17, 851. https://doi.org/10.3390/ph17070851
Virijević K, Živanović M, Pavić J, Dragačević L, Ljujić B, Miletić Kovačević M, Papić M, Živanović S, Milenković S, Radojević I, et al. Electrospun Gelatin Scaffolds with Incorporated Antibiotics for Skin Wound Healing. Pharmaceuticals. 2024; 17(7):851. https://doi.org/10.3390/ph17070851
Chicago/Turabian StyleVirijević, Katarina, Marko Živanović, Jelena Pavić, Luka Dragačević, Biljana Ljujić, Marina Miletić Kovačević, Miloš Papić, Suzana Živanović, Strahinja Milenković, Ivana Radojević, and et al. 2024. "Electrospun Gelatin Scaffolds with Incorporated Antibiotics for Skin Wound Healing" Pharmaceuticals 17, no. 7: 851. https://doi.org/10.3390/ph17070851
APA StyleVirijević, K., Živanović, M., Pavić, J., Dragačević, L., Ljujić, B., Miletić Kovačević, M., Papić, M., Živanović, S., Milenković, S., Radojević, I., & Filipović, N. (2024). Electrospun Gelatin Scaffolds with Incorporated Antibiotics for Skin Wound Healing. Pharmaceuticals, 17(7), 851. https://doi.org/10.3390/ph17070851









