Amoebicidal Effect of COVID Box Molecules against Acanthamoeba: A Study of Cell Death
Abstract
1. Introduction
2. Results
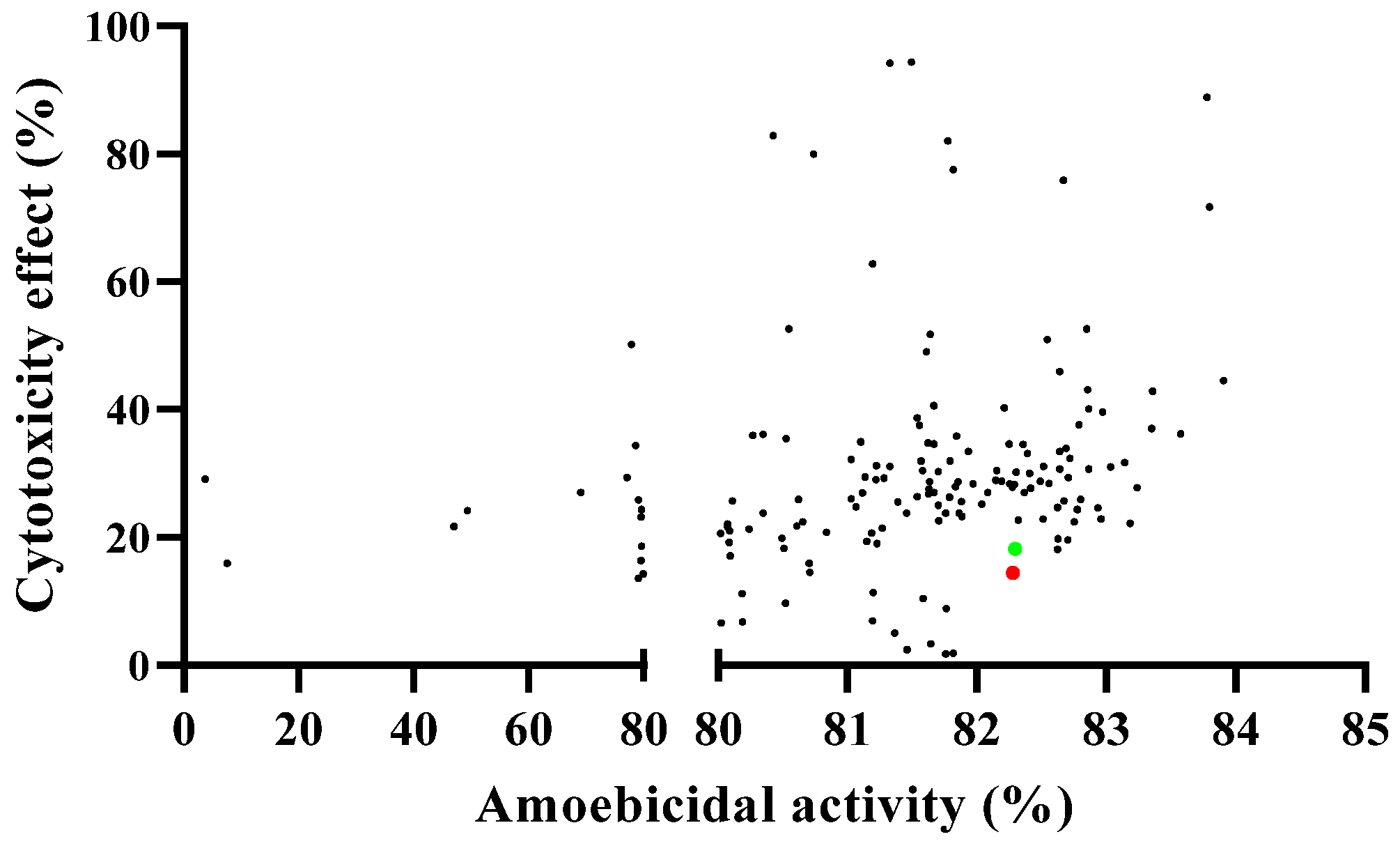
2.1. In Vitro Assay of COVID Box Molecules against Acanthamoeba castellanii Neff and Murine Macrophages
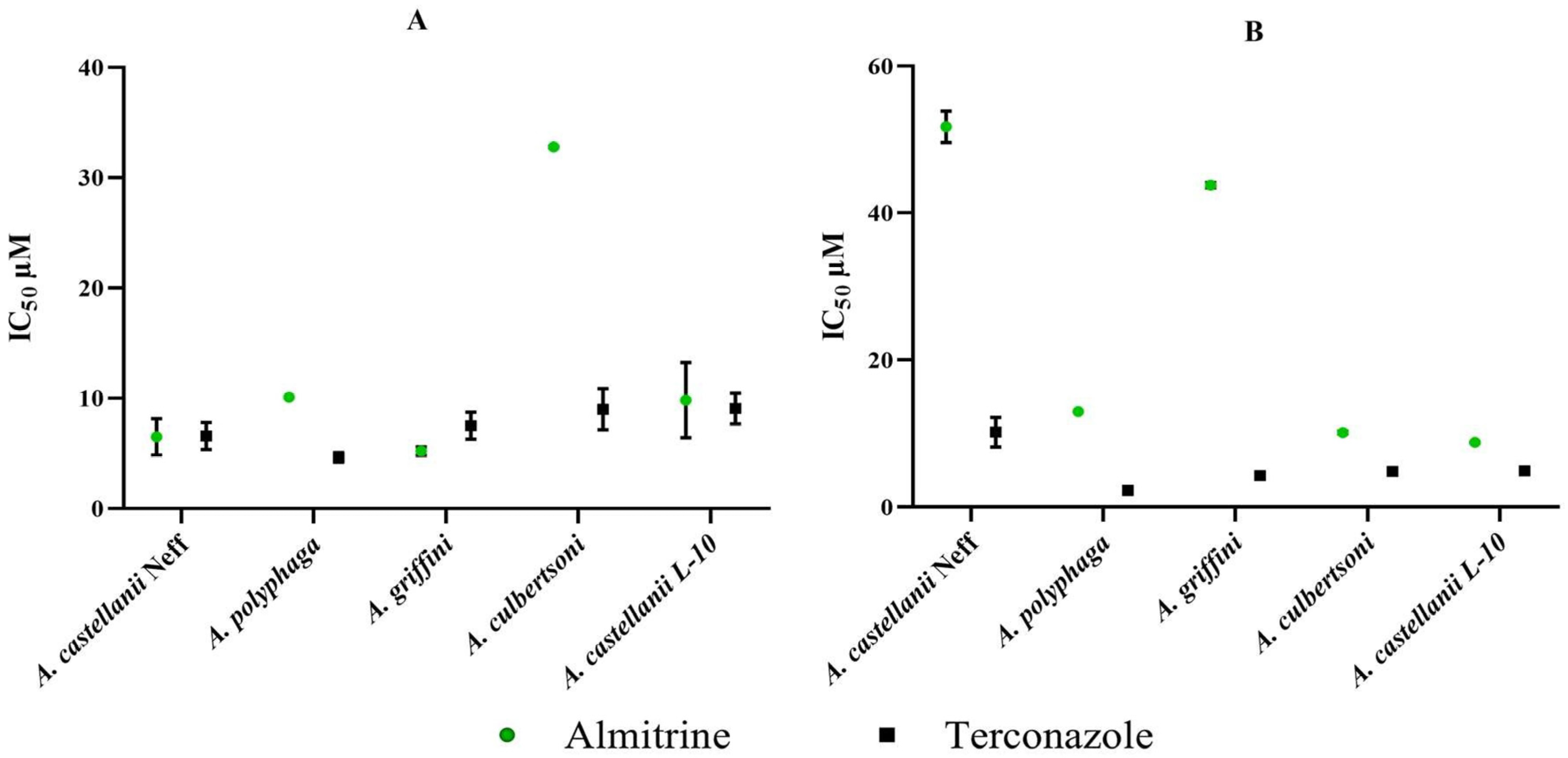
2.2. In Vitro Activity of the Most Active Drugs against Trophozoite and Cyst of Various Acanthamoeba Strains (µM)
2.3. Characterization of the Type of Cell Death Induced by Terconazole on Acanthamoeba culbertsoni
2.4. Disruption of the Cytoskeleton Organization in Acanthamoeba culbertsoni by the Terconazole
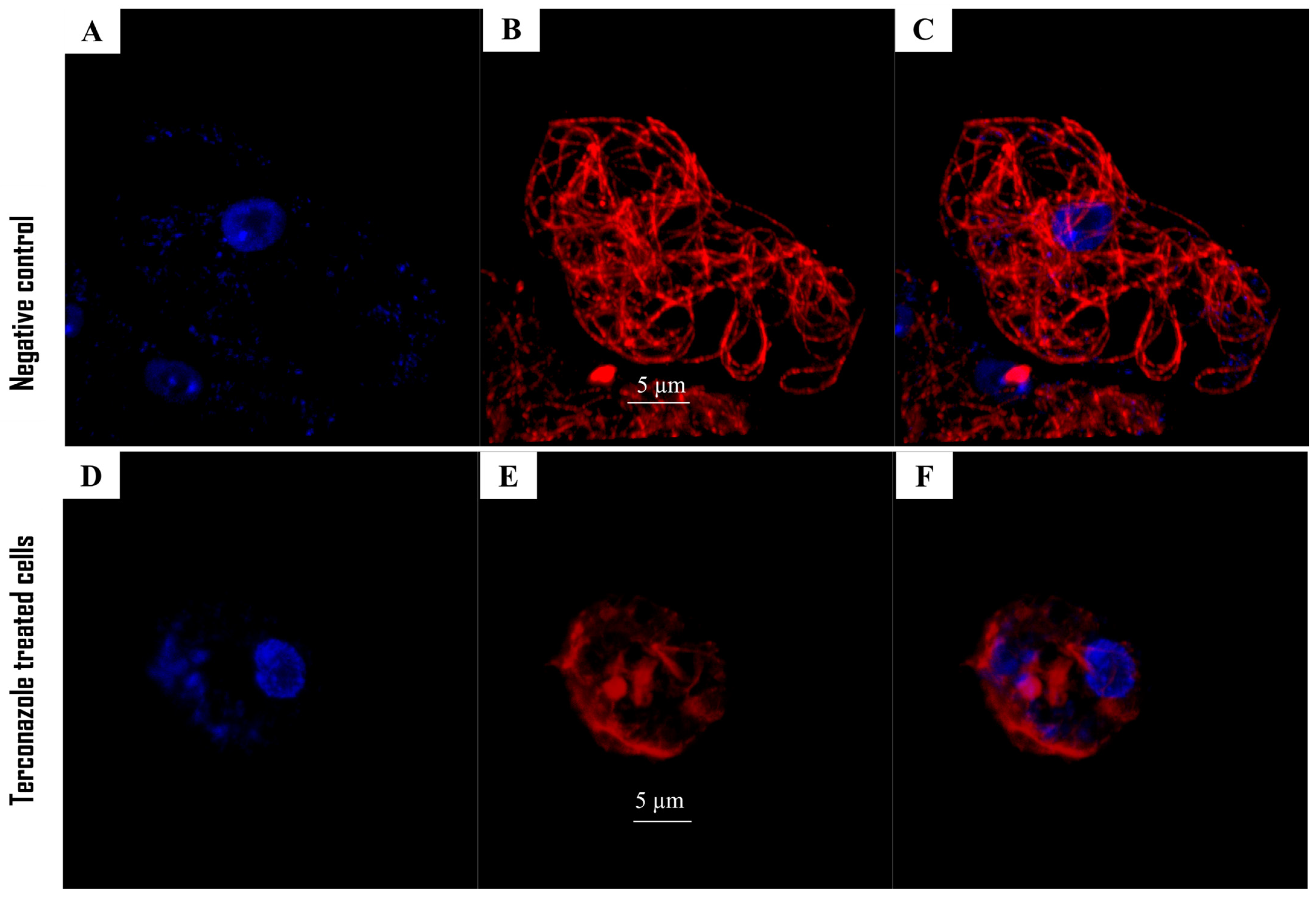
2.5. Disruption of Actin Protein in Acanthamoeba culbertsoni
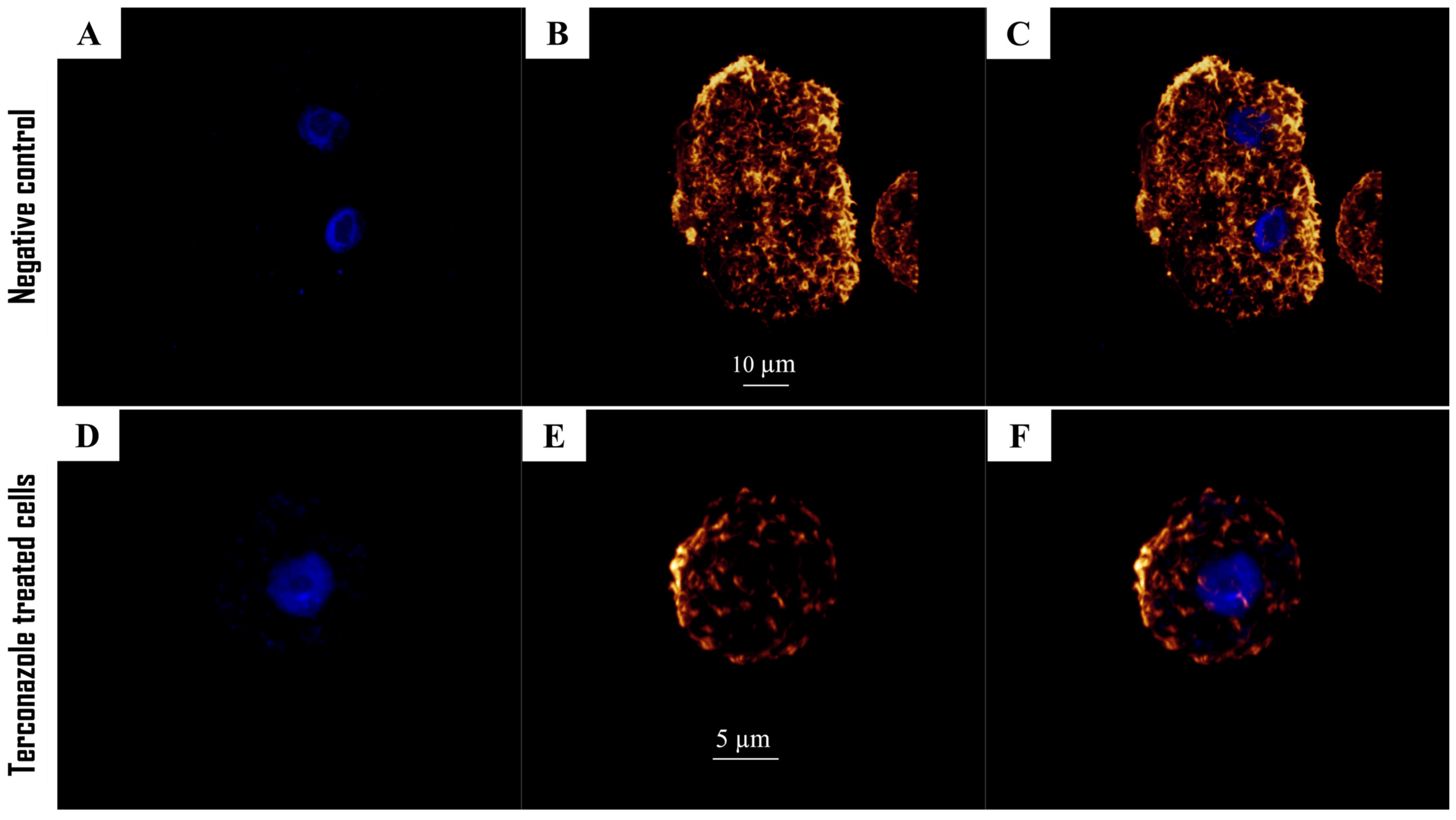
2.6. Disruption of the Microtubule Network in Acanthamoeba culbertsoni
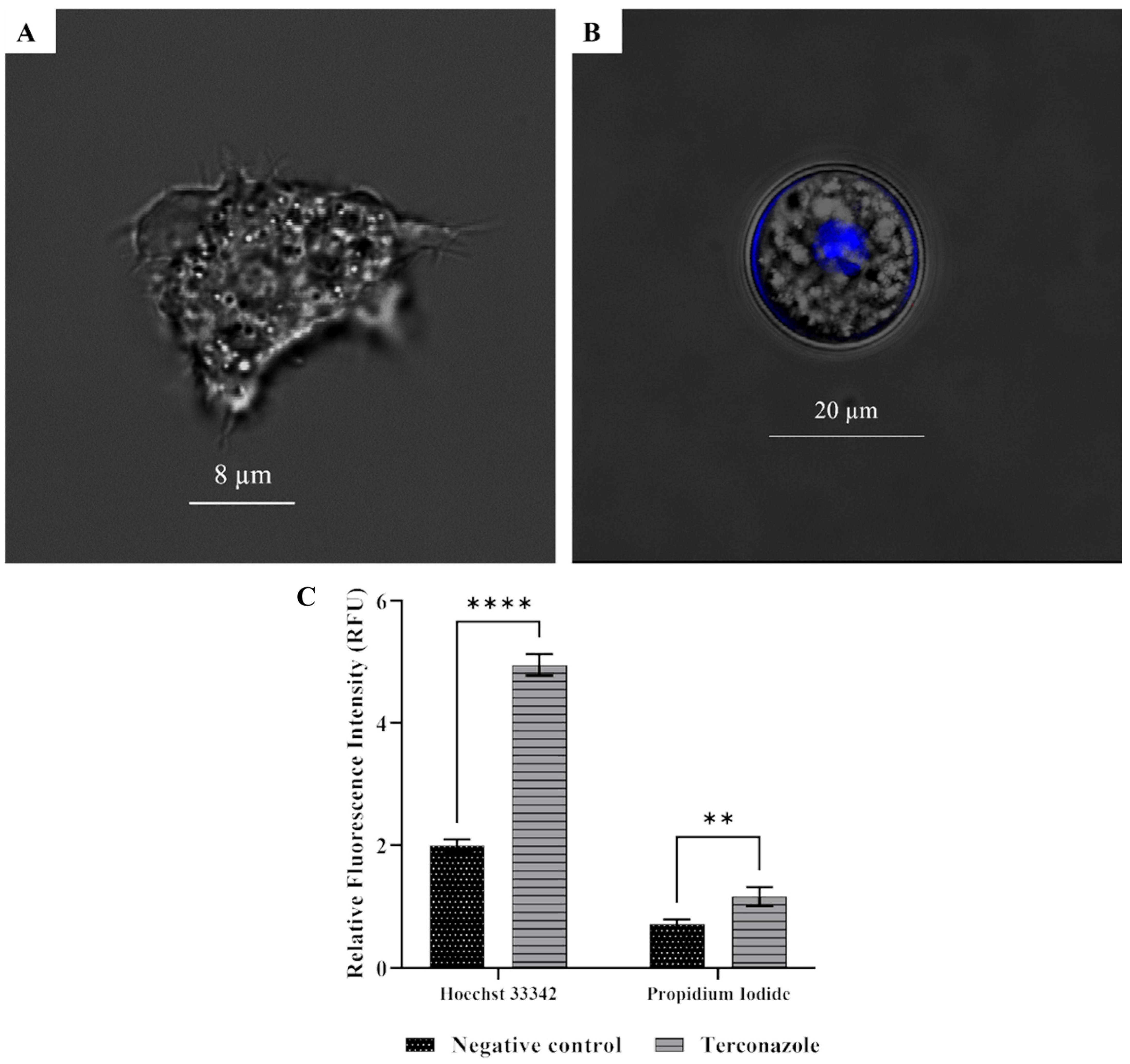
2.7. Induced Chromatin Condensation in Acanthamoeba culbertsoni
2.8. Damaged Cell Plasma Membrane Permeability in Acanthamoeba culbertsoni
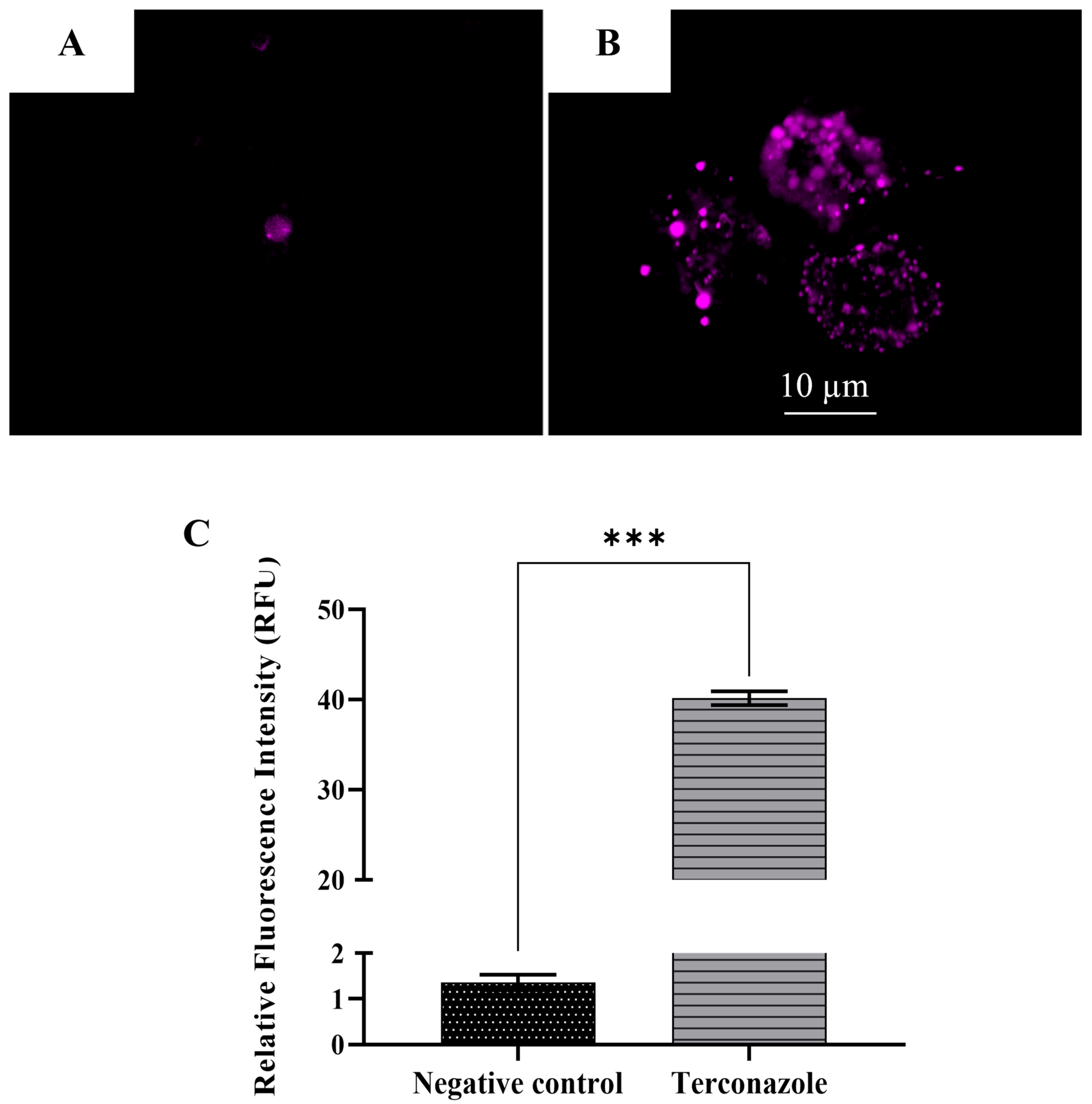
2.9. Validation of the Mitochondrial Malfunction after Incubation with the Terconazole
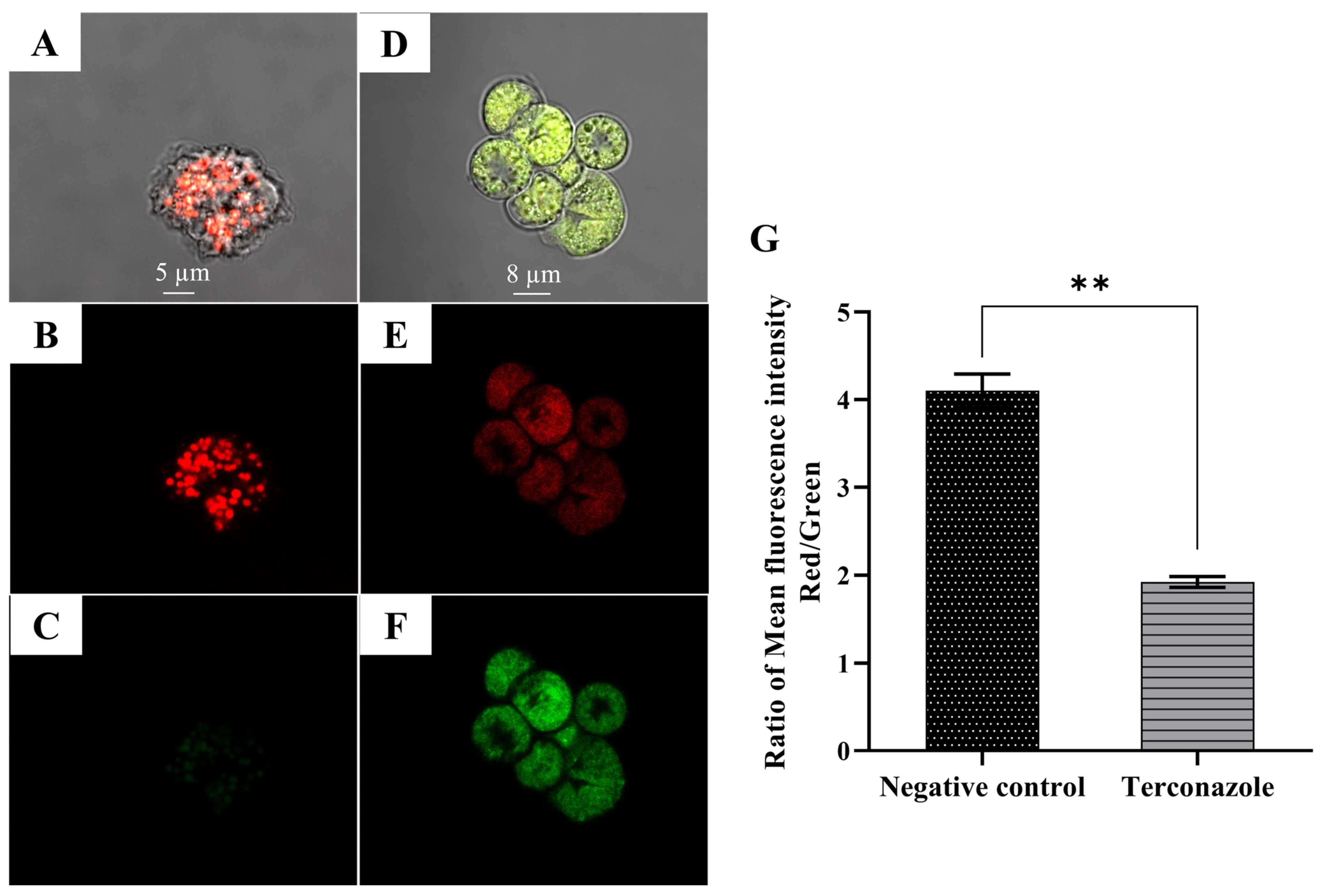
2.10. Induction of a Collapse in the Mitochondrial Membrane Potential (MMP)
2.11. Decrease in the ATP Production
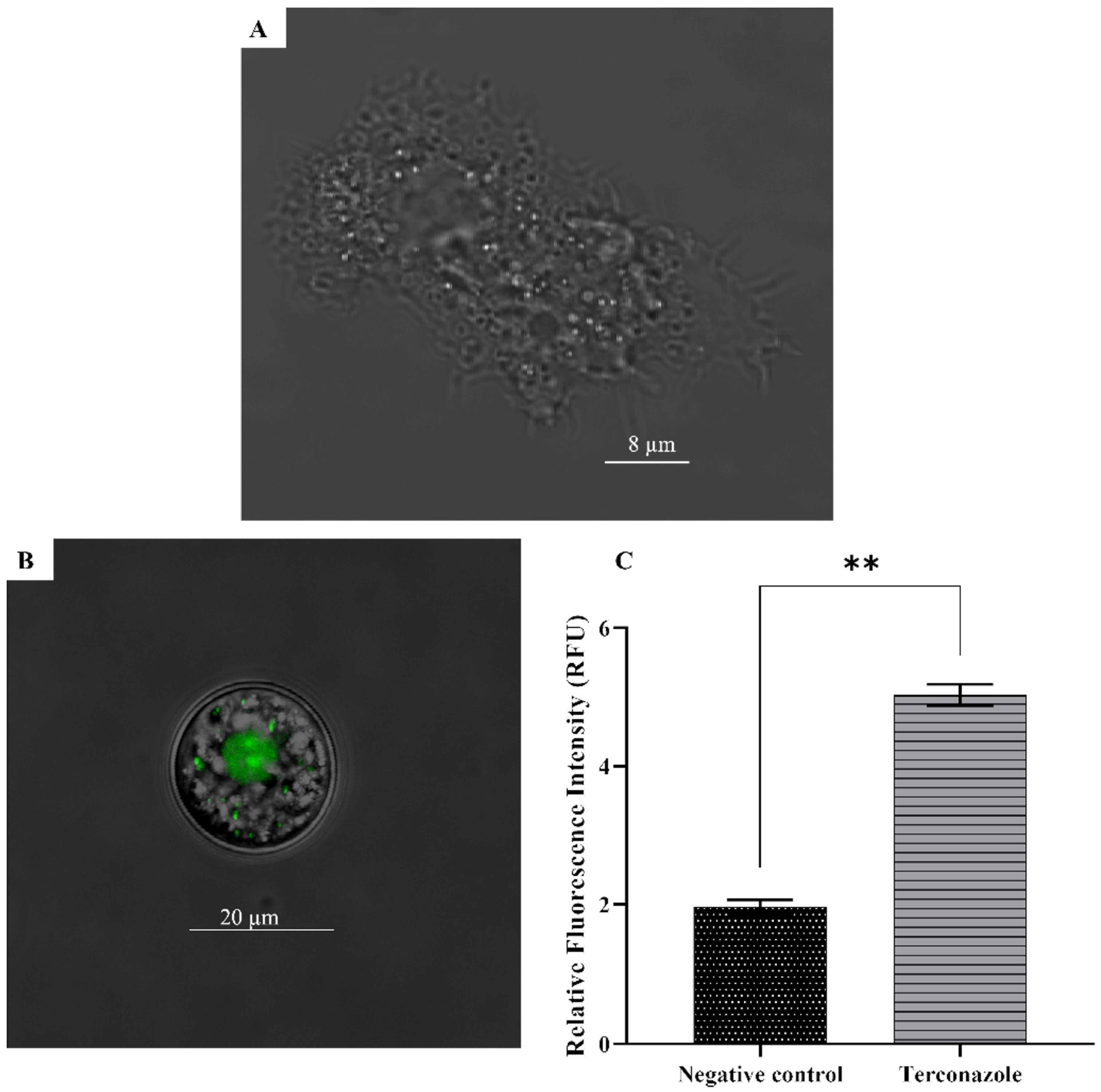
2.12. Increase in the Reactive Oxygen Species Production
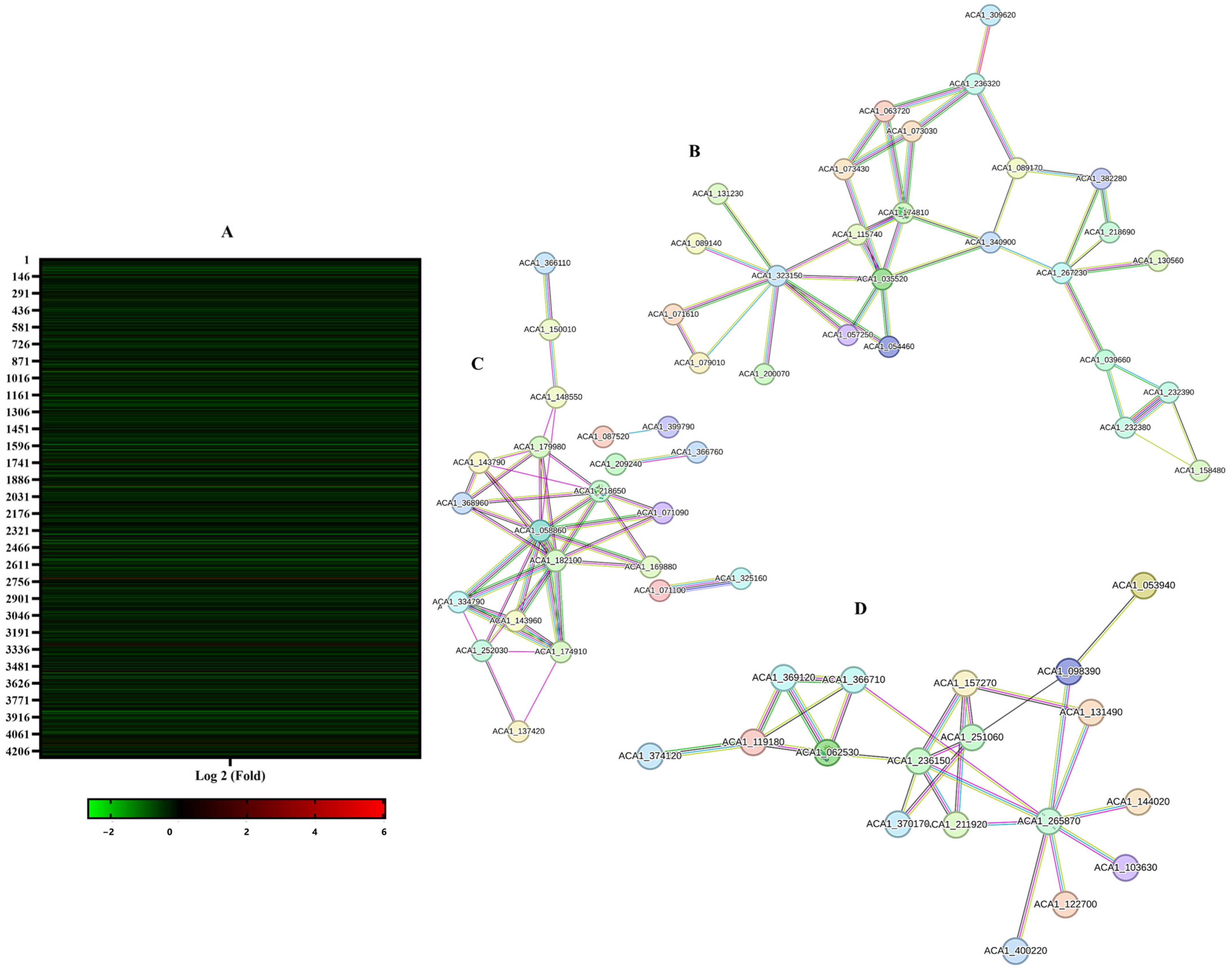
2.13. Highlighting Terconazole Effect on the Proteomic Profile of Acanthamoeba castellanii L10
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Drugs and Chemicals
5.2. Acanthamoeba Strains
5.3. In Vitro Effect against the Trophozoite Stage of Acanthamoeba
5.4. In Vitro Effect against the Cysts Stage
5.5. Evaluation of the Cytotoxicity against Murine Macrophage
5.6. Fluorescence Microscopy
5.6.1. Immunofluorescence Staining of Tubulin
5.6.2. Acting Staining Using Phalloidin-TRITC Conjugate
5.7. Mode of Action Evaluation
5.7.1. Double-Stain Assay for Programmed Cell Death Determination
5.7.2. Plasma Membrane Permeability
5.7.3. Analysis of Mitochondrial Membrane Potential Using JC-1 Kit
5.7.4. ATP Assays
5.7.5. Estimation of ROS Generation Using CellROX® Deep Red Staining
5.7.6. Comparative Label-Free Proteomics
5.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reyes-Batlle, M.; Córdoba-Lanús, E.; Domínguez-de-Barros, A.; Sifaoui, I.; Rodríguez-Expósito, R.L.; Mantesa-Rodríguez, S.; Piñero, J.E.; Lorenzo-Morales, J. Reliable and specific detection of Acanthamoeba spp. in dishcloths using quantitative real-time PCR assay. Food Microbiol. 2024, 122, 104562. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Khan, N.A. Biology and pathogenesis of Acanthamoeba. Parasites Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological characteristics and pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, N.; Sirajuddin, N.; Yin, X.; Huang, A.J.W.; Stuart, P.M. Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens 2021, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Chiboub, O.; Bethencourt-Estrella, C.J.; San Nicolás-Hernández, D.; Rodríguez Expósito, R.L.; Rizo-Liendo, A.; Piñero, J.E.; Lorenzo-Morales, J. Screening of the pathogen box for the identification of anti-Acanthamoeba agents. Exp. Parasitol. 2019, 201, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Botella, L.M. Drug repurposing as a current strategy in medicine discovery. Semer. Med. Fam. 2022, 48, 101790. [Google Scholar] [CrossRef] [PubMed]
- Barnes, C.B.G.; Dans, M.G.; Jonsdottir, T.K.; Crabb, B.S.; Gilson, P.R. PfATP4 inhibitors in the Medicines for Malaria Venture Malaria Box and Pathogen Box block the schizont-to-ring transition by inhibiting egress rather than invasion. Front. Cell. Infect. Microbiol. 2022, 12, 1060202. [Google Scholar] [CrossRef] [PubMed]
- Samby, K.; Willis, P.A.; Burrows, J.N.; Laleu, B.; Webborn, P.J.H. Actives from MMV Open Access Boxes? A suggested way forward. PLoS Pathog. 2021, 17, e1009384. [Google Scholar] [CrossRef]
- Lopez-Arencibia, A.; Sifaoui, I.; Reyes-Batlle, M.; Bethencourt-Estrella, C.J.; San Nicolas-Hernandez, D.; Lorenzo-Morales, J.; Pinero, J.E. Discovery of New Chemical Tools against Leishmania amazonensis via the MMV Pathogen Box. Pharmaceuticals 2021, 14, 1219. [Google Scholar] [CrossRef]
- Duffy, S.; Sykes, M.L.; Jones, A.J.; Shelper, T.B.; Simpson, M.; Lang, R.; Poulsen, S.; Sleebs, B.E.; Avery, V.M. Screening the Medicines for Malaria Venture Pathogen Box across Multiple Pathogens Reclassifies Starting Points for Open-Source Drug Discovery. Antimicrob. Agents Chemother. 2017, 61, e00379-17. [Google Scholar] [CrossRef]
- Spalenka, J.; Escotte-Binet, S.; Bakiri, A.; Hubert, J.; Renault, J.; Velard, F.; Duchateau, S.; Aubert, D.; Huguenin, A.; Villena, I. Discovery of New Inhibitors of Toxoplasma gondii via the Pathogen Box. Antimicrob. Agents Chemother. 2018, 62, e01640-17. [Google Scholar] [CrossRef]
- Dos Santos, B.R.; Ramos, A.B.d.S.B.; de Menezes, R.P.B.; Scotti, M.T.; Colombo, F.A.; Marques, M.J.; Reimao, J.Q. Repurposing the Medicines for Malaria Venture’s COVID Box to discover potent inhibitors of Toxoplasma gondii, and in vivo efficacy evaluation of almitrine bismesylate (MMV1804175) in chronically infected mice. PLoS ONE 2023, 18, e0288335. [Google Scholar] [CrossRef]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J. Exploring therapeutic approaches against Naegleria fowleri infections through the COVID box. Int. J. Parasitol. Drugs Drug Resist. 2024, 25, 100545. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef]
- Hua, Y.; Dai, X.; Xu, Y.; Xing, G.; Liu, H.; Lu, T.; Chen, Y.; Zhang, Y. Drug repositioning: Progress and challenges in drug discovery for various diseases. Eur. J. Med. Chem. 2022, 234, 114239. [Google Scholar] [CrossRef]
- Pareek, S.; Huang, Y.; Nath, A.; Huang, R.S. Chapter 6—The success story of drug repurposing in breast cancer. In Drug Repurposing in Cancer Therapy; To, K.K.W., Cho, W.C.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 173–190. [Google Scholar]
- Teixeira, M.M.; Carvalho, D.T.; Sousa, E.; Pinto, E. New Antifungal Agents with Azole Moieties. Pharmaceuticals 2022, 15, 1427. [Google Scholar] [CrossRef]
- Henriquez, F.L.; Ingram, P.R.; Muench, S.P.; Rice, D.W.; Roberts, C.W. Molecular Basis for Resistance of Acanthamoeba Tubulins to All Major Classes of Antitubulin Compounds. Antimicrob. Agents Chemother. 2007, 52, 1133. [Google Scholar] [CrossRef] [PubMed]
- Shing, B.; Balen, M.; Mckerrow, J.H.; Debnath, A. Acanthamoeba Keratitis: An update on amebicidal and cysticidal drug screening methodologies and potential treatment with azole drugs. Expert Rev. Anti Infect. Ther. 2022, 19, 1427. [Google Scholar] [CrossRef] [PubMed]
- Bahy, R.; Helal, D. Evaluation of the Antimycotic activity of Terconazole proniosomal Gel. Egypt. J. Med. Microbiol. 2022, 31, 121–126. [Google Scholar] [CrossRef]
- Lee, J.S.; Oh, Y.; Park, J.H.; Kyung, S.Y.; Kim, H.S.; Yoon, S. Terconazole, an Azole Antifungal Drug, Increases Cytotoxicity in Antimitotic Drug-Treated Resistant Cancer Cells with Substrate-Specific P-gp Inhibitory Activity. Int. J. Mol. Sci. 2022, 23, 13809. [Google Scholar] [CrossRef]
- Reigada, C.; Saye, M.; Valera-Vera, E.; Miranda, M.R.; Pereira, C.A. Repurposing of terconazole as an anti Trypanosoma cruzi agent. Heliyon 2019, 5, e01947. [Google Scholar] [CrossRef]
- Yang, S.; Yan, D.; Li, M.; Li, D.; Zhang, S.; Fan, G.; Peng, L.; Pan, S. Ergosterol depletion under bifonazole treatment induces cell membrane damage and triggers a ROS-mediated mitochondrial apoptosis in Penicillium expansum. Fungal Biol. 2022, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.A.; Yahya, N.S.W.; Chi, Y. Bio function of Cytochrome P450 on fungus: A review. IOP Conf. Ser. Earth Environ. Sci. 2022, 959, 12023. [Google Scholar] [CrossRef]
- Yoshida, Y. Cytochrome P450 of fungi: Primary target for azole antifungal agents. Curr. Top. Med. Mycol. 1988, 2, 388–418. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ko, P.; Huang, C.; Wen, P.; Chen, C.; Shih, M.; Lin, W.; Huang, F. Cytochrome P450 monooxygenase of Acanthamoeba castellanii participates in resistance to polyhexamethylene biguanide treatment. Parasite 2021, 28, 77. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Zhang, L.; Peng, J.; Zhang, J.; Li, H.; Zhang, P.; Calderone, R.; Liu, W.; Li, D. Mitochondrial Complex I Core Protein Regulates cAMP Signaling via Phosphodiesterase Pde2 and NAD Homeostasis in Candida albicans. Front. Microbiol. 2020, 11, 559975. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Yeomans, A.M.; Packham, G. Targeted inhibition of mRNA translation initiation factors as a novel therapeutic strategy for mature B-cell neoplasms. Explor. Target. Anti-Tumor Ther. 2020, 1, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Mo, D.; Liu, C.; Chen, Y.; Cheng, X.; Shen, J.; Zhao, L.; Zhang, J. The mitochondrial ribosomal protein mRpL4 regulates Notch signaling. EMBO Rep. 2023, 24, e55764. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, L.; Oberer, M.; Reibarkh, M.; Cencic, R.; Bordeleau, M.; Vogt, E.; Marintchev, A.; Tanaka, J.; Fagotto, F.; Altmann, M.; et al. Selective pharmacological targeting of a DEAD box RNA helicase. PLoS ONE 2008, 3, e1583. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X. DEAD-Box RNA Helicases in Cell Cycle Control and Clinical Therapy. Cells 2021, 10, 1540. [Google Scholar] [CrossRef]
- Yildizhan, H.; Barkan, N.P.; Karahisar Turan, S.; Demiralp, Ö.; Özel Demiralp, F.D.; Uslu, B.; Ōzkan, S.A. Chapter 1—Treatment strategies in cancer from past to present. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 1–37. [Google Scholar]
- Garcia-Diaz, M.; Bebenek, K. Multiple functions of DNA polymerases. CRC Crit. Rev. Plant Sci. 2007, 26, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Berdis, A.J. Inhibiting DNA Polymerases as a Therapeutic Intervention against Cancer. Front. Mol. Biosci. 2017, 4, 78. [Google Scholar] [CrossRef]
- Martín-Navarro, C.M.; Lorenzo-Morales, J.; Cabrera-Serra, M.G.; Rancel, F.; Coronado-Álvarez, N.M.; Piñero, J.E.; Valladares, B. The potential pathogenicity of chlorhexidine-sensitive Acanthamoeba strains isolated from contact lens cases from asymptomatic individuals in Tenerife, Canary Islands, Spain. J. Med. Microbiol. 2008, 57, 1399. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Wagner, C.; Chiboub, O.; De Agustino Rodríguez, J.; Rocha-Cabrera, P.; Valladares, B.; Piñero, J.E.; Lorenzo-Morales, J. Evaluation of the anti-Acanthamoeba activity of two commercial eye drops commonly used to lower eye pressure. Exp. Parasitol. 2017, 183, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sifaoui, I.; Reyes-Batlle, M.; López-Arencibia, A.; Chiboub, O.; Rodríguez-Martín, J.; Rocha-Cabrera, P.; Valladares, B.; Piñero, J.E.; Lorenzo-Morales, J. Toxic effects of selected proprietary dry eye drops on Acanthamoeba. Sci. Rep. 2018, 8, 8520. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Expósito, R.L.; Sifaoui, I.; Reyes-Batlle, M.; Fuchs, F.; Scheid, P.L.; Piñero, J.E.; Sutak, R.; Lorenzo-Morales, J. Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline. Antioxidants 2023, 12, 2081. [Google Scholar] [CrossRef]
- Arbon, D.; Zeniskova, K.; Subrtova, K.; Mach, J.; Stursa, J.; Machado, M.; Zahedifard, F.; Lestinova, T.; Hierro-Yap, C.; Neuzil, J.; et al. Repurposing of MitoTam: Novel Anti-Cancer Drug Candidate Exhibits Potent Activity against Major Protozoan and Fungal Pathogens. Antimicrob. Agents Chemother. 2022, 66, e0072722. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell Proteomics 2014, 13, 2513–2526. [Google Scholar] [CrossRef]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sifaoui, I.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; Sutak, R.; Piñero, J.E.; Lorenzo-Morales, J. Amoebicidal Effect of COVID Box Molecules against Acanthamoeba: A Study of Cell Death. Pharmaceuticals 2024, 17, 808. https://doi.org/10.3390/ph17060808
Sifaoui I, Rodríguez-Expósito RL, Reyes-Batlle M, Sutak R, Piñero JE, Lorenzo-Morales J. Amoebicidal Effect of COVID Box Molecules against Acanthamoeba: A Study of Cell Death. Pharmaceuticals. 2024; 17(6):808. https://doi.org/10.3390/ph17060808
Chicago/Turabian StyleSifaoui, Ines, Rubén L. Rodríguez-Expósito, María Reyes-Batlle, Robert Sutak, José E. Piñero, and Jacob Lorenzo-Morales. 2024. "Amoebicidal Effect of COVID Box Molecules against Acanthamoeba: A Study of Cell Death" Pharmaceuticals 17, no. 6: 808. https://doi.org/10.3390/ph17060808
APA StyleSifaoui, I., Rodríguez-Expósito, R. L., Reyes-Batlle, M., Sutak, R., Piñero, J. E., & Lorenzo-Morales, J. (2024). Amoebicidal Effect of COVID Box Molecules against Acanthamoeba: A Study of Cell Death. Pharmaceuticals, 17(6), 808. https://doi.org/10.3390/ph17060808










