Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective
Abstract
1. Introduction
2. Literature Searching Method
3. Role and Mechanisms of Polyphenols in the Treatment of Nervous System Diseases
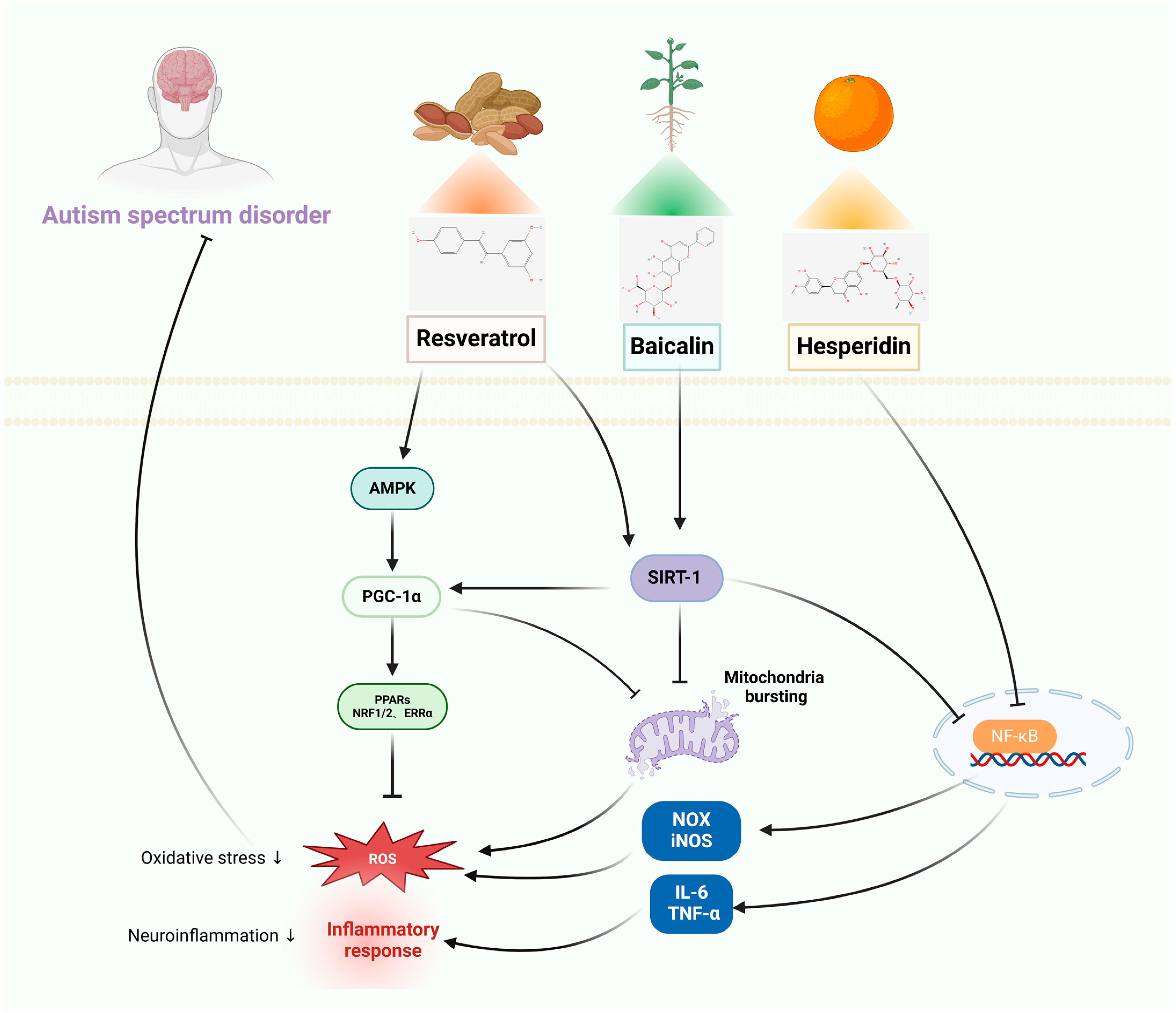
3.1. Autism-Spectrum Disorders
3.1.1. In Vitro Studies
3.1.2. In Vivo Studies
3.1.3. Clinical Studies
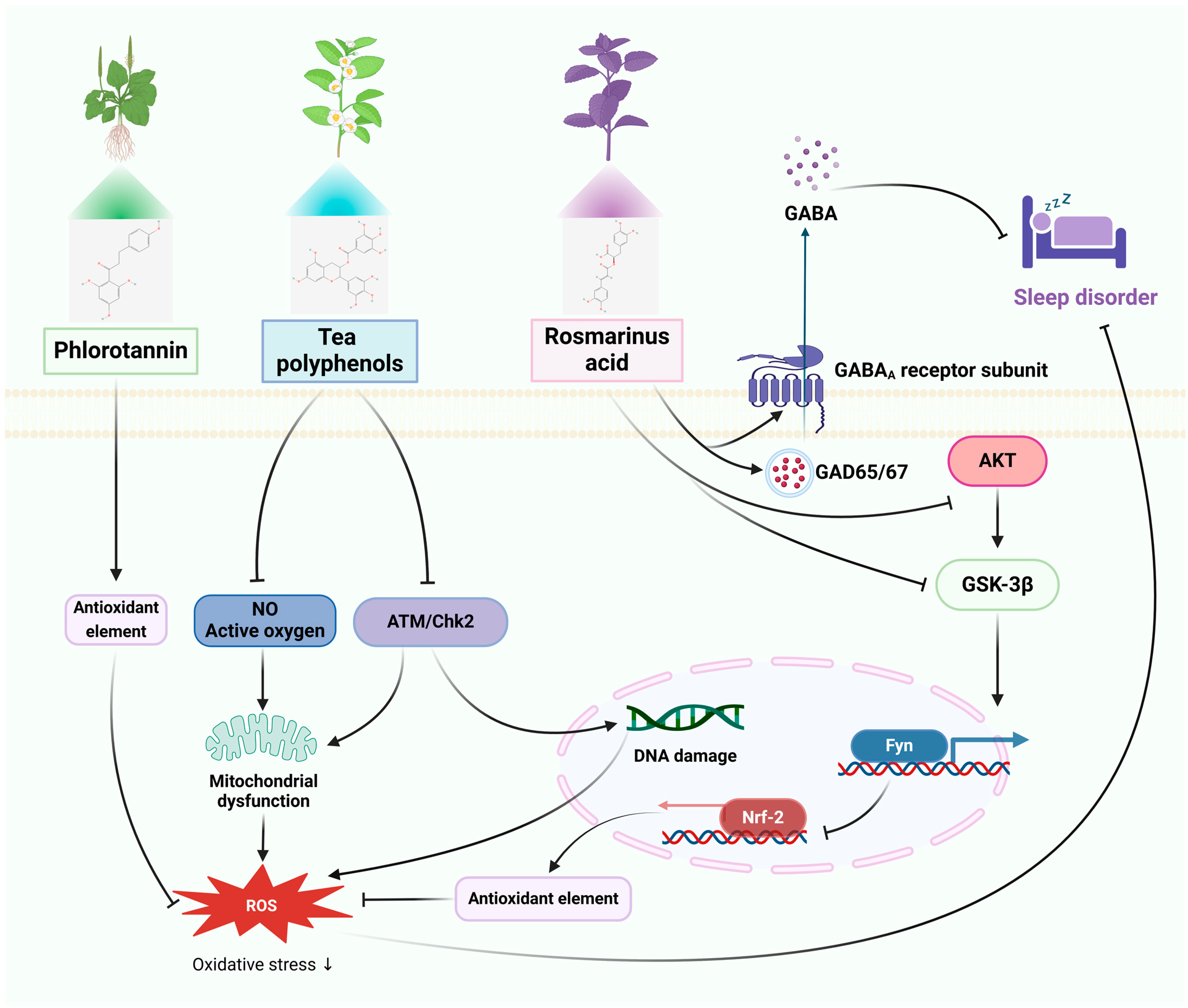
3.2. Sleep Disorders
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
3.2.3. Clinical Studies
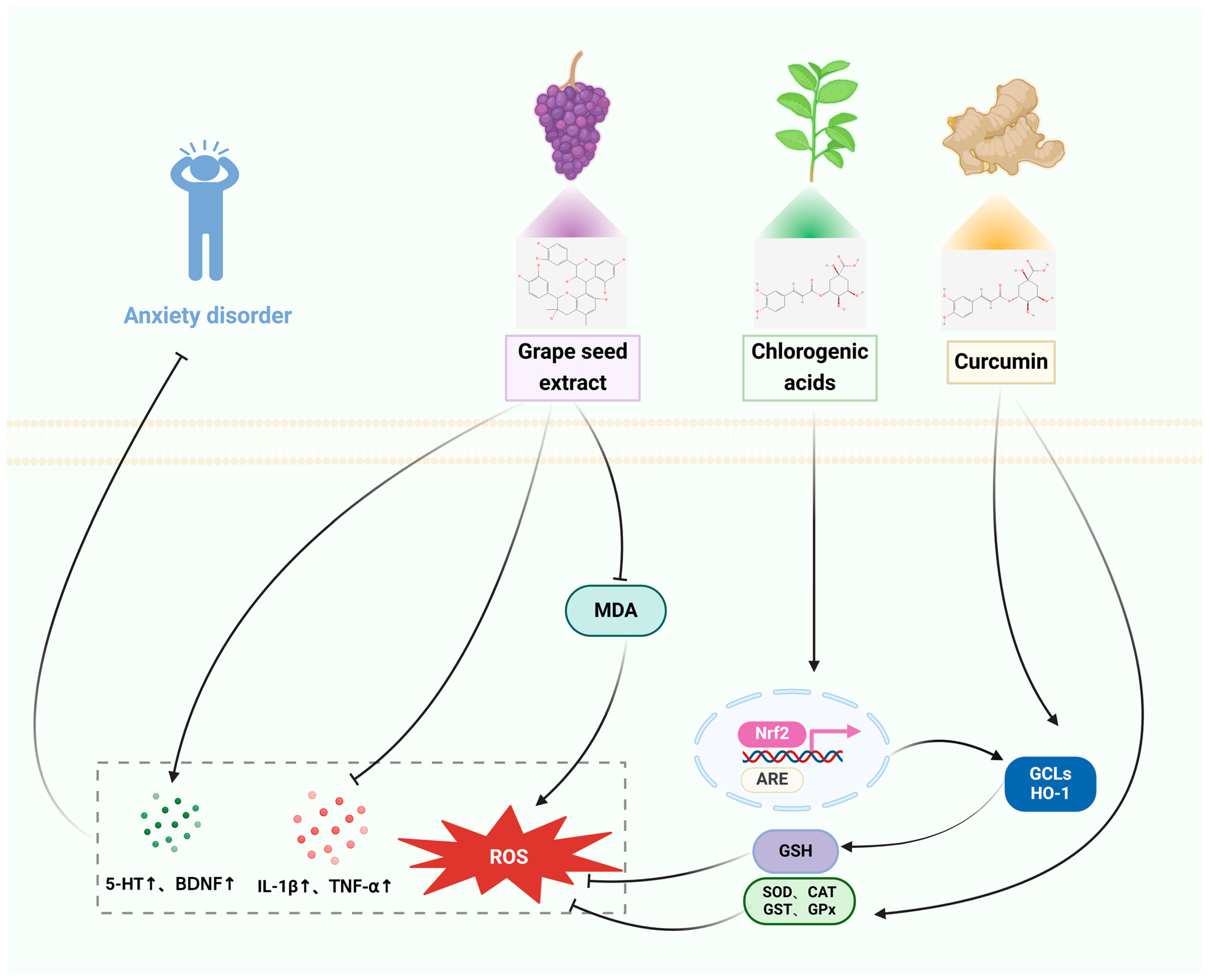
3.3. Anxiety Disorders
3.3.1. In Vitro Studies
3.3.2. In Vivo Studies
3.3.3. Clinical Studies
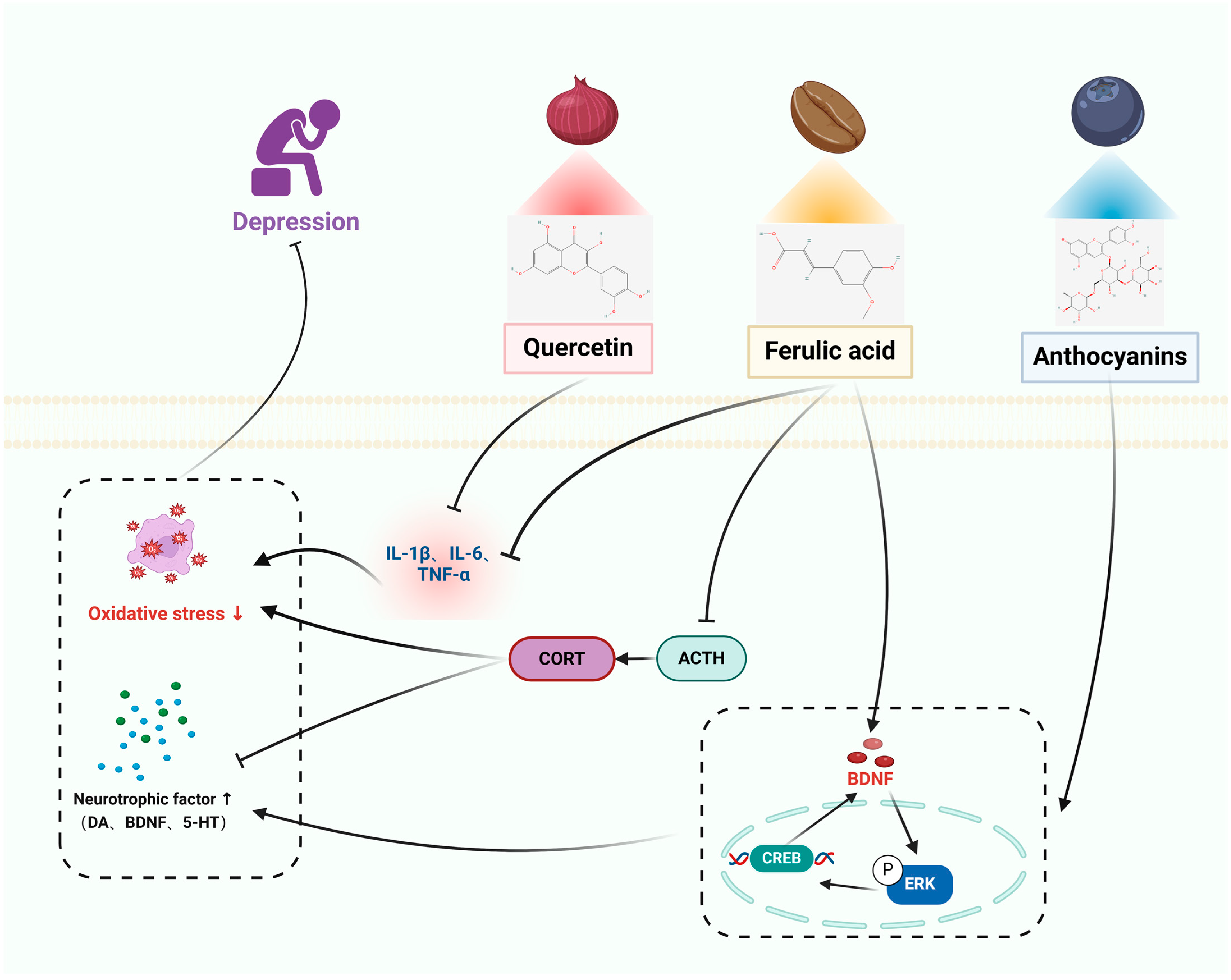
3.4. Depression
3.4.1. In Vitro Studies
3.4.2. In Vivo Studies
3.4.3. Clinical Studies
4. Conclusions and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kjærstad, H.L.; Varo, C.; Meluken, I.; Vieta, E.; Vinberg, M.; Kessing, L.V.; Miskowiak, K.W. Emotional cognition subgroups in unaffected first-degree relatives of patients with mood disorders. Psychol. Med. 2023, 53, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1223–1249. [CrossRef] [PubMed]
- Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 2020, 395, 709–733. [CrossRef] [PubMed]
- Jain, R.W.; Yong, V.W. B cells in central nervous system disease: Diversity, locations and pathophysiology. Nat. Rev. Immunol. 2022, 22, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Masuda, T.; Wheeler, M.A.; Quintana, F.J. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu. Rev. Immunol. 2021, 39, 251–277. [Google Scholar] [CrossRef] [PubMed]
- Yong, K.; Chin, R.F.M.; Shetty, J.; Hogg, K.; Burgess, K.; Lindsay, M.; McLellan, A.; Stone, J.; KamathTallur, K.; the Edinburgh Paediatric FND Study Group. Functional neurological disorder in children and young people: Incidence, clinical features, and prognosis. Dev. Med. Child Neurol. 2023, 65, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Keynejad, R.C.; Carson, A.J.; David, A.S.; Nicholson, T.R. Functional neurological disorder: Psychiatry’s blind spot. Lancet Psychiatry 2017, 4, e2–e3. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M., 3rd; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of neurodegenerative diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.W.; Tang, W.H.; Leonard, B.E. Treatment-induced mood switching in affective disorders. Acta Neuropsychiatr. 2022, 34, 55–68. [Google Scholar] [CrossRef]
- Bono, A.D.; Twaite, J.T.; Krch, D.; McCabe, D.L.; Scorpio, K.A.; Stafford, R.J.; Borod, J.C. Mood and emotional disorders associated with parkinsonism, huntington disease, and other movement disorders. In Disorders of Emotion in Neurologic Disease; Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 183, pp. 175–196. [Google Scholar]
- Briguglio, M.; Vitale, J.A.; Galentino, R.; Banfi, G.; Zanaboni Dina, C.; Bona, A.; Panzica, G.; Porta, M.; Dell’Osso, B.; Glick, I.D. Healthy eating, physical activity, and sleep hygiene (hepas) as the winning triad for sustaining physical and mental health in patients at risk for or with neuropsychiatric disorders: Considerations for clinical practice. Neuropsychiatr. Dis. Treat. 2020, 16, 55–70. [Google Scholar] [CrossRef]
- Leung, E.; Lau, E.W.; Liang, A.; de Dios, C.; Suchting, R.; Östlundh, L.; Masdeu, J.C.; Fujita, M.; Sanches, M.; Soares, J.C.; et al. Alterations in brain synaptic proteins and mrnas in mood disorders: A systematic review and meta-analysis of postmortem brain studies. Mol. Psychiatry 2022, 27, 1362–1372. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N. Diagnosis of autism. JAMA 2024, 331, 259. [Google Scholar] [CrossRef] [PubMed]
- The, L. Progress in the USA for autistic spectrum disorder. Lancet 2018, 391, 1750. [Google Scholar]
- Orefice, L.L. Outside-in: Rethinking the etiology of autism spectrum disorders. Science 2019, 366, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Rosenqvist, M.A.; Larsson, H.; Gillberg, C.; D’Onofrio, B.M.; Lichtenstein, P.; Lundström, S. Etiology of autism spectrum disorders and autistic traits over time. JAMA Psychiatry 2020, 77, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, J.S. Genetics. Insights into the pathogenesis of autism. Science 2008, 321, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.M.; Soke, G.N.; Sabourin, K.R.; Hepburn, S.; Katz, T.; Wiggins, L.D.; Schieve, L.A.; Levy, S.E. Sleep problems in 2- to 5-year-olds with autism spectrum disorder and other developmental delays. Pediatrics 2019, 143, e20180492. [Google Scholar] [CrossRef]
- Goldman, S.E.; Surdyka, K.; Cuevas, R.; Adkins, K.; Wang, L.; Malow, B.A. Defining the sleep phenotype in children with autism. Dev. Neuropsychol. 2009, 34, 560–573. [Google Scholar] [CrossRef]
- Souders, M.C.; Zavodny, S.; Eriksen, W.; Sinko, R.; Connell, J.; Kerns, C.; Schaaf, R.; Pinto-Martin, J. Sleep in children with autism spectrum disorder. Curr. Psychiatry Rep. 2017, 19, 34. [Google Scholar] [CrossRef]
- Hirota, T.; King, B.H. Autism spectrum disorder: A review. JAMA 2023, 329, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Buck, C.; Chick, C.F.; Anker, L.; Talbot, L.; Schneider, L.; Linkovski, O.; Cotto, I.; Parker-Fong, K.; Phillips, J.; et al. Sleep architecture is associated with core symptom severity in autism spectrum disorder. Sleep 2023, 46, zsac273. [Google Scholar] [CrossRef] [PubMed]
- Boyd, K.; Woodbury-Smith, M.; Szatmari, P. Managing anxiety and depressive symptoms in adults with autism-spectrum disorders. J. Psychiatry Neurosci. JPN 2011, 36, E35–E36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shah, H.E.; Bhawnani, N.; Ethirajulu, A.; Alkasabera, A.; Onyali, C.B.; Anim-Koranteng, C.; Mostafa, J.A. Iron deficiency-induced changes in the hippocampus, corpus striatum, and monoamines levels that lead to anxiety, depression, sleep disorders, and psychotic disorders. Cureus 2021, 13, e18138. [Google Scholar] [CrossRef] [PubMed]
- Durukan, İ.; Kara, K.; Almbaideen, M.; Karaman, D.; Gül, H. Alexithymia, depression and anxiety in parents of children with neurodevelopmental disorder: Comparative study of autistic disorder, pervasive developmental disorder not otherwise specified and attention deficit-hyperactivity disorder. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2018, 60, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Massa, S.M. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 2013, 12, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, M.; Wei, Y.; Li, H.; He, X.; Yang, Q.; Li, Z.; Duan, J.; Wu, Z.; Chen, Q.; et al. Inhalation aromatherapy via brain-targeted nasal delivery: Natural volatiles or essential oils on mood disorders. Front. Pharmacol. 2022, 13, 860043. [Google Scholar] [CrossRef]
- Xia, X.; Zhou, Y.; Gao, H. Prodrug strategy for enhanced therapy of central nervous system disease. Chem. Commun. 2021, 57, 8842–8855. [Google Scholar] [CrossRef]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. PTR 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, V.; Hediyal, T.A.; Anand, N.; Kendaganna, P.H.; Gorantla, V.R.; Mahalakshmi, A.M.; Ghanekar, R.K.; Yang, J.; Sakharkar, M.K.; Chidambaram, S.B. Polyphenols, autophagy and neurodegenerative diseases: A review. Biomolecules 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Rosales, T.K.O.; da Silva, F.F.A.; Bernardes, E.S.; Paulo Fabi, J. Plant-derived polyphenolic compounds: Nanodelivery through polysaccharide-based systems to improve the biological properties. Crit. Rev. Food Sci. Nutr. 2023, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Márquez, A.; Morante-Carriel, J.A.; Ramírez-Estrada, K.; Cusidó, R.M.; Palazon, J.; Bru-Martínez, R. Production of highly bioactive resveratrol analogues pterostilbene and piceatannol in metabolically engineered grapevine cell cultures. Plant Biotechnol. J. 2016, 14, 1813–1825. [Google Scholar] [CrossRef] [PubMed]
- Dossou, S.S.K.; Xu, F.-T.; Dossa, K.; Zhou, R.; Zhao, Y.-Z.; Wang, L.-H. Antioxidant lignans sesamin and sesamolin in sesame (Sesamum indicum L.): A comprehensive review and future prospects. J. Integr. Agric. 2023, 22, 14–30. [Google Scholar] [CrossRef]
- Jakobek, L.; Blesso, C. Beneficial effects of phenolic compounds: Native phenolic compounds vs metabolites and catabolites. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Relationship between dietary polyphenols and gut microbiota: New clues to improve cognitive disorders, mood disorders and circadian rhythms. Foods 2023, 12, 1309. [Google Scholar] [CrossRef]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, L. Neuroprotective polyphenols: A modulatory action on neurotransmitter pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and their impact on the prevention of neurodegenerative diseases and development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef] [PubMed]
- Nishigaki, A.; Kido, T.; Kida, N.; Kakita-Kobayashi, M.; Tsubokura, H.; Hisamatsu, Y.; Okada, H. Resveratrol protects mitochondrial quantity by activating sirt1/pgc-1α expression during ovarian hypoxia. Reprod. Med. Biol. 2020, 19, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Santos-Terra, J.; Deckmann, I.; Carello-Collar, G.; Nunes, G.D.; Bauer-Negrini, G.; Schwingel, G.B.; Fontes-Dutra, M.; Riesgo, R.; Gottfried, C. Resveratrol prevents cytoarchitectural and interneuronal alterations in the valproic acid rat model of autism. Int. J. Mol. Sci. 2022, 23, 4075. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, N.; Shiozaki, M.; Shibata, M.; Koike, M.; Uchiyama, Y.; Matsuura, N.; Gotow, T. Resveratrol affects undifferentiated and differentiated pc12 cells differently, particularly with respect to possible differences in mitochondrial and autophagic functions. Eur. J. Cell Biol. 2013, 92, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.F.; Ansari, M.A.; Nadeem, A.; Alzahrani, M.Z.; Bakheet, S.A.; Attia, S.M. Resveratrol improves neuroimmune dysregulation through the inhibition of neuronal toll-like receptors and cox-2 signaling in btbr t(+) itpr3(tf)/j mice. Neuromol. Med. 2018, 20, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, T.; Li, Y.; Han, D.; Hong, J.; Yang, N.; He, J.; Peng, R.; Mi, X.; Kuang, C.; et al. Baicalin ameliorates cognitive impairment and protects microglia from lps-induced neuroinflammation via the sirt1/hmgb1 pathway. Oxidative Med. Cell. Longev. 2020, 2020, 4751349. [Google Scholar] [CrossRef] [PubMed]
- Elesawy, R.O.; El-Deeb, O.S.; Eltokhy, A.K.; Arakeep, H.M.; Ali, D.A.; Elkholy, S.S.; Kabel, A.M. Postnatal baicalin ameliorates behavioral and neurochemical alterations in valproic acid-induced rodent model of autism: The possible implication of sirtuin-1/mitofusin-2/ bcl-2 pathway. Biomed. Pharmacother. 2022, 150, 112960. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.G.; Kim, S.; Boo, K.H.; Kim, J.H.; Kim, C.S. Anti-inflammatory effects of immature citrus unshiu fruit extracts via suppression of nf-κb and mapk signal pathways in lps-induced raw264.7 macrophage cells. Food Sci. Biotechnol. 2024, 33, 903–911. [Google Scholar] [CrossRef]
- Hussein, A.M.; Mahmoud, S.A.; Elazab, K.M.; Abouelnaga, A.F.; Abass, M.; Mosa, A.A.H.; Hussein, M.A.M.; Elsayed, M.E.G. Possible mechanisms of the neuroprotective actions of date palm fruits aqueous extracts against valproic acid-induced autism in rats. Curr. Issues Mol. Biol. 2023, 45, 1627–1643. [Google Scholar] [CrossRef]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin confers neuroprotection by regulating nrf2/tlr4/nf-κb signaling in an aβ mouse model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef]
- Feng, N.; Zhu, G.; Sivakumaran, v.; Zhang, M.; Bedja, D.; Takimoto, E.; Paolocci, N. Abstract 292: Brain derived neurotrophic factor induced upregulation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha signaling prevents hearts from heart failure progression against pressure overload. Circ. Res. 2014, 115, A292. [Google Scholar] [CrossRef]
- Zhen, A.X.; Hyun, Y.J.; Piao, M.J.; Fernando, P.; Kang, K.A.; Ahn, M.J.; Yi, J.M.; Kang, H.K.; Koh, Y.S.; Lee, N.H.; et al. Eckol inhibits particulate matter 2.5-induced skin keratinocyte damage via mapk signaling pathway. Mar. Drugs 2019, 17, 444. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Yoon, M.; Lee, J.; Moon, K.D.; Kim, D.; Kim, S.B.; Cho, S. A standardized phlorotannin supplement attenuates caffeine-induced sleep disruption in mice. Nutrients 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Ru, Q.; Xiong, Q.; Tian, X.; Chen, L.; Zhou, M.; Li, Y.; Li, C. Tea polyphenols attenuate methamphetamine-induced neuronal damage in pc12 cells by alleviating oxidative stress and promoting DNA repair. Front. Physiol. 2019, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Song, D.; Ho, C.T.; Zhang, X.; Zhang, C.; Cao, J.; Wu, Z. Omics analyses of gut microbiota in a circadian rhythm disorder mouse model fed with oolong tea polyphenols. J. Agric. Food Chem. 2019, 67, 8847–8854. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Liang, Y.; Niu, Y. Rosmarinic acid attenuates β-amyloid-induced oxidative stress via akt/gsk-3β/fyn-mediated nrf2 activation in pc12 cells. Free Radic. Biol. Med. 2018, 120, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.O.; Hong, J.T.; Oh, K.W. Rosmarinic acid potentiates pentobarbital-induced sleep behaviors and non-rapid eye movement (nrem) sleep through the activation of gaba(a)-ergic systems. Biomol. Ther. 2017, 25, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mokrzyński, K.; Krzysztyńska-Kuleta, O.; Wojtala, M.; Wnuk, D.; Sarna, M.; Sarna, T. Can l-ascorbic acid and trans-resveratrol protect hacat cells from fine particulate matter toxicity? Photochem. Photobiol. 2024, 100, 172–189. [Google Scholar] [CrossRef]
- Li, G.; Wang, G.; Shi, J.; Xie, X.; Fei, N.; Chen, L.; Liu, N.; Yang, M.; Pan, J.; Huang, W.; et al. Trans-resveratrol ameliorates anxiety-like behaviors and fear memory deficits in a rat model of post-traumatic stress disorder. Neuropharmacology 2018, 133, 181–188. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.; Seo, K.; Ki, S.H. 5-caffeoylquinic acid ameliorates oxidative stress-mediated cell death via nrf2 activation in hepatocytes. Pharm. Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef]
- Kwon, S.H.; Lee, H.K.; Kim, J.A.; Hong, S.I.; Kim, H.C.; Jo, T.H.; Park, Y.I.; Lee, C.K.; Kim, Y.B.; Lee, S.Y.; et al. Neuroprotective effects of chlorogenic acid on scopolamine-induced amnesia via anti-acetylcholinesterase and anti-oxidative activities in mice. Eur. J. Pharmacol. 2010, 649, 210–217. [Google Scholar] [CrossRef]
- Carvalho, A.C.; Franklin, G.; Dias, A.C.; Lima, C.F. Methanolic extract of hypericum perforatum cells elicited with agrobacterium tumefaciens provides protection against oxidative stress induced in human hepg2 cells. Ind. Crop. Prod. 2014, 59, 177–183. [Google Scholar] [CrossRef]
- Sevastre-Berghian, A.C.; Toma, V.A.; Sevastre, B.; Hanganu, D.; Vlase, L.; Benedec, D.; Oniga, I.; Baldea, I.; Olteanu, D.; Moldovan, R.; et al. Characterization and biological effects of hypericum extracts on experimentally-induced–anxiety, oxidative stress and inflammation in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2018, 69, 89–800. [Google Scholar]
- El-Tarras Ael, S.; Attia, H.F.; Soliman, M.M.; El Awady, M.A.; Amin, A.A. Neuroprotective effect of grape seed extract against cadmium toxicity in male albino rats. Int. J. Immunopathol. Pharmacol. 2016, 29, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kou, J.; Wang, C.; Yu, X.; Xie, X.; Pang, X. Curcumin inhibits apoe4-induced injury by activating peroxisome proliferator-activated receptor-γ (pparγ) in sh-sy5y cells. Iran. J. Basic Med. Sci. 2020, 23, 1576–1583. [Google Scholar] [PubMed]
- Haider, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Sadir, S.; Liaquat, L.; Naqvi, F.; Zuberi, N.A.; Shakeel, H.; Perveen, T. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats. Brain Res. Bull. 2015, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.W.; Lee, W.S.; Shin, S.C.; Kim, G.Y.; Choi, B.T.; Choi, Y.H. Anthocyanins downregulate lipopolysaccharide-induced inflammatory responses in bv2 microglial cells by suppressing the nf-κb and akt/mapks signaling pathways. Int. J. Mol. Sci. 2013, 14, 1502–1515. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-L.; Luo, Y.; Jin, S.-H.; Yuan, K.; Guo, Y. Ameliorative effect of anthocyanin on depression mice by increasing monoamine neurotransmitter and up-regulating bdnf expression. J. Funct. Foods 2020, 66, 103757. [Google Scholar] [CrossRef]
- Zingales, V.; Sirerol-Piquer, M.S.; Fernández-Franzón, M.; Ruiz, M.J. Role of quercetin on sterigmatocystin-induced oxidative stress-mediated toxicity. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 156, 112498. [Google Scholar] [CrossRef]
- Zheng, X.; Cheng, Y.; Chen, Y.; Yue, Y.; Li, Y.; Xia, S.; Li, Y.; Deng, H.; Zhang, J.; Cao, Y. Ferulic acid improves depressive-like behavior in prenatally-stressed offspring rats via anti-inflammatory activity and hpa axis. Int. J. Mol. Sci. 2019, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Nakahara, M.; Matsugi, E.; Soda, M.; Hattori, T.; Hara, K.; Usami, A.; Kusumoto, C.; Higashiyama, S.; Kitaichi, K. Protective effect of ferulic acid against hydrogen peroxide induced apoptosis in pc12 cells. Molecules 2020, 26, 90. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, A.; Okuyama, S.; Nakajima, M.; Furukawa, Y. Citrus flavonoid 3,5,6,7,8,3′,4′-heptamethoxyflavone induces bdnf via camp/erk/creb signaling and reduces phosphodiesterase activity in c6 cells. Pharmacol. Rep. PR 2019, 71, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Sawamoto, A.; Okuyama, S.; Yamamoto, K.; Amakura, Y.; Yoshimura, M.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-heptamethoxyflavone, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules 2016, 21, 541. [Google Scholar] [CrossRef] [PubMed]
- Hendouei, F.; Sanjari Moghaddam, H.; Mohammadi, M.R.; Taslimi, N.; Rezaei, F.; Akhondzadeh, S. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: A double-blind and placebo-controlled randomized trial. J. Clin. Pharm. Ther. 2020, 45, 324–334. [Google Scholar] [CrossRef]
- Barone, R.; Bastin, J.; Djouadi, F.; Singh, I.; Karim, M.A.; Ammanamanchi, A.; McCarty, P.J.; Delhey, L.; Shannon, R.; Casabona, A.; et al. Mitochondrial fatty acid β-oxidation and resveratrol effect in fibroblasts from patients with autism spectrum disorder. J. Pers. Med. 2021, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Asadi, S.; Panagiotidou, S. A case series of a luteolin formulation (neuroprotek®) in children with autism spectrum disorders. Int. J. Immunopathol. Pharmacol. 2012, 25, 317–323. [Google Scholar] [CrossRef]
- Taliou, A.; Zintzaras, E.; Lykouras, L.; Francis, K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin. Ther. 2013, 35, 592–602. [Google Scholar] [CrossRef]
- Bertolino, B.; Crupi, R.; Impellizzeri, D.; Bruschetta, G.; Cordaro, M.; Siracusa, R.; Esposito, E.; Cuzzocrea, S. Beneficial effects of co-ultramicronized palmitoylethanolamide/luteolin in a mouse model of autism and in a case report of autism. CNS Neurosci. Ther. 2017, 23, 87–98. [Google Scholar] [CrossRef]
- Ekici, B. Combination of steroid and flavonoid for the treatment of regressive autism. J. Neurosci. Rural Pract. 2020, 11, 216–218. [Google Scholar] [CrossRef]
- Um, M.Y.; Kim, J.Y.; Han, J.K.; Kim, J.; Yang, H.; Yoon, M.; Kim, J.; Kang, S.W.; Cho, S. Phlorotannin supplement decreases wake after sleep onset in adults with self-reported sleep disturbance: A randomized, controlled, double-blind clinical and polysomnographic study. Phytother. Res. PTR 2018, 32, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Unno, K.; Noda, S.; Kawasaki, Y.; Yamada, H.; Morita, A.; Iguchi, K.; Nakamura, Y. Reduced stress and improved sleep quality caused by green tea are associated with a reduced caffeine content. Nutrients 2017, 9, 777. [Google Scholar] [CrossRef]
- Zhang, S.; Takano, J.; Murayama, N.; Tominaga, M.; Abe, T.; Park, I.; Seol, J.; Ishihara, A.; Tanaka, Y.; Yajima, K.; et al. Subacute ingestion of caffeine and oolong tea increases fat oxidation without affecting energy expenditure and sleep architecture: A randomized, placebo-controlled, double-blinded cross-over trial. Nutrients 2020, 12, 3671. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.S.; Kennedy, K.E.R.; Alfonso-Miller, P.; Wills, C.C.A.; Grandner, M.A. A randomized, double-blind, placebo-controlled trial of a polyphenol botanical blend on sleep and daytime functioning. Int. J. Environ. Res. Public Health 2021, 18, 3044. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Rea, A.; Michel, S. Efficacy of a curcumin extract (curcugen™) on gastrointestinal symptoms and intestinal microbiota in adults with self-reported digestive complaints: A randomised, double-blind, placebo-controlled study. BMC Complement. Med. Ther. 2021, 21, 40. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Bonnländer, B.; Lang, S.C.; Pischel, I.; Forster, J.; Khan, J.; Jackson, P.A.; Wightman, E.L. Acute and chronic effects of green oat (avena sativa) extract on cognitive function and mood during a laboratory stressor in healthy adults: A randomised, double-blind, placebo-controlled study in healthy humans. Nutrients 2020, 12, 1598. [Google Scholar] [CrossRef]
- Schön, C.; Allegrini, P.; Engelhart-Jentzsch, K.; Riva, A.; Petrangolini, G. Grape seed extract positively modulates blood pressure and perceived stress: A randomized, double-blind, placebo-controlled study in healthy volunteers. Nutrients 2021, 13, 654. [Google Scholar] [CrossRef]
- Mestrom, A.; Charlton, K.E.; Thomas, S.J.; Larkin, T.A.; Walton, K.L.; Elgellaie, A.; Kent, K. Higher anthocyanin intake is associated with lower depressive symptoms in adults with and without major depressive disorder. Food Sci. Nutr. 2024, 12, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Shayani Rad, M.; Moohebati, M.; Mohajeri, S.A. Beneficial effects of celery seed extract (Apium graveolens), as a supplement, on anxiety and depression in hypertensive patients: A randomized clinical trial. Inflammopharmacology 2023, 31, 395–410. [Google Scholar] [CrossRef]
- Schallmo, M.P.; Kolodny, T.; Kale, A.M.; Millin, R.; Flevaris, A.V.; Edden, R.A.E.; Gerdts, J.; Bernier, R.A.; Murray, S.O. Weaker neural suppression in autism. Nat. Commun. 2020, 11, 2675. [Google Scholar] [CrossRef]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef] [PubMed]
- Usui, N.; Kobayashi, H.; Shimada, S. Neuroinflammation and oxidative stress in the pathogenesis of autism spectrum disorder. Int. J. Mol. Sci. 2023, 24, 5487. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Meguid, N.A.; El-Bana, M.A.; Tinkov, A.A.; Saad, K.; Dadar, M.; Hemimi, M.; Skalny, A.V.; Hosnedlová, B.; Kizek, R.; et al. Oxidative stress in autism spectrum disorder. Mol. Neurobiol. 2020, 57, 2314–2332. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.N.; Mondal, T.; Yao, Y.; Manley, K.; Lawrence, D.A. Oxidative stress and neuroimmune proteins in a mouse model of autism. Cell Stress Chaperones 2023, 28, 201–217. [Google Scholar] [CrossRef]
- Giulivi, C.; Zhang, Y.F.; Omanska-Klusek, A.; Ross-Inta, C.; Wong, S.; Hertz-Picciotto, I.; Tassone, F.; Pessah, I.N. Mitochondrial dysfunction in autism. JAMA 2010, 304, 2389–2396. [Google Scholar] [CrossRef]
- Nabi, S.U.; Rehman, M.U.; Arafah, A.; Taifa, S.; Khan, I.S.; Khan, A.; Rashid, S.; Jan, F.; Wani, H.A.; Ahmad, S.F. Treatment of autism spectrum disorders by mitochondrial-targeted drug: Future of neurological diseases therapeutics. Curr. Neuropharmacol. 2023, 21, 1042–1064. [Google Scholar] [CrossRef]
- Brenmoehl, J.; Hoeflich, A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 2013, 13, 755–761. [Google Scholar] [CrossRef]
- Rasha, F.; Mims, B.M.; Castro-Piedras, I.; Barnes, B.J.; Grisham, M.B.; Rahman, R.L.; Pruitt, K. The versatility of sirtuin-1 in endocrinology and immunology. Front. Cell Dev. Biol. 2020, 8, 589016. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, S.; Rumman, M.; Singh, B.; Pandey, S. Correction to: Role of silent information regulator 1 (sirt1) in regulating oxidative stress and inflammation. Inflammation 2021, 44, 2142. [Google Scholar] [CrossRef]
- Yadav, E.; Yadav, P.; Khan, M.M.U.; Singh, H.; Verma, A. Resveratrol: A potential therapeutic natural polyphenol for neurodegenerative diseases associated with mitochondrial dysfunction. Front. Pharmacol. 2022, 13, 922232. [Google Scholar] [CrossRef]
- Jardim, F.R.; de Rossi, F.T.; Nascimento, M.X.; da Silva Barros, R.G.; Borges, P.A.; Prescilio, I.C.; de Oliveira, M.R. Resveratrol and brain mitochondria: A review. Mol. Neurobiol. 2018, 55, 2085–2101. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Chen, S.D.; Jou, S.B.; Lin, T.K.; Chen, S.F.; Chen, N.C.; Hsu, C.Y. Sirtuin 1 regulates mitochondrial biogenesis and provides an endogenous neuroprotective mechanism against seizure-induced neuronal cell death in the hippocampus following status epilepticus. Int. J. Mol. Sci. 2019, 20, 3588. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.S.J.; Truong, T.T.T.; Bortolasci, C.C.; Spolding, B.; Panizzutti, B.; Swinton, C.; Kim, J.H.; Hernández, D.; Kidnapillai, S.; Gray, L.; et al. The potential of baicalin to enhance neuroprotection and mitochondrial function in a human neuronal cell model. Mol. Psychiatry 2024. [Google Scholar] [CrossRef] [PubMed]
- Poma, P. Nf-κb and disease. Int. J. Mol. Sci. 2020, 21, 9181. [Google Scholar] [CrossRef] [PubMed]
- Honarmand Tamizkar, K.; Badrlou, E.; Aslani, T.; Brand, S.; Arsang-Jang, S.; Ghafouri-Fard, S.; Taheri, M. Dysregulation of nf-κb-associated lncrnas in autism spectrum disorder. Front. Mol. Neurosci. 2021, 14, 747785. [Google Scholar] [CrossRef]
- Sarki, S.; Abdulmumin, T.; Murtala, M.; Abdulmumin, Y.; Muhammad, A.; Ismail, S.; Bichi, S.; Dalhatu, M.; Abubakar, A.; Shehu, A.; et al. Proximate composition, phytochemicals evaluation and characterization of aqueous fruit extract of balanites aegyptiaca (desert date palm). East Afr. Sch. J. Med Sci. 2022, 5, 176–184. [Google Scholar] [CrossRef]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective effects and therapeutic potential of the citrus flavonoid hesperetin in neurodegenerative diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.; Mandal, M.; Mishra, A. Effect of hesperidin on fluoride-induced neurobehavioral and biochemical changes in rats. J. Biochem. Mol. Toxicol. 2020, 34, e22575. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, C.; Pirozzi, C.; Coretti, L.; Cavaliere, G.; Lama, A.; Russo, R.; Lembo, F.; Mollica, M.P.; Meli, R.; Calignano, A.; et al. Palmitoylethanolamide counteracts autistic-like behaviours in btbr t+tf/j mice: Contribution of central and peripheral mechanisms. Brain Behav. Immun. 2018, 74, 166–175. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.; Xie, B.; Peng, Z.; Manning, J.R.; Zimmerman, R.; Wang, Q.; Wei, A.C.; Khalifa, M.; Reynolds, M.; et al. Myocardial brain-derived neurotrophic factor regulates cardiac bioenergetics through the transcription factor yin yang 1. Cardiovasc. Res. 2023, 119, 571–586. [Google Scholar] [CrossRef]
- Houten, S.M.; Wanders, R.J. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.; O’Neill, G.; Abu-Ghannam, N. Potential psychoactive effects of microalgal bioactive compounds for the case of sleep and mood regulation: Opportunities and challenges. Mar. Drugs 2022, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tuo, H.; Wang, S.; Zhao, L. The effects of dietary nutrition on sleep and sleep disorders. Mediat. Inflamm. 2020, 2020, 3142874. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Harvey, A.G.; Lockley, S.W.; Dijk, D.J. Circadian rhythms and disorders of the timing of sleep. Lancet 2022, 400, 1061–1078. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Que, M.; Wang, X.; Zhan, G.; Zhou, Z.; Luo, X.; Li, S. Exploring astrocyte-mediated mechanisms in sleep disorders and comorbidity. Biomedicines 2023, 11, 2476. [Google Scholar] [CrossRef] [PubMed]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from ecklonia cava (phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich fraction from ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of erk-creb-bdnf pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, R.; Liu, Y.; Wu, Z.; Weng, P. The interaction effect between tea polyphenols and intestinal microbiota: Role in ameliorating neurological diseases. J. Food Biochem. 2022, 46, e13870. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar]
- Ghazizadeh, J.; Hamedeyazdan, S.; Torbati, M.; Farajdokht, F.; Fakhari, A.; Mahmoudi, J.; Araj-Khodaei, M.; Sadigh-Eteghad, S. Melissa officinalis L. Hydro-alcoholic extract inhibits anxiety and depression through prevention of central oxidative stress and apoptosis. Exp. Physiol. 2020, 105, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic acid-human pharmacokinetics and health benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P.H.; Clément, O.; Valencia Garcia, S.; Brischoux, F.; Fort, P. New aspects in the pathophysiology of rapid eye movement sleep behavior disorder: The potential role of glutamate, gamma-aminobutyric acid, and glycine. Sleep Med. 2013, 14, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Leichsenring, F.; Leweke, F. Social anxiety disorder. N. Engl. J. Med. 2017, 376, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.B.; Sareen, J. Clinical practice. Generalized anxiety disorder. N. Engl. J. Med. 2015, 373, 2059–2068. [Google Scholar] [CrossRef] [PubMed]
- DeMartini, J.; Patel, G.; Fancher, T.L. Generalized anxiety disorder. Ann. Intern. Med. 2019, 170, Itc49–Itc64. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Sadeh, N. Traumatic stress, oxidative stress and post-traumatic stress disorder: Neurodegeneration and the accelerated-aging hypothesis. Mol. Psychiatry 2014, 19, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Emhan, A.; Selek, S.; Bayazıt, H.; Fatih Karababa, İ.; Katı, M.; Aksoy, N. Evaluation of oxidative and antioxidative parameters in generalized anxiety disorder. Psychiatry Res. 2015, 230, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ros) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Tanase, D.M.; Apostol, A.G.; Costea, C.F.; Tarniceriu, C.C.; Tudorancea, I.; Maranduca, M.A.; Floria, M.; Serban, I.L. Oxidative stress in arterial hypertension (htn): The nuclear factor erythroid factor 2-related factor 2 (nrf2) pathway, implications and future perspectives. Pharmaceutics 2022, 14, 534. [Google Scholar] [CrossRef]
- Narayanankutty, A.; Job, J.T.; Narayanankutty, V. Glutathione, an antioxidant tripeptide: Dual roles in carcinogenesis and chemoprevention. Curr. Protein Pept. Sci. 2019, 20, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Cao, L.; Wu, F.; Wang, L.; Wang, G.; Yu, Y.; Zhang, M.; Chen, L.; Wang, W.; Lv, W.; et al. The effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus-pituitary-adrenal axis. Neuropharmacology 2015, 97, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.H. Effects of epigallocatechin gallate on behavioral and cognitive impairments, hypothalamic-pituitary-adrenal axis dysfunction, and alternations in hippocampal bdnf expression under single prolonged stress. J. Med. Food 2018, 21, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Sur, B.; Kwon, S.; Hahm, D.H.; Lee, B. The anxiolytic-like effects of protocatechuic acid in an animal model of post-traumatic stress disorder. J. Med. Food 2022, 25, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Beidler, J.; Hong, M.Y. Resveratrol and depression in animal models: A systematic review of the biological mechanisms. Molecules 2018, 23, 2197. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, microbiome interactions and cellular effects on health in humans and animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Dębińska, A.; Sozańska, B. Dietary polyphenols-natural bioactive compounds with potential for preventing and treating some allergic conditions. Nutrients 2023, 15, 4823. [Google Scholar] [CrossRef] [PubMed]
- Nazam, N.; Jabir, N.R.; Ahmad, I.; Alharthy, S.A.; Khan, M.S.; Ayub, R.; Tabrez, S. Phenolic acids-mediated regulation of molecular targets in ovarian cancer: Current understanding and future perspectives. Pharmaceuticals 2023, 16, 274. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Aatif, M.; Muteeb, G.; Khan, K.; Siddiqui, F.A. Dietary polyphenols, plant metabolites, and allergic disorders: A comprehensive review. Pharmaceuticals 2024, 17, 670. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant activity, metabolism, and bioavailability of polyphenols in the diet of animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Iuchi, A.; Harada, H.; Hashimoto, S. Potential beneficial effects of wine flavonoids on allergic diseases. Diseases 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
| Compounds | Disease | Model | Effects and Mechanisms | References | ||
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | In Vitro | In Vivo | |||
| RES | Autism-spectrum disorder | KGN cells | Mice: VPA | Improvement of mitochondrial quantity through stimulating SIRT1/PGC-1α. | Prevents mPFC neuronal changes, antioxidant and neuroprotective effects, improves E/I balance-related parameters. | [43,44] |
| C2C12 cells | Mice: BTBR | Neuroprotection through inducing AMPK activation, regulating SIRT-1 protein activity, and promoting mitochondrial biogenesis. | Restoration of social interaction and enhancement of socialization in mice, improvement of neuroimmune disorders, suppression of molecules that promote inflammation and the signaling pathway involving TLR/NF-κB transcription factors. | [45,46] | ||
| BAI | BV-2 cells | Wistar rats: VPA | Improvement of neurocognitive deficits through reversing neuroinflammation, inhibition of HMGB1 release via the SIRT1/HMGB1 pathway, and reducing LPS-induced nuclear translocation of HMGB1. | Enhances postnatal growth and maturity, while also improving motor development, repetitive behaviors, and social impairments in rats who were exposed to VPA during prenatal stages. Improved functionality of mitochondria in neurons, increased sirtuin-1 (SIRT1) levels in brain tissue of VPA rats. | [47,48] | |
| AFE | RAW 264.7 cells | SD rats: VPA | Suppression of inflammation, blocking of LPS-induced NF-κB and MAPK signaling pathways in RAW264.7 macrophages. | Notable enhancements in neurobehavioral alterations seen in the raised plus-T maze, water maze, and rotating rod test, increases the expression of Nrf2 and HO-1, SIRT-1, and LC3, decreases the expression of NFκB. | [49,50] | |
| HES | BV-2 cells | SD rats: sodium fluoride | Antioxidant, anti-inflammatory, and anti-apoptosis, inhibition of the TLR4 /p-NF-κB signaling pathway. | Ameliorates neurobehavioral disorders and protects the nervous system, modulates Nrf2/Tlr4/NFκB signaling. | [51,52] | |
| PSs | Sleep disorder | HaCaT cells | Mice: caffeine | ROS inhibition, anti-oxidative stress, inhibition of the MAPK signaling pathway. | Relief of transient insomnia symptoms, promoting sleep by regulating GABA. | [53,54] |
| TPs | PC12 cells | Mice: inversion light/dark cycle | Anti-oxidative stress, increases p-ATM and p-Chk2 expression, activates DNA repair signaling pathway. | Enhancement of internal and peripheral circadian rhythm abnormalities and cognitive impairment, enhances the quantity of hypothalamic cell clusters, increases the expression of astrocytes and fibroblasts, and ameliorates structural abnormalities in the intestinal microbiota. | [55,56] | |
| RA | PC12 cells | SD rats: pentobarbital | Antioxidant stress, mediates Akt/GSK-3β/Fyn pathway activation of Nrf2. | Decreased sleep/wake cycle and REM sleep counts, increased sleep duration, increased glutamic acid decarboxylase and GABAA receptor expression. | [57,58] | |
| TRE | Anxiety disorder | HaCaT cells | SD rats: TDS | Reduces cytotoxicity and reduces apoptosis, protects against oxidative stress. | The TDS-induced decreases in the proportion of time spent in the middle of the arena, open-arm entrance, and time spent in the arena with open arms in the open field and raised cross maze tests were reversed. Reverses the index of adrenal activity and levels of corticotropin-releasing factor (CRF), while enhancing the phosphorylation of cyclic AMP response element-binding protein (pCREB) and levels of brain-derived neurotrophic factor (BDNF). | [59,60] |
| CGA | HepG2 cells | Mice: SCOP | Antioxidant stress, activation of Nrf2, ARE gene, and GCL, HO-1 and Sestrin2 expression. | Enhances short-term or working memory impairments in the scopolamine-induced Y-maze test, effectively counteracts cognitive impairments in the passive avoidance test in mice, and decreases the time taken to escape in the Morris water maze test, enhances GABA activity, and avoids neurological harm. | [61,62] | |
| HpE | HepG2 cells | Wistar rats: FG-7142 | Cryoprotection, activation of Nrf2 and increases GSH levels. | Improves anxiety behavior, modulates oxidative stress and inflammatory response, reduces IL-1α, IL-1β, MCP1, IFN, and MIP, reduces MDA levels, increases CAT and SOD activity, reduces CORT levels. | [63,64] | |
| GSE | SD rats: Cd | Increases glutathione reductase (GR) levels, restores GST and GPx expression, and decreases MDA levels to prevent oxidative damage. Restores 5-HTT expression. | [65] | |||
| CUR | SH-SY5Y cells | Wistar rats: immobilization stress | Inhibition of cellular inflammatory damage. Increased PPARγ protein expression, increased activity of ROS scavenging enzymes SOD and CAT. | Improves anxiety behavior, prevents stress-induced behavioral deficits, improves memory, reduces brain MDA levels, elevated CAT, GPx, SOD, and AChE activities. | [66,67] | |
| ANT | Depression | BV2 microglial cells | Mice: CUMS | Anti-inflammatory effect, blocking activation of NF-κB, PI3K/Akt, and MAPK signaling cascade responses in microglia. | Depression-like behavior was significantly improved after CUMS treatment. Mediation of the ERK/CREB/BDNF signaling pathway was enhanced, which upregulated BDNF and promoted neuronal dendrite development. | [68,69] |
| QUE | SH-SY5Y cells | Mice: CUS | Modulation of the NF-κB/HO pathway to inhibit NO and iNOS expression. Prevents NF-κB nuclear translocation and HO-1 downregulation. | Markedly decreased anxiety, relieved sadness, improved cognitive impairment, and restored normal motor functioning. Decreased concentrations of indicators of oxidative stress. The levels of TBARS, NO, and antioxidants (total thiols, catalase) were increased. Decreased production of pro-inflammatory cytokines (IL-6, TNF-α, IL-1β, and COX-2) in the hippocampus and injured hippocampal neurons. | [70,71] | |
| FA | PC12 cells | SD rats: PD | Resistance to oxidative stress in PC12 cells. Inhibits phosphorylation of ERK to attenuate H2O2-induced cellular damage. Increases BDNF by regulating microRNA-10b expression. | Amelioration of depressive-like behavior in rats descended from prenatal stress, inhibition of IL-6, IL-1β, and TNF-α, increase in IL-10 mRNA and protein expression, and significant reduction in adrenocorticotropic hormone (ATH) and adrenocorticotropic hormone (ATH) concentrations. | [71,72] | |
| HMF | C6 cells | Mice: CUMS | Neuroprotective effect, induction of m-BDNF expression to exert its neuroprotective effect/CREB signaling. Inhibits PDE4B or PDE4D. | Amelioration of corticosterone-induced weight loss and depressive-like behavior, up-regulation of BDNF in the hippocampus via the ERK1/2/MAP system. | [73,74] | |
| Compounds | Disease | Type of Study | Sample Size | Treatment Schedule | Finding | Reference |
|---|---|---|---|---|---|---|
| RES | Autism-spectrum disorder | Randomized double-blind controlled trials | 62 | 250 mg RES twice daily for 10 weeks | No significant impact on irritation, improved ASD hyperactivity/noncompliance. | [75] |
| Randomized trial ex vivo study | 10 | RES 2 mg kg-1 per day, up to 50 mg per day; 12 weeks total treatment | Resveratrol substantially boosted mtFAO activity, particularly in fibroblasts from individuals with severe symptoms. | [76] | ||
| Luteolin | Case series of study | 37 | A total of 200 mg of lutein once daily for at least 4 months | Improved speech recovery by 10%, social interaction by 25%, eye contact and attention by 50%, and gastrointestinal and allergy problems in 75% of subjects without side effects. | [77] | |
| Prospective open label trial | 50 | 200 mg Luteolin per 10 kg body weight for 26 weeks | Significantly enhanced children’s adaptive functioning and conduct, transient (1–8 weeks) irritation, no serious adverse effects. | [78] | ||
| Case report | 1 | Co-ultraPEA-LUT® at a dose of 700 mg + 70 mg | Improved clinical picture and stereotype reduction in a 10-year-old boy. | [79] | ||
| QUE | Not mentioned | 17 | Supplementation with 250 mg of quercetin per day for 16 months | Some autistic individuals improved their global progress score, social interaction, receptive language, and expressive language. | [80] | |
| PSs | Sleep disorder | Randomized double-blind placebo-controlled trial | 24 | 500 mg/day for 1 week | PSs significantly increased “sleep duration” ratings and decreased dyspnea during supine REM sleep without major side effects. | [81] |
| TPs | Double-blind crossover design | 20 | Tea consumption (≥300 mL/day) within 7 days | Reduces stress, improves sleep quality. | [82] | |
| Randomized, placebo-controlled, double-blind crossover trial | 12 | Oolong tea (100 mg caffeine, 21.4 mg gallic acid, 97 mg catechins, 125 mg polymerized polyphenols) was eaten for 14 days | Increased fat oxidation but no improvement in sleep. | [83] | ||
| RA | Randomized controlled parallel trials. | 89 | 65 mg daily for 30 days | EGCG and RA enhanced sleep and decreased insomnia. | [84] | |
| CUR | Anxiety disorder | Parallel double-blind randomized controlled trials | 77 | 500 mg CUR extract for 8 weeks | Notable enhancement in the Gastrointestinal Symptom Rating Scale (GSRS) and the Depression, Anxiety and Stress Scale-21 (DASS-21) | [85] |
| AS | Randomized double-blind placebo-controlled trial | 132 | 430 mg, 860 mg and 1290 mg over 29 days | Long-term supplementation may benefit cognitive function and modulate physiological responses to stressors. Significant reduction in observed anxiety symptoms using the STAI score. | [86] | |
| GSE | Randomized double-blind, placebo-controlled trials | 78 | 300 mg/day for 16 weeks | GSE relieved perceived stress. | [87] | |
| ANT | Depression | not mentioned | 93 | Different anthocyanin intake | Dietary deficiencies in ANT may cause major depression. | [88] |
| AG | Randomized triple-blind placebo-controlled crossover trial | 50 | 1.34 g/day for 4 weeks | Improves BAI and BDI and reduces depression symptoms such as sorrow, weeping, trance, sleeplessness, irritability, exhaustion, loss of libido, and thoughts of punishment. | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Zhou, Y.; Xi, Y.; Zhou, H.; Tang, Z.; Xiong, L.; Qin, D. Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective. Pharmaceuticals 2024, 17, 775. https://doi.org/10.3390/ph17060775
Wu X, Zhou Y, Xi Y, Zhou H, Tang Z, Xiong L, Qin D. Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective. Pharmaceuticals. 2024; 17(6):775. https://doi.org/10.3390/ph17060775
Chicago/Turabian StyleWu, Xinchen, Yang Zhou, Yujiang Xi, Haimei Zhou, Zhengxiu Tang, Lei Xiong, and Dongdong Qin. 2024. "Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective" Pharmaceuticals 17, no. 6: 775. https://doi.org/10.3390/ph17060775
APA StyleWu, X., Zhou, Y., Xi, Y., Zhou, H., Tang, Z., Xiong, L., & Qin, D. (2024). Polyphenols: Natural Food-Grade Biomolecules for the Treatment of Nervous System Diseases from a Multi-Target Perspective. Pharmaceuticals, 17(6), 775. https://doi.org/10.3390/ph17060775








