Novel Histone Deacetylase (HDAC) Inhibitor Induces Apoptosis and Suppresses Invasion via E-Cadherin Upregulation in Pancreatic Ductal Adenocarcinoma (PDAC)
Abstract
1. Introduction
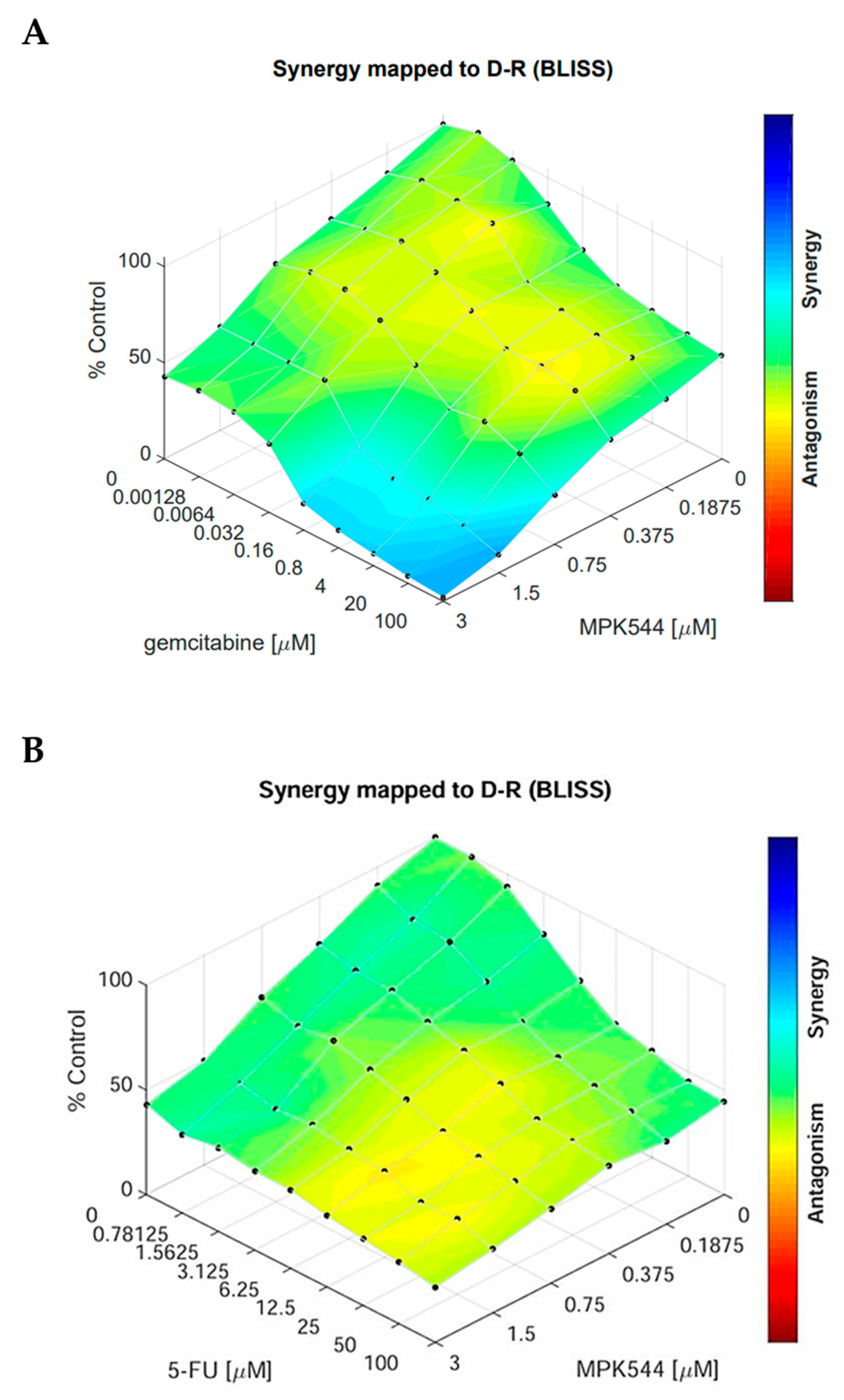
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Spheroid Formation
4.3. Cell Viability Assays
4.4. Western Blot Analysis
4.5. Quantitative Real-Time PCR (qRT-PCR)
4.6. Spherical Invasion Assay
4.7. Immunofluorescence of Whole Spheroids
4.8. Flow Cytometry
4.9. Combinatorial Drug Testing
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elrakaybi, A.; Ruess, D.A.; Lübbert, M.; Quante, M.; Becker, H. Epigenetics in Pancreatic Ductal Adenocarcinoma: Impact on Biology and Utilization in Diagnostics and Treatment. Cancers 2022, 14, 5926. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hosein, A.N.; Brekken, R.A.; Maitra, A. Pancreatic cancer stroma: An update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 487–505. [Google Scholar] [CrossRef]
- Nielsen, M.F.B.; Mortensen, M.B.; Detlefsen, S. Key players in pancreatic cancer-stroma interaction: Cancer-associated fibroblasts, endothelial and inflammatory cells. World J. Gastroenterol. 2016, 22, 2678–2700. [Google Scholar] [CrossRef]
- Bulle, A.; Lim, K.-H. Beyond just a tight fortress: Contribution of stroma to epithelial-mesenchymal transition in pancreatic cancer. Signal Transduct. Target. Ther. 2020, 5, 249. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Wang, S.; Gao, Q. An integrated overview of the immunosuppression features in the tumor microenvironment of pancreatic cancer. Front. Immunol. 2023, 14, 1258538. [Google Scholar] [CrossRef]
- Muller, M.; Haghnejad, V.; Schaefer, M.; Gauchotte, G.; Caron, B.; Peyrin-Biroulet, L.; Bronowicki, J.-P.; Neuzillet, C.; Lopez, A. The Immune Landscape of Human Pancreatic Ductal Carcinoma: Key Players, Clinical Implications, and Challenges. Cancers 2022, 14, 995. [Google Scholar] [CrossRef]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef]
- Xiang, X.-S.; Li, P.-C.; Wang, W.-Q.; Liu, L. Histone deacetylases: A novel class of therapeutic targets for pancreatic cancer. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188676. [Google Scholar] [CrossRef]
- Singh, T.; Kaur, P.; Singh, P.; Singh, S.; Munshi, A. Differential molecular mechanistic behavior of HDACs in cancer progression. Med. Oncol. 2022, 39, 171. [Google Scholar] [CrossRef]
- Kulka, L.A.M.; Fangmann, P.-V.; Panfilova, D.; Olzscha, H. Impact of HDAC Inhibitors on Protein Quality Control Systems: Consequences for Precision Medicine in Malignant Disease. Front. Cell Dev. Biol. 2020, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Barneda-Zahonero, B.; Parra, M. Histone deacetylases and cancer. Mol. Oncol. 2012, 6, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Seto, E.; Yoshida, M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [PubMed]
- Laschanzky, R.S.; Humphrey, L.E.; Ma, J.; Smith, L.M.; Enke, T.J.; Shukla, S.K.; Dasgupta, A.; Singh, P.K.; Howell, G.M.; Brattain, M.G.; et al. Selective Inhibition of Histone Deacetylases 1/2/6 in Combination with Gemcitabine: A Promising Combination for Pancreatic Cancer Therapy. Cancers 2019, 11, 1327. [Google Scholar] [CrossRef] [PubMed]
- Wawruszak, A.; Kalafut, J.; Okon, E.; Czapinski, J.; Halasa, M.; Przybyszewska, A.; Miziak, P.; Okla, K.; Rivero-Muller, A.; Stepulak, A. Histone Deacetylase Inhibitors and Phenotypical Transformation of Cancer Cells. Cancers 2019, 11, 148. [Google Scholar] [CrossRef] [PubMed]
- Krauß, L.; Schneider, C.; Hessmann, E.; Saur, D.; Schneider, G. Epigenetic control of pancreatic cancer metastasis. Cancer Metastasis Rev. 2023, 42, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.-H.; Zhang, X.; Yang, H.; Xu, X.-W.; Hu, Z.-L.; Yan, J.; Zheng, X.-L.; Wei, R.-R.; Zhang, Z.-Q.; Tang, S.-R.; et al. CUDC-907 displays potent antitumor activity against human pancreatic adenocarcinoma in vitro and in vivo through inhibition of HDAC6 to downregulate c-Myc expression. Acta Pharmacol. Sin. 2019, 40, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Luu, T. Epithelial-Mesenchymal Transition and its Regulation Mechanisms in Pancreatic Cancer. Front. Oncol. 2021, 11, 646399. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, S.-M.; Moon, J.-H.; Kim, J.H.; Shin, J.-S.; Hong, S.-W.; Shin, Y.J.; Lee, D.-H.; Lee, E.Y.; Hwang, I.-Y.; et al. SAHA, an HDAC inhibitor, overcomes erlotinib resistance in human pancreatic cancer cells by modulating E-cadherin. Tumour Biol. 2016, 37, 4323–4330. [Google Scholar] [CrossRef]
- Knoche, S.M.; Brumfield, G.L.; Goetz, B.T.; Sliker, B.H.; Larson, A.C.; Olson, M.T.; Poelaert, B.J.; Bavari, A.; Yan, Y.; Black, J.D.; et al. The histone deacetylase inhibitor M344 as a multifaceted therapy for pancreatic cancer. PLoS ONE 2022, 17, e0273518. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Pusceddu, S.; Ghidini, M.; Torchio, M.; Corti, F.; Tomasello, G.; Niger, M.; Prinzi, N.; Nichetti, F.; Coinu, A.; Di Bartolomeo, M.; et al. Comparative Effectiveness of Gemcitabine plus Nab-Paclitaxel and FOLFIRINOX in the First-Line Setting of Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis. Cancers 2019, 11, 484. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Radhakrishnan, P. Pancreatic Stellate Cells: The Key Orchestrator of The Pancreatic Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 57–70. [Google Scholar] [CrossRef]
- Cave, D.D.; Di Guida, M.; Costa, V.; Sevillano, M.; Ferrante, L.; Heeschen, C.; Corona, M.; Cucciardi, A.; Lonardo, E. TGF-β1 secreted by pancreatic stellate cells promotes stemness and tumourigenicity in pancreatic cancer cells through L1CAM downregulation. Oncogene 2020, 39, 4271–4285. [Google Scholar] [CrossRef]
- Marks, P.A.; Breslow, R. Dimethyl sulfoxide to vorinostat: Development of this histone deacetylase inhibitor as an anticancer drug. Nat. Biotechnol. 2007, 25, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.A. Discovery and development of SAHA as an anticancer agent. Oncogene 2007, 26, 1351–1356. [Google Scholar] [CrossRef]
- McClure, J.J.; Li, X.; Chou, C.J. Advances and Challenges of HDAC Inhibitors in Cancer Therapeutics. Adv. Cancer Res. 2018, 138, 183–211. [Google Scholar] [CrossRef]
- Stenzel, K.; Hamacher, A.; Hansen, F.K.; Gertzen, C.G.W.; Senger, J.; Marquardt, V.; Marek, L.; Marek, M.; Romier, C.; Remke, M.; et al. Alkoxyurea-Based Histone Deacetylase Inhibitors Increase Cisplatin Potency in Chemoresistant Cancer Cell Lines. J. Med. Chem. 2017, 60, 5334–5348. [Google Scholar] [CrossRef] [PubMed]
- Negmeldin, A.T.; Pflum, M.K.H. The structural requirements of histone deacetylase inhibitors: SAHA analogs modified at the C5 position display dual HDAC6/8 selectivity. Bioorg. Med. Chem. Lett. 2017, 27, 3254–3258. [Google Scholar] [CrossRef]
- Xie, B.; Hänsel, J.; Mundorf, V.; Betz, J.; Reimche, I.; Erkan, M.; Büdeyri, I.; Gesell, A.; Kerr, R.G.; Ariantari, N.P.; et al. Pseudopterosin and O-Methyltylophorinidine Suppress Cell Growth in a 3D Spheroid Co-Culture Model of Pancreatic Ductal Adenocarcinoma. Bioengineering 2020, 7, 57. [Google Scholar] [CrossRef]
- Aghdassi, A.; Sendler, M.; Guenther, A.; Mayerle, J.; Behn, C.-O.; Heidecke, C.-D.; Friess, H.; Büchler, M.; Evert, M.; Lerch, M.M.; et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut 2012, 61, 439–448. [Google Scholar] [CrossRef]
- Kaneta, Y.; Sato, T.; Hikiba, Y.; Sugimori, M.; Sue, S.; Kaneko, H.; Irie, K.; Sasaki, T.; Kondo, M.; Chuma, M.; et al. Loss of Pancreatic E-Cadherin Causes Pancreatitis-Like Changes and Contributes to Carcinogenesis. Cell. Mol. Gastroenterol. Hepatol. 2020, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Subramaniam, M.; Kari, V.; Pitel, K.S.; Baumgart, S.J.; Naylor, R.M.; Nagarajan, S.; Wegwitz, F.; Ellenrieder, V.; Hawse, J.R.; et al. Krüppel-like Transcription Factor KLF10 Suppresses TGFβ-Induced Epithelial-to-Mesenchymal Transition via a Negative Feedback Mechanism. Cancer Res. 2017, 77, 2387–2400. [Google Scholar] [CrossRef]
- Garajová, I.; Cavazzoni, A.; Verze, M.; Minari, R.; Pedrazzi, G.; Balsano, R.; Gelsomino, F.; Valle, R.D.; Digiacomo, G.; Giovannetti, E.; et al. It Takes Two to Tango: Potential Prognostic Impact of Circulating TGF-Beta and PD-L1 in Pancreatic Cancer. Life 2022, 12, 960. [Google Scholar] [CrossRef]
- Principe, D.R.; Timbers, K.E.; Atia, L.G.; Koch, R.M.; Rana, A. TGFβ Signaling in the Pancreatic Tumor Microenvironment. Cancers 2021, 13, 5086. [Google Scholar] [CrossRef]
- Alvarez, M.A.; Freitas, J.P.; Mazher Hussain, S.; Glazer, E.S. TGF-β Inhibitors in Metastatic Pancreatic Ductal Adenocarcinoma. J. Gastrointest. Cancer 2019, 50, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.D.; Akers, A.T.; Friedman, J.R.; Nolan, N.A.; Brown, K.C.; Dasgupta, P. Non-pungent long chain capsaicin-analogs arvanil and olvanil display better anti-invasive activity than capsaicin in human small cell lung cancers. Cell Adhes. Migr. 2017, 11, 80–97. [Google Scholar] [CrossRef]
- Krauß, L.; Urban, B.C.; Hastreiter, S.; Schneider, C.; Wenzel, P.; Hassan, Z.; Wirth, M.; Lankes, K.; Terrasi, A.; Klement, C.; et al. HDAC2 Facilitates Pancreatic Cancer Metastasis. Cancer Res. 2022, 82, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Alqosaibi, A.I.; Abdel-Ghany, S. Vorinostat induces G2/M cell cycle arrest in breast cancer cells via upregulation of PTEN. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1503–1511. [Google Scholar] [CrossRef]
- Niu, J.; Wang, X.; Qu, J.; Mager, D.E.; Straubinger, R.M. Pharmacodynamic modeling of synergistic birinapant/paclitaxel interactions in pancreatic cancer cells. BMC Cancer 2020, 20, 1024. [Google Scholar] [CrossRef]
- Urdiciain, A.; Erausquin, E.; Meléndez, B.; Rey, J.A.; Idoate, M.A.; Castresana, J.S. Tubastatin A, an inhibitor of HDAC6, enhances temozolomide–induced apoptosis and reverses the malignant phenotype of glioblastoma cells. Int. J. Oncol. 2019, 54, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.B.; Arkus, N.; Gunn, J.; Korc, M. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces growth inhibition and enhances gemcitabine-induced cell death in pancreatic cancer. Clin. Cancer Res. 2007, 13, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.S.; Moccia, T.; Iannelli, F.; Testa, C.; Vitagliano, C.; Minopoli, M.; Camerlingo, R.; de Riso, G.; de Cecio, R.; Bruzzese, F.; et al. HDAC class I inhibitor domatinostat sensitizes pancreatic cancer to chemotherapy by targeting cancer stem cell compartment via FOXM1 modulation. J. Exp. Clin. Cancer Res. 2022, 41, 83. [Google Scholar] [CrossRef] [PubMed]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Shustov, A.; Coiffier, B.; Horwitz, S.; Sokol, L.; Pro, B.; Wolfson, J.; Balser, B.; Eisch, R.; Popplewell, L.; Prince, H.M.; et al. Romidepsin is effective and well tolerated in older patients with peripheral T-cell lymphoma: Analysis of two phase II trials. Leuk. Lymphoma 2017, 58, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.A.; Horwitz, S.; Masszi, T.; van Hoof, A.; Brown, P.; Doorduijn, J.; Hess, G.; Jurczak, W.; Knoblauch, P.; Chawla, S.; et al. Belinostat in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J. Clin. Oncol. 2015, 33, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, C.S.; Meng, R.Y.; Jin, H.; Chai, O.H.; Kim, S.M. Regulation of Hippo-YAP/CTGF signaling by combining an HDAC inhibitor and 5-fluorouracil in gastric cancer cells. Toxicol. Appl. Pharmacol. 2024, 482, 116786. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.J.; Dunleavey, J.M.; Xiao, L.; Ollila, D.W.; Troester, M.A.; Otey, C.A.; Li, W.; Barker, T.H.; Dudley, A.C. Suppression of TGFβ-mediated conversion of endothelial cells and fibroblasts into cancer associated (myo)fibroblasts via HDAC inhibition. Br. J. Cancer 2018, 118, 1359–1368. [Google Scholar] [CrossRef]
- Mishra, V.K.; Wegwitz, F.; Kosinsky, R.L.; Sen, M.; Baumgartner, R.; Wulff, T.; Siveke, J.T.; Schildhaus, H.-U.; Najafova, Z.; Kari, V.; et al. Histone deacetylase class-I inhibition promotes epithelial gene expression in pancreatic cancer cells in a BRD4- and MYC-dependent manner. Nucleic Acids Res. 2017, 45, 6334–6349. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, J.; Zhao, J.; Yun, W.; Xie, C.; Taub, J.W.; Azmi, A.; Mohammad, R.M.; Dong, Y.; Kong, W.; et al. Class I and class II histone deacetylases are potential therapeutic targets for treating pancreatic cancer. PLoS ONE 2012, 7, e52095. [Google Scholar] [CrossRef]
- Gruber, W.; Peer, E.; Elmer, D.P.; Sternberg, C.; Tesanovic, S.; Del Burgo, P.; Coni, S.; Canettieri, G.; Neureiter, D.; Bartz, R.; et al. Targeting class I histone deacetylases by the novel small molecule inhibitor 4SC-202 blocks oncogenic hedgehog-GLI signaling and overcomes smoothened inhibitor resistance. Int. J. Cancer 2018, 142, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Hahn, S.; Oh, B.J.; Kim, H.; Han, I.W.; Shin, S.H.; Kim, G.; Jin, S.-M.; Kim, J.H. Anti-cancer effects of metformin in a 3D co-culture model of pancreatic ductal adenocarcinoma. Am. J. Cancer Res. 2023, 13, 1806–1825. [Google Scholar] [PubMed]
- Semaan, A.; Bernard, V.; Kim, D.U.; Lee, J.J.; Huang, J.; Kamyabi, N.; Stephens, B.M.; Qiao, W.; Varadhachary, G.R.; Katz, M.H.; et al. Characterisation of circulating tumour cell phenotypes identifies a partial-EMT sub-population for clinical stratification of pancreatic cancer. Br. J. Cancer 2021, 124, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.J.; Meneceur, S.; Hommel, K.; Schulz, W.A.; Niegisch, G. Downregulation of Cell Cycle and Checkpoint Genes by Class I HDAC Inhibitors Limits Synergism with G2/M Checkpoint Inhibitor MK-1775 in Bladder Cancer Cells. Genes 2021, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Rajoria, P.; Chopra, M. HDAC6: A unique HDAC family member as a cancer target. Cell. Oncol. 2022, 45, 779–829. [Google Scholar] [CrossRef]
- Kutil, Z.; Skultetyova, L.; Rauh, D.; Meleshin, M.; Snajdr, I.; Novakova, Z.; Mikesova, J.; Pavlicek, J.; Hadzima, M.; Baranova, P.; et al. The unraveling of substrate specificity of histone deacetylase 6 domains using acetylome peptide microarrays and peptide libraries. FASEB J. 2019, 33, 4035–4045. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Xiang, S.; Zhang, M.; Fang, B.; Huang, H.; Kwon, O.K.; Zhao, Y.; Yang, Z.; Bai, W.; Bepler, G.; et al. Histone deacetylase 6 (HDAC6) deacetylates extracellular signal-regulated kinase 1 (ERK1) and thereby stimulates ERK1 activity. J. Biol. Chem. 2018, 293, 1976–1993. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.; Wang, C.; Zhang, S.; Wang, Z.; Li, M.; Wang, Y.; Wang, X.; Yang, X. HDAC6, modulated by miR-206, promotes endometrial cancer progression through the PTEN/AKT/mTOR pathway. Sci. Rep. 2020, 10, 3576. [Google Scholar] [CrossRef]
- Yoo, J.; Jeon, Y.H.; Lee, D.H.; Kim, G.W.; Lee, S.W.; Kim, S.Y.; Park, J.; Kwon, S.H. HDAC6-selective inhibitors enhance anticancer effects of paclitaxel in ovarian cancer cells. Oncol. Lett. 2021, 21, 201. [Google Scholar] [CrossRef]
- Olah, G.A.; Fung, A.P. Hexahydro-2-(1H)-Azocinone. Org. Synth. 2003, 63, 188. [Google Scholar]
- Giovannini, A.; Savoia, D.; Umani-Ronchi, A. Organometallic ring-opening reactions of N-acyl and N-alkoxycarbonyl lactams. Synthesis of cyclic imines. J. Org. Chem. 1989, 54, 228–234. [Google Scholar] [CrossRef]
- Ugwuegbulam, C.O.; Foy, J.E. Process for the Preparation of Anti-Malarial Drugs. U.S. Patent 6,479,660, 12 November 2002. [Google Scholar]
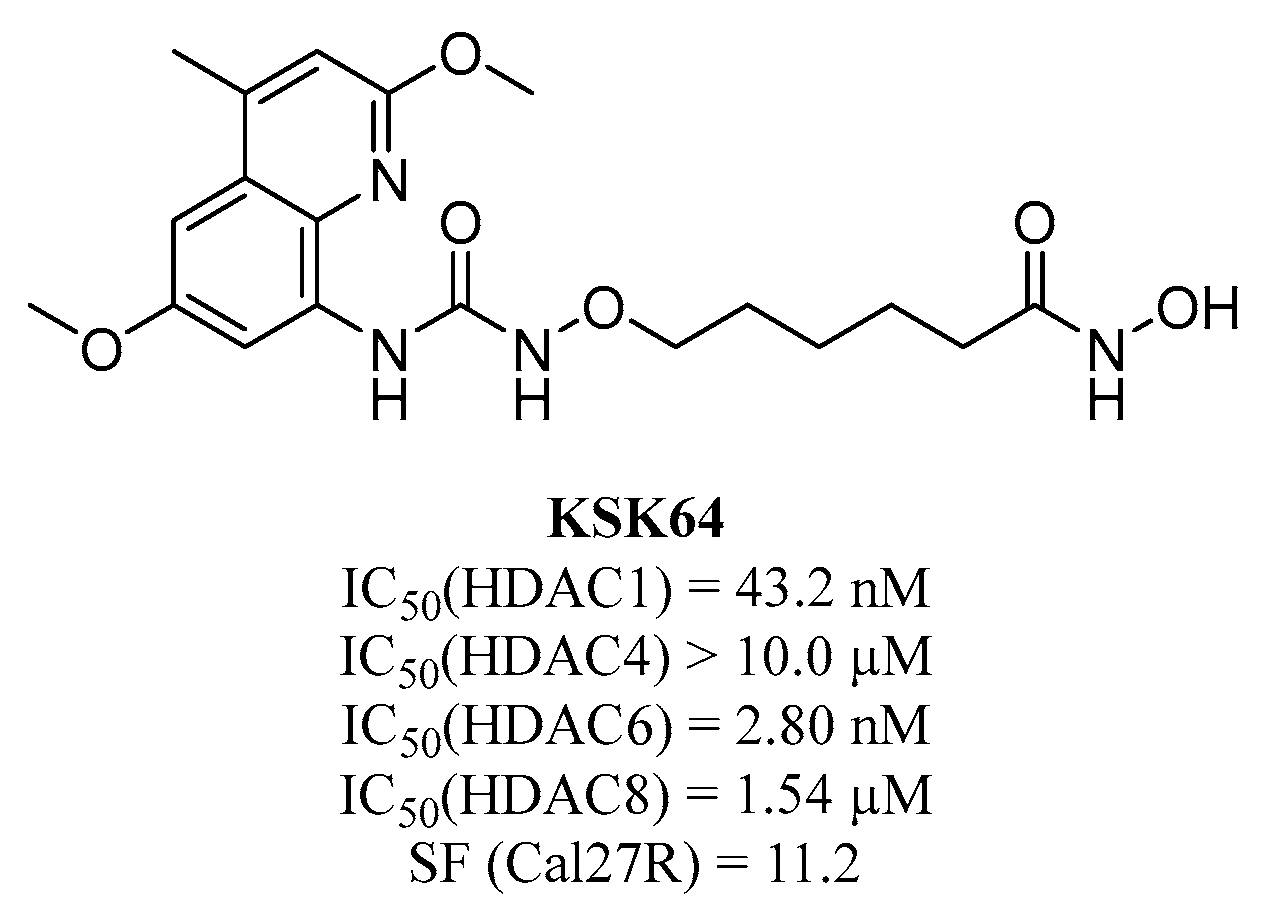
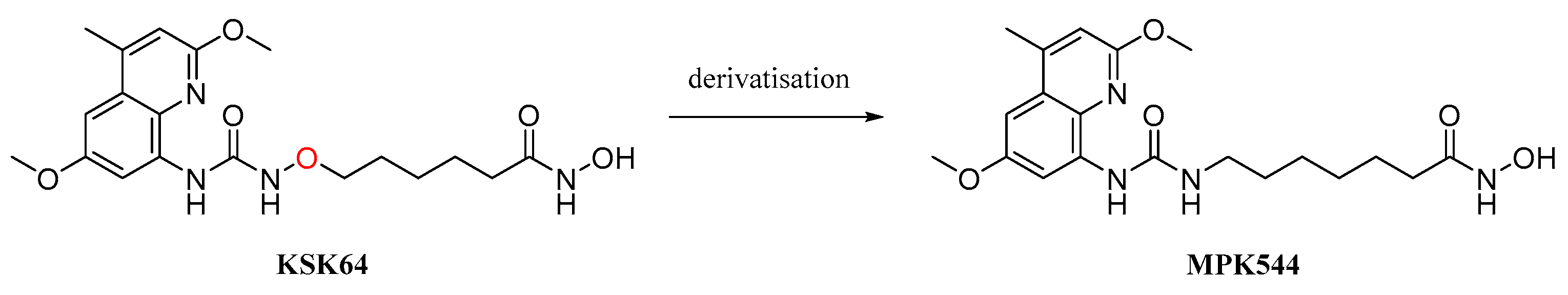
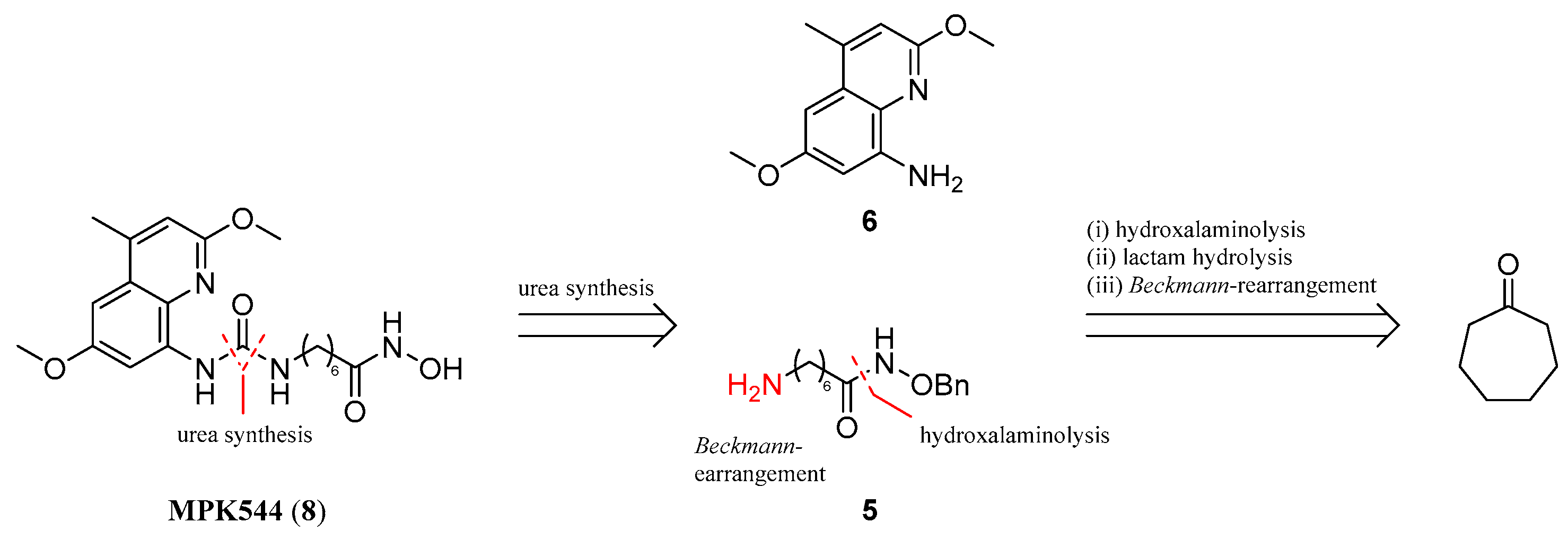
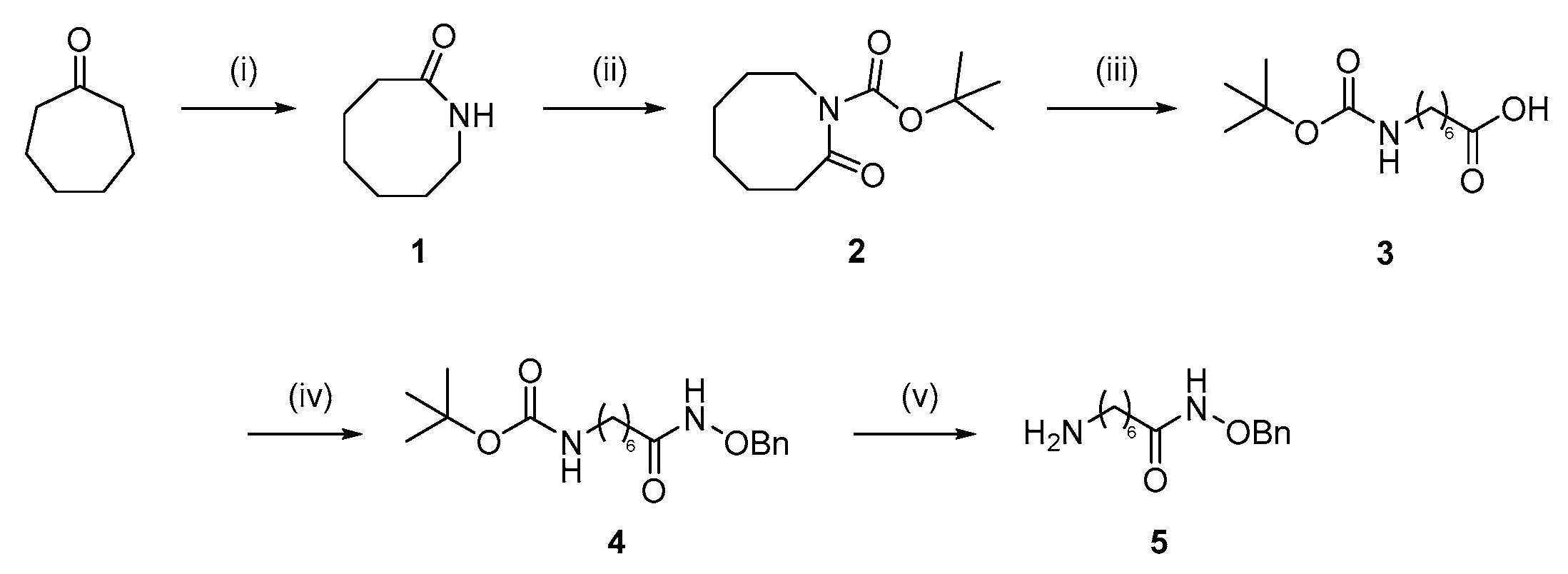
 ; 7 µM MPK544
; 7 µM MPK544  ). Representative Western blots are shown (B). Error bars indicate standard errors of mean (of at least three independent experiments, with; ** = p ≤ 0.01; and **** = p ≤ 0.0001).
). Representative Western blots are shown (B). Error bars indicate standard errors of mean (of at least three independent experiments, with; ** = p ≤ 0.01; and **** = p ≤ 0.0001).
 ; 7 µM MPK544
; 7 µM MPK544  ). Representative Western blots are shown (B). Error bars indicate standard errors of mean (of at least three independent experiments, with; ** = p ≤ 0.01; and **** = p ≤ 0.0001).
). Representative Western blots are shown (B). Error bars indicate standard errors of mean (of at least three independent experiments, with; ** = p ≤ 0.01; and **** = p ≤ 0.0001).
 ; 20 ng/mL TGF-β with 4 µM SAHA
; 20 ng/mL TGF-β with 4 µM SAHA  ; 20 ng/mL TGF-β with 10 µM tubastatin *). Error bars indicate standard errors of mean (of at least three independent experiments, with * = p ≤ 0.05; ** = p ≤ 0.01; and **** = p ≤ 0.0001).
; 20 ng/mL TGF-β with 10 µM tubastatin *). Error bars indicate standard errors of mean (of at least three independent experiments, with * = p ≤ 0.05; ** = p ≤ 0.01; and **** = p ≤ 0.0001).
 ; 20 ng/mL TGF-β with 4 µM SAHA
; 20 ng/mL TGF-β with 4 µM SAHA  ; 20 ng/mL TGF-β with 10 µM tubastatin *). Error bars indicate standard errors of mean (of at least three independent experiments, with * = p ≤ 0.05; ** = p ≤ 0.01; and **** = p ≤ 0.0001).
; 20 ng/mL TGF-β with 10 µM tubastatin *). Error bars indicate standard errors of mean (of at least three independent experiments, with * = p ≤ 0.05; ** = p ≤ 0.01; and **** = p ≤ 0.0001).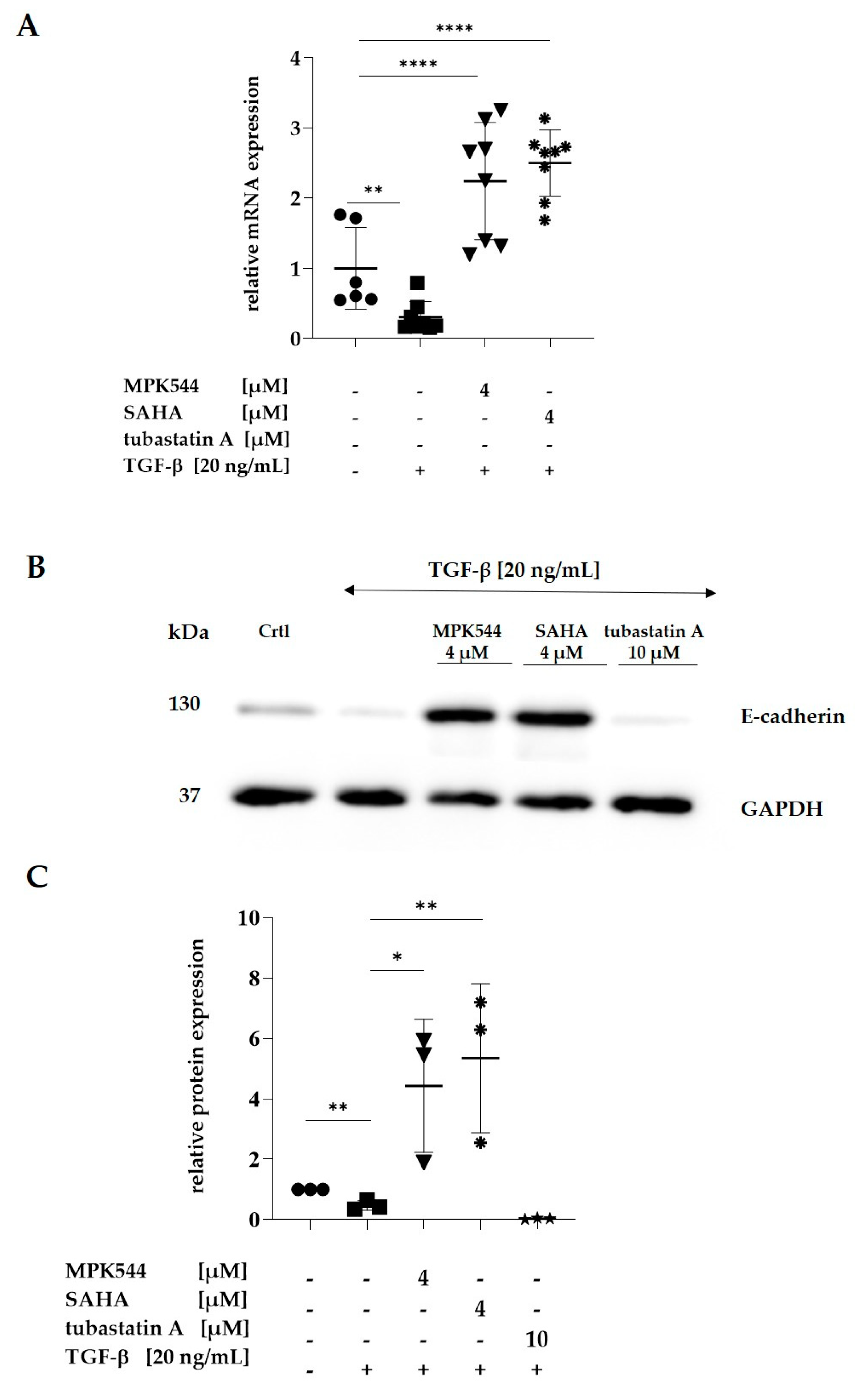
 ). E-cadherin expression was measured in at least 3000 cells. Error bars indicate standard errors of mean (n = 3 independent experiments with * = p ≤ 0.05.
). E-cadherin expression was measured in at least 3000 cells. Error bars indicate standard errors of mean (n = 3 independent experiments with * = p ≤ 0.05.
 ). E-cadherin expression was measured in at least 3000 cells. Error bars indicate standard errors of mean (n = 3 independent experiments with * = p ≤ 0.05.
). E-cadherin expression was measured in at least 3000 cells. Error bars indicate standard errors of mean (n = 3 independent experiments with * = p ≤ 0.05.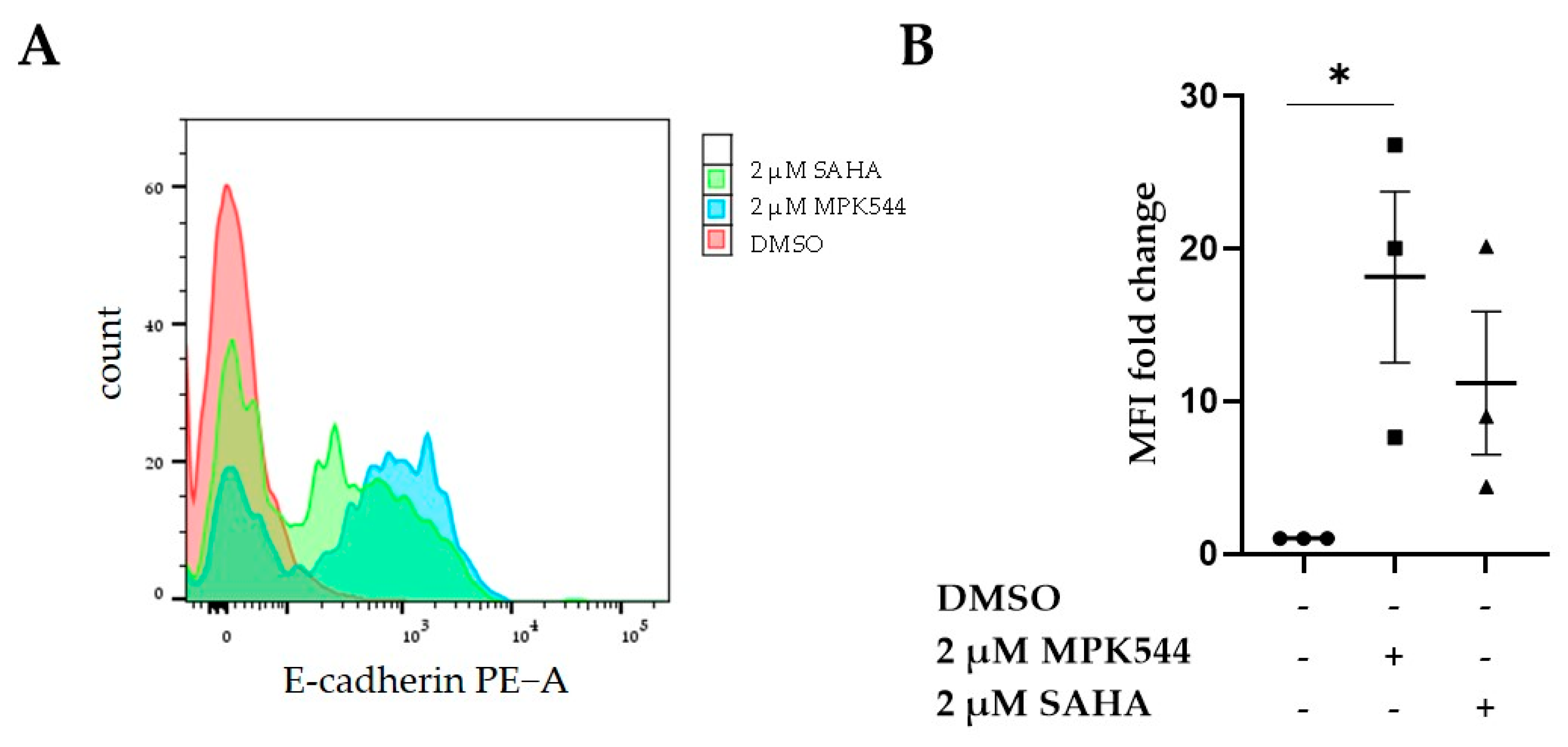
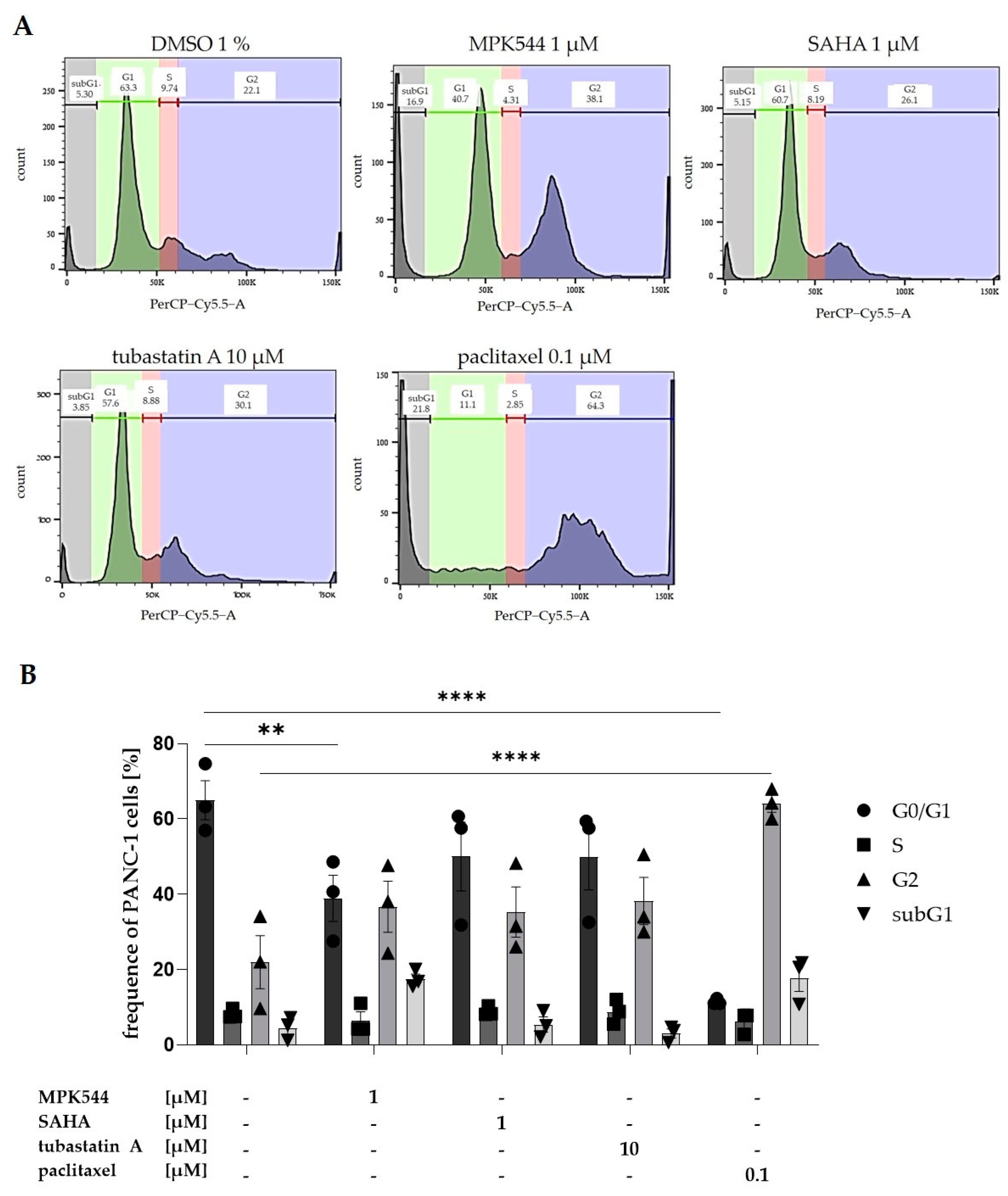
 ). Error bars indicate the standard errors of the mean (n = 3 independent experiments with **** = p ≤ 0.0001).
). Error bars indicate the standard errors of the mean (n = 3 independent experiments with **** = p ≤ 0.0001).
 ). Error bars indicate the standard errors of the mean (n = 3 independent experiments with **** = p ≤ 0.0001).
). Error bars indicate the standard errors of the mean (n = 3 independent experiments with **** = p ≤ 0.0001).

| HDAC Inhibitors | PANC-1 | PSC | Co-Culture Spheroid |
|---|---|---|---|
| IC50 in µM | |||
| MPK544 | 2.95 ± 0.45 | 0.68 ± 0.48 | 0.90 ± 0.15 |
| KSK64 | 7.04 ± 0.58 | 14.87 ± 4.55 | 3.30 ± 0.58 |
| SAHA | 3.65 ± 0.43 | 3.50 ± 0.99 | 0.85 ± 0.25 |
| tubastatin A | >100 | >100 | >100 |
| HDAC 2 | HDAC 4 | HDAC 6 |
|---|---|---|
| IC50 in µM | ||
| 0.058 ± 0.02 | >100 | 0.097 ± 0.062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiedlauske, K.; Deipenbrock, A.; Pflieger, M.; Hamacher, A.; Hänsel, J.; Kassack, M.U.; Kurz, T.; Teusch, N.E. Novel Histone Deacetylase (HDAC) Inhibitor Induces Apoptosis and Suppresses Invasion via E-Cadherin Upregulation in Pancreatic Ductal Adenocarcinoma (PDAC). Pharmaceuticals 2024, 17, 752. https://doi.org/10.3390/ph17060752
Schiedlauske K, Deipenbrock A, Pflieger M, Hamacher A, Hänsel J, Kassack MU, Kurz T, Teusch NE. Novel Histone Deacetylase (HDAC) Inhibitor Induces Apoptosis and Suppresses Invasion via E-Cadherin Upregulation in Pancreatic Ductal Adenocarcinoma (PDAC). Pharmaceuticals. 2024; 17(6):752. https://doi.org/10.3390/ph17060752
Chicago/Turabian StyleSchiedlauske, Katja, Alina Deipenbrock, Marc Pflieger, Alexandra Hamacher, Jan Hänsel, Matthias U. Kassack, Thomas Kurz, and Nicole E. Teusch. 2024. "Novel Histone Deacetylase (HDAC) Inhibitor Induces Apoptosis and Suppresses Invasion via E-Cadherin Upregulation in Pancreatic Ductal Adenocarcinoma (PDAC)" Pharmaceuticals 17, no. 6: 752. https://doi.org/10.3390/ph17060752
APA StyleSchiedlauske, K., Deipenbrock, A., Pflieger, M., Hamacher, A., Hänsel, J., Kassack, M. U., Kurz, T., & Teusch, N. E. (2024). Novel Histone Deacetylase (HDAC) Inhibitor Induces Apoptosis and Suppresses Invasion via E-Cadherin Upregulation in Pancreatic Ductal Adenocarcinoma (PDAC). Pharmaceuticals, 17(6), 752. https://doi.org/10.3390/ph17060752







