Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders
Abstract
1. Introduction
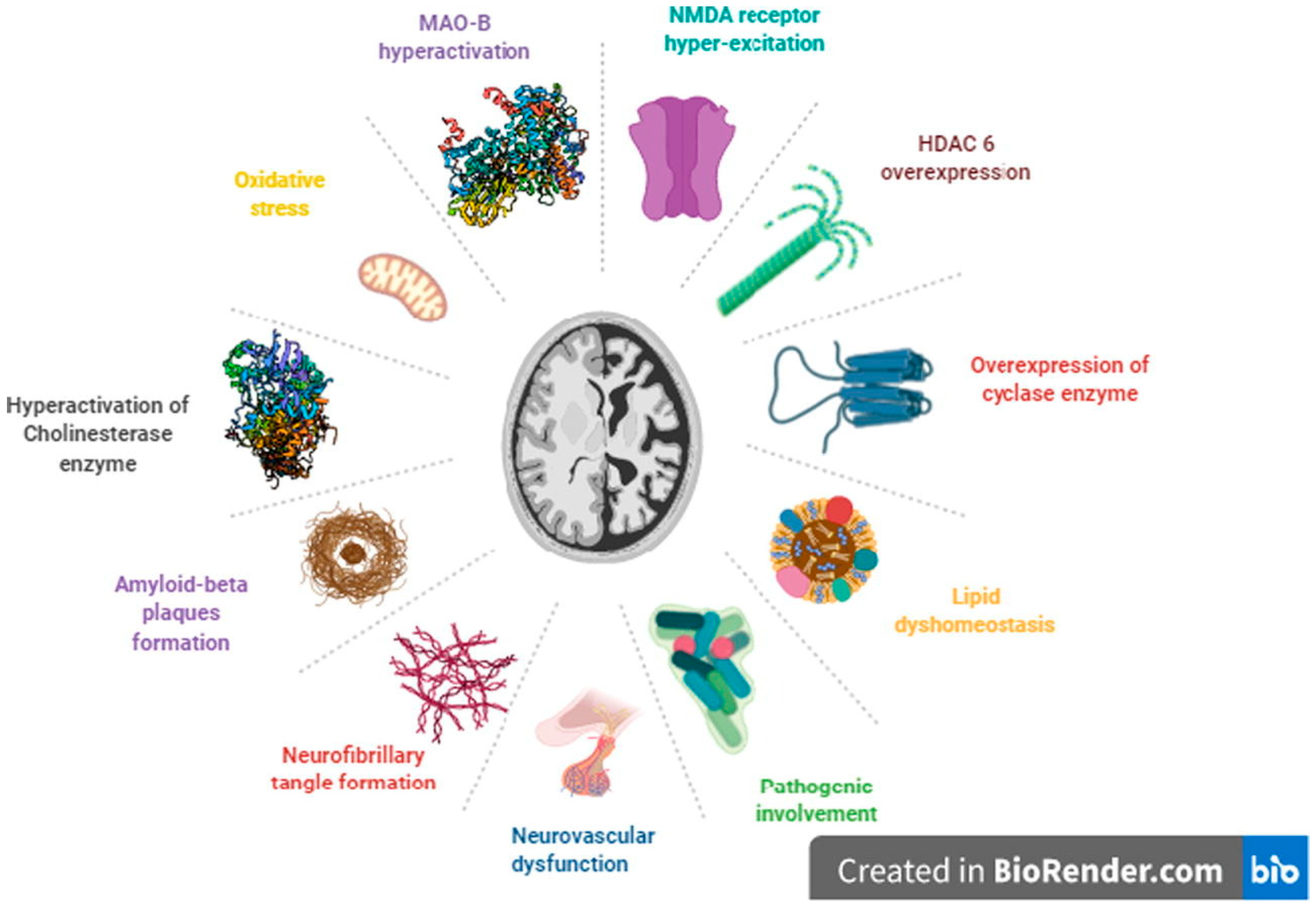
2. Alzheimer’s Disease (AD)
3. Multi-Target Drugs
3.1. Chemical-Based Drugs
3.2. Immune System-Modulating Drugs
3.3. Nanobodies
3.3.1. Fab Fragments
3.3.2. Domain Antibodies
3.3.3. Single-Chain Variable Fragments (scFv)
3.4. Antibody Targeting
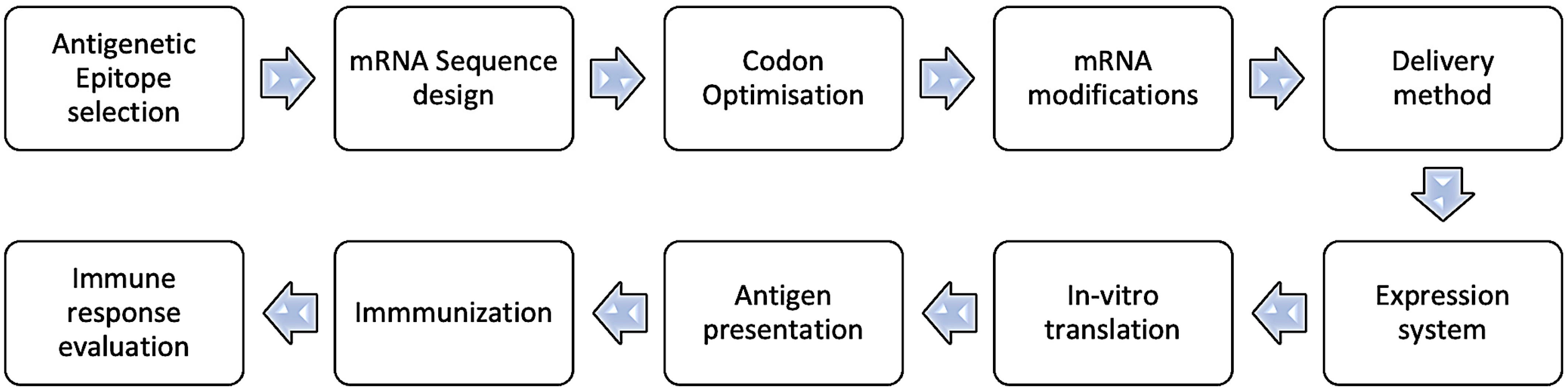
- 1.
- Select an Antigenic Epitope: Identify a specific sequence or epitope known to be antigenic, crucial for antibody–antigen interaction in AD.
- 2.
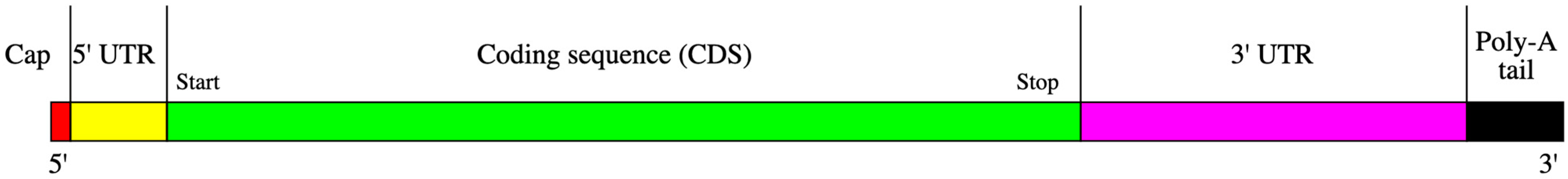
- Design mRNA Sequence: Create an mRNA sequence encoding the chosen epitope, incorporating a 5′ cap and a 3′ poly-A tail to align with transcription, such as starting with a 5′ cap and including a 3′ poly-A tail. Ensure the sequence is in-frame with the ribosome so that translation produces the desired epitope.
- 3.
- Codon Optimization: Optimize the mRNA sequence for effective translation in the desired host cell, mainly by selecting codons frequently used by the host.
- 4.
- Consider mRNA Modifications: To boost stability and translation, integrate modified nucleotides such as pseudouridine or 5-methylcytidine into the mRNA sequence, which minimizes immune recognition. Alternatively, the replacement of uridine with pseudouridine is also a practical approach.
- 5.
- Delivery Method: Decide on the delivery approach for the mRNA to the target cells, such as electroporation, lipid nanoparticles, or viral vectors.
- 6.
- Expression System: Select an efficient expression system for effective mRNA translation and epitope production, such as a suitable cell line or organism.
- 7.
- In Vitro Translation: Verify the mRNA’s ability to produce the desired epitope through in vitro translation systems.
- 8.
- Antigen Presentation: Process and present the translated antigenic peptide on the cell surface via major histocompatibility complex (MHC) molecules for immune recognition.
- 9.
- Immunization: Use the peptide to immunize, stimulating an immune response as part of a vaccine or immunotherapy.
- 10.
- Immune Response Evaluation: Assess the immune response by measuring antibody or T-cell reactions against the peptide using enzyme-linked immunosorbent assay (ELISA), flow cytometry, or cytokine assays.
3.5. mRNA-Based Antibodies
4. AI-Driven Multi-Target Drugs
5. Drug Delivery across the BBB
Strategies That Aid Drugs Cross the Blood–Brain Barrier
- 1.
- Invasive techniques include intra-cerebral injection, convection-enhanced delivery, and intra-cerebroventricular infusion [121].
- 2.
- BBB disruption with bradykinin analogs, ultrasonography, and osmotic pressure [122].
- 3.
- Physiological procedures involving transporter-mediated delivery, receptor-mediated transcytosis, and adsorptive-mediated transcytosis [123].
- 4.
- Pharmacological techniques involving liposome-mediated drug delivery or chemically modifying pharmaceuticals to lipophilic molecules [124].
- 5.
- Opsonization and drug delivery by nanoparticles across the BBB, wherein the drug is adsorbed onto the particles passively [125].
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ciurea, A.V.; Mohan, A.G.; Covache-Busuioc, R.A.; Costin, H.P.; Glavan, L.A.; Corlatescu, A.D.; Saceleanu, V.M. Unraveling molecular and genetic insights into neurodegenerative diseases: Advances in understanding Alzheimer’s, Parkinson’s, and Huntington’s diseases and amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2023, 24, 10809. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic brain injury and risk of neurodegenerative disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Kakoti, B.B.; Bezbaruah, R.; Ahmed, N. Therapeutic drug repositioning with special emphasis on neurodegenerative diseases: Threats and issues. Front. Pharmacol. 2022, 13, 1007315. [Google Scholar] [CrossRef]
- Han, C.; Chaineau, M.; Chen, C.X.; Beitel, L.K.; Durcan, T.M. Open science meets stem cells: A new drug discovery approach for neurodegenerative disorders. Front. Neurosci. 2018, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimer’s Dement. 2022, 8, e12295. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.; Yao, Z.; Khleifat, A.A.L.; Tantiangco, H.; Tamburin, S.; Albertyn, C.; Thakur, L.; Llewellyn, D.J.; Oxtoby, N.P.; Lourida, I.; et al. Artificial intelligence for dementia drug discovery and trials optimization. Alzheimer’s Dement. 2023, 19, 5922–5933. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.; Bose, N.; Nisenbaum, L.; Partrick, K.A.; Fillit, H.M. The critical role of biomarkers for drug development targeting the biology of aging. J. Prev. Alzheimer’s Dis. 2023, 10, 729–742. [Google Scholar] [CrossRef]
- Li, S.; Yi, Y.; Cui, K.; Zhang, Y.; Chen, Y.; Han, D.; Sun, L.; Zhang, X.; Chen, F.; Zhang, Y.; et al. A single-chain variable fragment antibody inhibits aggregation of phosphorylated tau and ameliorates tau toxicity in vitro and in vivo. J. Alzheimer’s Dis. 2021, 79, 1613–1629. [Google Scholar] [CrossRef]
- Medeiros, R.; Baglietto-Vargas, D.; LaFerla, F.M. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci. Ther. 2011, 17, 514–524. [Google Scholar] [CrossRef]
- Kumari, S.; Mehta, S.L.; Li, P.A. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: Preventive effects of selenium. PLoS ONE 2012, 7, e39382. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Song, C.; Qin, T.; Zhai, Z.; Cai, J.; Dai, J.; Sun, T.; Xu, Y. Moschus ameliorates glutamate-induced cellular damage by regulating autophagy and apoptosis pathway. Sci. Rep. 2023, 13, 18586. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G. The pathobiology of Alzheimer’s disease. Annu. Rev. Med. 1989, 40, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Roher, A.E.; Lowenson, J.D.; Clarke, S.; Woods, A.S.; Cotter, R.J.; Gowing, E.; Ball, M.J. beta-amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: Implications for the pathology of Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 10836–10840. [Google Scholar] [CrossRef] [PubMed]
- Ow, S.Y.; Dunstan, D.E. A brief overview of amyloids and Alzheimer’s disease. Protein Sci. 2014, 23, 1315–1331. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Gawas, C.; Ahmad, I.Z.; Wani, M.; Tabassum, H. Neurodegenerative disorders: Assessing the impact of natural vs drug-induced treatment options. Aging Med. (Milton). 2023, 6, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Waiker, D.K.; Bhardwaj, B.; Saraf, P.; Shrivastava, S.K. The molecular mechanism, targets, and novel molecules in the treatment of Alzheimer’s disease. Bioorganic Chem. 2022, 119, 105562. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.L.; Tiew, J.K.; Fong, Y.H.; Leong, H.W.; Chan, Y.M.; Chan, Z.L.; Kong, E.W.J. Current pharmacotherapy and multi-target approaches for Alzheimer’s disease. Pharmaceuticals 2022, 15, 1560. [Google Scholar] [CrossRef]
- Medina-Franco, J.L.; Giulianotti, M.A.; Welmaker, G.S.; Houghten, R.A. Shifting from the single to the multitarget paradigm in drug discovery. Drug Discov. Today 2013, 18, 495–501. [Google Scholar] [CrossRef]
- Talevi, A. Multi-target pharmacology: Possibilities and limitations of the "skeleton key approach" from a medicinal chemist perspective. Front. Pharmacol. 2015, 6, 205. [Google Scholar] [CrossRef] [PubMed]
- Makhoba, X.H.; Viegas, C., Jr.; Mosa, R.A.; Viegas, F.P.D.; Pooe, O.J. Potential impact of the multi-target drug approach in the treatment of some complex diseases. Drug Des. Devel Ther. 2020, 14, 3235–3249. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W. Single-target versus multi-target drugs versus combinations of drugs with multiple targets: Preclinical and clinical evidence for the treatment or prevention of epilepsy. Front. Pharmacol. 2021, 12, 730257. [Google Scholar] [CrossRef] [PubMed]
- Kieburtz, K. Treating neurodegenerative disease before illness: A challenge for the 21st century. Lancet Neurol. 2016, 15, 540–541. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Safia; Haque, E.; Mir, S.S. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, G.; Qin, Z.; Yin, M.; Chen, W.; Zhang, Y.; Liu, X. Safinamide protects against amyloid β (Aβ)-induced oxidative stress and cellular senescence in M17 neuronal cells. Bioengineered 2022, 13, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Dodge, H.H.; Arnold, S.E. One step forward to personalized medicine? Alzheimer’s Dement 2023, 9, e12435. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kale, N.K.; Borah, S.; Deokar, S.S.; et al. Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis: An updated review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Aisen, P.S.; DuBois, B.; Frölich, L.; Jack, C.R., Jr.; Jones, R.W.; Morris, J.C.; Raskin, J.; Dowsett, S.A.; Scheltens, P. Drug development in Alzheimer’s disease: The path to 2025. Alzheimer’s Res. Ther. 2016, 8, 39. [Google Scholar] [CrossRef]
- Kabir, M.T.; Sufian, M.A.; Uddin, M.S.; Begum, M.M.; Akhter, S.; Islam, A.; Amran, M.S.; Md Ashraf, G. NMDA receptor antagonists: Repositioning of memantine as a multitargeting agent for Alzheimer’s therapy. Curr. Pharm. Des. 2019, 25, 3506–3518. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Tewari, D.; Mathew, B.; Aleya, L. Emerging signal regulating potential of small molecule biflavonoids to combat neuropathological insults of Alzheimer’s disease. Sci. Total Environ. 2020, 700, 134836. [Google Scholar] [CrossRef] [PubMed]
- Deardorff, W.J.; Grossberg, G.T. A fixed-dose combination of memantine extended-release and donepezil in the treatment of moderate-to-severe Alzheimer’s disease. Drug Des. Devel Ther. 2016, 10, 3267–3279. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.T.; Uddin, M.S.; Mamun, A.A.; Jeandet, P.; Aleya, L.; Mansouri, R.A.; Ashraf, G.M.; Mathew, B.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Combination drug therapy for the management of Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 3272. [Google Scholar] [CrossRef] [PubMed]
- Morphy, R.; Kay, C.; Rankovic, Z. From magic bullets to designed multiple ligands. Drug Discov. Today 2004, 9, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.H.; Shi, Z.; Tan, C.; Jiang, Y.; Go, M.L.; Low, B.C.; Chen, Y.Z. In-silico approaches to multi-target drug discovery: Computer aided multi-target drug design, multi-target virtual screening. Pharm. Res. 2010, 27, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.W.; Savonenko, A.V.; Melnikova, T.; Kim, H.; Price, D.L.; Li, T.; Wong, P.C. Modeling an anti-amyloid combination therapy for Alzheimer’s. Sci. Transl. Med. 2010, 2, 13ra1. [Google Scholar] [CrossRef] [PubMed]
- Pepeu, G.; Giovannini, M.G. Cholinesterase inhibitors and beyond. Curr. Alzheimer Res. 2009, 6, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Acetylcholinesterase inhibitors: A patent review (2008-present). Expert. Opin. Ther. Pat. 2012, 22, 871–886. [Google Scholar] [CrossRef]
- Zheng, H.; Fridkin, M.; Youdim, M. From single target to multitarget/network therapeutics in Alzheimer’s therapy. Pharmaceuticals 2014, 7, 113–135. [Google Scholar] [CrossRef]
- Plazas, E.; Hagenow, S.; Avila Murillo, M.; Stark, H.; Cuca, L.E. Isoquinoline alkaloids from the roots of Zanthoxylum rigidum as multi-target inhibitors of cholinesterase, monoamine oxidase A and Aβ(1-42) aggregation. Bioorg Chem. 2020, 98, 103722. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, H.; Gu, Q.; Xu, J. Synthesis and evaluation of tetrahydroisoquinoline-benzimidazole hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 167, 133–145. [Google Scholar] [CrossRef] [PubMed]
- González-Naranjo, P.; Pérez-Macias, N.; Pérez, C.; Roca, C.; Vaca, G.; Girón, R.; Sánchez-Robles, E.; Martín-Fontelles, M.I.; de Ceballos, M.L.; Martin-Requero, A.; et al. Indazolylketones as new multitarget cannabinoid drugs. Eur. J. Med. Chem. 2019, 166, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, L.; Karelson, M.; Dobchev, D.A. Multitarget approach to drug candidates against Alzheimer’s disease related to AChE, SERT, BACE1 and GSK3β protein targets. Molecules 2020, 25, 1846. [Google Scholar] [CrossRef] [PubMed]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and astrocyte function and communication: What do we know in humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.; Miyauchi, J.; Tsirka, S.E. Microglia: An active player in the regulation of synaptic activity. Neural Plast. 2013, 2013, 627325. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Dobrin, N.; Brehar, F.M.; Popa, C.; Covache-Busuioc, R.A.; Glavan, L.A.; Costin, H.P.; Bratu, B.G.; Corlatescu, A.D.; Popa, A.A.; et al. From recognition to remedy: The significance of biomarkers in neurodegenerative disease pathology. Int. J. Mol. Sci. 2023, 24, 16119. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Thangavel, R.; Natteru, P.A.; Selvakumar, G.P.; Saeed, D.; Zahoor, H.; Zaheer, S.; Iyer, S.S.; Zaheer, A. Neuroinflammation induces neurodegeneration. J. Neurol. Neurosurg. Spine 2016, 1, 1003. [Google Scholar] [PubMed]
- Doty, K.R.; Guillot-Sestier, M.V.; Town, T. The role of the immune system in neurodegenerative disorders: Adaptive or maladaptive? Brain Res. 2015, 1617, 155–173. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Schafer, D.; Vincent, A.; Blachère, N.E.; Bar-Or, A. Neuroinflammation: Ways in which the immune system affects the brain. Neurotherapeutics 2015, 12, 896–909. [Google Scholar] [CrossRef]
- Strzelec, M.; Detka, J.; Mieszczak, P.; Sobocińska, M.K.; Majka, M. Immunomodulation-a general review of the current state-of-the-art and new therapeutic strategies for targeting the immune system. Front. Immunol. 2023, 14, 1127704. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ma, H.; Yang, Y.; Liao, Y.; Lin, C.; Zheng, J.; Yu, M.; Lan, J. Microglia in Alzheimer’s disease: Pathogenesis, mechanisms, and therapeutic potentials. Front. Aging Neurosci. 2023, 15, 1201982. [Google Scholar] [CrossRef] [PubMed]
- Yeh, F.L.; Hansen, D.V.; Sheng, M. TREM2, microglia, and neurodegenerative diseases. Trends Mol. Med. 2017, 23, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Gratuze, M.; Leyns, C.E.G.; Holtzman, D.M. New insights into the role of TREM2 in Alzheimer’s disease. Mol. Neurodegener. 2018, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, A.R.; Marriott, I. The interleukin-10 family of cytokines and their role in the CNS. Front. Cell Neurosci. 2018, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Disease Treatment. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment (accessed on 24 February 2024).
- Silva, J.D.; Taglialatela, G.; Jupiter, D.C. Reduced prevalence of dementia in patients prescribed tacrolimus, sirolimus, or cyclosporine. J. Alzheimer’s Dis. 2023, 95, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.; Zawia, N.H. Fenamates as potential therapeutics for neurodegenerative disorders. Cells 2021, 10, 702. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Song, C.; Shi, J.; Zhang, P.; Zhang, Y.; Xu, J.; Zhao, L.; Zhang, R.; Wang, H.; Chen, H. Immunotherapy for Alzheimer’s disease: Targeting β-amyloid and beyond. Transl. Neurodegener. 2022, 11, 18. [Google Scholar] [CrossRef]
- Khorassani, F.; Hilas, O. Bapineuzumab, an investigational agent for Alzheimer’s disease. Pharm. Ther. 2013, 38, 89–91. [Google Scholar]
- Abushouk, A.I.; Elmaraezy, A.; Aglan, A.; Salama, R.; Fouda, S.; Fouda, R.; AlSafadi, A.M. Bapineuzumab for mild to moderate Alzheimer’s disease: A meta-analysis of randomized controlled trials. BMC Neurol. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, A.; Bramanti, P.; Mazzon, E. Role of vitamin E in the treatment of Alzheimer’s disease: Evidence from animal models. Int. J. Mol. Sci. 2017, 18, 2504. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Grundman, M. Vitamin E and Alzheimer disease: The basis for additional clinical trials. Am. J. Clin. Nutr. 2000, 71, 630s–636s. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Kajihara, R. Neurotrophins and other growth factors in the pathogenesis of Alzheimer’s disease. Life 2023, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Budni, J.; Bellettini-Santos, T.; Mina, F.; Garcez, M.L.; Zugno, A.I. The involvement of BDNF, NGF and GDNF in aging and Alzheimer’s disease. Aging Dis. 2015, 6, 331–341. [Google Scholar]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Abskharon, R.; Pan, H.; Sawaya, M.R.; Seidler, P.M.; Olivares, E.J.; Chen, Y.; Murray, K.A.; Zhang, J.; Lantz, C.; Bentzel, M.; et al. Structure-based design of nanobodies that inhibit seeding of Alzheimer’s patient-extracted tau fibrils. Proc. Natl. Acad. Sci. USA 2023, 120, e2300258120. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Pang, Y.; Li, L.; Pang, Y.; Zhang, J.; Wang, X.; Raes, G. Applications of nanobodies in brain diseases. Front. Immunol. 2022, 13, 978513. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Moos, T.; Thomsen, M.S.; Burkhart, A.; Hede, E.; Laczek, B. Targeted transport of biotherapeutics at the blood-brain barrier. Expert. Opin. Drug Deliv. 2023, 20, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.M.; De Haard, H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Van Audenhove, I.; Gettemans, J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, Y.; Ruan, S.; Hu, Y. Current anti-amyloid-β therapy for Alzheimer’s disease treatment: From clinical research to nanomedicine. Int. J. Nanomed. 2003, 18, 7825–7845. [Google Scholar] [CrossRef] [PubMed]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s disease (AD): From risk factors to therapeutic targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef]
- Saeed, A.F.; Wang, R.; Ling, S.; Wang, S. Antibody engineering for pursuing a healthier future. Front. Microbiol. 2017, 8, 495. [Google Scholar] [CrossRef]
- Nilvebrant, J.; Tessier, P.M.; Sidhu, S.S. Engineered autonomous human variable domains. Curr. Pharm. Des. 2016, 22, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.J.; Lazar, G.A. Next generation antibody drugs: Pursuit of the ‘high-hanging fruit’. Nat. Rev. Drug Discov. 2018, 17, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Amano, A.; Sanjo, N.; Araki, W.; Anraku, Y.; Nakakido, M.; Matsubara, E.; Tomiyama, T.; Nagata, T.; Tsumoto, K.; Kataoka, K.; et al. Peripheral administration of nanomicelle-encapsulated anti-Aβ oligomer fragment antibody reduces various toxic Aβ species in the brain. J. Nanobiotechnol. 2023, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Tammer, A.H.; Coia, G.; Cappai, R.; Fuller, S.; Masters, C.L.; Hudson, P.; Underwood, J.R. Generation of a recombinant Fab antibody reactive with the Alzheimer’s disease-related Abeta peptide. Clin. Exp. Immunol. 2002, 129, 453–463. [Google Scholar] [CrossRef]
- Krah, S.; Schröter, C.; Zielonka, S.; Empting, M.; Valldorf, B.; Kolmar, H. Single-domain antibodies for biomedical applications. Immunopharmacol. Immunotoxicol. 2016, 38, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.T.; Ma, C.; Li, G.J.; Zheng, X.Y.; Hao, Y.T.; Yang, Y.; Wang, X. Application of antibody fragments against Aβ with emphasis on combined application with nanoparticles in Alzheimer’s disease. Front. Pharmacol. 2021, 12, 654611. [Google Scholar] [CrossRef] [PubMed]
- Si, Z.Z.; Zou, C.J.; Mei, X.; Li, X.F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.X.; Wu, L.; Liu, Y. Targeting neuroinflammation in Alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar] [PubMed]
- Satheeshkumar, P.K. Expression of single chain variable fragment (scFv) molecules in plants: A comprehensive update. Mol. Biotechnol. 2020, 62, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Montoliu-Gaya, L.; Villegas, S. Production of therapeutic single-chain variable fragments (ScFv) in Pichia pastoris. Methods Mol. Biol. 2022, 2313, 151–167. [Google Scholar]
- Fan, X.; Xu, L.; Zhang, J.; Wang, Y.; Wu, Z.; Sun, W.; Yao, X.; Wang, X.; Guan, S.; Shan, Y. Mechanism exploration of amyloid-β-42 disaggregation by single-chain variable fragments of Alzheimer’s disease therapeutic antibodies. Int. J. Mol. Sci. 2023, 24, 8371. [Google Scholar] [CrossRef]
- Martin-Peña, A.; Rincon-Limas, D.E.; Fernandez-Funez, P. Anti-Aβ single-chain variable fragment antibodies restore memory acquisition in a Drosophila model of Alzheimer’s disease. Sci. Rep. 2017, 7, 11268. [Google Scholar] [CrossRef]
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and tolerability of BAN2401--a clinical study in Alzheimer’s disease with a protofibril selective Abeta antibody. Alzheimer’s Res. Ther. 2016, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef] [PubMed]
- Im, D.; Heo, C.E.; Son, M.K.; Park, C.R.; Kim, H.I.; Choi, J.M. Kinetic modulation of amyloid-beta (1-42) aggregation and toxicity by structure-based rational design. J. Am. Chem. Soc. 2022, 144, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Thacker, D.; Willas, A.; Dear, A.J.; Linse, S. Role of hydrophobicity at the N-terminal region of Abeta42 in secondary nucleation. ACS Chem. Neurosci. 2022, 13, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Ravetch, J.V.; Bolland, S. IgG Fc receptors. Annu. Rev. Immunol. 2001, 19, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.; Janeway, C. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Pub: New York, NY, USA, 2001; p. xviii. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.A.; Cavanaugh, M.; Clark, K.; Karsch-Mizrachi, I.; Lipman, D.J.; Ostell, J.; Sayers, E.W. GenBank. Nucleic Acids Res. 2013, 41, D36–D42. [Google Scholar] [CrossRef] [PubMed]
- Mooers, B.H.M. Shortcuts for faster image creation in PyMOL. Protein Sci. 2020, 29, 268–276. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Pal, A.; Pyne, N.; Paul, S. In-silico designing of a multi-epitope vaccine against SARS-CoV2 and studying the interaction of the vaccine with alpha, beta, delta and Omicron variants of concern. Curr. Drug Discov. Technol. 2023, 20, e090922208713. [Google Scholar] [CrossRef] [PubMed]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein-protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef] [PubMed]
- Mauger, D.M.; Cabral, B.J.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Mao, M.; Wang, L.J. Integrated clustering signature of genomic heterogeneity, stemness and tumor microenvironment predicts glioma prognosis and immunotherapy response. Aging (Albany NY) 2023, 15, 9086–9104. [Google Scholar] [CrossRef] [PubMed]
- Lührs, T.; Ritter, C.; Adrian, M.; Riek-Loher, D.; Bohrmann, B.; Döbeli, H.; Schubert, D.; Riek, R. 3D structure of Alzheimer’s amyloid-beta(1-42) fibrils. Proc. Natl. Acad. Sci. USA 2005, 102, 17342–17347. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D.M.; Walsh, D.M.; Ye, C.P.; Diehl, T.; Vasquez, S.; Vassilev, P.M.; Teplow, D.B.; Selkoe, D.J. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 1999, 19, 8876–8884. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef]
- Lim, C.M.; González Díaz, A.; Fuxreiter, M.; Pun, F.W.; Zhavoronkov, A.; Vendruscolo, M. Multiomic prediction of therapeutic targets for human diseases associated with protein phase separation. Proc. Natl. Acad. Sci. USA 2023, 120, e2300215120. [Google Scholar] [CrossRef]
- Merchant, J.P.; Zhu, K.; Henrion, M.Y.R.; Zaidi, S.S.A.; Lau, B.; Moein, S.; Alamprese, M.L.; Pearse, R.V., 2nd; Bennett, D.A.; Ertekin-Taner, N.; et al. Predictive network analysis identifies JMJD6 and other potential key drivers in Alzheimer’s disease. Commun. Biol. 2023, 6, 503. [Google Scholar] [CrossRef] [PubMed]
- Silva-Spínola, A.; Baldeiras, I.; Arrais, J.P.; Santana, I. The road to personalized medicine in Alzheimer’s disease: The use of artificial intelligence. Biomedicines 2022, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Arrué, L.; Cigna-Méndez, A.; Barbosa, T.; Borrego-Muñoz, P.; Struve-Villalobos, S.; Oviedo, V.; Martínez-García, C.; Sepúlveda-Lara, A.; Millán, N.; Márquez Montesinos, J.C.E.; et al. New drug design avenues targeting Alzheimer’s disease by pharmacoinformatics-aided tools. Pharmaceutics 2022, 14, 1914. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chowdhury, S.; Kumar, S. In silico repurposing of antipsychotic drugs for Alzheimer’s disease. BMC Neurosci. 2017, 18, 76. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Whittlesey, K.J.; Shea, L.D. Nerve growth factor expression by PLG-mediated lipofection. Biomaterials 2006, 27, 2477–2486. [Google Scholar] [CrossRef]
- Gärtner, A.; Collin, L.; Lalli, G. Nucleofection of primary neurons. Methods Enzymol. 2006, 406, 374–388. [Google Scholar] [PubMed]
- Luo, D.; Saltzman, W.M. Enhancement of transfection by physical concentration of DNA at the cell surface. Nat. Biotechnol. 2000, 18, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Drug targeting to the brain. Pharm. Res. 2007, 24, 1733–1744. [Google Scholar] [CrossRef]
- Fortin, D.; Gendron, C.; Boudrias, M.; Garant, M.P. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in the treatment of cerebral metastasis. Cancer 2007, 109, 751–760. [Google Scholar] [CrossRef]
- Jones, A.R.; Shusta, E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.O.; Swindell, C.S.; Anthony, F.H.; Witman, P.A.; Devanesan, P.; Webb, N.L.; Baker, S.; Wolff, A.; Donehower, R. Tumor targeting by conjugation of DHA to paclitaxel. J. Control Release 2001, 74, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef]
- Niewoehner, J.; Bohrmann, B.; Collin, L.; Urich, E.; Sade, H.; Maier, P.; Rueger, P.; Stracke, J.O.; Lau, W.; Tissot, A.C.; et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 2014, 81, 49–60. [Google Scholar] [CrossRef]
- Biden, J. FACT SHEET: The United States Announces New Investments and Resources to Advance President Biden’s National Biotechnology and Biomanufacturing Initiative. White House. 2022. Available online: https://www.whitehouse.gov/briefing-room/statements-releases/2022/09/14/fact-sheet-the-united-states-announces-new-investments-and-resources-to-advance-president-bidens-national-biotechnology-and-biomanufacturing-initiative/ (accessed on 24 February 2024).
| Drug Name | Target(s) | Function(s) | Stage of Development |
|---|---|---|---|
| Aβ oligomer inhibitors (e.g., BAN2401, aducanumab) | Amyloid-β oligomers | Prevent or disassemble toxic clumps of amyloid-β | Clinical trials (aducanumab recently received FDA approval) |
| BACE1 inhibitors (e.g., verubecestat, MK-8931) | β-Secretase 1 (BACE1) | Reduce production of amyloid-β by inhibiting the enzyme that cleaves its precursor | Clinical trials (some promising results, others halted due to lack of efficacy) |
| Tau aggregates inhibitors (e.g., P-tau217 PET tracers, LMTX) | Tau protein aggregates | Prevent or remove tangles of misfolded tau protein | Preclinical/early clinical trials (imaging agents more advanced than therapeutic agents) |
| Cholinesterase inhibitors (e.g., donepezil, rivastigmine, galantamine) | Acetylcholinesterase (AChE) | Increase levels of the neurotransmitter acetylcholine, which is depleted in AD | Approved for symptomatic treatment of mild-to-moderate AD |
| NMDA receptor modulators (e.g., memantine) | N-methyl-D-aspartate (NMDA) receptors | Protect neurons from excitotoxicity and improve cognitive function | Approved for moderate-to-severe AD |
| Multi-target drugs (e.g., J147, AV-1750, CTS-5559) | Combinations of targets from above (e.g., AChE + NMDA, BACE1 + tau) | Address multiple aspects of AD pathology for potentially greater efficacy | Preclinical/early clinical trials (potentially more effective but require careful design and validation) |
| Type of Nanobodies | Description | Mechanism of Action | Advantage | Disadvantage |
|---|---|---|---|---|
| Fab fragments | Modified antigen-binding fragments of conventional antibodies | Bind to specific targets, trigger immune response | High affinity, good specificity | Large size, limited tissue penetration |
| Domain antibodies | Single variable domains from antibodies with only the heavy chain (VH) | Bind to specific targets, inhibit specific pathways | Smaller than Fab fragments, they have potentially better tissue penetration | Less potent than Fab fragments, limited repertoire |
| Single-chain variable fragments (scFv) | Engineered fusion of heavy and light chain variable domains | Bind to specific targets, can be engineered for additional functions | Smaller than Fab fragments, customizable | Lower affinity than Fab fragments, limited potential stability |
| Element | Description | Position |
|---|---|---|
| cap | A modified 5′-cap 1 structure (m7G+m3′-5′-ppp-5′-Am) | 1–2 |
| 5′-UTR | The 5′-untranslated region derived from human alpha globin RNA with an optimized Kozak sequence. | 3–54 |
| sig | S glycoprotein signal peptide (extended leader sequence) guides translocation of the nascent polypeptide chain into the endoplasmic reticulum. | 55–102 |
| ORF | Codon-optimized sequence: GAAΨΨ ΨCGCC AΨGAΨ AGCGG CΨAΨG AAGΨG CAΨCA ΨGGCA GCGGC AGCGG CAGCG GCAGC GAGAΨ GΨGG GCAGC AACAA AGGC | 103–187 |
| 3′-UTR | The 3′ untranslated region comprises two sequence elements derived from the amino-terminal enhancer of split (AES) mRNA and the mitochondrial encoded 12S ribosomal RNA to confer RNA stability and high total protein expression: GCΨAG CΨGCC CCΨΨΨ CCCGΨ CCΨGG GΨACC CCGAG ΨCΨCC CCCGA CCΨCG GGΨCC CAGGΨ AΨGC ΨCCCA CCΨCC ACCΨG CCCCA CΨCAC CACCΨ CΨGCΨ AGΨΨC CAGAC ACCΨCC CAAGC ACGCA GCAAΨ GCAGC ΨCAAA ACGCΨ ΨAGCC ΨAGCC ACACC CCCAC GGGAA ACAGC AGΨGA ΨΨAAC CΨΨΨA GCAAΨ AAACG AAAGΨ ΨΨAAC ΨAAGC ΨAΨAC ΨAACC CCAGG GΨΨGG ΨCAAΨ ΨΨCGΨ GCCAG CCACA CCCΨG GAGCΨ AGC | 188–456 |
| poly(A) | A 110-nucleotide poly(A)-tail consisting of a stretch of 30 adenosine residues, followed by a 10-nucleotide linker sequence and another 70 adenosine residues: AAAAA AAAAA AAAAA AAAAA AAAAA AAAAA GCAΨA ΨGACΨ AAAAA AAAAA AAAAA AAAAA AAAAA AAAAA AAAAAA AAAAA AAAAA AAAAA AAAAA AAAAA AAAAA AAAA | 457–566 |
| Target Antigen: Aβ42 | DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA | 42 amino acids | ||||
| Linked epitopes: (http://tools.iedb.org/bcell/) | No. | Start | End | Peptide | Length | EFRHDSGYEVHH -GSGSGSGS- EDVGSNKG |
| 1 | 3 | 14 | EFRHDSGYEVHH | 12 | ||
| 2 | 22 | 29 | EDVGSNKG | 8 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niazi, S.K.; Magoola, M.; Mariam, Z. Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders. Pharmaceuticals 2024, 17, 741. https://doi.org/10.3390/ph17060741
Niazi SK, Magoola M, Mariam Z. Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders. Pharmaceuticals. 2024; 17(6):741. https://doi.org/10.3390/ph17060741
Chicago/Turabian StyleNiazi, Sarfaraz K., Matthias Magoola, and Zamara Mariam. 2024. "Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders" Pharmaceuticals 17, no. 6: 741. https://doi.org/10.3390/ph17060741
APA StyleNiazi, S. K., Magoola, M., & Mariam, Z. (2024). Innovative Therapeutic Strategies in Alzheimer’s Disease: A Synergistic Approach to Neurodegenerative Disorders. Pharmaceuticals, 17(6), 741. https://doi.org/10.3390/ph17060741







