Evaluation of Solubility, Dissolution Rate, and Oral Bioavailability of β-Cyclodextrin and Hydroxypropyl β-Cyclodextrin as Inclusion Complexes of the Tyrosine Kinase Inhibitor, Alectinib
Abstract
1. Introduction
2. Results
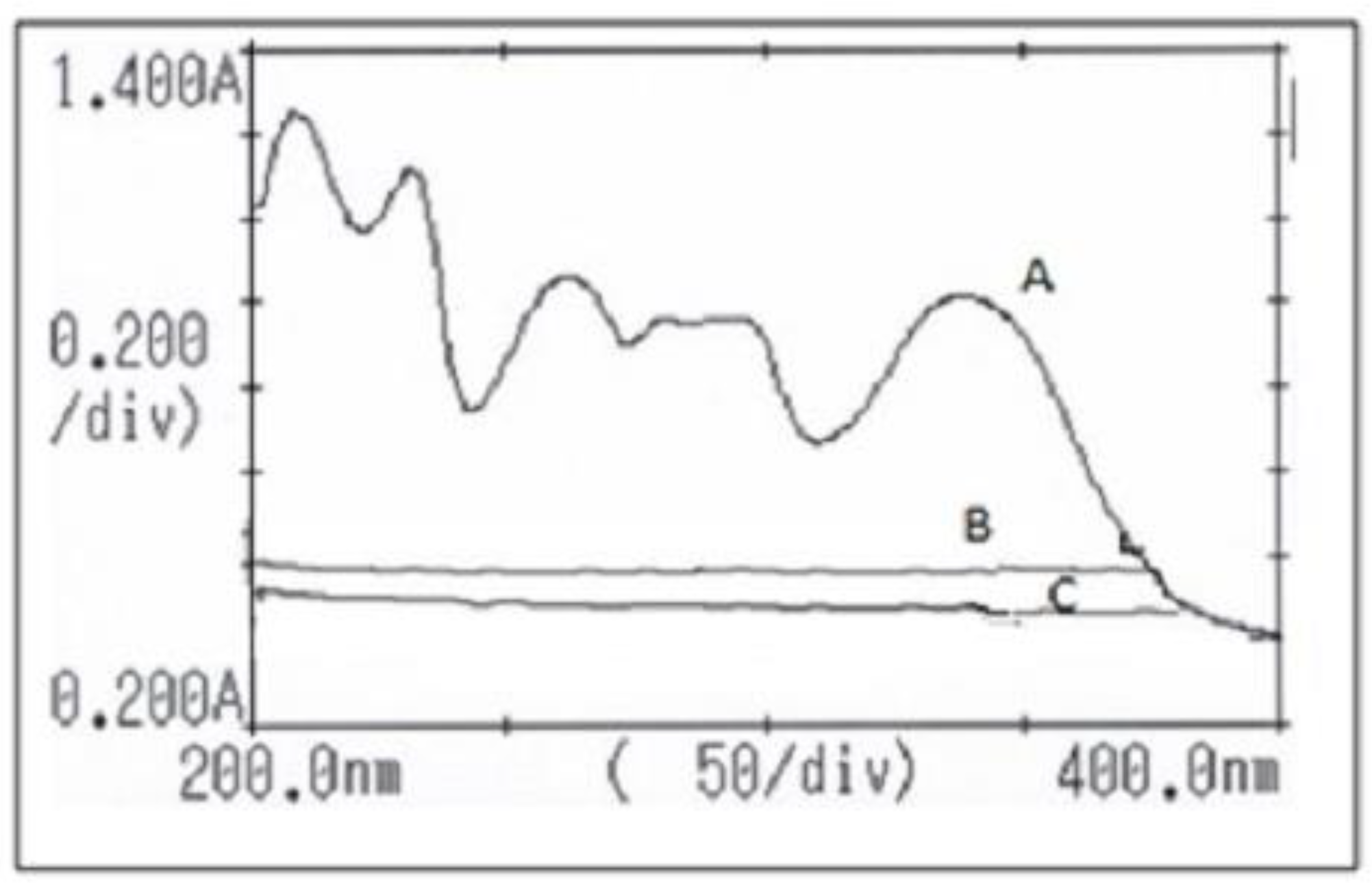
2.1. Method of Analysis
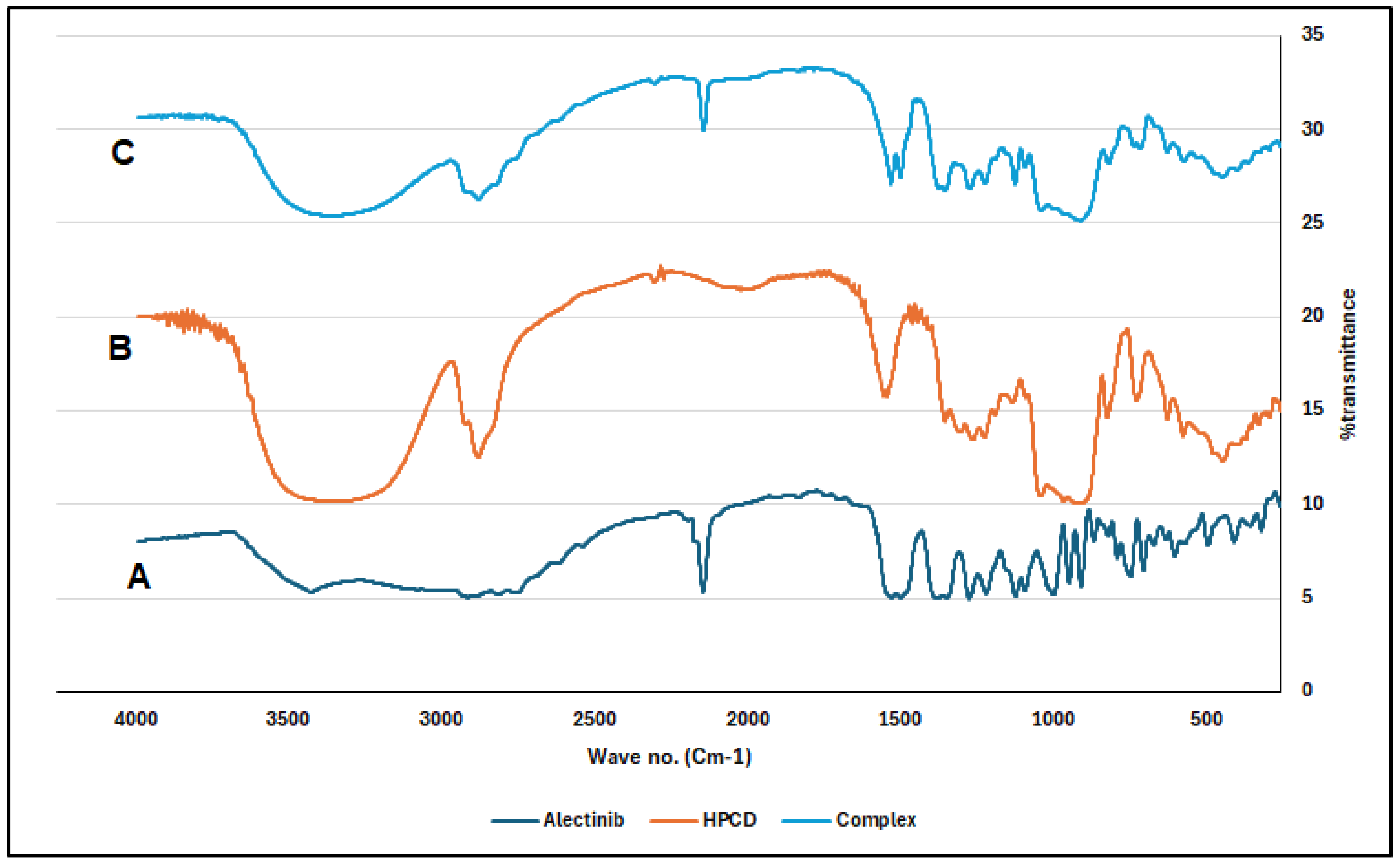
2.2. Characterization of the Complex
2.3. Phase Solubility Study
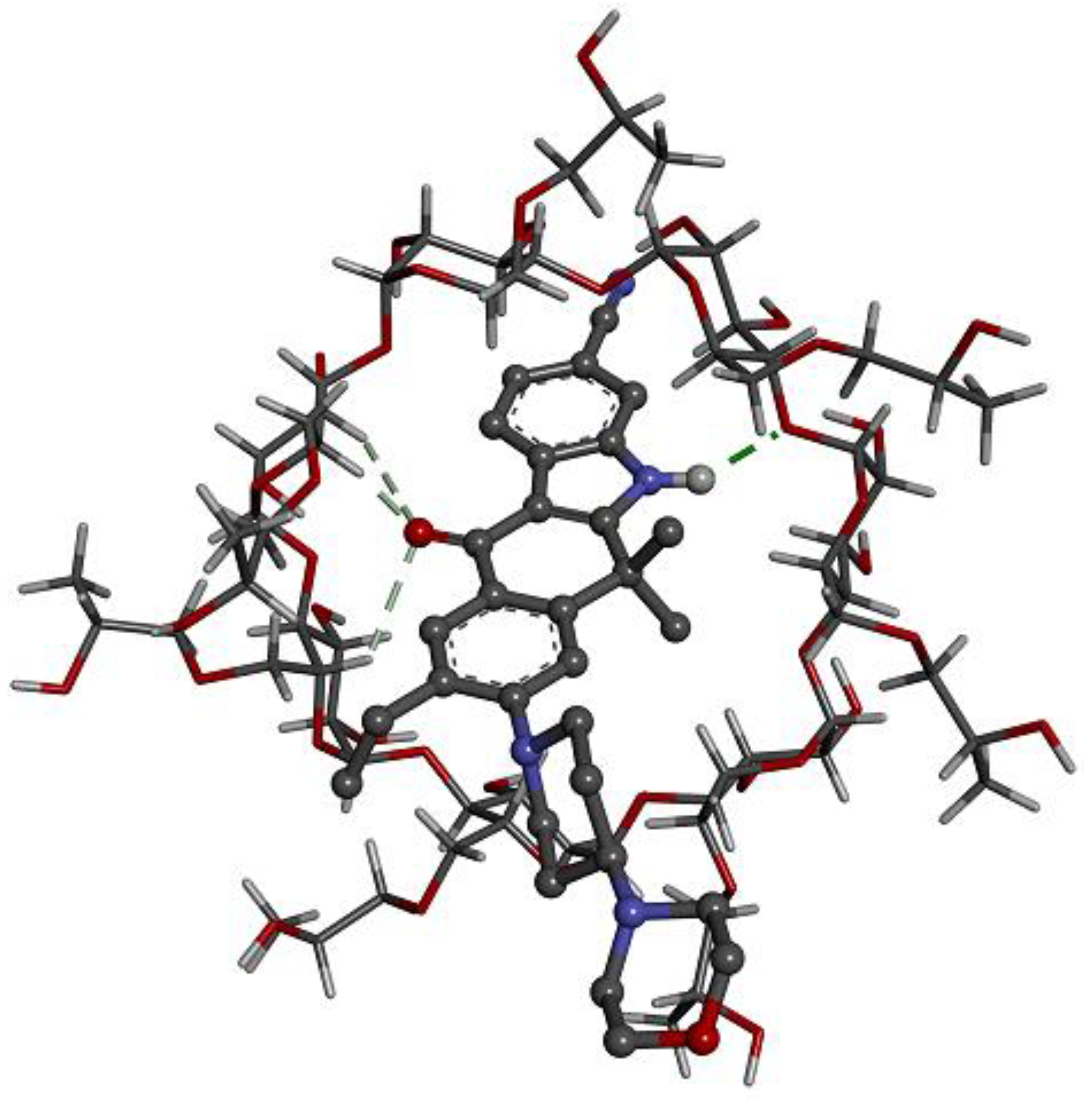
2.4. Computer Simulations of the Inclusion Complex
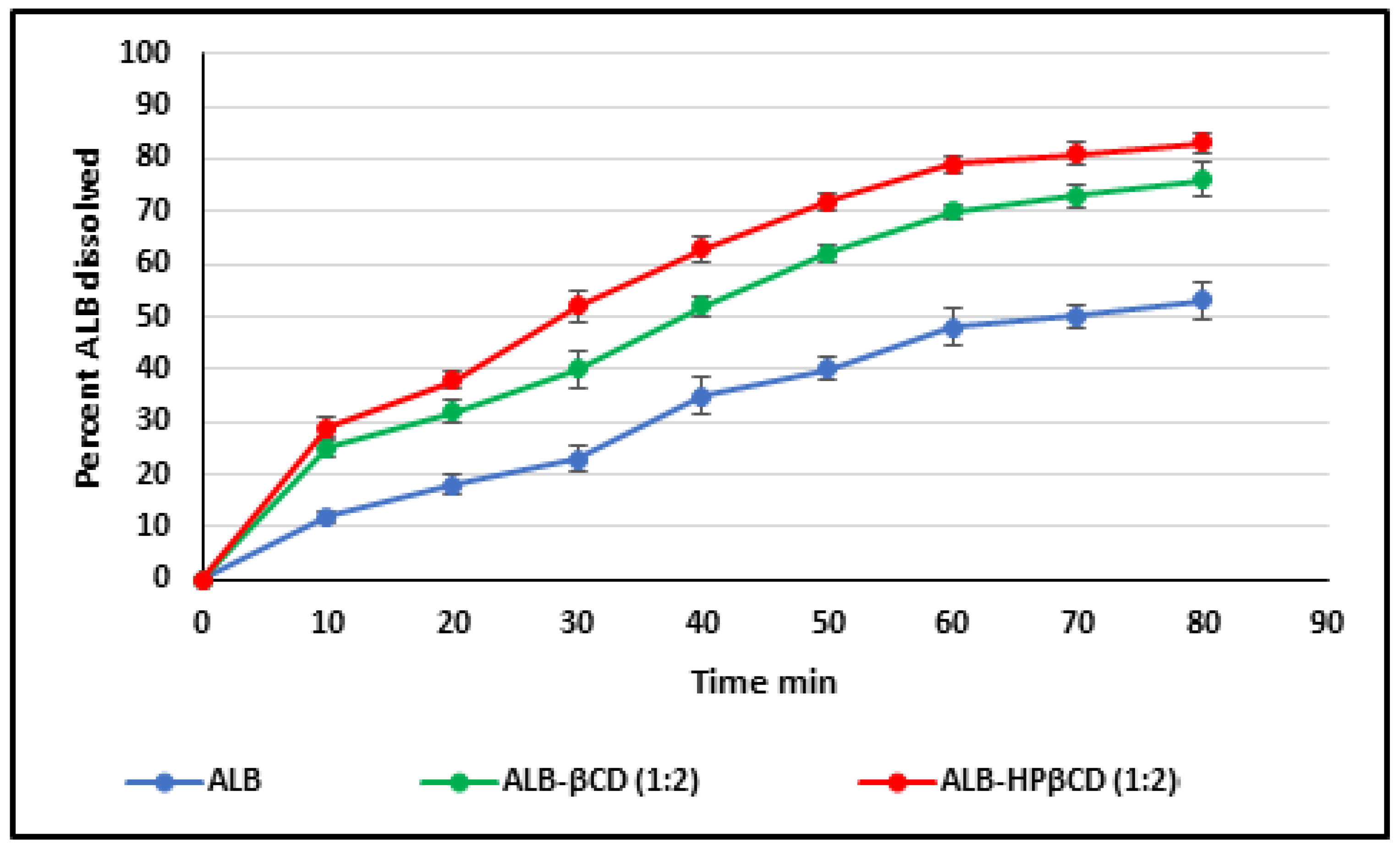
2.5. Dissolution Study
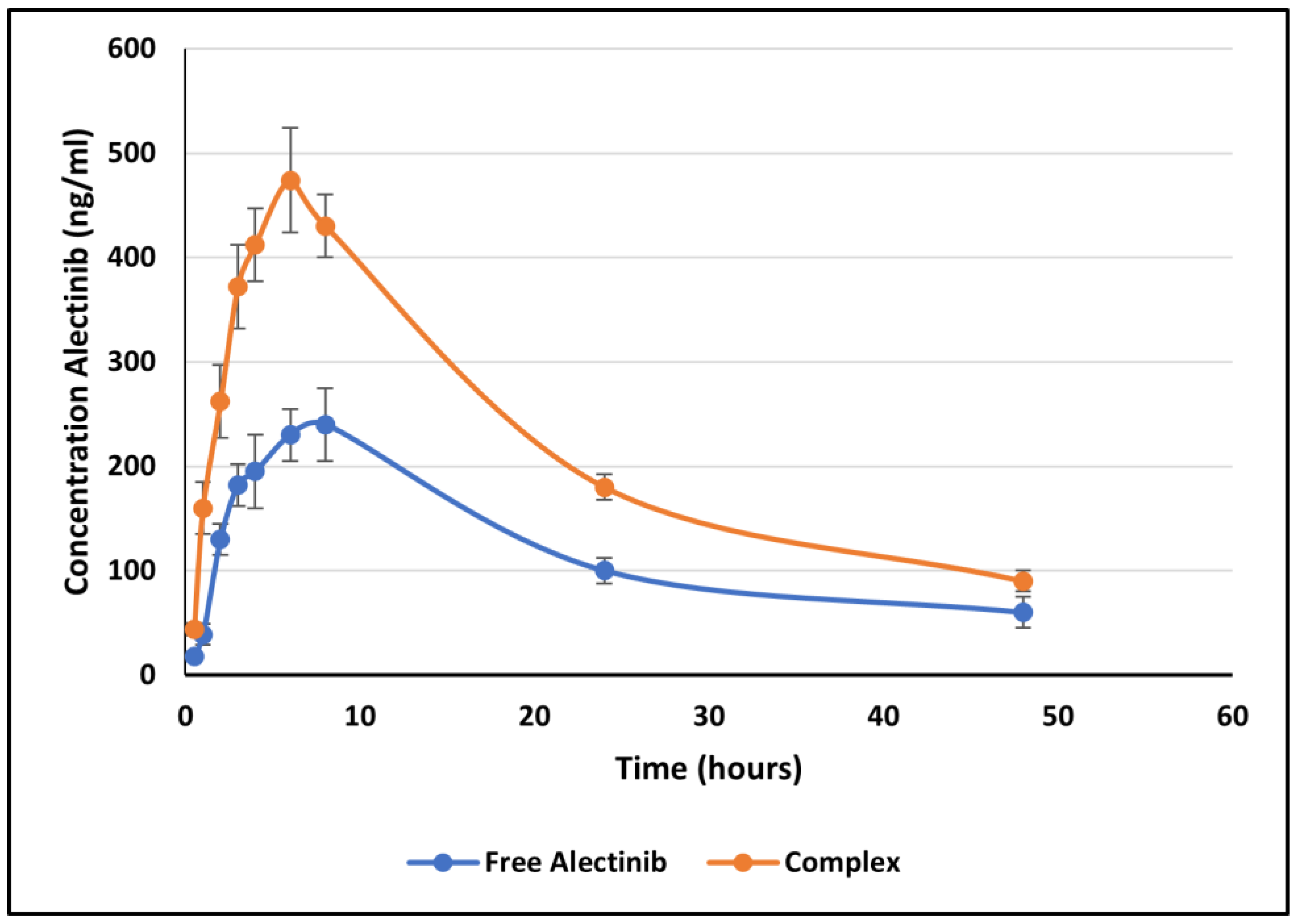
2.6. In Vivo Bioavailability Study
2.6.1. Method of Analysis and Validation
2.6.2. Bioavailability Parameters
3. Discussion
4. Methodology
4.1. Materials and Chemicals
4.2. Development of the Method of Analysis Based on UV Absorbance
4.3. Preparation of the ALB–CD Complex
4.4. Characterization of the Prepared Complexes
4.5. Phase Solubility Study
4.6. Computer Simulations of the Inclusion Complexes
4.7. Dissolution Test
4.8. In Vivo Study
4.8.1. LC–MS/MS for the Detection of ALB in Rat Plasma
4.8.2. Extraction Method
4.8.3. Method Validation
4.8.4. Percent Recovery
4.9. Pharmacokinetic Study
4.10. Non-Compartmental Analysis of Plasma Concentration–Time Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- WHO Lung Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 2 January 2024).
- Chen, J.W.; Dhahbi, J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identi-fication, and gene expression analysis using overlapping feature selection methods. Sci. Rep. 2021, 11, 13323. [Google Scholar] [CrossRef] [PubMed]
- Dubin, S.; Griffin, D. Lung cancer in non-smokers. Mo. Med. 2020, 117, 375–379. [Google Scholar] [PubMed]
- Alberg, A.J.; Brock, M.V.; Ford, J.G.; Samet, J.M.; Spivack, S.D. Epidemiology of lung cancer. Chest 2013, 143, e1S–e29S. [Google Scholar] [CrossRef] [PubMed]
- Jona, A.; Miltenyi, Z.; Pinczes, L.; Kerek, P.; Bittner, N.; Szilasi, M.; Barna, S.; Illes, A. Pulmonary Toxicity of Hodgkin Lymphoma Treatment: A Prospective Single-Center Study. J. Hematol. 2021, 10, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Pepper, N.B.; Oertel, M.; Kittel, C.; Kröger, K.J.; Elsayad, K.; Haverkamp, U.; Eich, H.T. Impact of radiation techniques on lung toxicity in patients with mediastinal Hodgkin’s lymphoma. Strahlenther. Onkol. 2021, 197, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Abu Qubo, A.; Numan, J.; Snijder, J.; Padilla, M.; Austin, J.H.; Capaccione, K.M.; Pernia, M.; Bustamante, J.; O’Connor, T.; Salvatore, M.M. Idiopathic pulmonary fibrosis and lung cancer: Future directions and challenges. Breathe 2022, 18, 220147. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Fushiya, N.; Koike, K.; Nishino, H.; Tajiri, H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br. J. Cancer 2012, 107, 988–993. [Google Scholar] [CrossRef]
- Sakamoto, H.; Tsukaguchi, T.; Hiroshima, S.; Kodama, T.; Kobayashi, T.; Fukami, T.A.; Oikawa, N.; Tsukuda, T.; Ishii, N.; Aoki, Y. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011, 19, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shi, Y.; Liu, Y.; Wang, Z.; Ma, Y.; Shi, X.; Lu, L.; Wang, Z.; Li, H.; Zhang, Y. Efficacy and safety of alectinib in ALK-positive non-small cell lung cancer and blood markers for prognosis and efficacy: A retrospective cohort study. Transl. Lung Cancer Res. 2022, 11, 2521–2538. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Guo, X.; Tang, X.; Li, S.; Gong, G.; Gao, S.; Zhang, Y.; Lin, S. Comparative safety of anaplastic lym-phoma kinase tyrosine kinase inhibitors in advanced anaplastic lymphoma kinase-mutated non-small cell lung cancer: Systematic review and network meta-analysis. Lung Cancer 2023, 184, 107319. [Google Scholar] [CrossRef] [PubMed]
- Sgambato, A.; Casaluce, F.; Maione, P.; Gridelli, C. Targeted therapies in non-small cell lung cancer: A focus on ALK/ROS1 tyrosine kinase inhibitors. Expert Rev. Anticancer Ther. 2018, 18, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Kodama, T.; Tsukaguchi, T.; Yoshida, M.; Kondoh, O.; Sakamoto, H. Selective ALK inhibitor alectinib with potent an-titumor activity in models of crizotinib resistance. Cancer Lett. 2014, 351, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Miao, E.; Eichholz, J.E.; Lebow, E.S.; Flynn, J.; Zhang, Z.; Walch, H.; Hubbeling, H.; Beal, K.; Moss, N.S.; Kenny, K.Y. Characterization of Central Nervous System Clinico-Genomic Outcomes in ALK-Positive Non-Small Cell Lung Cancer Patients with Brain Metastases Treated with Alectinib. Lung Cancer 2023, 178, 57–65. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.-X.; Jiang, B.-Y.; Wu, Y.-L.; Zhong, W.-Z. Feasibility and safety of neoadjuvant alectinib in a patient with ALK-positive locally advanced NSCLC. Onco Targets Ther. 2021, 14, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 49806720, Alectinib. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Alectinib (accessed on 20 May 2024).
- Morcos, P.N.; Guerini, E.; Parrott, N.; Dall, G.; Blotner, S.; Bogman, K.; Sturm, C.; Balas, B.; Martin-Facklam, M.; Phipps, A. Effect of food and esomeprazole on the pharmacokinetics of alectinib, a highly selective ALK inhibitor, in healthy sub-jects. Clin. Pharmacol. Drug Dev. 2017, 6, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Lanser, D.A.; de Leeuw, S.P.; Oomen-de Hoop, E.; de Bruijn, P.; Paats, M.S.; Dumoulin, D.W.; Koolen, S.L.; Dingemans, A.-M.C.; Mathijssen, R.H.; Veerman, G.M. Influence of Food With Different Fat Concentrations on Alectinib Exposure: A Randomized Crossover Pharmacokinetic Trial. J. Natl. Compr. Cancer Netw. 2023, 21, 645–651.e1. [Google Scholar] [CrossRef]
- Leeson, P.D.; Davis, A.M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 2004, 47, 6338–6348. [Google Scholar] [CrossRef]
- Wenlock, M.C.; Austin, R.P.; Barton, P.; Davis, A.M.; Leeson, P.D. A comparison of physiochemical property profiles of de-velopment and marketed oral drugs. J. Med. Chem. 2003, 46, 1250–1256. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Rizwan, M.; Irfanuddin, M.; Shobha Malini, D. Bioavailability enhancement strategies: Basics, formulation approaches and regulatory considerations. Curr. Drug Deliv. 2011, 8, 691–702. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Maheriya, P. Cyclodextrin: A Promising Candidate in Enhancing Oral Bioavailability of poorly Water Soluble Drugs. MOJ Bioequiv. Availab. 2017, 3, 60–63. [Google Scholar] [CrossRef]
- Hedayati, N.; Montazer, M.; Mahmoudirad, M.; Toliyat, T. Ketoconazole and Ketoconazole/β-cyclodextrin performance on cotton wound dressing as fungal skin treatment. Carbohydr. Polym. 2020, 240, 116267. [Google Scholar] [CrossRef] [PubMed]
- Beig, A.; Miller, J.M.; Dahan, A. The interaction of nifedipine with selected cyclodextrins and the subsequent solubility-permeability trade-off. Eur. J. Pharm. Biopharm. 2013, 85, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Ainurofiq, A.; Choiri, S.; Azhari, M.A. Improvement of Meloxicam Solubility Using a β-Cyclodextrin Complex Prepared via the Kneading Method and Incorporated into an Orally Disintegrating Tablet. Adv. Pharm. Bull. 2016, 6, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Mukne, A.P.; Nagarsenker, M. Triamterene-β-cyclodextrin systems: Preparation, characterization and in vivo evaluation. AAPS PharmSciTech 2004, 5, 142–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, E.J.; Choi, S.A.; Min, K.A.; Jee, J.-P.; Jin, S.G.; Cho, K.H. Development of Alectinib-Suspended SNEDDS for Enhanced Solubility and Dissolution. Pharmaceutics 2022, 14, 1694. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Joshi, A.; Singh, R.; Dubey, K. Enhanced Solubility And Dissolution By Surface-Modified Solid Dispersion of Alectinib Hydrochloride. Int. J. App. Pharm. 2023, 15, 257–265. [Google Scholar] [CrossRef]
- Corina-Cristina, C.; Corina-Cristina, A.; Monciu, C.M. Preparation and characterization of inclusion complexes between repaglinide and β-cyclodextrin, 2-hydroxypropyl-β-cyclodextrin and randomly methylated β-cyclodextrin. Pharmacia 2010, 58, 78–88. [Google Scholar]
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Inc., Wallingford CT (2016). ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-m10-bioanalytical-method-validation-step-5_en.pdf (accessed on 3 March 2022).
- Cardoso, E.; Mercier, T.; Wagner, A.D.; Homicsko, K.; Michielin, O.; Ellefsen-Lavoie, K.; Cagnon, L.; Diezi, M.; Buclin, T.; Widmer, N. Quantification of the next-generation oral anti-tumor drugs dabrafenib, trametinib, vemurafenib, cobimetinib, pazopanib, regorafenib and two metabolites in human plasma by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1083, 124–136. [Google Scholar] [CrossRef]



| Type of Complex | Ratio of ALB:CD | Percent EE (%) |
|---|---|---|
| Kneading Method | ||
| ALB–βCD | 1:1 | 42.5 ± 2.0 |
| ALB–βCD | 1:2 | 64.0 ± 2.5 |
| ALB–βCD | 1:3 | 63.5 ± 1.0 |
| ALB–βCD | 1:4 | 58.6 ± 1.6 |
| ALB–HP βCD | 1:1 | 45.6 ± 1.3 |
| ALB–HP βCD | 1:2 | 65.2 ± 1.5 |
| ALB–HP βCD | 1:3 | 62.5 ± 0.8 |
| ALB–HP βCD | 1:4 | 56.8 ± 1.0 |
| Solvent evaporation method | ||
| ALB–βCD | 1:1 | 38.5 ± 0.5 |
| ALB–βCD | 1:2 | 54.6 ± 2.6 |
| ALB–βCD | 1:3 | 50.5 ± 1.0 |
| ALB–βCD | 1:4 | 35.4 ± 3.0 |
| ALB–HP βCD | 1:1 | 35.0 ± 1.2 |
| ALB–HP βCD | 1:2 | 49.0 ± 2.6 |
| ALB–HP βCD | 1:3 | 45.3 ± 1.5 |
| ALB–HP βCD | 1:4 | 42.6 ± 2.1 |
| Spray Drying | ||
| ALB–βCD | 1:1 | 58.3 ± 2.3 |
| ALB–βCD | 1:2 | 61.2 ± 0.6 |
| ALB–βCD | 1:3 | 60.5 ± 0.5 |
| ALB–βCD | 1:4 | 55.3 ± 2.4 |
| ALB–HP βCD | 1:1 | 55.6 ± 0.5 |
| ALB–HP βCD | 1:2 | 63.5 ± 1.5 |
| ALB–HP βCD | 1:3 | 55.1 ± 2.7 |
| ALB–HP βCD | 1:4 | 49.2 ± 1.0 |
| Model | Zero Order | First Order | Peppas Model | Higuchi Model | Hixson–Crowell Model |
|---|---|---|---|---|---|
| Free ALB | |||||
| R2 | 0.985 | 0.961 | 0.977 | 0.980 | 0.972 |
| ALB released from the complex (ALB–HPβCD 1:2) | |||||
| R2 | 0.931 | 0.989 | 0.972 | 0.970 | 0.900 |
| Samples (n = 6) | LLOQ 15.0 ng/mL | QCLow 130.0 ng/mL | QCMid 2200.0 ng/mL | QCHigh 3000.0 ng/mL |
|---|---|---|---|---|
| Mean | 14.70 | 131.63 | 2183.01 | 2967.55 |
| SD | 0.39 | 4.48 | 65.47 | 24.41 |
| CV% | 2.6 | 3.6 | 2.9 | 0.8 |
| Accuracy | 98.2% | 101.3% | 99.2% | 98.9% |
| Formula | Cmax (ng/mL) | Tmax (h) | AUC-48 (ng. h/mL) | AUC-Inf (ng. h/mL) | Kel (h−1) |
|---|---|---|---|---|---|
| F1 (free alectinib) | 240 ± 26.95 | 7.33 ± 1.03 | 5946.75 ± 265 | 7280.02 ± 306 | 0.045 ± 0.008 |
| F2 (complex) | 474 ± 50.97 | 5.1 ± 1.3 | 10520 ± 310 | 12320 ± 415 | 0.050 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, B.J.M.; Saadallah, M.A.; Al-Ani, I.H.; El-Tanani, M.K.; Al Azzam, K.M.; Abdallah, H.H.; Al-Hajji, F. Evaluation of Solubility, Dissolution Rate, and Oral Bioavailability of β-Cyclodextrin and Hydroxypropyl β-Cyclodextrin as Inclusion Complexes of the Tyrosine Kinase Inhibitor, Alectinib. Pharmaceuticals 2024, 17, 737. https://doi.org/10.3390/ph17060737
Majeed BJM, Saadallah MA, Al-Ani IH, El-Tanani MK, Al Azzam KM, Abdallah HH, Al-Hajji F. Evaluation of Solubility, Dissolution Rate, and Oral Bioavailability of β-Cyclodextrin and Hydroxypropyl β-Cyclodextrin as Inclusion Complexes of the Tyrosine Kinase Inhibitor, Alectinib. Pharmaceuticals. 2024; 17(6):737. https://doi.org/10.3390/ph17060737
Chicago/Turabian StyleMajeed, Bashar J. M., Mohammed A. Saadallah, Israa H. Al-Ani, Mohamed K. El-Tanani, Khaldun M. Al Azzam, Hassan H. Abdallah, and Feras Al-Hajji. 2024. "Evaluation of Solubility, Dissolution Rate, and Oral Bioavailability of β-Cyclodextrin and Hydroxypropyl β-Cyclodextrin as Inclusion Complexes of the Tyrosine Kinase Inhibitor, Alectinib" Pharmaceuticals 17, no. 6: 737. https://doi.org/10.3390/ph17060737
APA StyleMajeed, B. J. M., Saadallah, M. A., Al-Ani, I. H., El-Tanani, M. K., Al Azzam, K. M., Abdallah, H. H., & Al-Hajji, F. (2024). Evaluation of Solubility, Dissolution Rate, and Oral Bioavailability of β-Cyclodextrin and Hydroxypropyl β-Cyclodextrin as Inclusion Complexes of the Tyrosine Kinase Inhibitor, Alectinib. Pharmaceuticals, 17(6), 737. https://doi.org/10.3390/ph17060737







