Abstract
Selective COX-1 inhibitors are preferential therapeutic targets for platelet aggregation and clotting responses. In this study, we examined the selective COX-1-inhibitory activities of four newly synthesized compounds, 10–13, along with their abilities to inhibit platelet aggregation against ADP and collagen. The target compounds 10–13 were synthesized using the conventional method, sonication, and microwave-assisted methods. Microanalytical and spectral data were utilized to elucidate the structures of the new compounds 10–13. Additionally, a spectral NMR experiment [NOESY] was conducted to emphasize the configuration around the double bond of the imine group C=N. The obtained results revealed no observed correlation between any of the neighboring protons, suggesting that the configuration at the C=N double bond is E. Biological results revealed that all the screened compounds 10–13 might serve as selective COX-1 inhibitors. They showed IC50 values ranging from 0.71 μM to 4.82 μM against COX-1 and IC50 values ranging from 9.26 μM to 15.24 μM against COX-2. Their COX-1 selectivity indices ranged between 2.87 and 18.69. These compounds show promise as promising anti-platelet aggregation agents. They effectively prevented platelet aggregation induced by ADP with IC50 values ranging from 0.11 μM to 0.37 μM, surpassing the standard aspirin with an IC50 value of 0.49 μM. Additionally, they inhibited the platelet aggregation induced by collagen with IC50 values ranging from 0.12 μM to 1.03 μM, demonstrating superior efficacy compared to aspirin, which has an IC50 value of 0.51 μM. In silico molecular modeling was performed for all the target compounds within the active sites of COX-1 and COX-2 to rationalize their selective inhibitory activities towards COX-1. It was found that the binding interactions of the designed compounds within the COX-1 active site had remained unaffected by the presence of celecoxib. Molecular modeling and DFT calculations using the B3LYP/6-31+G (d,p) level were performed to study the stability of E-forms with respect to Z-forms for the investigated compounds. A strong correlation was observed between the experimental observations and the quantum chemical descriptors.
1. Introduction
Cyclooxygenase (COX) serves as the pivotal enzyme in the rate-limiting step in prostanoid biosynthesis. Prostanoids, which act as autocoid mediators, play crucial roles in physiological and pathological processes. Cyclooxygenase (COX) comprises identical homodimers subunits: cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) [1,2,3]. During catalysis, it has been found that only one subunit is activated at a time. COX-2 is notably implicated in the pathophysiology of inflammation and tumorigenesis [4,5] while COX-1 primarily regulates platelet functions. It facilitates platelet activation, adhesion, and aggregation, thereby contributing the clotting response [6,7,8].
Coxibs are widely used anti-inflammatory drugs and are renowned for their selective inhibition of COX-2. Surprisingly, it has been found that coxibs can tightly bind to COX-1 active sites and impede the binding interactions of COX-1 inhibitors like aspirin. Aspirin, known to inhibit both COX-1 and COX-2, carries a toxic acetyl tail that acetylates the serine residue of COX-1 [9]. This acetylation leads to permanent damage to the COX-1 active site. COX-2 is inhibited by the same mechanism; however, higher doses of aspirin are required for effective COX-2 inhibition. Moreover, several studies have suggested that lower doses of aspirin are adequate for inhibiting platelet COX-1. However, a significant limitation of aspirin use is that coxibs interfere with its anti-platelet aggregation effect when administered concurrently [10,11,12]. These findings have prompted us to develop novel COX-1 inhibitors, distinct from aspirin, capable of controlling platelet aggregation even in the presence of coxibs. Another limitation is the scarcity of studies focusing on the design of selective COX-1 inhibitors. From the available research, we have selected two promising selective COX-1 inhibitors to serve as models in the design of our target compounds. Compound I is a benzene sulfonamide derivative with an IC50 value of 12 μM against COX-1 and a selectivity index of approximately 100 folds. Compound II is a stilbene derivative with an IC50 value of 15 μM against COX-1 and a selectivity index of around 200 folds [13]. Drawing from the mixed pharmacophore theory [14], novel Schiff base analogues were designed based on two pharmacophoric regions (Figure 1). Region I is a benzene sulfonamide moiety inspired by the structure of compound I. Region II is a stilbene-like Schiff base moiety akin to compound II.
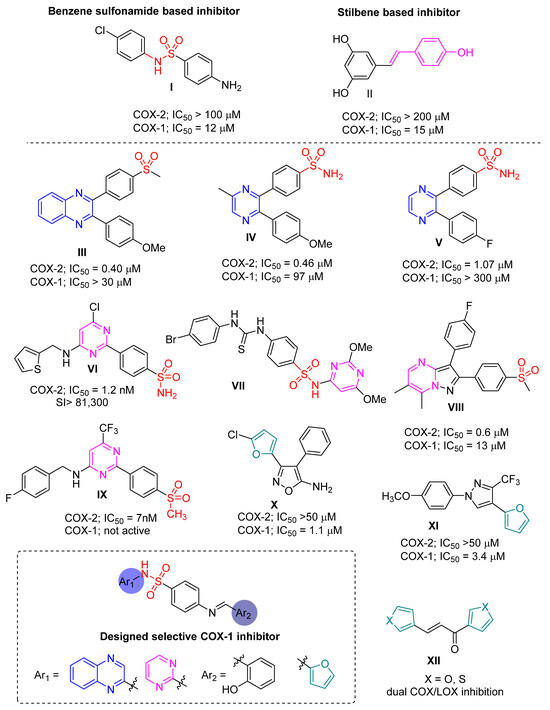
Figure 1.
The design of selective COX-1 inhibitors based on previous selective examples having different pharmacophoric groups.
Heterocycles like pyrimidine, quinoxaline, and furan [15,16,17,18,19,20] are crucial in developing selective cyclooxygenase (COX) inhibitors, which are essential in medicinal chemistry for managing inflammation and pain. COX-2 is a primary target, and selective inhibitors aim to minimize side effects. Pyrimidine derivatives selectively inhibit COX-2 while sparing COX-1, reducing gastrointestinal risks. Quinoxalines exhibit potent anti-inflammatory properties and are investigated for COX inhibition. Furan derivatives also show promise in preclinical COX inhibition studies. These heterocyclic structures improve selectivity and aid in optimizing drug properties, advancing the development of safer and more effective anti-inflammatory drugs. As representative examples, compounds III–V as quinoxaline derivatives have shown selective activity against COX-2 rather than COX-1. Also, pyrimidine scaffold has exhibited good potential against COX enzymes such as compounds VI–IX, with these compounds being mainly more active towards COX-2. Also, it has been found that the insertion of the furan moiety leads to a good enhancement in reactivity towards the COX-1 enzyme; for example, compounds X (COX-1; IC50 = 1.1 μM) [21], XI (COX-1; IC50 = 3.4 μM) [22], and XII have been reported as dual inhibitors for COX/LOX enzymes [23]. To explore the combination of the different pharmacophoric groups into one compound is a challenge. In this text we have designed, synthesized, and evaluated a novel selective COX-1 inhibitor having the pyrimidine, quinoxaline, and furan motifs for the first time.
It is worth noting that recently, in the field of drug discovery, computational chemistry has progressed in correlating the experimental observations and the theoretical data of new candidates [24]. The density functional theory (DFT) is one of the most important theoretical methods that are used in calculating a great variety of molecular properties [25,26,27,28]. The aim of this study was to utilize DFT to investigate the difference in energy between the E- and Z-forms of novel Schiff base analogues. These analogues might selectively recognize the platelet COX-1 active site.
2. Results and Discussion
2.1. Chemistry
Scheme 1 illustrates the synthetic route developed for the preparation of four new Schiff base sulfonamide analogues, designated as 10–13. The biological significance of Schiff base analogues has motivated numerous researchers to devise various methods for their synthesis [29]. In this study, our objective was to synthesize the target compounds 10–13 through the conventional method, sonication, and microwave-assisted methods [30,31,32,33].
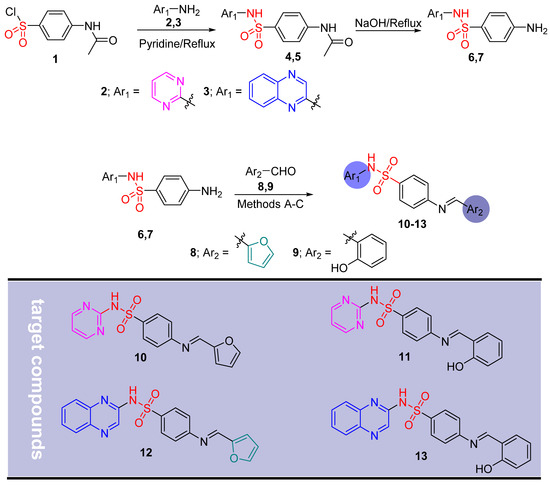
Scheme 1.
Synthesis of target compounds 10–13.
Microanalyses and analyses of spectral data (IR, 1H NMR,13CNMR, and EI-MS) were carried out to confirm the chemical structures of the new compounds 10–13. The IR spectra displayed NH stretching at around 3400–3500 cm−1 and bending at around 1550 cm−1, the appearance of O=S=O asymmetric stretching at around 1350 cm−1 and symmetric stretching at around 1140 cm−1, and the appearance of =C-H stretching at around 2900 cm−1. There was also an appearance of C=N at around 1650 cm−1. Additionally, the IR spectra of compounds 11 and 13 showed O-H stretching at around 3150 cm−1. 1H-NMR spectra of the target compounds 10, 11, 12, and 13 showed NH singlets at δH [11.30–11.68 ppm] in addition to aromatic multiplets at δH [6.80–8.20 ppm]. Regarding the 1H NMR spectra of compounds 11 and 13, singlets at δH [12.50 ppm] were detected that referred to the hydroxyl group (OH).
An additional spectral NMR experiment, specifically a NOESY (Nuclear Overhauser Effect Spectroscopy), was conducted to highlight the configuration around the double bond of the imine group (C=N). The obtained results revealed no observed correlation between any of the neighboring protons in the space, suggesting that the configuration at the C=N double bond is E. (Supplementary Materials).
2.2. Biological Activity
2.2.1. Cyclooxygenase Inhibition Assays
The cyclooxygenase-inhibitory activities of the four newly compounds 10–13 were investigated against COX-1 and COX-2. The COX-1 selectivity indices were also calculated. The results are listed in Table 1.

Table 1.
The cyclooxygenase-inhibitory activities of new compounds 10–13.
The tested compounds displayed IC50 values ranging from 0.71 to 4.82 μM against COX-1 and IC50 values ranging from 9.26 to 15.24 μM against COX-2. Their selectivity indices for COX-1 ranged between 2.87 and 18.69. Fortunately, it was found that the designed compounds showed, collectively, a selective COX-1 inhibition range superior to the celecoxib for standard compounds I and II. Compound 12 was identified as the most active sulfonamide analogue among the designed compounds with 20, 16.9, and 21 folds compared to the reference (the celecoxib, standard compounds I and II). The chemical structure of compound 12, having a quinoxaline/furan scaffold-based Schiff base sulfonamide, was the best in the reactivity order 12 > 10 > 13 > 11. Therefore, the compounds could be considered promising lead molecules and selective COX-1 inhibitors.
2.2.2. The Anti-Platelet Aggregation Assay
Furthermore, the target compounds 10–13 were capable of inhibiting the platelet aggregation induced by 10 μM ADP and 0.33 U/mL collagen. The platelet aggregation inhibition rates and the corresponding IC50 values are listed in Table 2.

Table 2.
Anti-platelet aggregation assay of the tested compounds.
Compounds 10–13 showed promising anti-platelet aggregation activity. The IC50 ranges against ADP were from 0.11 to 0.37 μM, and against collagen, they were from 0.12 to 1.03 μM. These ranges were superior to the standard aspirin with IC50 = 0.49 μM against ADP and IC50 = 0.51 μM against collagen. The compound 12, having quinoxaline and furan moieties, showed the best reactivity with 4.45 folds compared to aspirin in the case of ADP; also, on the other hand, it exhibited more activity than aspirin with 4.25 folds in the case of collagen. The second compound that showed better reactivity was compound 11, in the case of ADP; the compound had, in its chemical structure, pyrimidine and 2-hydroxy-susbitituted benzene. The third compound in the reactivity order against ADP was 10, having pyrimidine and a furan nucleus. In the case of collagen, the reactivity order was as follows: 12 > 10 > 11. Compound 13-based sulfonamide, having the quinoxaline and 2-hydroxy-susbitituted benzene, was not active in both ADP and collagen.
2.3. In Silico Studies
2.3.1. Molecular Docking
To rationalize the selectivity of the investigated compounds towards COX-1, the novel compounds were screened for their binding abilities towards the cylooxygenase binding sites (COX-1 and COX-2), highlighting both binding interactions and binding energies (Table 3). The tested compounds were docked inside the COX-1 active site with good binding energies ranging from −16.44 Kcal/mol to −20.70 Kcal/mol (Figure 2). Interestingly, the binding interactions of the target compounds were not hindered by celecoxib. They formed four strong hydrogen bond interactions with the key amino acids Gln 192, His 90, Ser 353, and Ser 516 within the platelet COX-1 active site. The binding energy of the most active compound 12 was −20.70 Kcal/mol, which was superior to the binding energy of celecoxib, −16.91 Kcal/mol.

Table 3.
Binding energies and binding interactions of the target compounds in COX-1 and COX-2.
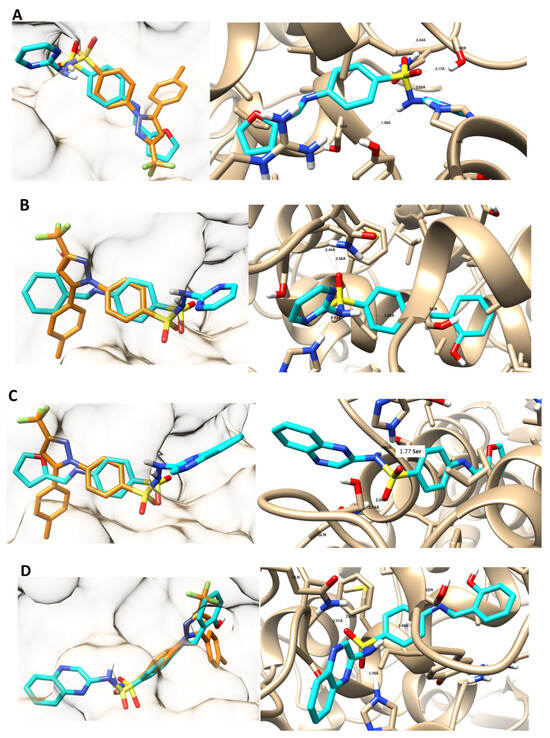
Figure 2.
Binding dispositions of the co-crystallized ligand: celecoxib (orange-colored) and the docked compounds (cyan-colored) towards platelet COX-1 active site. (A): 10, (B): 11, (C): 12, and (D): 13. The panel on the left shows the surface representation while the right panel shows the interactive binding mode with the main amino acids.
Celecoxib as a selective COX-2 inhibitor formed three hydrogen bonds with the key amino acids Leu 338, Gln 178, and Ser 339 within COX-2 active sites with a binding energy of −14.45 Kcal/mol. Unlike celecoxib, compound 10 formed one hydrogen bond with Ser 516 with a binding energy of −9.87 Kcal/mol. Compound 11 formed binding interactions with Arg 499 and His 75 with a binding energy of −8.94 Kcal/mol. Compound 12 formed one hydrogen bond and an Arene-cation with Arg 499 and one hydrogen bond with His 75 with a binding energy of −5.89 Kcal/mol. Compound 13 formed two hydrogen bonds with Arg 106 and Gln 178 with a binding energy of −8.84 Kcal/mol.
To sum up, the target compounds extended deeply in the characteristic pocket of the platelet COX-1 receptor cavity formed by Gln 192, His 90, Ser 353, and Ser 516. Their binding interactions were not hindered by celecoxib.
Docking calculation was validated using the AutoDock Vina 1 software. The RMSD was lower than 2.
2.3.2. Bioinformatics Study
Bioinformatics studies were also performed to investigate the physicochemical properties and drug-like properties of compounds 10–13.
According to Lipinski rule of five (Ro5) [35], the target compounds displayed good drug-likeness model scores and promising physicochemical properties Figure 3. The investigated compounds had one to two H-bond donors (HBD) and six H-bond acceptors (HBA). The number of rotatable bonds (nrotb) was five, indicating controlled conformational changes for good oral bioavailability. Additionally, the Log P values of the target compounds were between 1.67 and 4.10. The polar surface area (PSA) values were between 97.46 and 104.55, suggesting the good absorption and permeability of the tested compounds (Table 4). Consequentially, the newly synthesized compounds were promising drug-like candidates with Molecular Weight ≤ 500, Log P ≤ 4.15, HBA ≤ 10, HBD ≤ 5, and nrotb ≤ 10 [35,36,37].
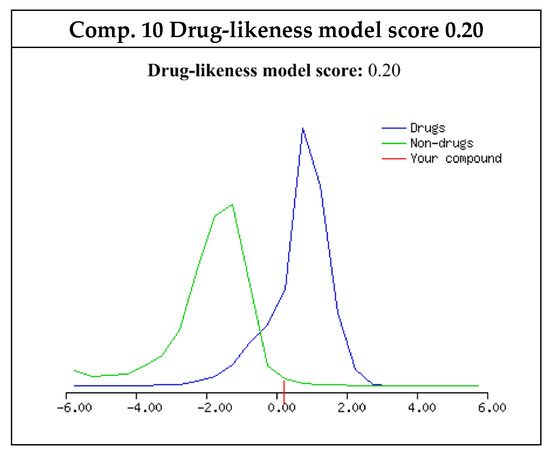
Figure 3.
Plots of drug-likeness score for target compound 10 using Molsoft. The drug-like behavior curve is colored blue and non-drug like behavior curve is colored green.

Table 4.
In silico ADME pharmacokinetic properties of the target compounds 10–13.
2.3.3. Density Functional Theory Study
Structures of the tested compounds were optimized using density functional theory (DFT) in combination with Beck’s three-parameter exchange functional along with the Lee–Yang–Parr non-local correlation functional (B3LYP) [41,42,43] with the 6-31+G(d,p) basis set, which is implemented in the Gaussian 09 program package [44,45]. The quantum chemical calculation studies were performed to make a comparison between the stabilities of the newly synthesized compounds in Z- and E-forms. The quantum chemical calculations displayed that compounds 13, 10, 11, and 12 were more stable in the E-forms than the Z-forms, by about 0.021, 0.053, 0.023, and 0.003 a.u., respectively, which agree with the experimental findings. Figure 4 shows the optimized molecular structures of the investigated compounds with minimum energies obtained from the calculations.
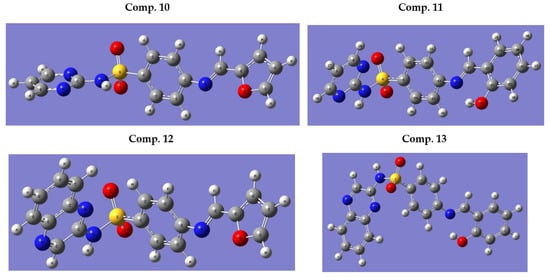
Figure 4.
The optimized molecular structures of E-form for the novel compounds obtained from DFT calculation.
3. Materials and Methods
3.1. Chemistry
“Stuart melting point apparatus SMP10 was used for melting points determination (China). Melting points were uncorrected. Bruker Vector 22 Infrared spectrophotometer (υmax in cm−1) (Burladingen, Germany) was used to measure infrared (IR) spectra. Nuclear Magnetic Resonance spectra were recorded on Bruker spectrometer (400 MHz) (Burladingen, Germany). Electron impact mass spectra (EI-MS) were recorded on a Finnigan MAT 312 mass spectrometer (Burladingen, Germany). Sonication was performed by Daihan (Wiseclean, D-40 kHz) sonicator (China). Microwave assisted reactions were accomplished using Milestone Startsynth lab station microwave (Via Fatebenefratelli 1/5-24010 Sorisole (BG), Italy)”.
- N-(4-(N-Pyrimidin-2-ylsulfamoyl)phenyl)acetamide 4 and N-(4-(N-quinoxalin-2-ylsulfamoyl) phenyl)acetamide 5
In a 50 mL flask, p-acetamidobenzenesulfonyl chloride 1 (2.34 g, 10 mmol) was added to the appropriate amine 2 or 3 (0.94 g, 10 mmol) in anhydrous pyridine (3.0 mL). A reflux condenser was connected to this system with a desiccant tube. After magnetic stirring for 4 h at 55 °C, the mixture was concentrated under vacuum for pyridine removal (bath at approximately 40–50 °C). This crude product was directly used in the following hydrolysis reaction [30].
- 4-Amino-N-(pyrimidin-2-yl)benzenesulfonamide 6 and N-(4-(N-pyrimidin-2-ylsulfamoyl) phenyl)acetamid 7
The crude product obtained in the previous step was treated with NaOH solution (2M, 25 mL). The mixture was refluxed for 2 h at 100 °C under nitrogen. The mixture was allowed to cool to room temperature, neutralized with concentrated HCl to pH 6.0. Afterwards, the mixture was cooled in an ice bath until complete precipitation of the final product. The precipitated was filtered under vacuum, washed, and dried. The final product was re-crystallized from boiling acetonitrile/water (2:1) mixture [30].
General procedure for the synthesis of final compounds 10–13.
- Method A: Conventional method—The appropriate aldehyde 8 or 9 (0.002 moL) was added to an equimolar amount of benzene sulfonamide derivatives 6 or 7 (0.5 gm, 0.002 moL) in 10 mL 95% ethanol. The reaction mixture was stirred and refluxed until completion. The reaction mixture was poured on ice for precipitation [31]. The final product was re-crystallized from ethanol to yield the target compounds as listed in Table 5.
 Table 5. The reaction times and yields of target compounds 10–13.
Table 5. The reaction times and yields of target compounds 10–13. - Method B: Sonicated Reaction—Benzene sulfonamide derivatives 6 or 7 (0.5 gm, 0.002 moL) and the appropriate aldehyde 8 or 9 (0.002 moL) were suspended in 5 mL of absolute ethanol. The mixture was subjected to ultrasound irradiation at 60–65 °C for suitable time until the starting material was no longer detectable via TLC. Upon completion, the reaction mixture was poured on ice [32]. The formed precipitate was filtered and re-crystallized from ethanol to yield the target compounds as listed in Table 5.
- Method C: Microwave-assisted reaction—An equimolar mixture of benzene sulfonamide derivatives 6 or 7 (0.5 gm, 0.002 moL) and the appropriate aldehyde 8 or 9 (0.002 moL) in 5 mL 95% ethanol was irradiated by the microwave reactor at 850 watts and 100 °C until completion. The mixture was cooled and poured on ice and the separated precipitate was filtered and washed with cold absolute ethanol [33]. The resulting product was then re-crystallized from absolute ethanol to yield the target compounds as listed in Table 5.
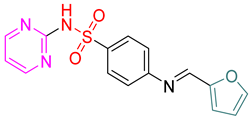
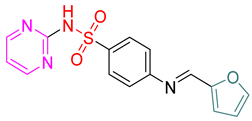
- (E)-4-(Furan-2-ylmethyleneamino)-N-(pyrimidin-2-yl)benzenesulfonamide 10
Yellow crystals; m.p. (250–252) °C; IR (KBr, cm−1): 3448, 2924, 1604, 1500, 1442, 1342, 1141; EI-MS (m/z, %): 328 (M+); 1H-NMR (400 MHz) (DMSO-d6): δH 11.30 (s, 1H), 8.97 (s, 1H), 8.31–8.26 (m, 2H) 7.59–7.54 (m, 3H), 6.84–6.80 (m,1H), 6.61–6.63 (d, 2H, J = 8 Hz), 6.11–6.13 (d, 2H, J = 8 Hz); 13CNMR (DMSO-d6): δC160.83, 158.20, 152.05, 129.61, 129.17, 113.11, 112.45. Anal.Calcd. for C15H12N4O3S:C, 54.87; H, 3.68; N, 17.06. Found: C, 55.07; H, 4.08; N, 17.46.
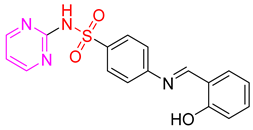
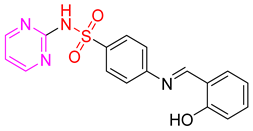
- (E)-4-(2-Hydroxybenzylideneamino)-N-(pyrimidin-2-yl)benzenesulfonamide 11
Yellow crystals; m.p. (251–253) °C; IR (KBr, cm−1): 3446, 3170, 2904, 1627, 1595, 1495, 1330, 1172; EI-MS (m/z, %): 354 (M+); 1H-NMR (400 MHz) (DMSO-d6): δH12.56 (s, 1H), 11.36 (s, 1H), 8.98 (s, 1H) 8.52–8.50 (d, 2H, J = 8 Hz), 8.06–8.04 (dd, 1H, J = 8, 2 Hz),7.71–7.64 (m, 2H), 7.62–7.54 (m, 1H), 7.48–7.44 (m, 1H), 7.00–7.02 (d, 2H, J = 8 Hz), 6.57–6.59 (d, 2H, J = 8 Hz); 13CNMR (DMSO- d6): δC161.19, 160.72, 158.73, 153.51, 129.67, 125.28, 119.97, 112.60.Anal.Calcd. for C17H14N4O3S:C, 57.62; H, 3.98; N, 15.81. Found: C, 57.22; H, 4.18; N, 16.10.
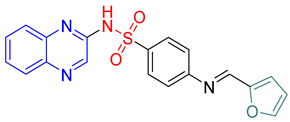
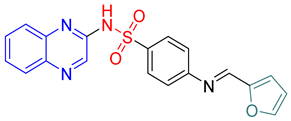
- (E)-4-(Furan-2-ylmethyleneamino)-N-(quinoxalin-2-yl)benzenesulfonamide 12
Light brown crystals; m.p. (255–257) °C; IR (KBr, cm−1): 3425, 2924, 1650, 1534, 1481, 1388, 1110; EI-MS (m/z, %): 378 (M+); 1H-NMR (400 MHz) (DMSO-d6): δH 11.68 (s, 1H), 8.98 (s, 1H), 8.58 (s, 1H) 7.93–7.80 (m, 2H), 7.78–7.71 (m,3H), 7.62–7.58 (m, 2H), 6.61–6.59 (d, 2H, J = 8 Hz), 6.19–6.21 (d, 2H, J = 8 Hz); 13CNMR (DMSO-d6): δC153.80, 146.90, 139.18, 138.35, 131.15, 130.55, 129.15, 127.90, 127.54. 112.69.Anal.Calcd. for C19H14N4O3S:C, 60.31; H, 3.73; N, 14.81. Found: C, 60.60; H, 4.12; N, 15.14.
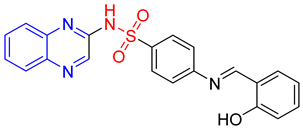
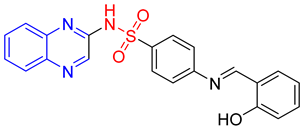
- (E)-4-(2-Hydroxybenzylideneamino)-N-(quinoxalin-2-yl)benzenesulfonamide 13
Light brown crystals; m.p. (254–256) °C; IR (KBr, cm−1): 3425, 3147, 3047, 1600, 1551, 1450, 1373, 1195; EI-MS (m/z, %): 404 (M+); 1H-NMR (400 MHz) (DMSO d6): δH 12.50 (s, 1H), 11.58 (s, 1H), 8.96 (s, 1H) 8.58 (s, 1H),8.20–8.18 (m,2H), 7.96–7.92 (m, 2H), 7.81–7.72 (m, 1H), 7.69–7.59 (m, 2H), 7.57–7.43 (m, 1H), 7.01–6.99 (d, 2H, J = 8 Hz), 6.60–6.58 (d, 2H, J = 8 Hz); 13CNMR (DMSO-d6): δC161.20, 153.87, 146.73, 136.92, 131.20, 130.58, 129.18, 127.65, 119.96, 117.69, 112.70.Anal.Calcd. for C21H16N4O3S:C, 62.36; H, 3.99; N, 13.85. Found: C, 62.00; H, 4.19; N, 14.25.
3.2. Biological Evaluation
The experimental protocols were approved by the Ethics Committee of the Suez Canal University with code 202403R1.
3.2.1. Cyclooxygenase Inhibition Assays
The ability of the test compounds to inhibit human recombinant COX-1 and human recombinant COX-2 (IC50 value, µM) was assigned using an enzyme immune assay (EIA) kit (item no. 560131, Cayman Chemical, Ann Arbor, MI, USA) according to the previously reported method [46].
3.2.2. The Anti-Platelet Aggregation Assays
The anti-platelet aggregation assays were performed according to the method reported by XIE et al. [47] (Supplementary Materials).
3.3. In Silico Studies
3.3.1. Molecular Docking
The X-ray crystal structures of two proteins with the co-crystallized ligands crystal structure of cyclooxygenase-1 in complex with celecoxib (PDB 3KK6) [48] and crystal structure of cyclooxygenase-2 in complex with celecoxib (PDB 3LN1) [49] were downloaded from the Protein Data Bank. The in silico studies were carried out using AutoDock Vina software [50] with Chimera-UCSF [51]. Chimera was used to tantalize both the interactive analysis as well as binding disposition.
3.3.2. Bioinformatics Study
Bioinformatics study of the novel compounds were calculated using Molinspiration [38], SwissADME [40] website and MolSoft web-based software [39].
3.3.3. Density Functional Theory Study
The DFT study was performed according to the method reported by Awad et al. in 2018 [52].
4. Conclusions
Four new Schiff base analogues 10–13 were synthesized using the conventional method, sonication, and microwave-assisted methods. Biological results revealed that all the screened compounds hold promise as potential anti-platelet aggregation agents. They displayed IC50 values ranging from 0.11 to 0.37 μM against ADP and IC50 values ranging from 0.12 to 1.03 μM against collagen. These ranges for compounds 10–13 were superior to those of the standard aspirin with IC50 = 0.49 μM against ADP, but only compound 12 (IC50 = 0.12 μM) was found more potent than the standard aspirin with IC50 = 0.51 μM against collagen. Additionally, they exhibited notable COX-1-inhibitory activity, with IC50 values ranging from 0.71 μM to 4.82 μM and COX-1 selectivity indices ranging from 2.87 to 18.69. These findings suggest that compounds 10–13 hold potential as dual-function agents with anti-platelet aggregation and COX-1-inhibitory properties, warranting further investigation into their therapeutic applications. In silico molecular modeling was performed for all the target compounds inside the platelet COX-1 active site, and their binding interactions were not hindered by celecoxib. The geometric molecular structures were deduced through computational chemistry calculations with the B3LYP/6-31+G (d,p) level to analyze the stable forms of the novel compounds. The calculations showed that E-forms are more stable than Z-form for the investigated compounds, which are experimentally in a good correlation with the NOESY.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17060710/s1, NMR spectrum and the anti-platelet aggregation assays.
Author Contributions
Conceptualization, Y.M.A.A., P.A.H., and M.E.; methodology, Y.M.A.A., P.A.H., and M.E.; software, M.S.N.; validation and molecular docking, M.S.N.; formal analysis, Y.M.A.A., P.A.H., and M.E.; investigation, Y.M.A.A., P.A.H., and M.E.; resources, Y.M.A.A., P.A.H., and M.E.; data curation, Y.M.A.A., P.A.H., M.S.N., S.R., A.B., and M.E.; writing—original draft preparation, Y.M.A.A., P.A.H., and M.E.; writing—review and editing, all; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to extend their sincere appreciation to the Researchers Supporting Project (RSP2024R64), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to extend their sincere appreciation to the Researchers Supporting Project (RSP2024R64), King Saud University, Riyadh, Saudi Arabia. The authors acknowledge Mohamed K. Awad (Theoretical Applied Chemistry Unit (TACU), Chemistry Department, Faculty of Science, Tanta University, 6632110 Tanta, Egypt for performing the DFT calculations.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fitzpatrick, F.A. Cyclooxygenase enzymes: Regulation and function. Curr. Pharm. Des. 2004, 10, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Maenthaisong, R.; Tacconelli, S. Cyclooxygenase-2: Biology of Prostanoid Biosynthesis and Metabolism. eLS 2012. [Google Scholar] [CrossRef]
- Vane, J.; Bakhle, Y.; Botting, R. CYCLOOXYGENASES 1 AND 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tighe, S.; Zhu, Y.-T. COX-2 Signaling in the Tumor Microenvironment. In Tumor Microenvironment: Molecular Players—Part B; Birbrair, A., Ed.; Springer International Publishing: Cham, Germany, 2020; pp. 87–104. [Google Scholar]
- Gómez-Valenzuela, F.; Escobar, E.; Pérez-Tomás, R.; Montecinos, V.P. The Inflammatory Profile of the Tumor Microenvironment, Orchestrated by Cyclooxygenase-2, Promotes Epithelial-Mesenchymal Transition. Front. Oncol. 2021, 11, 686792. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.A.; Kirkby, N.S.; Ahmetaj-Shala, B.; Armstrong, P.C.; Crescente, M.; Ferreira, P.; Pires, M.E.L.; Vaja, R.; Warner, T.D. Cyclooxygenases and the cardiovascular system. Pharmacol. Ther. 2021, 217, 107624. [Google Scholar] [CrossRef] [PubMed]
- Geraldo, R.B.; Sathler, P.C.; Lourenço, A.L.; Saito, M.S.; Cabral, L.M.; Rampelotto, P.H.; Castro, H.C. Platelets: Still a therapeutical target for haemostatic disorders. Int. J. Mol. Sci. 2014, 15, 17901–17919. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Tacconelli, S.; Contursi, A.; Ballerini, P.; Patrignani, P. Chapter Three—Cyclooxygenases and platelet functions. In Advances in Pharmacology; Zeldin, D.C., Seubert, J.M., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 133–165. [Google Scholar]
- Maria, I.T. Therapeutic Uses of Aspirin. In Pain Management; Theodoros, A., Christos, N., Eds.; IntechOpen: Rijeka, Croatia, 2023; p. 13. [Google Scholar]
- Ahrens, I.; Schwarz, M.; Peter, K.; Bode, C. Therapeutic Inhibitors of Platelet Aggregation–from Aspirin to Integrin Blockersf. Transfus. Med. Hemotherapy 2007, 34, 44–54. [Google Scholar] [CrossRef]
- Gladding, P.A.; Webster, M.W.; Farrell, H.B.; Zeng, I.S.; Park, R.; Ruijne, N. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am. J. Cardiol. 2008, 101, 1060–1063. [Google Scholar] [CrossRef] [PubMed]
- Patrono, C.; Rocca, B. Aspirin and other COX-1 inhibitors. Antiplatelet Agents 2012, 210, 137–164. [Google Scholar]
- Perrone, M.G.; Scilimati, A.; Simone, L.; Vitale, P. Selective COX-1 inhibition: A therapeutic target to be reconsidered. Curr. Med. Chem. 2010, 17, 3769–3805. [Google Scholar] [CrossRef]
- Taha, M.O. Mixing pharmacophore modeling and classical QSAR analysis as powerful tool for lead discovery. Virtual Screen. 2012, 1. [Google Scholar] [CrossRef]
- ur Rashid, H.; Martines, M.A.U.; Duarte, A.P.; Jorge, J.; Rasool, S.; Muhammad, R.; Ahmad, N.; Umar, M.N. Research developments in the syntheses, anti-inflammatory activities and structure–activity relationships of pyrimidines. RSC Adv. 2021, 11, 6060–6098. [Google Scholar] [CrossRef] [PubMed]
- Orjales, A.; Mosquera, R.; Lopez, B.; Olivera, R.; Labeaga, L.; Núñez, M.T. Novel 2-(4-methylsulfonylphenyl) pyrimidine derivatives as highly potent and specific COX-2 inhibitors. Bioorg. Med. Chem. 2008, 16, 2183–2199. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Saibaba, V.; Ravikumar, V.; Rudrawar, S.V.; Daga, P.; Rao, C.S.; Akhila, V.; Hegde, P.; Rao, Y.K. Synthesis and biological evaluation of 2, 3-diarylpyrazines and quinoxalines as selective COX-2 inhibitors. Bioorg. Med. Chem. 2004, 12, 1881–1893. [Google Scholar] [CrossRef] [PubMed]
- Caturla, F.; Jiménez, J.-M.; Godessart, N.; Amat, M.; Cárdenas, A.; Soca, L.; Beleta, J.; Ryder, H.; Crespo, M.I. Synthesis and biological evaluation of 2-phenylpyran-4-ones: A new class of orally active cyclooxygenase-2 inhibitors. J. Med. Chem. 2004, 47, 3874–3886. [Google Scholar] [CrossRef] [PubMed]
- Almansa, C.; de Arriba, A.F.; Cavalcanti, F.L.; Gómez, L.A.; Miralles, A.; Merlos, M.; García-Rafanell, J.; Forn, J. Synthesis and SAR of a new series of COX-2-selective inhibitors: Pyrazolo [1, 5-a] pyrimidines. J. Med. Chem. 2001, 44, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Ghorab, M.M.; SA El-Gaby, M.; S Alsaid, M.; AMM Elshaier, Y.; M Soliman, A.; F El-Senduny, F.; A Badria, F.; YA Sherif, A. Novel thiourea derivatives bearing sulfonamide moiety as anticancer agents through COX-2 inhibition. Curr. Med. Chem. Anticancer. Agents 2017, 17, 1411–1425. [Google Scholar] [CrossRef]
- Vitale, P.; Perrone, M.G.; Malerba, P.; Lavecchia, A.; Scilimati, A. Selective COX-1 inhibition as a target of theranostic novel diarylisoxazoles. Eur. J. Med. Chem. 2014, 74, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Vitale, P.; Malerba, P.; Altomare, A.; Rizzi, R.; Lavecchia, A.; Di Giovanni, C.; Novellino, E.; Scilimati, A. Diarylheterocycle Core Ring Features Effect in Selective COX-1 Inhibition. ChemMedChem 2012, 7, 629–641. [Google Scholar] [CrossRef]
- Ye, Y.; Wu, W.; Shin, V.; Bruce, I.; Wong, B.; Cho, C. Dual inhibition of 5-LOX and COX-2 suppresses colon cancer formation promoted by cigarette smoke. Carcinogenesis 2005, 26, 827–834. [Google Scholar] [CrossRef]
- Houk, K.; Liu, F. Holy grails for computational organic chemistry and biochemistry. Acc. Chem. Res. 2017, 50, 539–543. [Google Scholar] [CrossRef]
- Atlam, F.M.; Awad, M.K.; El-Bastawissy, E.A. Computational simulation of the effect of quantum chemical parameters on the molecular docking of HMG-CoA reductase drugs. J. Mol. Struct. 2014, 1075, 311–326. [Google Scholar] [CrossRef]
- Awad, M.; Masoud, M.; Shaker, M.A.; Ali, A.E.; El-Tahawy, M. MP2 and DFT theoretical studies of the geometry, vibrational and electronic absorption spectra of 2-aminopyrimidine. Res. Chem. Intermediat. 2013, 39, 2741–2761. [Google Scholar] [CrossRef]
- Kabanda, M.M.; Murulana, L.C.; Ozcan, M.; Karadag, F.; Dehri, I.; Obot, I.; Ebenso, E.E. Quantum chemical studies on the corrosion inhibition of mild steel by some triazoles and benzimidazole derivatives in acidic medium. Int. J. Electrochem. Sci. 2012, 7, 5035–5056. [Google Scholar] [CrossRef]
- Udhayakala, P.; Jayanthi, A.; Rajendiran, T. Adsorption and quantum chemical studies on the inhibition potentials of some formazan derivatives. Pharma Chem. 2011, 3, 528–539. [Google Scholar]
- Weng, Q.; Yi, J.; Chen, X.; Luo, D.; Wang, Y.; Sun, W.; Kang, J.; Han, Z. Controllable synthesis and biological application of schiff bases from D-glucosamine and terephthalaldehyde. ACS Omega 2020, 5, 24864–24870. [Google Scholar] [CrossRef] [PubMed]
- Borges, Á.D.L.; Ponte, G.D.; Federman Neto, A.; Carvalho, I. Síntese de sulfadiazina e sulfadiazina de prata em escala semi-micro: Prática experimental em síntese de fármacos. Quim. Nova. 2005, 28, 727–731. [Google Scholar] [CrossRef]
- Sinha, D.; Tiwari, A.K.; Singh, S.; Shukla, G.; Mishra, P.; Chandra, H.; Mishra, A.K. Synthesis, characterization and biological activity of Schiff base analogues of indole-3-carboxaldehyde. Eur. J. Med. Chem. 2008, 43, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Alfooty, K.; Khalifah, S. An efficient sonochemical synthesis of novel Schiff’s bases, thiazolidine, and pyrazolidine incorporating 1, 8-naphthyridine moiety and their cytotoxic activity against HePG2 cell lines. Sci. World J. 2014, 2014, 587059. [Google Scholar] [CrossRef]
- Naglah, A.M.; Awad, H.M.; Bhat, M.A.; Al-Omar, M.A.; Amr, A.E.-G.E. Microwave-assisted synthesis and antimicrobial activity of some novel isatin schiff bases linked to nicotinic acid via certain amino acid bridge. J. Chem. 2015, 2015, 364841. [Google Scholar] [CrossRef]
- Kassab, S.E.; Khedr, M.A.; Ali, H.I.; Abdalla, M.M. Discovery of new indomethacin-based analogs with potentially selective cyclooxygenase-2 inhibition and observed diminishing to PGE2 activities. Eur. J. Med. Chem. 2017, 141, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.E.; Pickett, S.D. Computational methods for the prediction of ‘drug-likeness’. Drug Discov. Today 2000, 5, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics Software. 2018. Available online: https://www.molinspiration.com/ (accessed on 26 May 2024).
- Molsoft Molecules In Silico. Available online: https://www.molsoft.com/ (accessed on 26 May 2024).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. II. The effect of the Perdew–Wang generalized-gradient correlation correction. J. Chem. Phys. 1992, 97, 9173–9177. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R., II; Keith, T.; Millam, J. (Eds.) GaussView; Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Roschek Jr, B.; Fink, R.C.; Li, D.; McMichael, M.; Tower, C.M.; Smith, R.D.; Alberte, R.S. Pro-inflammatory enzymes, cyclooxygenase 1, cyclooxygenase 2, and 5-lipooxygenase, inhibited by stabilized rice bran extracts. J. Med. Food 2009, 12, 615–623. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, L.; Ding, X.; Kong, Y.; Li, Z. Design, synthesis and evaluation of 1, 4-benzodioxine derivatives as novel platelet aggregation inhibitors. Future Med. Chem. 2018, 10, 367–378. [Google Scholar] [CrossRef]
- Rimon, G.; Sidhu, R.S.; Lauver, D.A.; Lee, J.Y.; Sharma, N.P.; Yuan, C.; Frieler, R.A.; Trievel, R.C.; Lucchesi, B.R.; Smith, W.L. Coxibs interfere with the action of aspirin by binding tightly to one monomer of cyclooxygenase-1. Proc. Natl. Acad. Sci. USA 2010, 107, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorg. Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Awad, M.K.; Abdel-Aal, M.F.; Atlam, F.M.; Hekal, H.A. Design, synthesis, molecular modeling, and biological evaluation of novel α-aminophosphonates based quinazolinone moiety as potential anticancer agents: DFT, NBO and vibrational studies. J. Mol. Struct. 2018, 1173, 128–141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).