Meta-Analysis of the Safety and Efficacy of Direct Oral Anticoagulants for the Treatment of Left Ventricular Thrombus
Abstract
1. Introduction
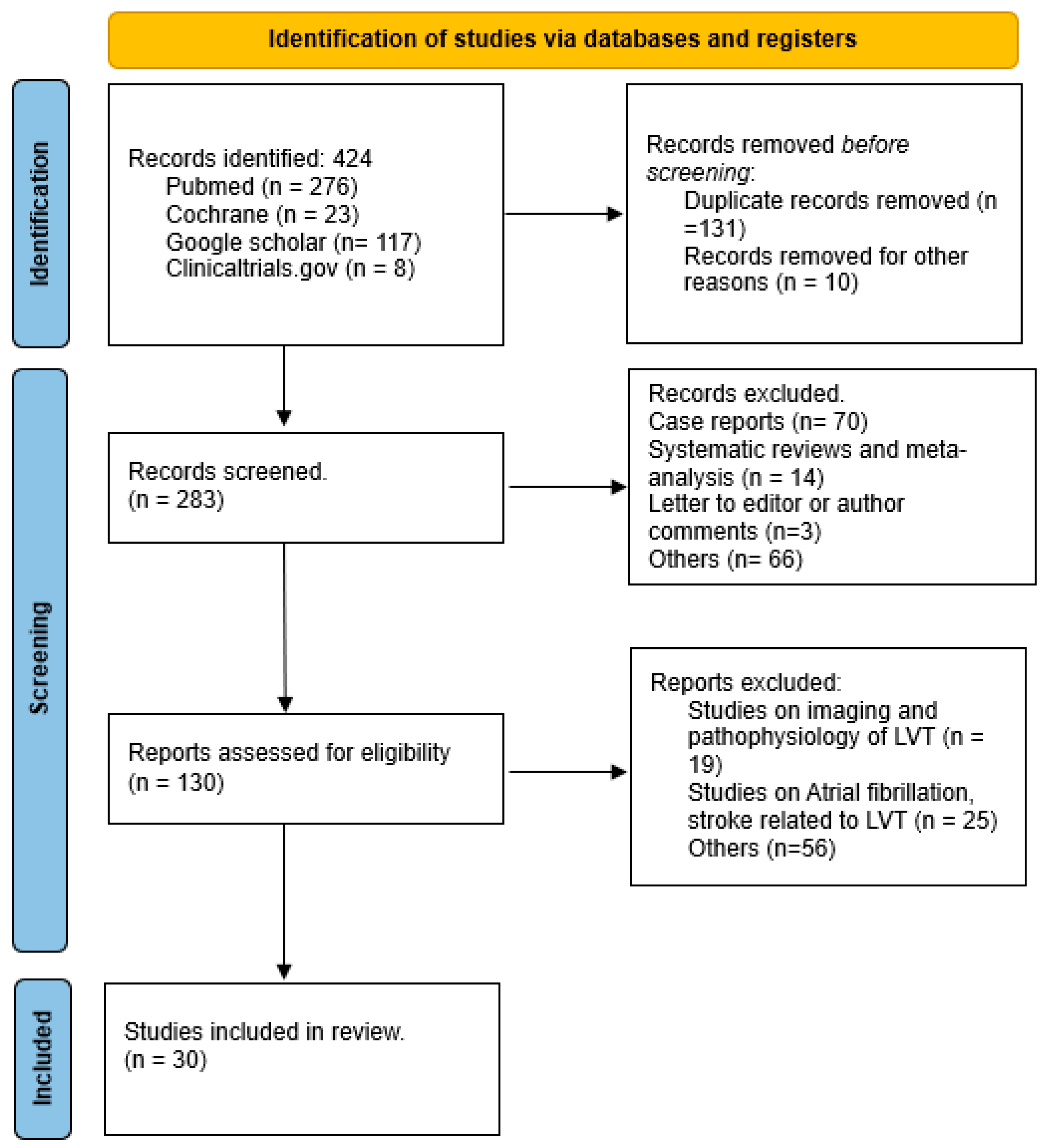
2. Methods
2.1. Data Sources and Searches
2.2. Study Selection
2.3. Outcome Measures and Quality Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
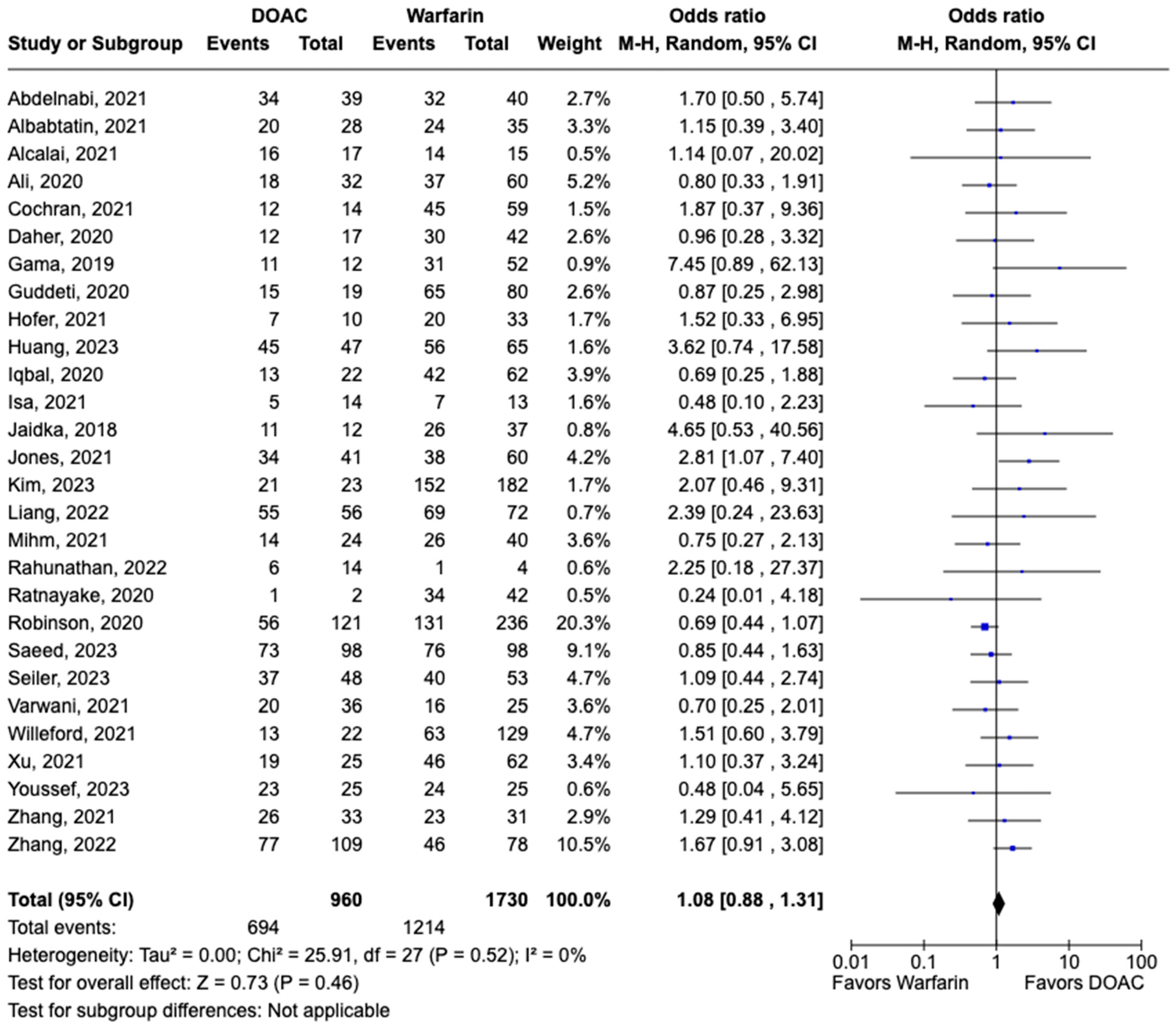
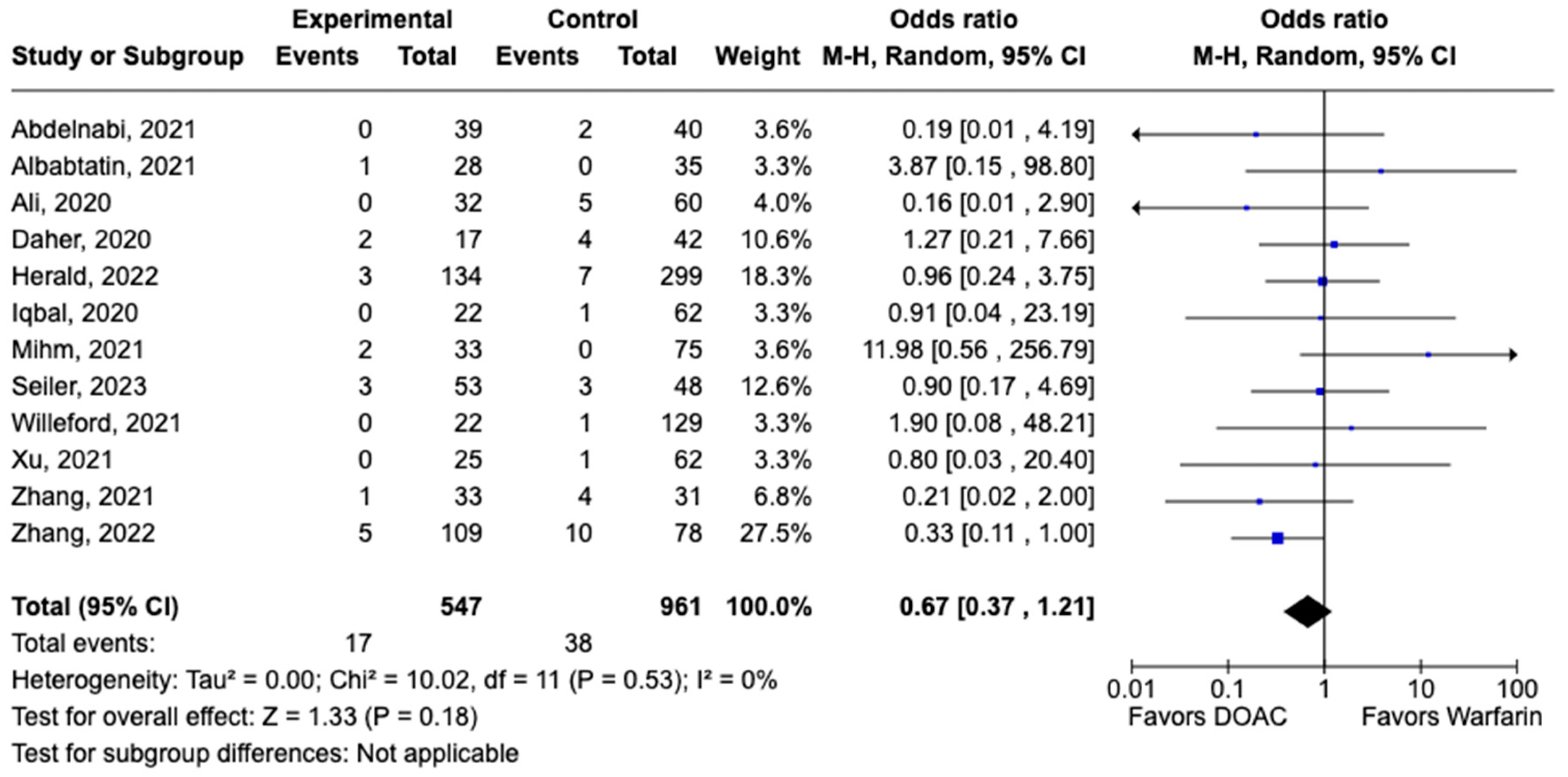
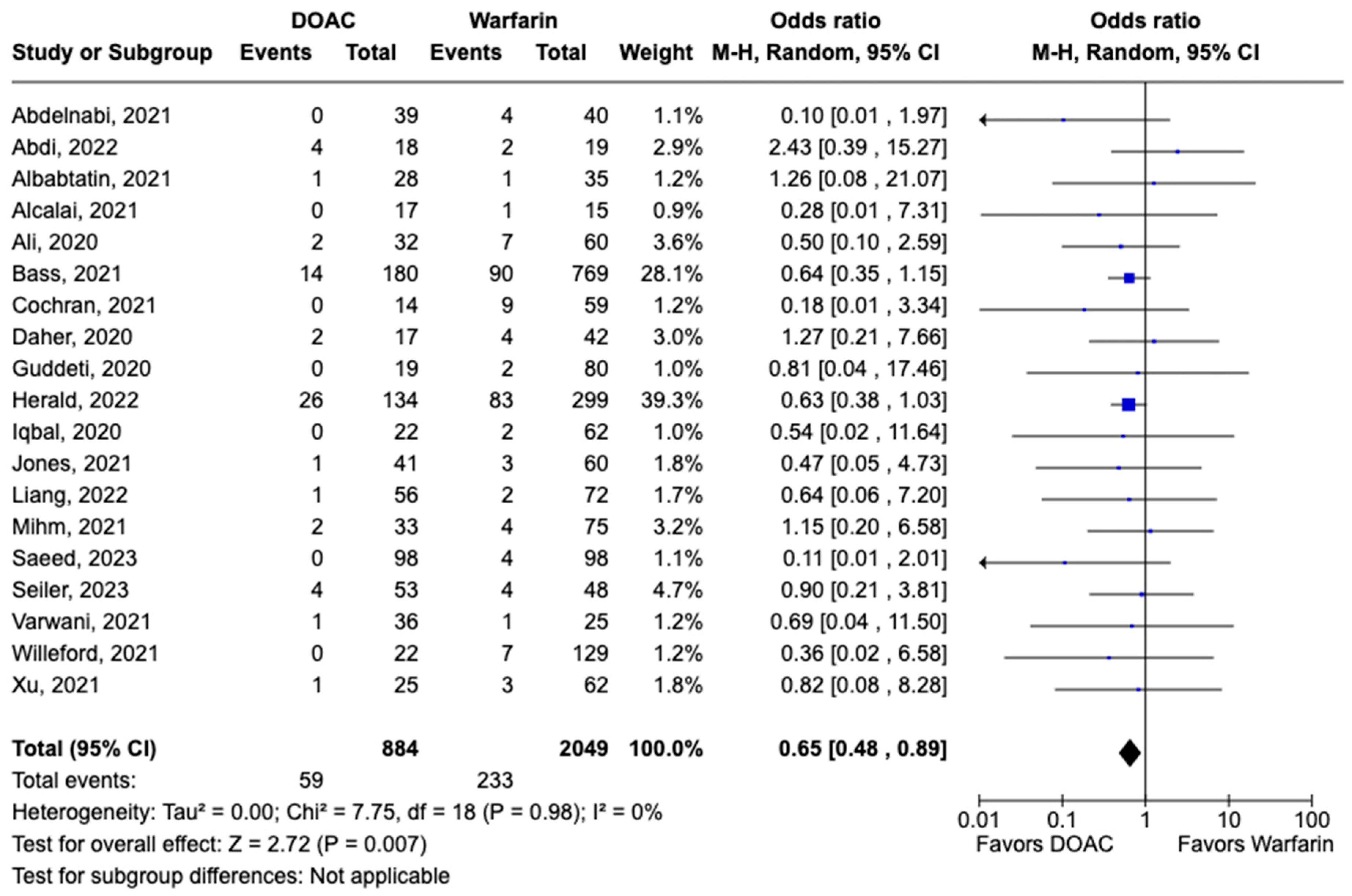
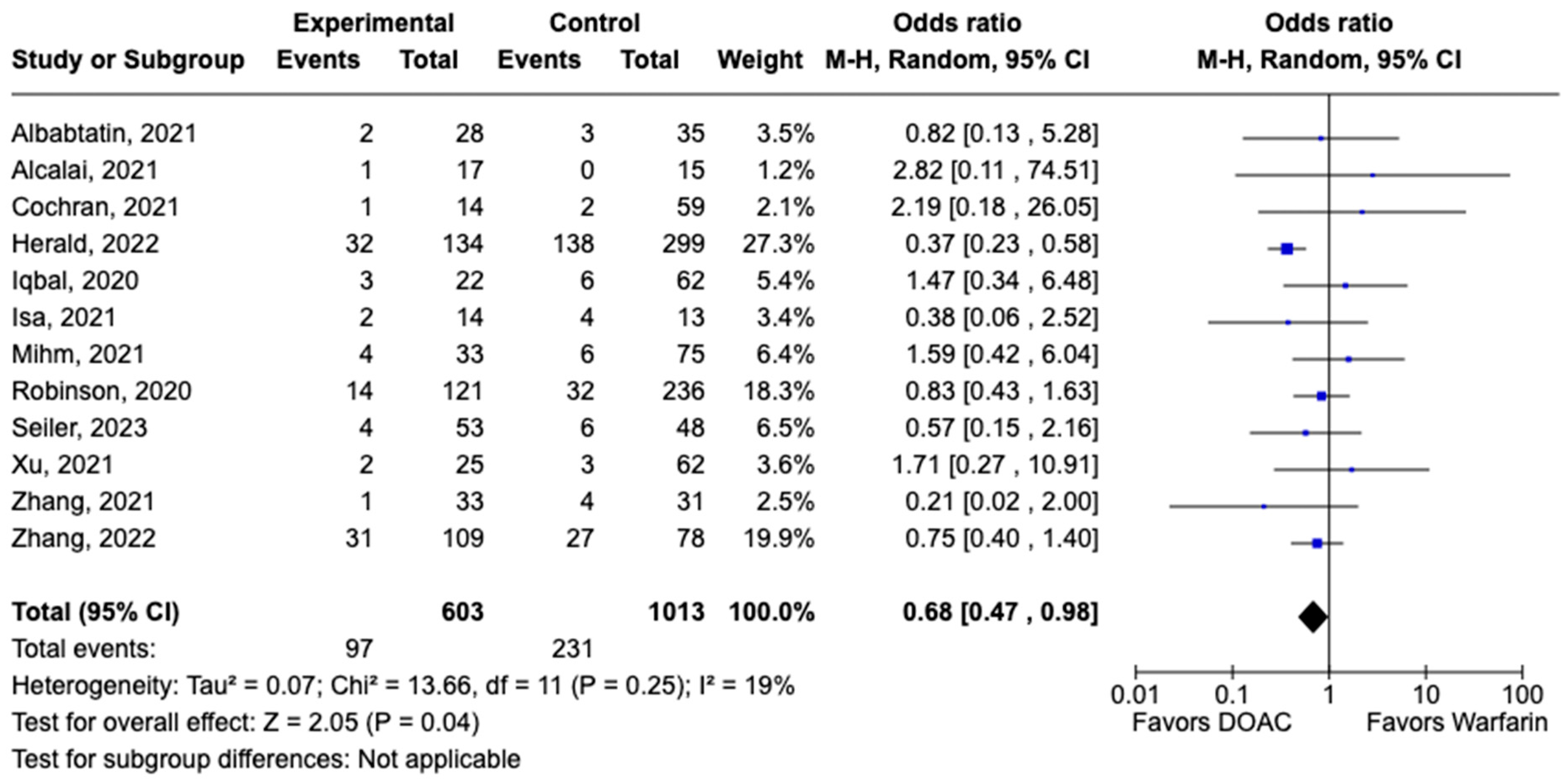
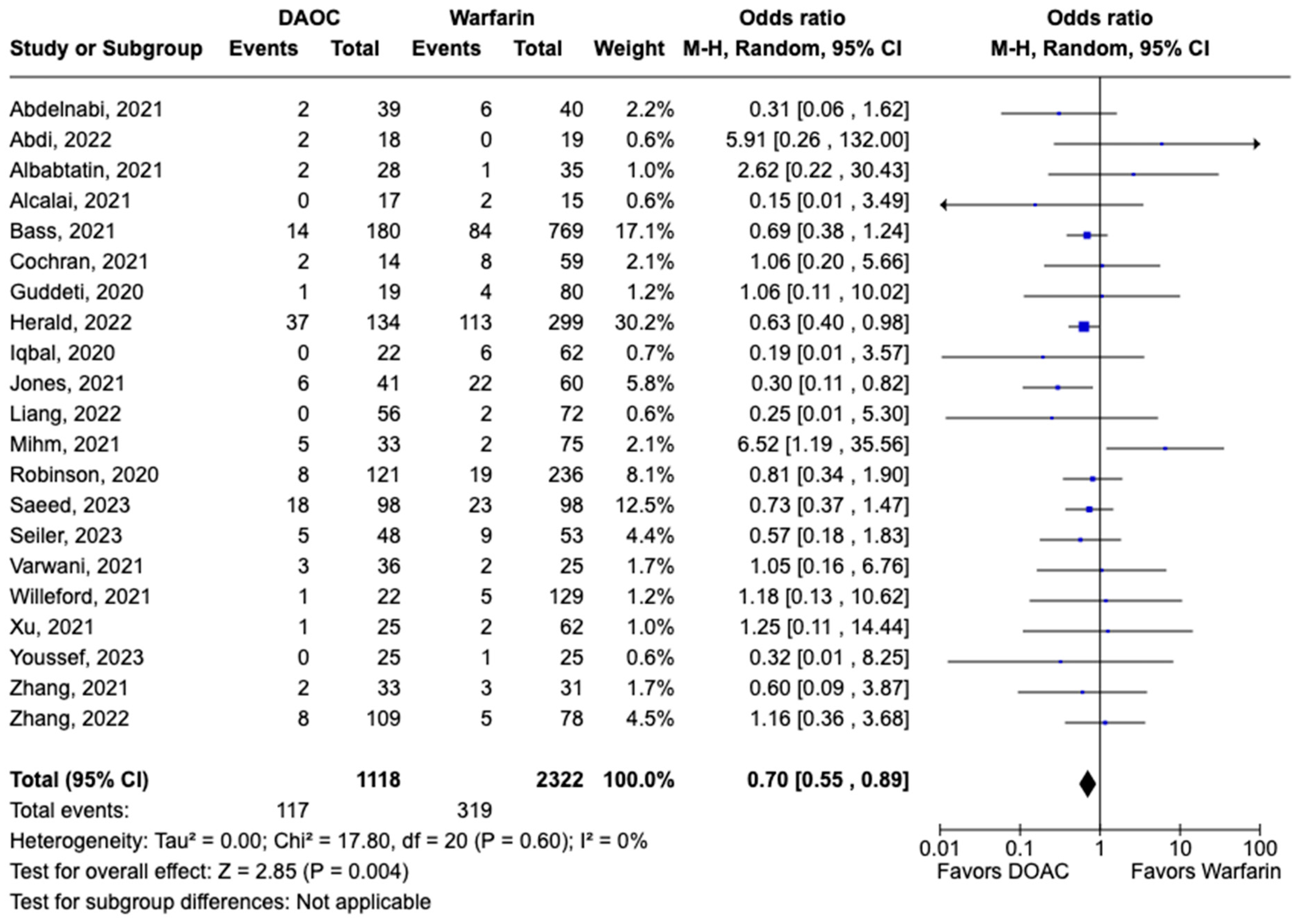
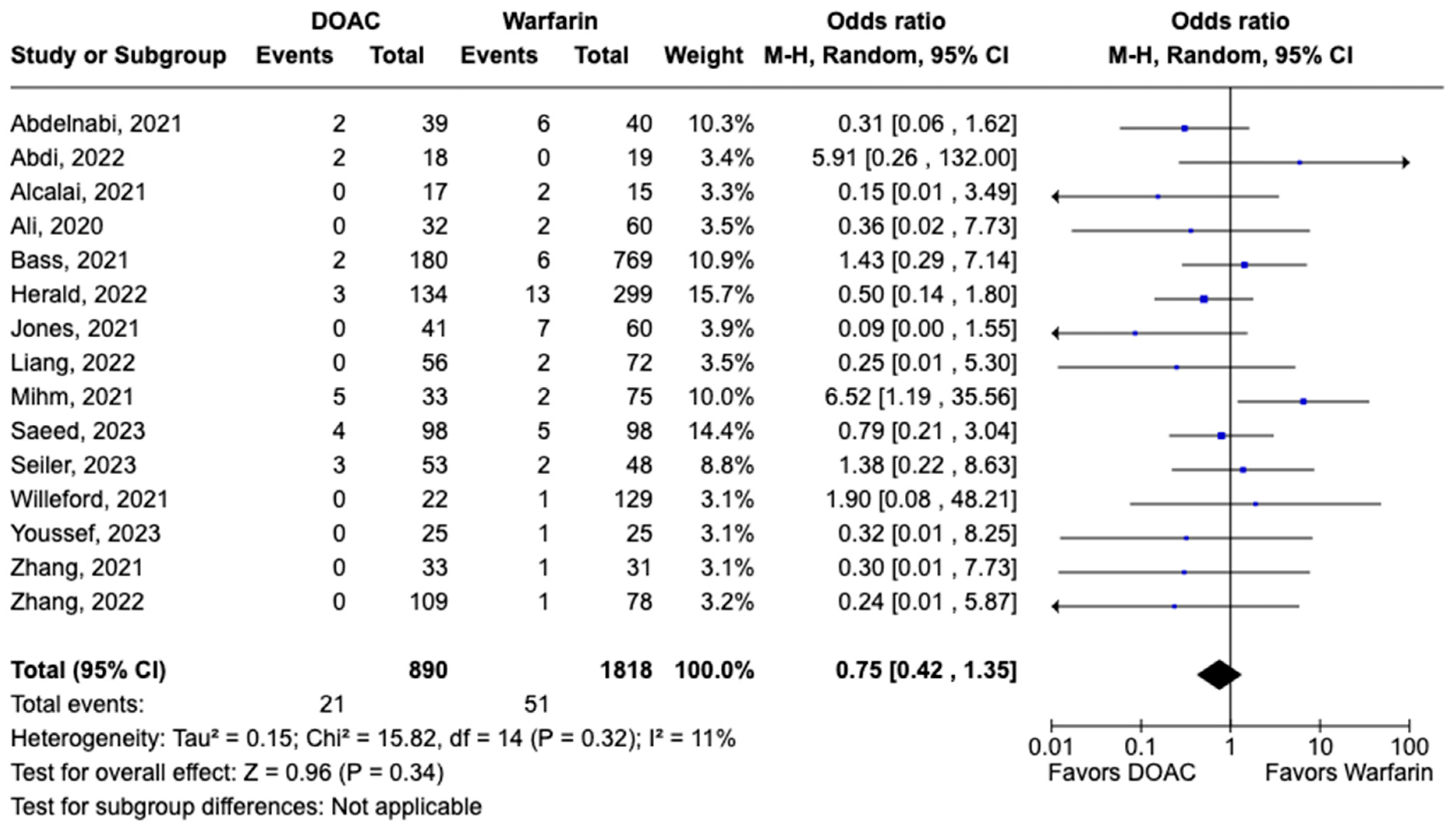
3.1. Efficacy Outcomes
3.2. Safety Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| AHA | American Heart Association |
| BARC | Bleeding Academic Research Consortium |
| CI | Confidence interval |
| CMRi | Cardiac magnetic resonance imaging |
| DCM | Dilated cardiomyopathy |
| DOAC | Direct oral anticoagulant |
| ESC | European Society of Cardiology |
| GUSTO | Global Use of Streptokinase and t-PA for Occluded Coronary Arteries |
| HF | Heart failure |
| LVT | Left ventricular thrombus |
| meta-analysis | Meta-analysis |
| MACE | Major adverse cardiovascular events |
| MeSH | Medical Subject Headings |
| MI | Myocardial infarction |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized clinical trial |
| RoB 2 | Risk-of-Bias 2 |
| STEMI | ST segment elevation myocardial infarction |
| TE | Thromboembolic events |
| TIA | Transient ischemic attack |
| TTE | Transthoracic echocardiography |
| TTR | Time to achieve therapeutic range |
| VKA | Vitamin K antagonist |
References
- McCarthy, C.P.; Vaduganathan, M.; McCarthy, K.J.; Januzzi, J.L.; Bhatt, D.L.; McEvoy, J.W. Left Ventricular Thrombus After Acute Myocardial Infarction: Screening, Prevention, and Treatment. JAMA Cardiol. 2018, 3, 642–649. [Google Scholar] [CrossRef]
- Falk, R.H.; Foster, E.; Coats, M.H. Ventricular Thrombi and Thromboembolism in Dilated Cardiomyopathy: A Prospective Follow-up Study. Am. Heart J. 1992, 123, 136–142. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Gay, J.A.; VanVoorhees, L.; DiBianco, R.; Fletcher, R.D. Frequency and Embolic Potential of Left Ventricular Thrombus in Dilated Cardiomyopathy: Assessment by 2-Dimensional Echocardiography. Am. J. Cardiol. 1983, 52, 1281–1285. [Google Scholar] [CrossRef]
- Cruz Rodriguez, J.B.; Okajima, K.; Greenberg, B.H. Management of Left Ventricular Thrombus: A Narrative Review. Ann. Transl. Med. 2021, 9, 520. [Google Scholar] [CrossRef]
- Massussi, M.; Scotti, A.; Lip, G.Y.H.; Proietti, R. Left Ventricular Thrombosis: New Perspectives on an Old Problem. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 158–167. [Google Scholar] [CrossRef]
- Visser, C.A.; Kan, G.; Meltzer, R.S.; Dunning, A.J.; Roelandt, J. Embolic Potential of Left Ventricular Thrombus after Myocardial Infarction: A Two-Dimensional Echocardiographic Study of 119 Patients. J. Am. Coll. Cardiol. 1985, 5, 1276–1280. [Google Scholar] [CrossRef]
- Lattuca, B.; Bouziri, N.; Kerneis, M.; Portal, J.-J.; Zhou, J.; Hauguel-Moreau, M.; Mameri, A.; Zeitouni, M.; Guedeney, P.; Hammoudi, N.; et al. Antithrombotic Therapy for Patients with Left Ventricular Mural Thrombus. J. Am. Coll. Cardiol. 2020, 75, 1676–1685. [Google Scholar] [CrossRef]
- O’Gara, P.T.; Kushner, F.G.; Ascheim, D.D.; Casey, D.E.; Chung, M.K.; de Lemos, J.A.; Ettinger, S.M.; Fang, J.C.; Fesmire, F.M.; Franklin, B.A.; et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013, 127, e362–e425. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes: Developed by the Task Force on the Management of Acute Coronary Syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Levine, G.N.; McEvoy, J.W.; Fang, J.C.; Ibeh, C.; McCarthy, C.P.; Misra, A.; Shah, Z.I.; Shenoy, C.; Spinler, S.A.; Vallurupalli, S.; et al. Management of Patients at Risk for and With Left Ventricular Thrombus: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e205–e223. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The Nuts and Bolts of PROSPERO: An International Prospective Register of Systematic Reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized Bleeding Definitions for Cardiovascular Clinical Trials: A Consensus Report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef]
- Ottawa Hospital Research Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 31 March 2024).
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Abdi, I.A.; Karataş, M.; Öcal, L.; Elmi Abdi, A.; Farah Yusuf Mohamud, M. Retrospective Analysis of Left Ventricular Thrombus Among Heart Failure Patients with Reduced Ejection Fraction at a Single Tertiary Care Hospital in Somalia. Open Access Emerg. Med. 2022, 14, 591–597. [Google Scholar] [CrossRef]
- Albabtain, M.A.; Alhebaishi, Y.; Al-Yafi, O.; Kheirallah, H.; Othman, A.; Alghosoon, H.; Arafat, A.A.; Alfagih, A. Rivaroxaban versus Warfarin for the Management of Left Ventricle Thrombus. Egypt. Heart J. 2021, 73, 41. [Google Scholar] [CrossRef]
- Ali, Z.; Isom, N.; Dalia, T.; Sami, F.; Mahmood, U.; Shah, Z.; Gupta, K. Direct Oral Anticoagulant Use in Left Ventricular Thrombus. Thrombosis J. 2020, 18, 29. [Google Scholar] [CrossRef]
- Bass, M.E.; Kiser, T.H.; Page, R.L.; McIlvennan, C.K.; Allen, L.A.; Wright, G.; Shakowski, C. Comparative Effectiveness of Direct Oral Anticoagulants and Warfarin for the Treatment of Left Ventricular Thrombus. J. Thromb. Thrombolysis 2021, 52, 517–522. [Google Scholar] [CrossRef]
- Cochran, J.M.; Jia, X.; Kaczmarek, J.; Staggers, K.A.; Rifai, M.A.; Hamzeh, I.R.; Birnbaum, Y. Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus: A Retrospective, Multicenter Study and Meta-Analysis of Existing Data. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 173–178. [Google Scholar] [CrossRef]
- Daher, J.; Da Costa, A.; Hilaire, C.; Ferreira, T.; Pierrard, R.; Guichard, J.B.; Romeyer, C.; Isaaz, K. Management of Left Ventricular Thrombi with Direct Oral Anticoagulants: Retrospective Comparative Study with Vitamin K Antagonists. Arch. Cardiovasc. Dis. Suppl. 2021, 13, 74. [Google Scholar] [CrossRef]
- Gama, F.; Freitas, P.; Trabulo, M.; Ferreira, A.; Andrade, M.J.; Matos, D.; Strong, C.; Ribeiras, R.; Ferreira, J.; Mendes, M. 459 Direct Oral Anticoagulants Are an Effective Therapy for Left Ventricular Thrombus Formation. Eur. Heart J. 2019, 40, ehz747.0118. [Google Scholar] [CrossRef]
- Guddeti, R.R.; Anwar, M.; Walters, R.W.; Apala, D.; Pajjuru, V.; Kousa, O.; Gujjula, N.R.; Alla, V.M. Treatment of Left Ventricular Thrombus With Direct Oral Anticoagulants: A Retrospective Observational Study. Am. J. Med. 2020, 133, 1488–1491. [Google Scholar] [CrossRef]
- Herald, J.; Goitia, J.; Duan, L.; Chen, A.; Lee, M.-S. Safety and Effectiveness of Direct Oral Anticoagulants Versus Warfarin for Treating Left Ventricular Thrombus. Am. J. Cardiovasc. Drugs 2022, 22, 437–444. [Google Scholar] [CrossRef]
- Hofer, F.; Kazem, N.; Schweitzer, R.; Horvat, P.; Winter, M.-P.; Koller, L.; Hengstenberg, C.; Sulzgruber, P.; Niessner, A. The Prognostic Impact of Left Ventricular Thrombus Resolution after Acute Coronary Syndrome and Risk Modulation via Antithrombotic Treatment Strategies. Clin. Cardiol. 2021, 44, 1692–1699. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, X.; Wang, J.; Liang, L.; Tian, P.; Chen, Y.; Zhai, M.; Huang, Y.; Zhou, Q.; Xin, A.; et al. Clinical Profile, Treatment, and Prognosis of Left Ventricular Thrombus in Dilated Cardiomyopathy. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231179683. [Google Scholar] [CrossRef]
- Iqbal, H.; Straw, S.; Craven, T.P.; Stirling, K.; Wheatcroft, S.B.; Witte, K.K. Direct Oral Anticoagulants Compared to Vitamin K Antagonist for the Management of Left Ventricular Thrombus. ESC Heart Fail. 2020, 7, 2032–2041. [Google Scholar] [CrossRef]
- Isa, W.Y.H.W.; Hwong, N.; Mohamed Yusof, A.K.; Yusof, Z.; Loong, N.S.; Wan-Arfah, N.; Naing, N.N. Apixaban versus Warfarin in Patients with Left Ventricular Thrombus: A Pilot Prospective Randomized Outcome Blinded Study Investigating Size Reduction or Resolution of Left Ventricular Thrombus. J. Clin. Prev. Cardiol. 2020, 9, 150. [Google Scholar] [CrossRef]
- Jaidka, A.; Zhu, T.; Lavi, S.; Johri, A. Treatment of Left Ventricular Thrombus Using Warfarin versus Direct Oral Anticoagulants Following Anterior Myocardial Infarction. Can. J. Cardiol. 2018, 34, S143. [Google Scholar] [CrossRef]
- Jones, D.A.; Wright, P.; Alizadeh, M.A.; Fhadil, S.; Rathod, K.S.; Guttmann, O.; Knight, C.; Timmis, A.; Baumbach, A.; Wragg, A.; et al. The Use of Novel Oral Anticoagulants Compared to Vitamin K Antagonists (Warfarin) in Patients with Left Ventricular Thrombus after Acute Myocardial Infarction. Eur. Heart J. Cardiovasc. Pharmacother. 2021, 7, 398–404. [Google Scholar] [CrossRef]
- Kim, S.-E.; Lee, C.J.; Oh, J.; Kang, S.-M. Factors Influencing Left Ventricular Thrombus Resolution and Its Significance on Clinical Outcomes. ESC Heart Fail. 2023, 10, 1987–1995. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Z.; Zhou, Y.; Shen, H.; Chai, M.; Ma, X.; Han, H.; Shao, Q.; Li, Q. Efficacy and Safety of Direct Oral Anticoagulants in the Treatment of Left Ventricular Thrombus After Acute Anterior Myocardial Infarction in Patients Who Underwent Percutaneous Coronary Intervention. Curr. Vasc. Pharmacol. 2022, 20, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Mihm, A.E.; Hicklin, H.E.; Cunha, A.L.; Nisly, S.A.; Davis, K.A. Direct Oral Anticoagulants versus Warfarin for the Treatment of Left Ventricular Thrombosis. Intern. Emerg. Med. 2021, 16, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Rahunathan, N.; Hurdus, B.; Straw, S.; Iqbal, H.; Witte, K.; Wheatcroft, S. Improving the Management of Left Ventricular Thrombus in a Tertiary Cardiology Centre: A Quality Improvement Project. BMJ Open Qual. 2023, 12, e002111. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, C.; Liu, B.; Benatar, J.; Stewart, R.A.H.; Somaratne, J.B. Left Ventricular Thrombus after ST Segment Elevation Myocardial Infarction: A Single-Centre Observational Study. N. Z. Med. J. 2020, 133, 45–54. [Google Scholar]
- Robinson, A.A.; Trankle, C.R.; Eubanks, G.; Schumann, C.; Thompson, P.; Wallace, R.L.; Gottiparthi, S.; Ruth, B.; Kramer, C.M.; Salerno, M.; et al. Off-Label Use of Direct Oral Anticoagulants Compared With Warfarin for Left Ventricular Thrombi. JAMA Cardiol. 2020, 5, 685. [Google Scholar] [CrossRef]
- Seiler, T.; Vasiliauskaite, E.; Grüter, D.; Young, M.; Attinger-Toller, A.; Madanchi, M.; Cioffi, G.M.; Tersalvi, G.; Müller, G.; Stämpfli, S.F.; et al. Direct Oral Anticoagulants Versus Vitamin K Antagonists for the Treatment of Left Ventricular Thrombi—Insights from a Swiss Multicenter Registry. Am. J. Cardiol. 2023, 194, 113–121. [Google Scholar] [CrossRef]
- Varwani, M.H.; Shah, J.; Ngunga, M.; Jeilan, M. Treatment and Outcomes in Patients with Left Ventricular Thrombus—Experiences from the Aga Khan University Hospital, Nairobi-Kenya. Pan Afr. Med. J. 2021, 39, 212. [Google Scholar] [CrossRef]
- Willeford, A.; Zhu, W.; Stevens, C.; Thomas, I.C. Direct Oral Anticoagulants Versus Warfarin in the Treatment of Left Ventricular Thrombus. Ann. Pharmacother. 2021, 55, 839–845. [Google Scholar] [CrossRef]
- Xu, Z.; Li, X.; Li, X.; Gao, Y.; Mi, X. Direct Oral Anticoagulants versus Vitamin K Antagonists for Patients with Left Ventricular Thrombus. Ann. Palliat. Med. 2021, 10, 9427–9434. [Google Scholar] [CrossRef]
- Zhang, Z.; Si, D.; Zhang, Q.; Qu, M.; Yu, M.; Jiang, Z.; Li, D.; Yang, P.; Zhang, W. Rivaroxaban versus Vitamin K Antagonists (Warfarin) Based on the Triple Therapy for Left Ventricular Thrombus after ST-Elevation Myocardial Infarction. Heart Vessel. 2022, 37, 374–384. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Z.; Zheng, H.; Qu, M.; Li, S.; Yang, P.; Si, D.; Zhang, W. Rivaroxaban in Heart Failure Patients with Left Ventricular Thrombus: A Retrospective Study. Front. Pharmacol. 2022, 13, 1008031. [Google Scholar] [CrossRef] [PubMed]
- Cochrane RevMan. RevMan: Systematic Review and Meta-Analysis Tool for Researchers Worldwide. Available online: https://revman.cochrane.org/info (accessed on 13 January 2024).
- ScienceDirect Topics. Mantel Haenszel Test—An Overview. Available online: https://www.sciencedirect.com/topics/medicine-and-dentistry/mantel-haenszel-test (accessed on 31 March 2024).
- Abdelnabi, M.; Saleh, Y.; Fareed, A.; Nossikof, A.; Wang, L.; Morsi, M.; Eshak, N.; Abdelkarim, O.; Badran, H.; Almaghraby, A. Comparative Study of Oral Anticoagulation in Left Ventricular Thrombi (No-LVT Trial). J. Am. Coll. Cardiol. 2021, 77, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Alcalai, R.; Butnaru, A.; Moravsky, G.; Yagel, O.; Rashad, R.; Ibrahimli, M.; Planer, D.; Amir, O.; Elbaz-Greener, G.; Leibowitz, D. Apixaban vs. Warfarin in Patients with Left Ventricular Thrombus: A Prospective Multicentre Randomized Clinical Trial. Eur. Heart J. Cardiovasc. Pharmacother. 2022, 8, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.A.; Alrefae, M.A.; Khalil, H.H.; Abdullah, H.I.; Khalifa, Z.S.; Al Shaban, A.A.; Wali, H.A.; AlRajab, M.R.; Saleh, O.M.; Nashy, B.N. Apixaban in Patients With Post-Myocardial Infarction Left Ventricular Thrombus: A Randomized Clinical Trial. CJC Open 2023, 5, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Use of XARELTO in Ventricular Thrombus. Available online: https://www.janssenscience.com/products/xarelto/medical-content/use-of-xarelto-in-ventricular-thrombus (accessed on 15 April 2024).
- Rehan, A.; Kanwar, M.; Rosman, H.; Ahmed, S.; Ali, A.; Gardin, J.; Cohen, G. Incidence of Post Myocardial Infarction Left Ventricular Thrombus Formation in the Era of Primary Percutaneous Intervention and Glycoprotein IIb/IIIa Inhibitors. A Prospective Observational Study. Cardiovasc. Ultrasound 2006, 4, 20. [Google Scholar] [CrossRef]
- Phan, J.; Nguyen, T.; French, J.; Moses, D.; Schlaphoff, G.; Lo, S.; Juergens, C.; Dimitri, H.; Richards, D.; Thomas, L. Incidence and Predictors of Left Ventricular Thrombus Formation Following Acute ST-Segment Elevation Myocardial Infarction: A Serial Cardiac MRI Study. Int. J. Cardiol. Heart Vasc. 2019, 24, 100395. [Google Scholar] [CrossRef] [PubMed]
- Gianstefani, S.; Douiri, A.; Delithanasis, I.; Rogers, T.; Sen, A.; Kalra, S.; Charangwa, L.; Reiken, J.; Monaghan, M.; MacCarthy, P. Incidence and Predictors of Early Left Ventricular Thrombus after ST-Elevation Myocardial Infarction in the Contemporary Era of Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2014, 113, 1111–1116. [Google Scholar] [CrossRef]
- Saeedi, R.; Johns, K.; Frohlich, J.; Bennett, M.T.; Bondy, G. Lipid Lowering Efficacy and Safety of Ezetimibe Combined with Rosuvastatin Compared with Titrating Rosuvastatin Monotherapy in HIV-Positive Patients. Lipids Health Dis. 2015, 14, 57. [Google Scholar] [CrossRef]
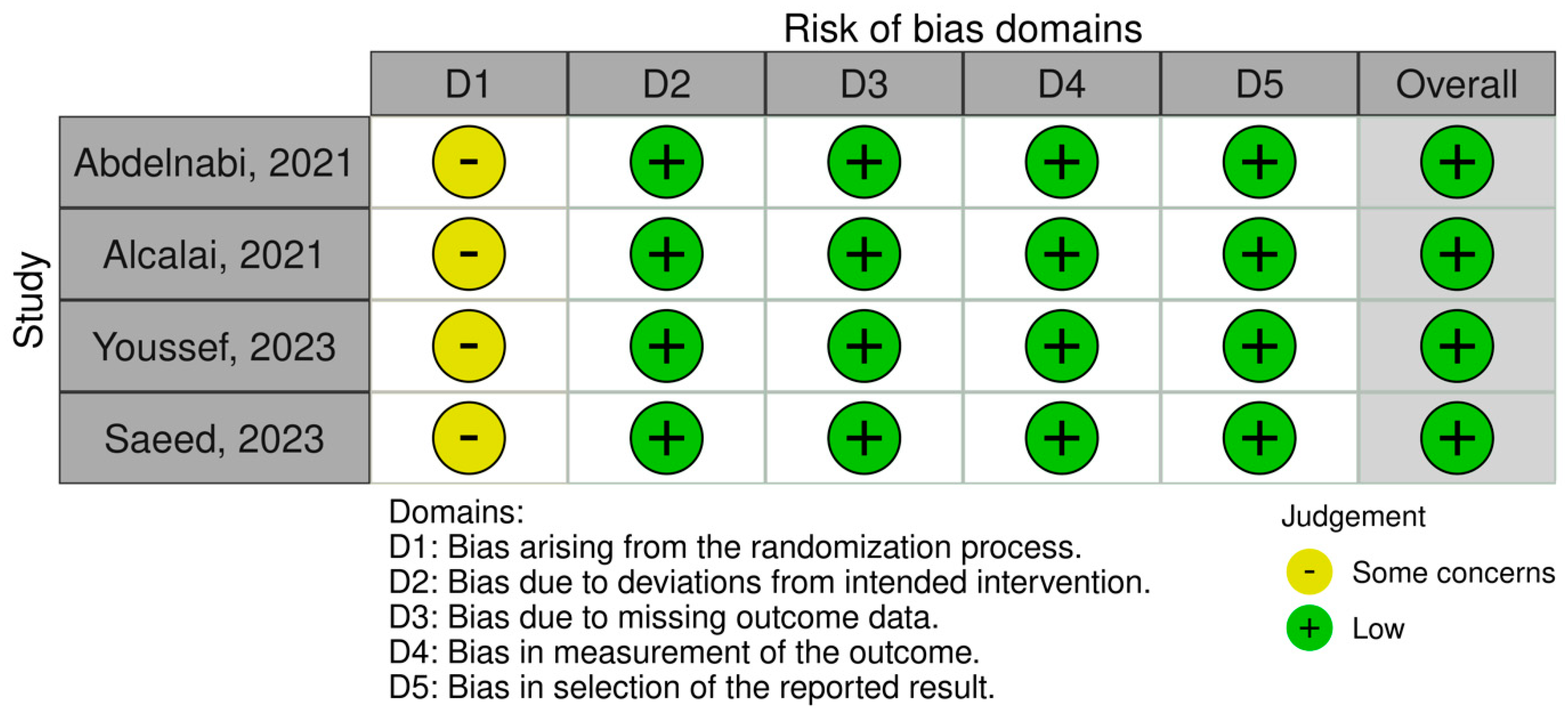
| Selection | Comparability | Outcome | Overall Score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Selection of Subjects Truly Representative/Not | Selection of Controls Drawn from the Same Cohort/Not | Ascertainment of Exposure Drawn from Secure Record/Self-Report | Demonstration of Outcome of Interest Absent/Present | Controlled for Baseline Characteristics Yes/No | Controlled for Other Factors Yes/No | Assessment of Outcome Drawn from Secure Record/Self-Report | Follow Up Length >3/<3 Months | Adequacy of Follow-Up <20%/>80% Lost to Follow-Up | |
| Abdi, 2022 [16] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0, 30% lost to follow-up | Good |
| Albabtain, 2021 [17] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | Good |
| Ali, 2020 [18] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Bass, 2022 [19] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | NR | 1 | Good |
| Cochran, 2021 [20] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | Good |
| Daher, 2020 [21] | 1 | 1 | 1 | 0 | 0 | 0 | 1 | NR | 1 | Fair |
| Gama, 2019 [22] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | NR | 1 | Good |
| Guddeti, 2020 [23] | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | Good |
| Herald, 2022 [24] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Hofer, 2021 [25] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | Good |
| Huang, 2023 [26] | 0, included only DCM patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Iqbal, 2020 [27] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Isa, 2020 [28] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Jaidka, 2018 [29] | 0, only AMI patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | Good |
| Jones, 2021 [30] | 0, only AMI patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Kim, 2023 [31] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Liang, 2022 [32] | 0, only AMI patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Mihm, 2021 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | Good |
| Rahunathan, 2023 [34] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Ratnayake, 2020 [35] | 0, only AMI patients | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | Fair |
| Robinson, 2020 [36] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Seiler, 2023 [37] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Varwani, 2021 [38] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Willeford, 2021 [39] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Xu, 2021 [40] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Zhang, 2021 [41] | 0, only AMI patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Zhang, 2022 [42] | 0, only HF patients | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Good |
| Author, Year | Type of Study | Total Participants, n | DOAC/VKA Group, n | DOAC/VKA Group Mean Age, Years | Women, n (DOAC/Warfarin) | CAD, % (DOAC/Warfarin) | CHF, % (DOAC/Warfarin) |
|---|---|---|---|---|---|---|---|
| Abdelnabi, 2021 [45] | RCT | 79 | 39/40 | NR | NR | NR | NR |
| Abdi, 2022 [16] | Observational | 40 | 18/19 | NR | NR | NR | NR |
| Albabtain, 2021 [17] | Observational | 63 | 28/35 | 58/59 | 4/1 | NR | NR |
| Alcalai, 2021 [46] | RCT | 35 | 18/17 | 56/59 | 5/2 | 18/22 | NR |
| Ali, 2020 [18] | Observational | 92 | 32/60 | 59/58 | 6/11 | NR | 78/75 |
| Bass, 2022 [19] | Observational | 949 | 180/769 | 63/62 | 55/224 | 43/57 | 68/75 |
| Cochran, 2021 [20] | Observational | 73 | 14/59 | 52/62 | 3/14 | 53/61 | 73/81 |
| Daher, 2020 [21] | Observational | 59 | 17/42 | 57/61 | 3/7 | 88/74 | NR |
| Gama, 2019 [22] | Observational | 66 | 13/53 | 69/69 | NR | NR | NR |
| Guddeti, 2020 [23] | Observational | 99 | 19/80 | 61/61 | 4/25 | 58/66 | 100/96 |
| Herald, 2022 [24] | Observational | 433 | 134/299 | 66/65 | 18/57 | 35/36 | 88/88 |
| Hofer, 2021 [25] | Observational | 43 | 10/33 | NR | NR | NR | NR |
| Huang, 2023 [26] | Observational | 122 | 47/65 | 49/39 | 9/12 | NR | NR |
| Iqbal, 2020 [27] | Observational | 84 | 22/62 | 62/62 | 2/7 | NR | 95/94 |
| Isa, 2020 [28] | RCT | 27 | 14/13 | 55/55 | 1/1 | NR | NR |
| Jaidka, 2018 [29] | Observational | 49 | 12/37 | 57/61 | 3/9 | 0/8 | NR |
| Jones, 2021 [30] | Observational | 111 | 41/60 | 59/67 | 7/9 | NR | NR |
| Kim, 2023 [31] | Observational | 205 | 23/182 | NR | NR | NR | NR |
| Liang, 2022 [32] | Observational | 128 | 56/72 | 55/55 | 5/10 | NR | NR |
| Mihm, 2021 [33] | Observational | 108 | 33/75 | 63/60 | 7/9 | NR | NR |
| Rahunathan, 2023 [34] | Observational | 18 | 14/4 | 59/64 | 2/1 | NR | NR |
| Ratnayake, 2020 [35] | Observational | 44 | 2/42 | NR | NR | NR | NR |
| Robinson, 2020 [36] | Observational | 357 | 121/236 | 58/58 | 27/66 | NR | NR |
| Saeed, 2023 [48] | Observational | 196 | 98/98 | 56/56 | 17/22 | NR | 13/11 |
| Seiler, 2023 [37] | Observational | 101 | 48/54 | 64/62 | 6/12 | NR | 6/10 |
| Varwani, 2021 [38] | Observational | 92 | 58/34 | NR | NR | NR | NR |
| Willeford, 2021 [39] | Observational | 151 | 22/129 | 54/56 | 5/25 | NR | 86/85 |
| Xu, 2021 [40] | Observational | 87 | 25/62 | 59/62 | 6/15 | NR | NR |
| Youssef, 2023 [47] | RCT | 100 | 25/25 | 52/54 | NR | NR | NR |
| Zhang, 2021 [41] | Observational | 64 | 33/31 | 60/61 | 9/8 | NR | NR |
| Zhang, 2022 [42] | Observational | 187 | 109/78 | 65/63 | 24/12 | 97/61 | 59/39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vorla, M.; Kalra, D.K. Meta-Analysis of the Safety and Efficacy of Direct Oral Anticoagulants for the Treatment of Left Ventricular Thrombus. Pharmaceuticals 2024, 17, 708. https://doi.org/10.3390/ph17060708
Vorla M, Kalra DK. Meta-Analysis of the Safety and Efficacy of Direct Oral Anticoagulants for the Treatment of Left Ventricular Thrombus. Pharmaceuticals. 2024; 17(6):708. https://doi.org/10.3390/ph17060708
Chicago/Turabian StyleVorla, Mounica, and Dinesh K. Kalra. 2024. "Meta-Analysis of the Safety and Efficacy of Direct Oral Anticoagulants for the Treatment of Left Ventricular Thrombus" Pharmaceuticals 17, no. 6: 708. https://doi.org/10.3390/ph17060708
APA StyleVorla, M., & Kalra, D. K. (2024). Meta-Analysis of the Safety and Efficacy of Direct Oral Anticoagulants for the Treatment of Left Ventricular Thrombus. Pharmaceuticals, 17(6), 708. https://doi.org/10.3390/ph17060708







