Barbaloin Protects Pentylenetetrazol-Induced Cognitive Deficits in Rodents via Modulation of Neurotransmitters and Inhibition of Oxidative-Free-Radicals-Led Inflammation
Abstract
1. Introduction
2. Results
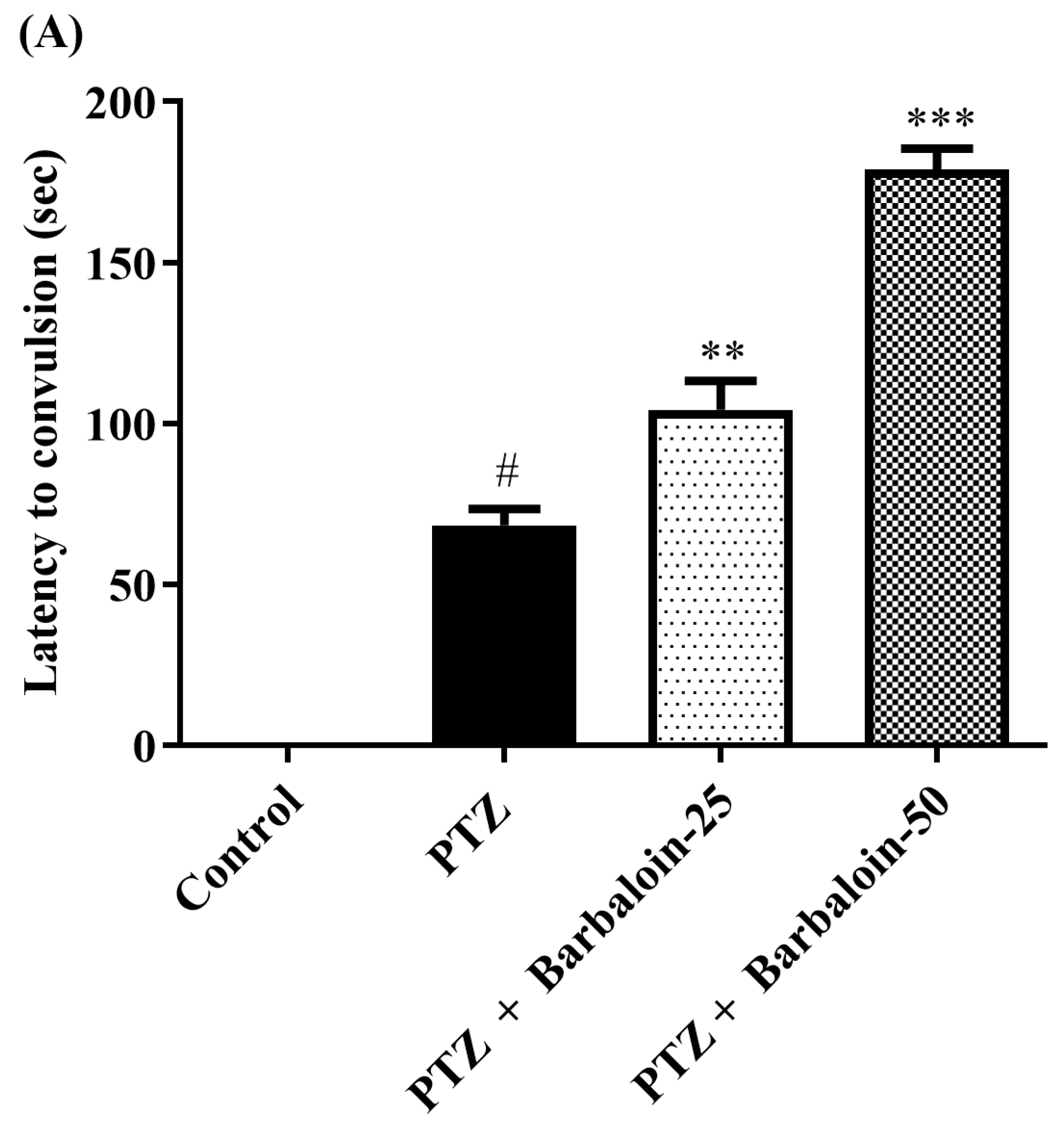
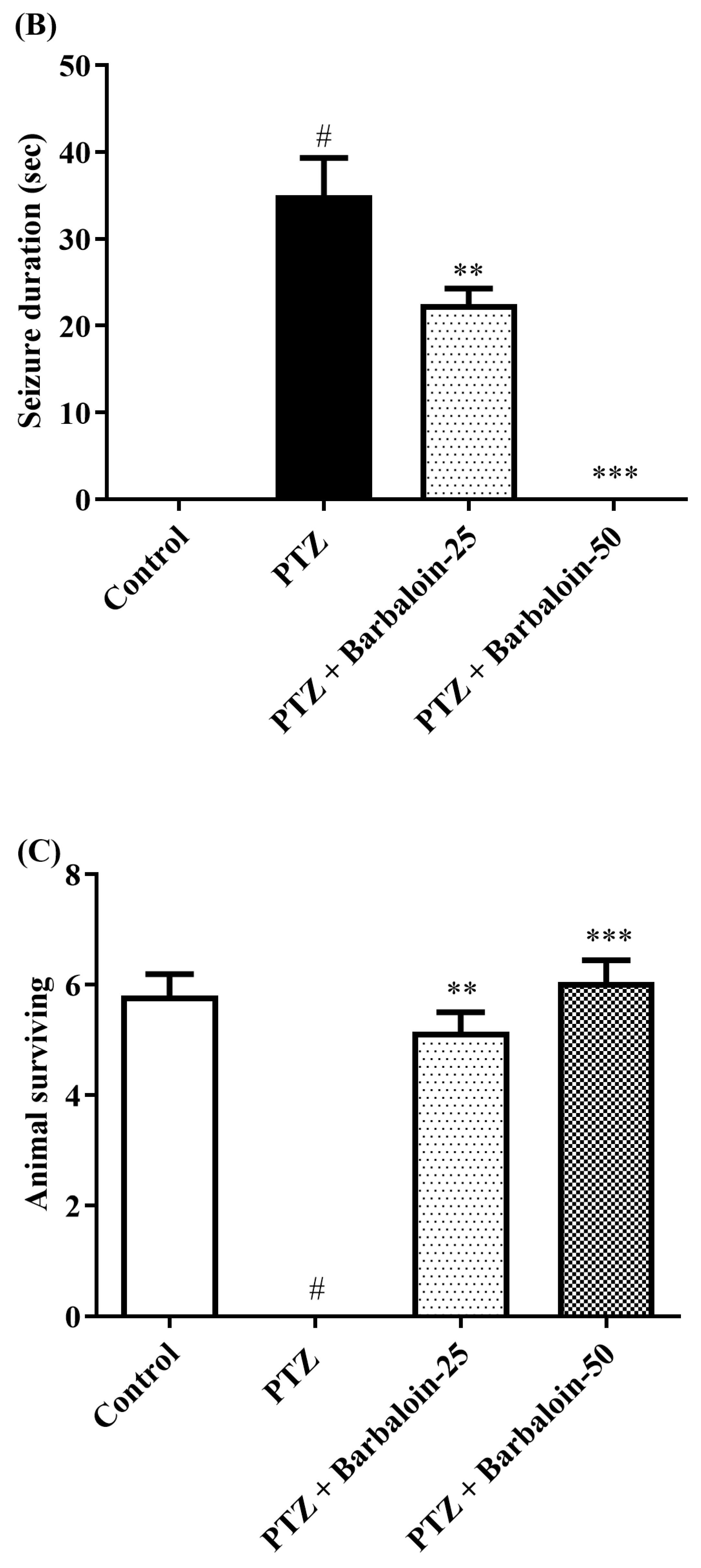
2.1. Kindling Score
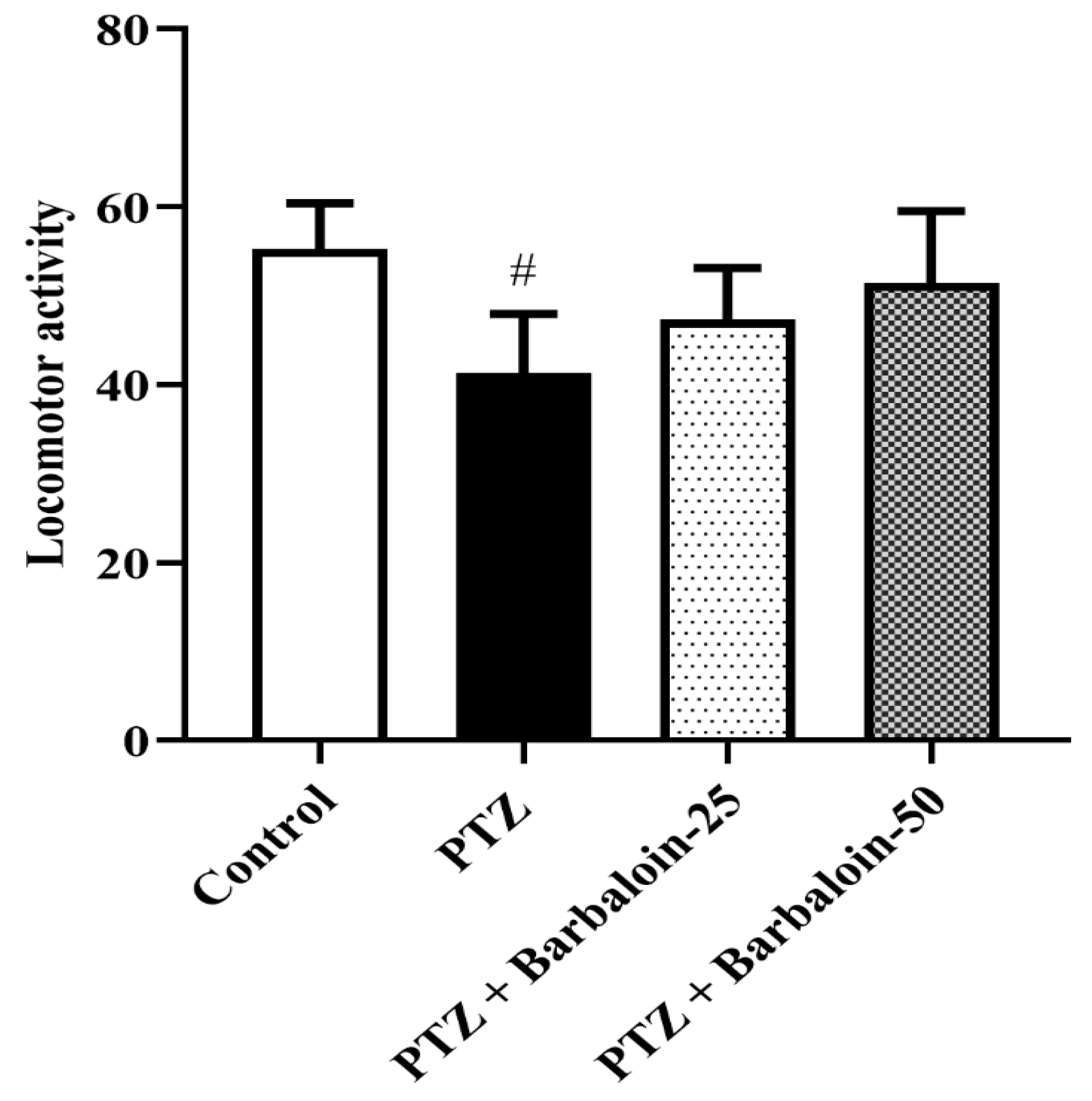
2.2. Open Field Test (OFT)
2.3. Novel Object Recognition Test (NORT)
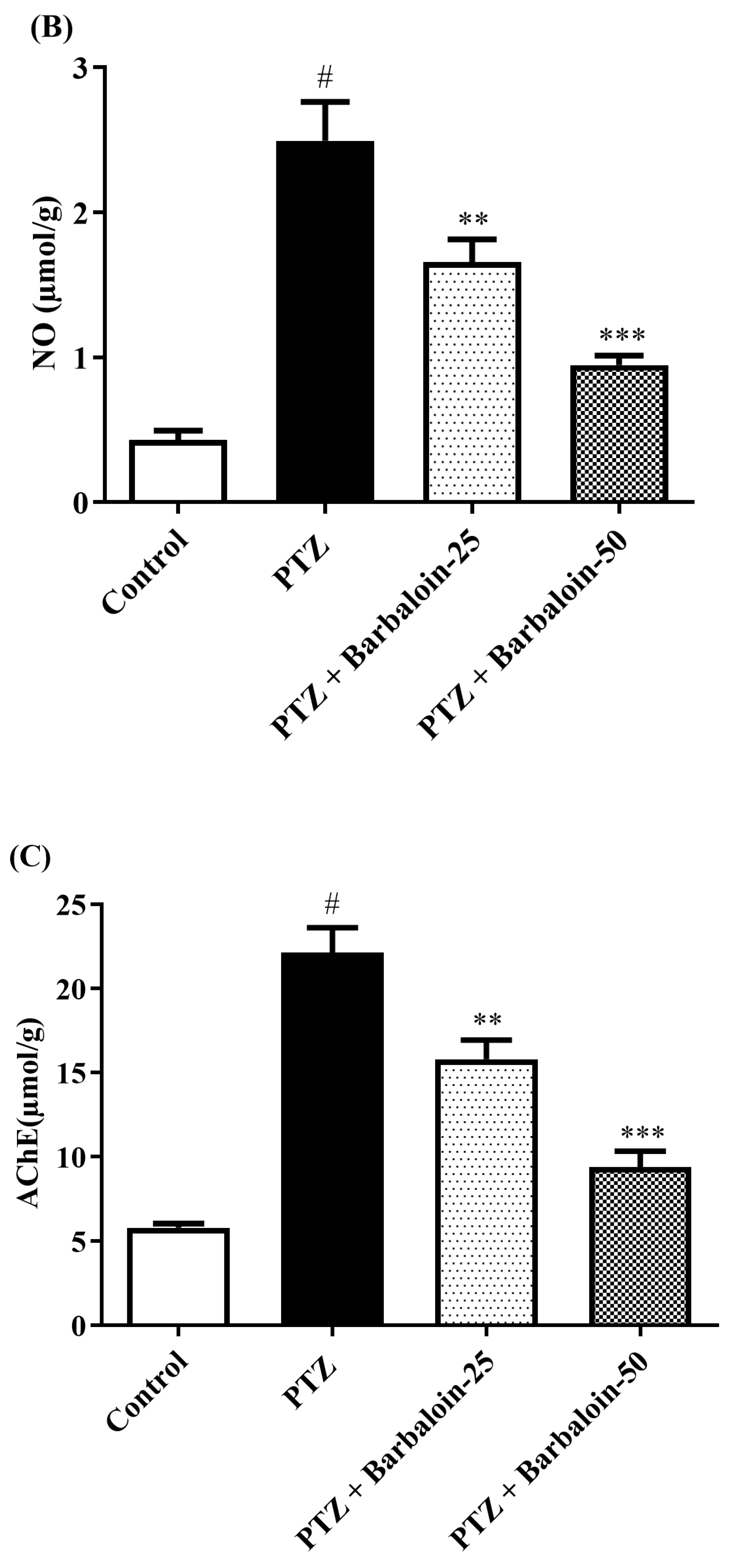
2.4. Malondialdehyde (MDA), Nitric Oxide (NO), AChE Estimation
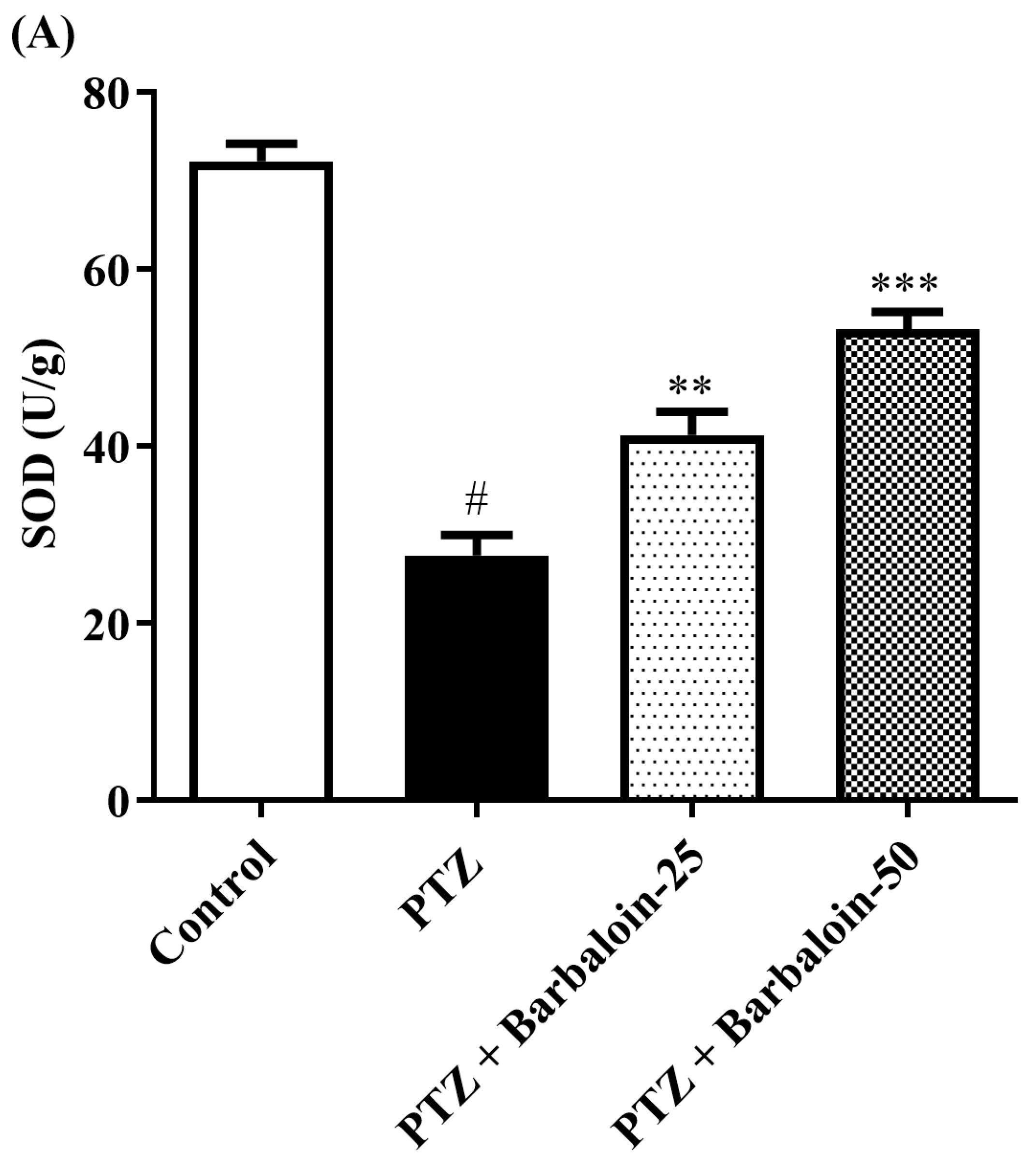
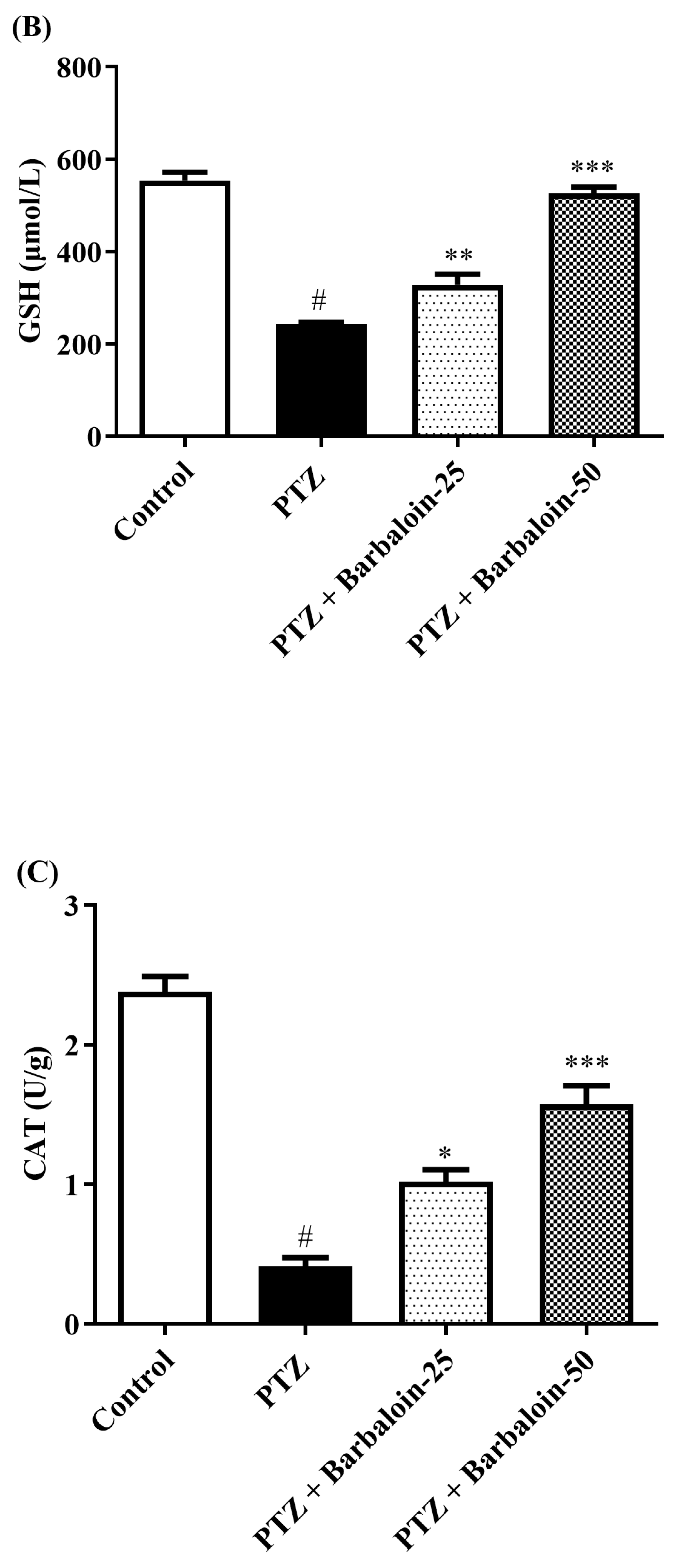
2.5. SOD, GSH, Catalase (CAT) Estimation
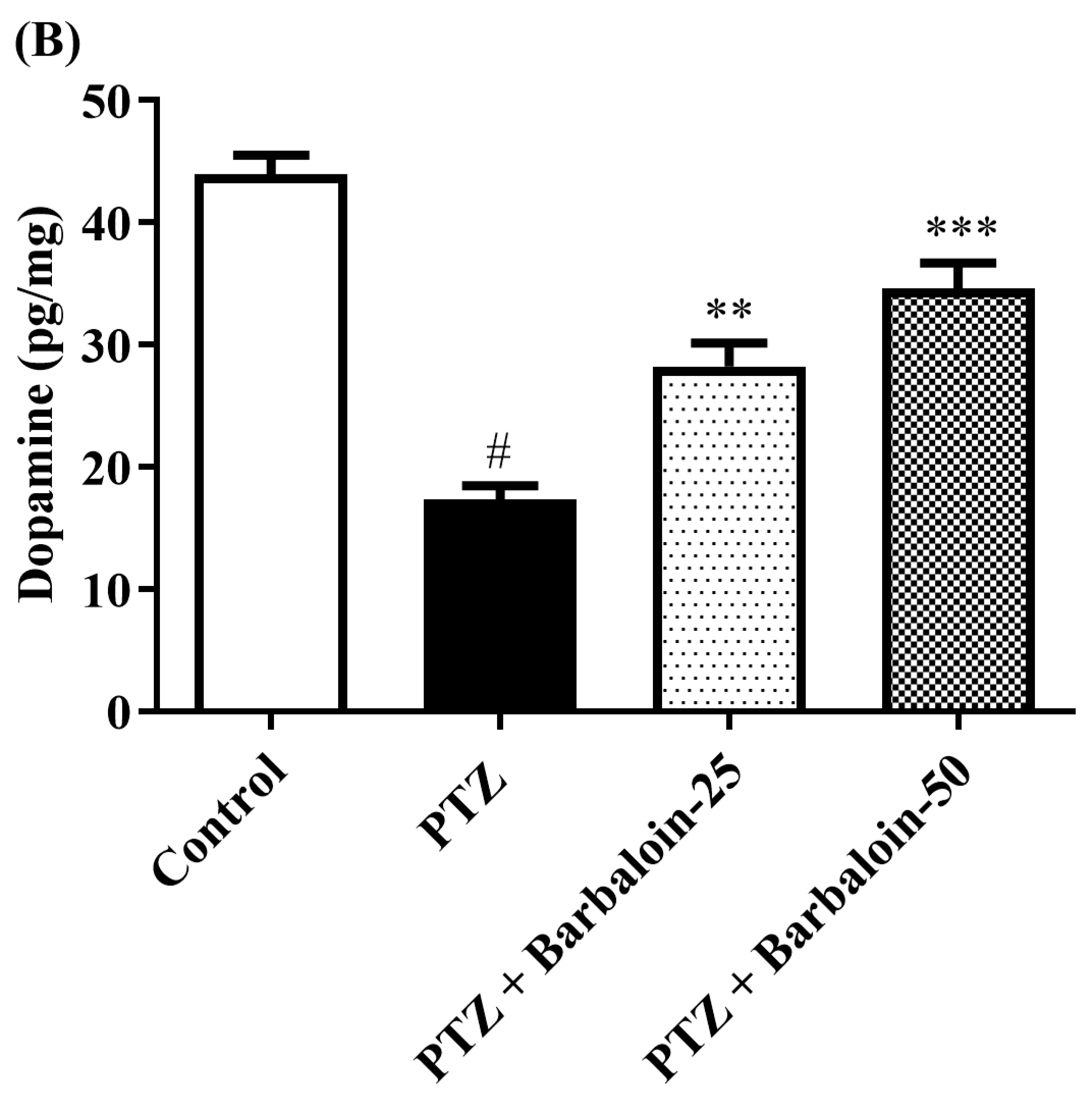
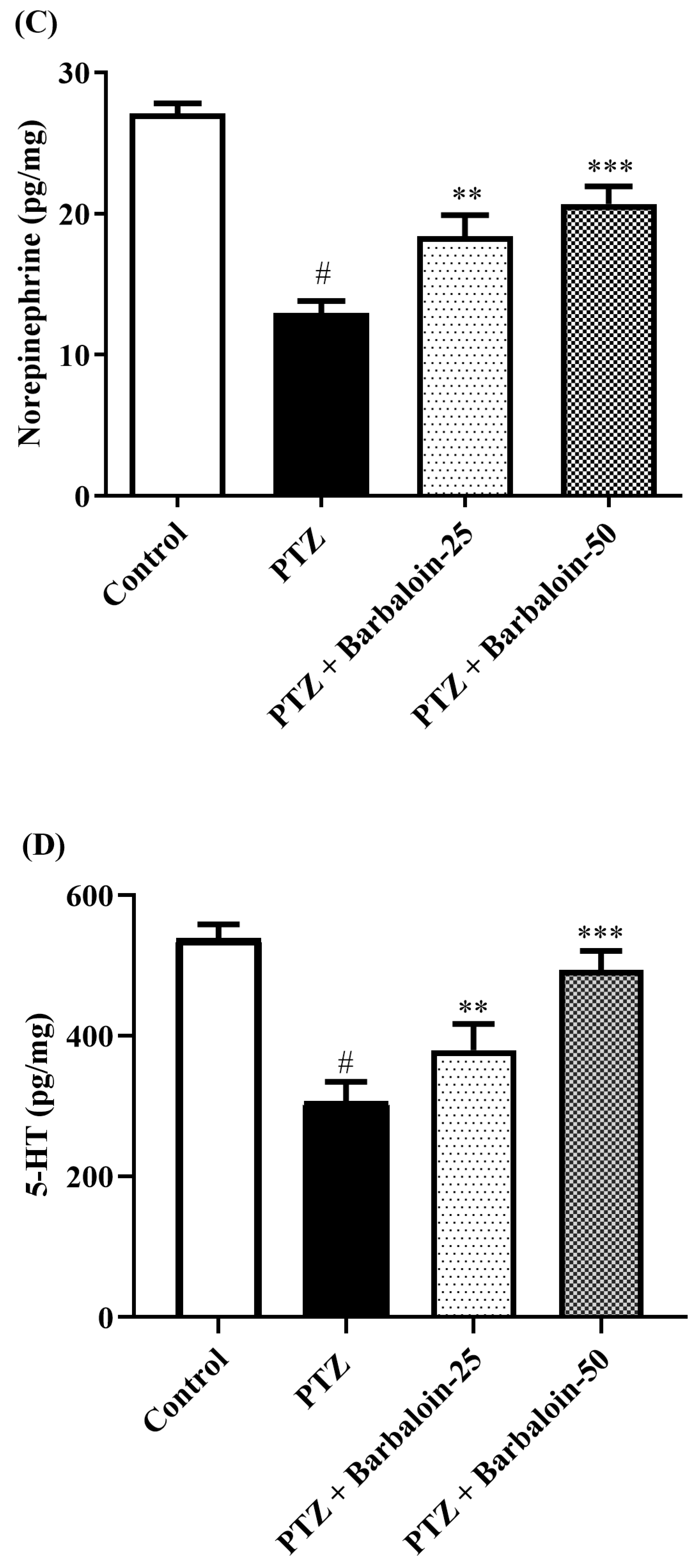
2.6. Brain GABA, Dopamine (DA), Norepinephrine (NE), and 5-Hydroxytryptamine (5-HT) Contents
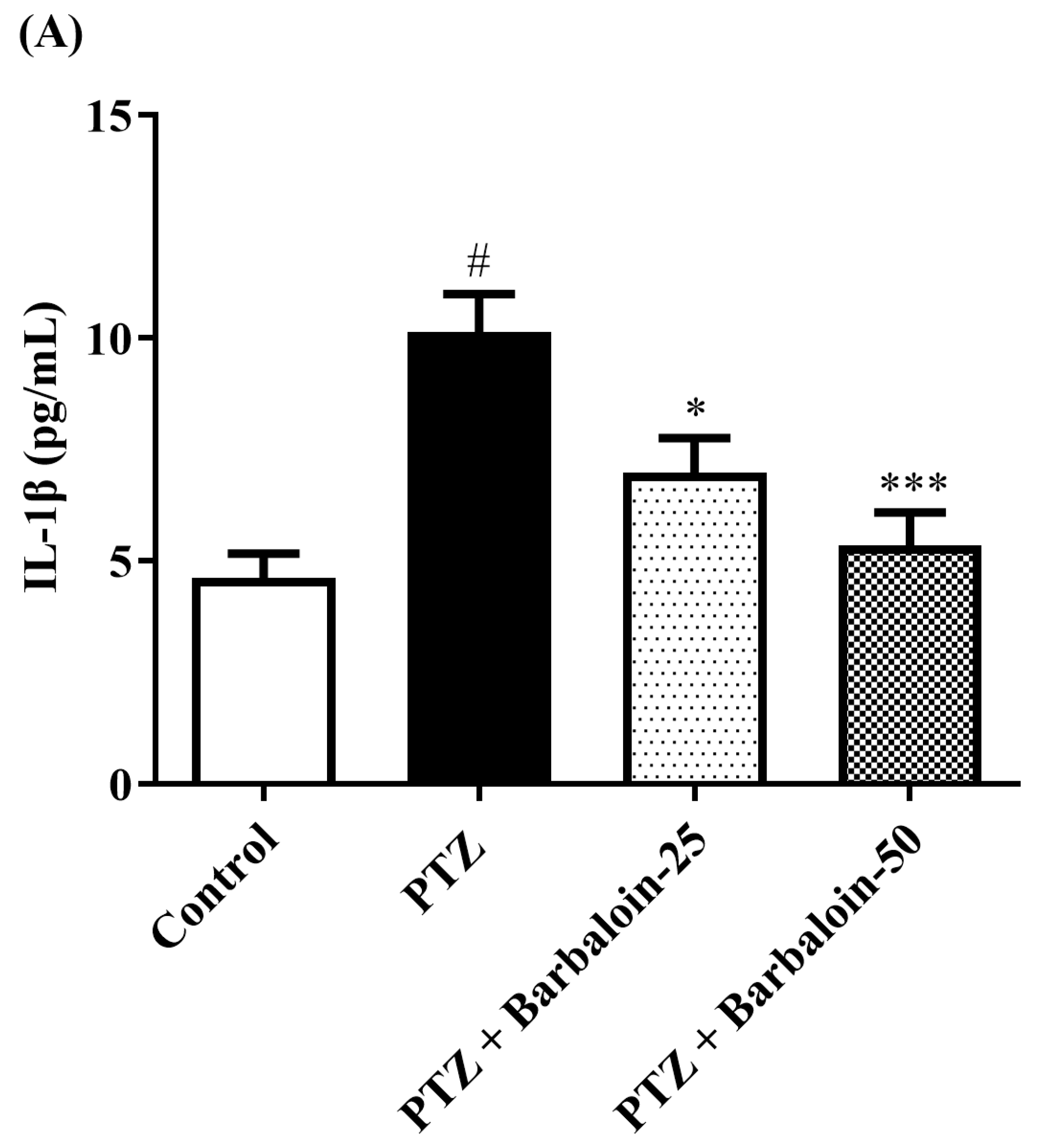
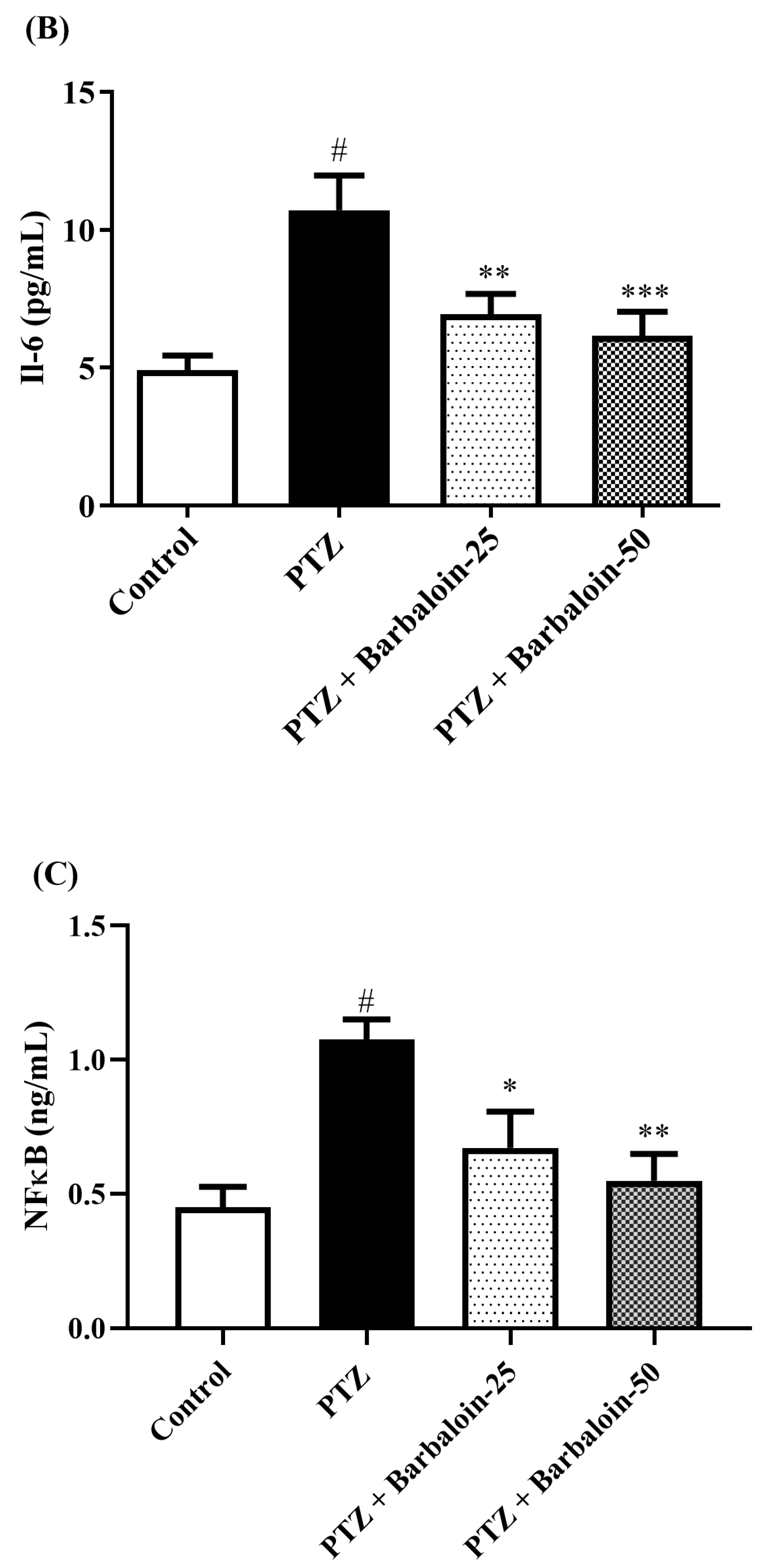
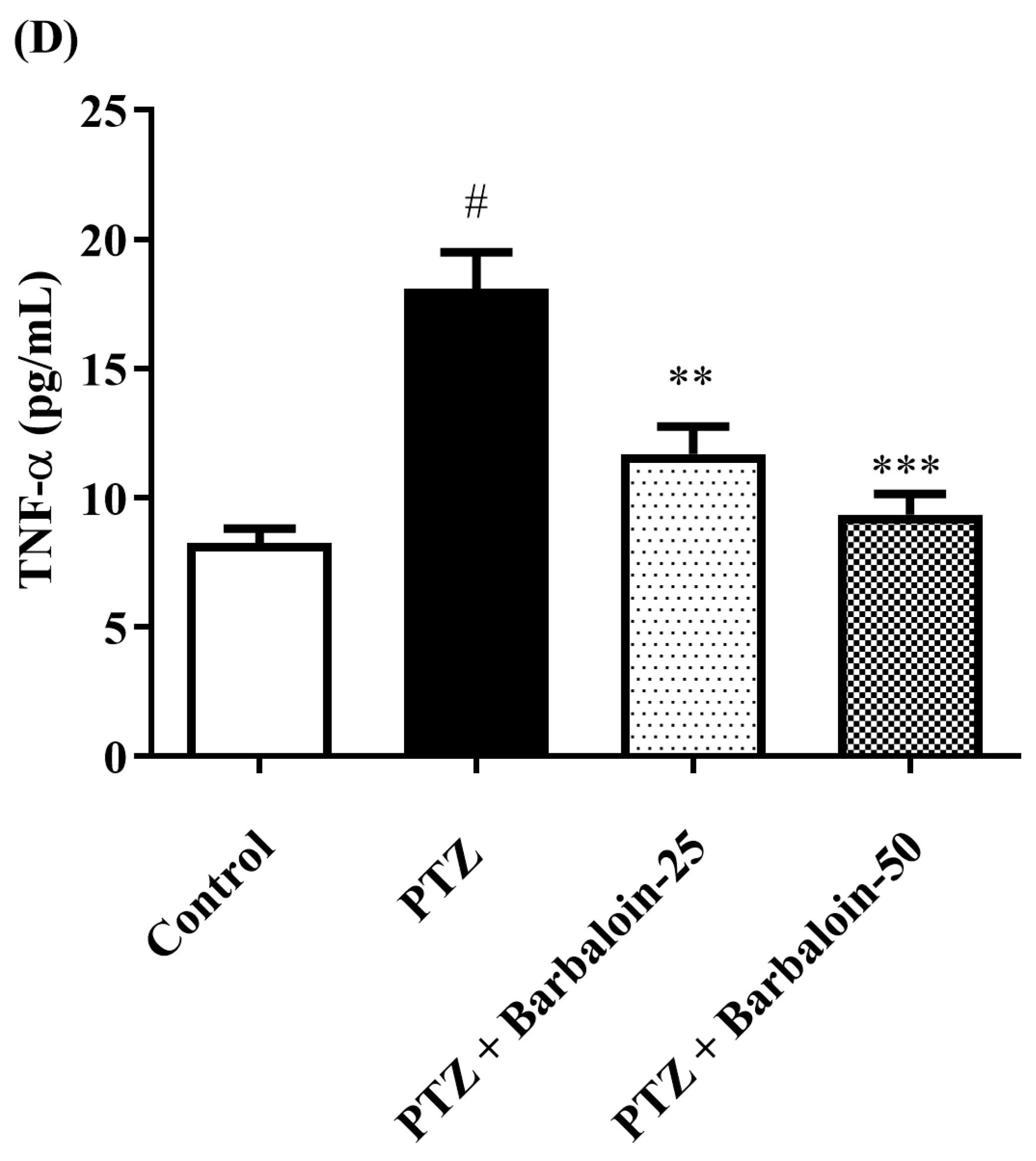
2.7. Estimation of Cytokines
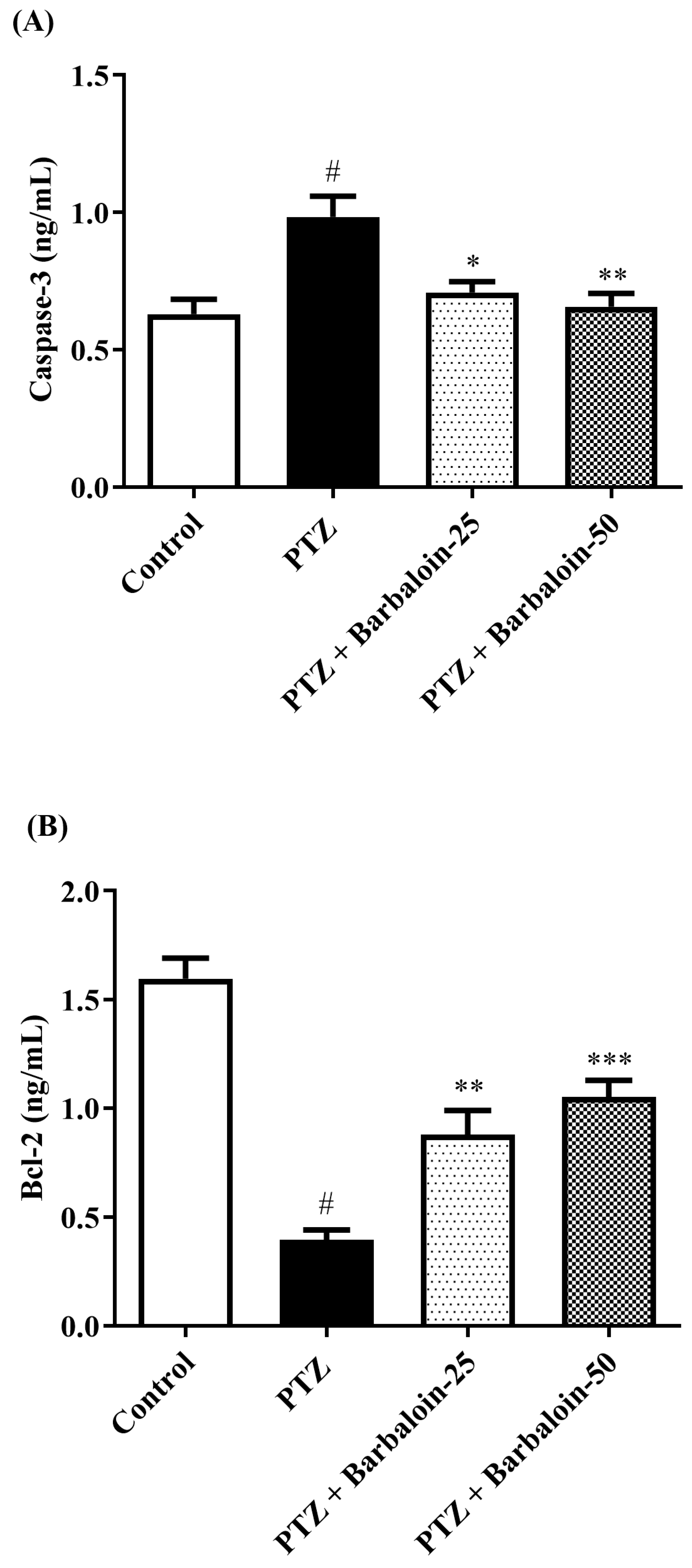
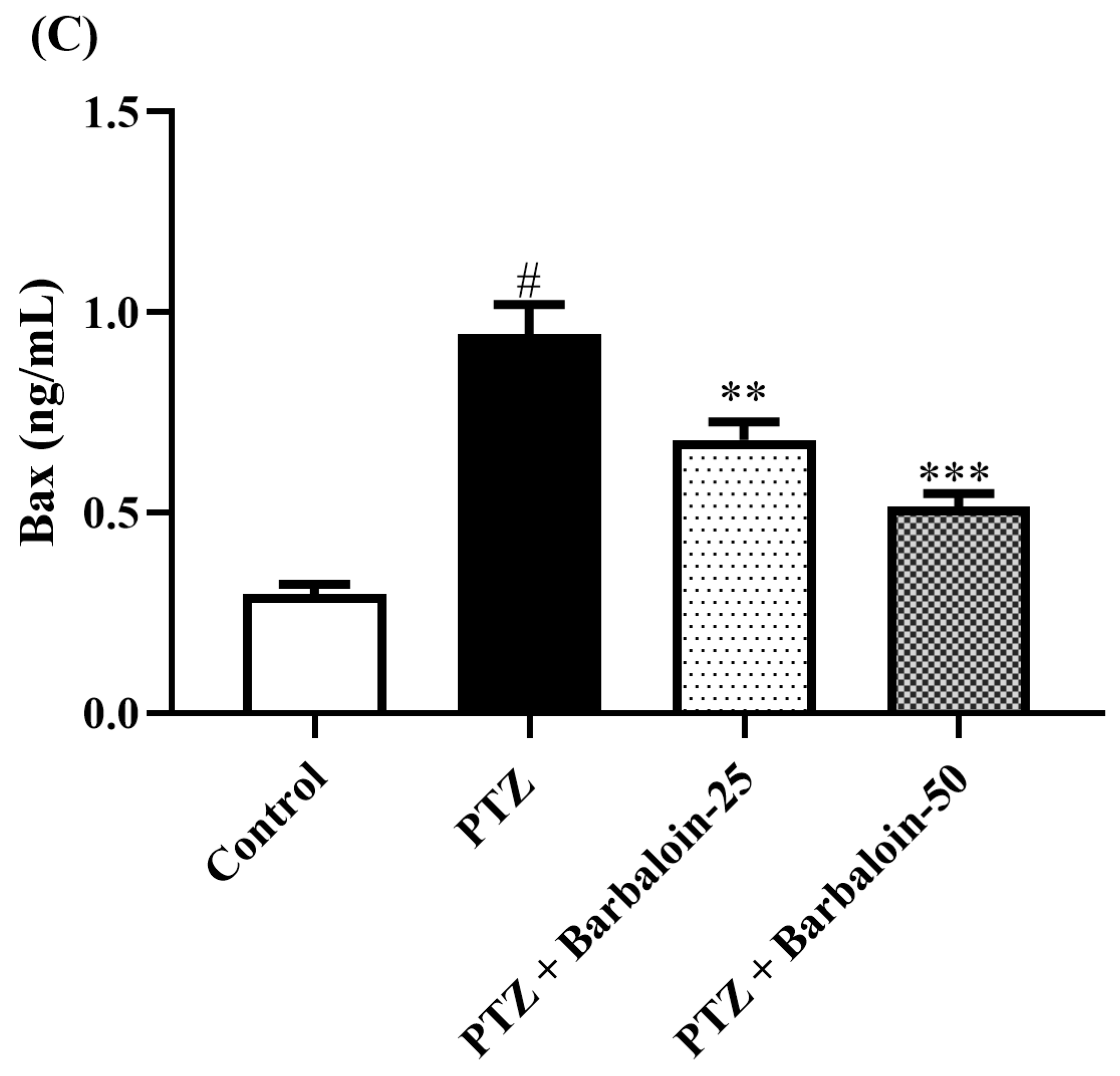
2.8. Caspase-3 Bcl-2 and Bax Contents
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs
4.3. Epileptic Seizure Scoring and a Rat Model Induced by PTZ
4.4. Research Design
4.5. Behavioral Tests
4.5.1. OFT
4.5.2. NORT
4.6. Oxidative Stress Estimation
4.7. AChE Estimation
4.8. Neurotransmitter Levels
4.9. Biological Inflammation
4.10. Analysis of Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohamed, H.K.; Eltony, S.A. Effect of acute pentylenetetrazol injection induced epileptic seizures on rat dentate gyrus at different postnatal ages. Anat. Cell Biol. 2020, 53, 84–94. [Google Scholar] [CrossRef]
- Boison, D.; Steinhäuser, C. Epilepsy and astrocyte energy metabolism. Glia 2018, 66, 1235–1243. [Google Scholar] [CrossRef]
- Cerri, C.; Caleo, M.; Bozzi, Y. Chemokines as new inflammatory players in the pathogenesis of epilepsy. Epilepsy Res. 2017, 136, 77–83. [Google Scholar] [CrossRef]
- Gales, J.M.; Prayson, R.A. Chronic inflammation in refractory hippocampal sclerosis-related temporal lobe epilepsy. Ann. Diagn. Pathol. 2017, 30, 12–16. [Google Scholar] [CrossRef]
- Salgado, P.R.R.; da Fonsêca, D.V.; de Melo, C.G.F.; Leite, F.C.; Alves, A.F.; Ferreira, P.B.; Piuvezam, M.R.; de Sousa, D.P.; de Almeida, R.N. Comparison of behavioral, neuroprotective, and proinflammatory cytokine modulating effects exercised by (+)-cis-EC and (-)-cis-EC stereoisomers in a PTZ-induced kindling test in mice. Fundam. Clin. Pharmacol. 2018, 32, 507–515. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Wu, Y.; Li, T.; Wang, W. Salidroside shows anticonvulsant and neuroprotective effects by activating the Nrf2-ARE pathway in a pentylenetetrazol-kindling epileptic model. Brain Res. Bull. 2020, 164, 14–20. [Google Scholar] [CrossRef]
- Carmona-Aparicio, L.; Pérez-Cruz, C.; Zavala-Tecuapetla, C.; Granados-Rojas, L.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Hernández-Damián, J.; Pedraza-Chaverri, J.; Sampieri, A., 3rd; Coballase-Urrutia, E.; et al. Overview of Nrf2 as Therapeutic Target in Epilepsy. Int. J. Mol. Sci. 2015, 16, 18348–18367. [Google Scholar] [CrossRef]
- Shimada, T.; Takemiya, T.; Sugiura, H.; Yamagata, K. Role of inflammatory mediators in the pathogenesis of epilepsy. Mediat. Inflamm. 2014, 2014, 901902. [Google Scholar] [CrossRef]
- Hashemian, M.; Anissian, D.; Ghasemi-Kasman, M.; Akbari, A.; Khalili-Fomeshi, M.; Ghasemi, S.; Ahmadi, F.; Moghadamnia, A.A.; Ebrahimpour, A. Curcumin-loaded chitosan-alginate-STPP nanoparticles ameliorate memory deficits and reduce glial activation in pentylenetetrazol-induced kindling model of epilepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 462–471. [Google Scholar] [CrossRef]
- Franke, H.; Kittner, H. Morphological alterations of neurons and astrocytes and changes in emotional behavior in pentylenetetrazol-kindled rats. Pharmacol. Biochem. Behav. 2001, 70, 291–303. [Google Scholar] [CrossRef]
- Mortazavi, F.; Ericson, M.; Story, D.; Hulce, V.D.; Dunbar, G.L. Spatial learning deficits and emotional impairments in pentylenetetrazole-kindled rats. Epilepsy Behav. 2005, 7, 629–638. [Google Scholar] [CrossRef]
- Zhu, X.; Dong, J.; Han, B.; Huang, R.; Zhang, A.; Xia, Z.; Chang, H.; Chao, J.; Yao, H. Neuronal Nitric Oxide Synthase Contributes to PTZ Kindling-Induced Cognitive Impairment and Depressive-Like Behavior. Front. Behav. Neurosci. 2017, 11, 203. [Google Scholar] [CrossRef]
- Dhir, A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr. Protoc. Neurosci. 2012, 58, 9–37. [Google Scholar] [CrossRef]
- Ahmadian, S.R.; Ghasemi-Kasman, M.; Pouramir, M.; Sadeghi, F. Arbutin attenuates cognitive impairment and inflammatory response in pentylenetetrazol-induced kindling model of epilepsy. Neuropharmacology 2019, 146, 117–127. [Google Scholar] [CrossRef]
- Kaur, H.; Patro, I.; Tikoo, K.; Sandhir, R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015, 89, 40–50. [Google Scholar] [CrossRef]
- Choudhary, K.M.; Mishra, A.; Poroikov, V.V.; Goel, R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013, 704, 33–40. [Google Scholar] [CrossRef]
- Safar, M.M.; Shahin, N.N.; Mohamed, A.F.; Abdelkader, N.F. Suppression of BACE1 and amyloidogenic/RAGE axis by sitagliptin ameliorates PTZ kindling-induced cognitive deficits in rats. Chem. Biol. Interact. 2020, 328, 109144. [Google Scholar] [CrossRef]
- Shimada, T.; Yamagata, K. Pentylenetetrazole-Induced Kindling Mouse Model. J. Vis. Exp. 2018, 136, e56573. [Google Scholar] [CrossRef]
- Pracucci, E.; Pillai, V.; Lamers, D.; Parra, R.; Landi, S. Neuroinflammation: A Signature or a Cause of Epilepsy? Int. J. Mol. Sci. 2021, 22, 6981. [Google Scholar] [CrossRef]
- Utech, M.; Mennigen, R.; Bruewer, M. Endocytosis and recycling of tight junction proteins in inflammation. J. Biomed. Biotechnol. 2010, 2010, 484987. [Google Scholar] [CrossRef]
- Javaid, S.; Alqahtani, F.; Ashraf, W.; Anjum, S.M.M.; Rasool, M.F.; Ahmad, T.; Alasmari, F.; Alasmari, A.F.; Alqarni, S.A.; Imran, I. Tiagabine suppresses pentylenetetrazole-induced seizures in mice and improves behavioral and cognitive parameters by modulating BDNF/TrkB expression and neuroinflammatory markers. Biomed. Pharmacother. 2023, 160, 114406. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Yang, F. Barbaloin Treatment Contributes to the Rebalance of Glucose and Lipid Homeostasis of Gestational Diabetes Mellitus Mice. Dose Response 2020, 18, 1559325820984910. [Google Scholar] [CrossRef]
- El-Shemy, H.A.; Aboul-Soud, M.A.; Nassr-Allah, A.A.; Aboul-Enein, K.M.; Kabash, A.; Yagi, A. Antitumor properties and modulation of antioxidant enzymes’ activity by Aloe vera leaf active principles isolated via supercritical carbon dioxide extraction. Curr. Med. Chem. 2010, 17, 129–138. [Google Scholar] [CrossRef]
- Patel, D.K.; Patel, K.; Tahilyani, V. Barbaloin: A concise report of its pharmacological and analytical aspects. Asian Pac. J. Trop. Biomed. 2012, 2, 835–838. [Google Scholar] [CrossRef]
- Gai, L.; Chu, L.; Xia, R.; Chen, Q.; Sun, X. Barbaloin Attenuates Mucosal Damage in Experimental Models of Rat Colitis by Regulating Inflammation and the AMPK Signaling Pathway. Med. Sci. Monit. 2019, 25, 10045–10056. [Google Scholar] [CrossRef]
- Jiang, K.; Guo, S.; Yang, C.; Yang, J.; Chen, Y.; Shaukat, A.; Zhao, G.; Wu, H.; Deng, G. Barbaloin protects against lipopolysaccharide (LPS)-induced acute lung injury by inhibiting the ROS-mediated PI3K/AKT/NF-κB pathway. Int. Immunopharmacol. 2018, 64, 140–150. [Google Scholar] [CrossRef]
- Omer, A.B.; Afzal, O.; Altamimi, A.S.A.; Patil, S.; AlGhamdi, S.A.; Alghamdi, A.M.; Alzarea, S.I.; Almalki, W.H.; Kazmi, I. Neuroprotective Effect of Barbaloin on Streptozotocin-Induced Cognitive Dysfunction in Rats via Inhibiting Cholinergic and Neuroinflammatory Cytokines Pathway-TNF-α/IL-1β/IL-6/NF-κB. ACS Omega 2023, 8, 8110–8118. [Google Scholar] [CrossRef]
- Kazmi, I.; Afzal, M.; Imam, F.; Alzarea, S.I.; Patil, S.; Mhaiskar, A.; Shah, U.; Almalki, W.H. Barbaloin’s Chemical Intervention in Aluminum Chloride Induced Cognitive Deficits and Changes in Rats through Modulation of Oxidative Stress, Cytokines, and BDNF Expression. ACS Omega 2024, 9, 6976–6985. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Chehaibi, K.; Trabelsi, I.; Mahdouani, K.; Slimane, M.N. Correlation of Oxidative Stress Parameters and Inflammatory Markers in Ischemic Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 2585–2593. [Google Scholar] [CrossRef]
- Shin, E.J.; Jeong, J.H.; Chung, Y.H.; Kim, W.K.; Ko, K.H.; Bach, J.H.; Hong, J.S.; Yoneda, Y.; Kim, H.C. Role of oxidative stress in epileptic seizures. Neurochem. Int. 2011, 59, 122–137. [Google Scholar] [CrossRef]
- Kandeda, A.K.; Taiwe, G.S.; Moto, F.C.O.; Ngoupaye, G.T.; Nkantchoua, G.C.N.; Njapdounke, J.S.K.; Omam, J.P.O.; Pale, S.; Kouemou, N.; Ngo Bum, E. Antiepileptogenic and Neuroprotective Effects of Pergularia daemia on Pilocarpine Model of Epilepsy. Front. Pharmacol. 2017, 8, 440. [Google Scholar] [CrossRef]
- Kavaye Kandeda, A.; Okomolo Moto, F.C.; Mbomo Ayissi, R.E.; Omam Omam, J.P.; Ojong, L.; Ngo Bum, E. Pergularia daemia hydro-ethanolic extract protects against pentylenetetrazole kindling-induced seizures, oxidative stress, and neuroinflammation in mice. J. Ethnopharmacol. 2021, 279, 114338. [Google Scholar] [CrossRef]
- Rauca, C.; Zerbe, R.; Jantze, H. Formation of free hydroxyl radicals after pentylenetetrazol-induced seizure and kindling. Brain Res. 1999, 847, 347–351. [Google Scholar] [CrossRef]
- Ueda, Y.; Doi, T.; Takaki, M.; Nagatomo, K.; Nakajima, A.; Willmore, L.J. Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: In vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res. 2009, 1266, 1–7. [Google Scholar] [CrossRef]
- Verrotti, A.; Scardapane, A.; Franzoni, E.; Manco, R.; Chiarelli, F. Increased oxidative stress in epileptic children treated with valproic acid. Epilepsy Res. 2008, 78, 171–177. [Google Scholar] [CrossRef]
- Karikas, G.A.; Schulpis, K.H.; Bartzeliotou, A.; Regoutas, S.; Thanopoulou, C.; Papaevangelou, V.; Giannoulia-Karantana, A.; Papassotiriou, I.; Fytou-Pallikari, A. Early effects of sodium valproate monotherapy on serum paraoxonase/arylesterase activities. Scand. J. Clin. Lab. Investig. 2009, 69, 31–35. [Google Scholar] [CrossRef]
- Uma Devi, P.; Pillai, K.K.; Vohora, D. Modulation of pentylenetetrazole-induced seizures and oxidative stress parameters by sodium valproate in the absence and presence of N-acetylcysteine. Fundam. Clin. Pharmacol. 2006, 20, 247–253. [Google Scholar] [CrossRef]
- Méndez-Armenta, M.; Nava-Ruíz, C.; Juárez-Rebollar, D.; Rodríguez-Martínez, E.; Gómez, P.Y. Oxidative stress associated with neuronal apoptosis in experimental models of epilepsy. Oxid. Med. Cell Longev. 2014, 2014, 293689. [Google Scholar] [CrossRef]
- Goel, R.; Saxena, P. Pycnogenol Protects against Pentylenetetrazole-Induced Oxidative Stress and Seizures in Mice. Curr. Clin. Pharmacol. 2019, 14, 68–75. [Google Scholar] [CrossRef]
- Farlow, M.R. Do cholinesterase inhibitors slow progression of Alzheimer’s disease? Int. J. Clin. Pract. Suppl. 2002, 127, 37–44. [Google Scholar]
- Giacobini, E. Cholinesterases: New roles in brain function and in Alzheimer’s disease. Neurochem. Res. 2003, 28, 515–522. [Google Scholar] [CrossRef]
- Alachkar, A.; Azimullah, S.; Lotfy, M.; Adeghate, E.; Ojha, S.K.; Beiram, R.; Łażewska, D.; Kieć-Kononowicz, K.; Sadek, B. Antagonism of Histamine H3 receptors Alleviates Pentylenetetrazole-Induced Kindling and Associated Memory Deficits by Mitigating Oxidative Stress, Central Neurotransmitters, and c-Fos Protein Expression in Rats. Molecules 2020, 25, 1575. [Google Scholar] [CrossRef]
- Uddin, M.S.; Mamun, A.A.; Kabir, M.T.; Nasrullah, M.; Wahid, F.; Begum, M.M. Neurochemistry of neurochemicals: Messengers of brain functions. J. Intellect. Disabil. Diagn. Treat. 2018, 5, 137–151. [Google Scholar] [CrossRef]
- Chimakurthy, J.; Talasila, M. Effects of curcumin on pentylenetetrazole-induced anxiety-like behaviors and associated changes in cognition and monoamine levels. Psychol. Neurosci. 2010, 3, 238–244. [Google Scholar] [CrossRef]
- Visweswari, G.; Prasad, K.S.; Chetan, P.S.; Lokanatha, V.; Rajendra, W. Evaluation of the anticonvulsant effect of Centella asiatica (gotu kola) in pentylenetetrazol-induced seizures with respect to cholinergic neurotransmission. Epilepsy Behav. 2010, 17, 332–335. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.; Arafa, N.; El-Khadragy, M.; Kassab, R. The neuroprotective role of Nigella sativa extract on ciprofloxacin and pentylenetetrazole treated rats. Afr. J. Pharm. Pharmacol. 2013, 7, 660–1670. [Google Scholar] [CrossRef][Green Version]
- Tchekalarova, J.; Pechlivanova, D.; Atanasova, T.; Markova, P.; Lozanov, V.; Stoynev, A. Diurnal variations in depression-like behavior of Wistar and spontaneously hypertensive rats in the kainate model of temporal lobe epilepsy. Epilepsy Behav. 2011, 20, 277–285. [Google Scholar] [CrossRef]
- Essawy, A.E.; El-Sayed, S.A.; Tousson, E.; Abd El-Gawad, H.S.; Alhasani, R.H.; Abd Elkader, H.A.E. Anti-kindling effect of Ginkgo biloba leaf extract and L-carnitine in the pentylenetetrazol model of epilepsy. Environ. Sci. Pollut. Res. Int. 2022, 29, 48573–48587. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Sadeghian, A.; Roohi, N.; Shojaei, A.; Mirnajafi-Zadeh, J. Epilepsy and dopaminergic system. Physiol. Pharmacol. 2017, 21, 1–14. [Google Scholar]
- Yuan, X.; Fu, Z.; Ji, P.; Guo, L.; Al-Ghamdy, A.O.; Alkandiri, A.; Habotta, O.A.; Abdel Moneim, A.E.; Kassab, R.B. Selenium Nanoparticles Pre-Treatment Reverse Behavioral, Oxidative Damage, Neuronal Loss and Neurochemical Alterations in Pentylenetetrazole-Induced Epileptic Seizures in Mice. Int. J. Nanomed. 2020, 15, 6339–6353. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, T.; Dong, K. Effect of formononetin from Trifolium pratense L. on oxidative stress, energy metabolism and inflammatory response after cerebral ischemia-reperfusion injury in mice. Food Sci. Technol. 2021, 42, e57821. [Google Scholar] [CrossRef]
- Font-Nieves, M.; Sans-Fons, M.G.; Gorina, R.; Bonfill-Teixidor, E.; Salas-Pérdomo, A.; Márquez-Kisinousky, L.; Santalucia, T.; Planas, A.M. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J. Biol. Chem. 2012, 287, 6454–6468. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.S.; Obeidat, S.T.; Aleid, G.M.; Al-Bagawi, A.H.; Fareid, M.A.; Hameed, R.A.; Mohamed, K.M.; Abdelfattah, M.S.; Fehaid, A.; Hussein, M.M.; et al. Green Synthetized Selenium Nanoparticles Using Syzygium aromaticum (Clove) Extract Reduce Pentylenetetrazol-Induced Epilepsy and Associated Cortical Damage in Rats. Appl. Sci. 2023, 13, 1050. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Liang, J.; Yan, J.X.; Ye, Y.C.; Wang, J.J.; Chen, C.; Sun, H.T.; Chen, F.; Tu, Y.; Li, X.H. TBHQ improved neurological recovery after traumatic brain injury by inhibiting the overactivation of astrocytes. Brain Res. 2020, 1739, 146818. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Sun, A.Y. Oxidative mechanisms involved in kainate-induced cytotoxicity in cortical neurons. Neurochem. Res. 1994, 19, 1557–1564. [Google Scholar] [CrossRef]
- Henshall, D.C.; Clark, R.S.; Adelson, P.D.; Chen, M.; Watkins, S.C.; Simon, R.P. Alterations in bcl-2 and caspase gene family protein expression in human temporal lobe epilepsy. Neurology 2000, 55, 250–257. [Google Scholar] [CrossRef]
- Hansen, N.; Widman, G.; Witt, J.A.; Wagner, J.; Becker, A.J.; Elger, C.E.; Helmstaedter, C. Seizure control and cognitive improvement via immunotherapy in late onset epilepsy patients with paraneoplastic versus GAD65 autoantibody-associated limbic encephalitis. Epilepsy Behav. 2016, 65, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Waggas, A.M.; Al-Hasani, R.H. Neurophysiological study on possible protective and therapeutic effects of Sidr (Zizyphus spina-christi L.) leaf extract in male albino rats treated with pentylenetetrazol. Saudi J. Biol. Sci. 2010, 17, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, L.; Pandey, R.; Singh, P.; Ali, M.; Kaushik, R.; Soni, P. Neuroprotective Effect of Chlorogenic Acid against Pentylenetetrazol Induced Kindled Epilepsy in Mice. Int. J. Drug Deliv. Technol. 2023, 13, 1030–1036. [Google Scholar] [CrossRef]
- Gáll, Z.; Kelemen, K.; Tolokán, A.; Zolcseak, I.; Sável, I.; Bod, R.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Anticonvulsant Action and Long-Term Effects of Chronic Cannabidiol Treatment in the Rat Pentylenetetrazole-Kindling Model of Epilepsy. Biomedicines 2022, 10, 1811. [Google Scholar] [CrossRef]
- Dix, S.L.; Aggleton, J.P. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav. Brain Res. 1999, 99, 191–200. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Yousef, M.I.; Abdou, H.M.; Abd Elkader, H.-T.A.E.A.; Hussein, H.K.; Abou Samra, W.E.M. Neuroprotective Potential of Spirulina Platensis Against Aluminium Chloride-Induced Neural Degeneration. Curr. Top. Nutraceutical Res. 2020, 18, 310. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Djeuzong, E.; Kandeda, A.K.; Djiogue, S.; Stéphanie, L.; Nguedia, D.; Ngueguim, F.; Djientcheu, J.P.; Kouamouo, J.; Dimo, T. Antiamnesic and Neuroprotective Effects of an Aqueous Extract of Ziziphus jujuba Mill. (Rhamnaceae) on Scopolamine-Induced Cognitive Impairments in Rats. Evid. Based Complement. Altern. Med. 2021, 2021, 5577163. [Google Scholar] [CrossRef]
- Shahid Nadeem, M.; Khan, J.A.; Al-Abbasi, F.A.; AlGhamdi, S.A.; Alghamdi, A.M.; Sayyed, N.; Gupta, G.; Kazmi, I. Protective Effect of Hirsutidin against Rotenone-Induced Parkinsonism via Inhibition of Caspase-3/Interleukins-6 and 1β. ACS Omega 2023, 8, 13016–13025. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altyar, A.E.; Afzal, M.; Ghaboura, N.; Alharbi, K.S.; Alenezi, S.K.; Sayyed, N.; Kazmi, I. Barbaloin Protects Pentylenetetrazol-Induced Cognitive Deficits in Rodents via Modulation of Neurotransmitters and Inhibition of Oxidative-Free-Radicals-Led Inflammation. Pharmaceuticals 2024, 17, 699. https://doi.org/10.3390/ph17060699
Altyar AE, Afzal M, Ghaboura N, Alharbi KS, Alenezi SK, Sayyed N, Kazmi I. Barbaloin Protects Pentylenetetrazol-Induced Cognitive Deficits in Rodents via Modulation of Neurotransmitters and Inhibition of Oxidative-Free-Radicals-Led Inflammation. Pharmaceuticals. 2024; 17(6):699. https://doi.org/10.3390/ph17060699
Chicago/Turabian StyleAltyar, Ahmad Essam, Muhammad Afzal, Nehmat Ghaboura, Khalid Saad Alharbi, Sattam Khulaif Alenezi, Nadeem Sayyed, and Imran Kazmi. 2024. "Barbaloin Protects Pentylenetetrazol-Induced Cognitive Deficits in Rodents via Modulation of Neurotransmitters and Inhibition of Oxidative-Free-Radicals-Led Inflammation" Pharmaceuticals 17, no. 6: 699. https://doi.org/10.3390/ph17060699
APA StyleAltyar, A. E., Afzal, M., Ghaboura, N., Alharbi, K. S., Alenezi, S. K., Sayyed, N., & Kazmi, I. (2024). Barbaloin Protects Pentylenetetrazol-Induced Cognitive Deficits in Rodents via Modulation of Neurotransmitters and Inhibition of Oxidative-Free-Radicals-Led Inflammation. Pharmaceuticals, 17(6), 699. https://doi.org/10.3390/ph17060699








