Microbicides for Topical HIV Immunoprophylaxis: Current Status and Future Prospects
Abstract
1. Introduction
2. Materials and Methods
3. Local Immune Response and Mucous Membranes
4. Microbicides
4.1. Surfactants/Membrane Disruptors
4.2. Vaginal Milieu Protectors
4.3. Entry Inhibitors
4.4. HIV Replication Inhibitors
4.4.1. Clinical Trials of Topical and Oral Pre-Exposure Prophylaxis (PrEP)
4.4.2. Drugs in Preclinical and Early Clinical Phases
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- UNAIDS Data 2023. Available online: https://www.unaids.org/en/resources/documents/2023/2023_unaids_data (accessed on 14 May 2024).
- WHO HIV Data and Statistics. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics (accessed on 14 May 2024).
- Frolova, O.P.; Butylchenko, O.V.; Gadzhieva, P.G.; Timofeeva, M.Y.; Basangova, V.A.; Petrova, V.O.; Fadeeva, I.A.; Kashutina, M.I.; Zabroda, N.N.; Basov, A.A.; et al. Medical Care for Tuberculosis-HIV-Coinfected Patients in Russia with Respect to a Changeable Patients’ Structure. Trop. Med. Infect. Dis. 2022, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Moskaleychik, F.F.; Laga, V.Y.; Delgado, E.; Vega, Y.; Fernandez-Garcia, A.; Perez-Alvarez; Kornilaeva, G.V.; Pronin, A.Y.; Zhernov, Y.V.; Thomson, M.M.; et al. Rapid spread of the HIV-1 circular recombinant CRF02-AG in Russia and neighboring countries. Vopr. Virusol. 2015, 60, 14–19. (In Russian) [Google Scholar]
- Karamov, E.; Epremyan, K.; Siniavin, A.; Zhernov, Y.; Cuevas, M.T.; Delgado, E.; Sánchez-Martínez, M.; Carrera, C.; Kornilaeva, G.; Turgiev, A.; et al. HIV-1 genetic diversity in recently diagnosed infections in Moscow: Predominance of AFSU, frequent branching in clusters, and circulation of the Iberian subtype G variant. AIDS Res. Hum. Retroviruses 2018, 34, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Zhernov, Y.V.; Kremb, S.; Helfer, M.; Schindler, M.; Harir, M.; Mueller, C.; Hertkorn, N.; Avvakumova, N.P.; Konstantinov, A.I.; Brack-Werner, R.; et al. Supramolecular combinations of humic polyanions as potent microbicides with polymodal anti-HIV-activities. New J. Chem. 2017, 41, 212–224. [Google Scholar] [CrossRef]
- Zhernov, Y. Natural humic substances interfere with multiple stages of the replication cycle of human immunodeficiency virus. J. Allergy Clin. Immunol. 2018, 141, AB233. [Google Scholar] [CrossRef]
- Zhernov, Y.V.; Khaitov, M.R. Microbicides for topical immunoprevention of HIV infection. Bull. Sib. Med. 2019, 18, 49–59. [Google Scholar] [CrossRef]
- Zhernov, Y.V.; Konstantinov, A.I.; Zherebker, A.; Nikolaev, E.; Orlov, A.; Savinykh, M.I.; Kornilaeva, G.V.; Karamov, E.V.; Perminova, I.V. Antiviral activity of natural humic substances and shilajit materials against HIV-1: Relation to structure. Environ. Res. 2021, 193, 110312. [Google Scholar] [CrossRef]
- Iwasaki, A. Antiviral immune responses in the genital tract: Clues for vaccines. Nat. Rev. Immunol. 2010, 10, 699–711. [Google Scholar] [CrossRef]
- Pudney, J.; Quayle, A.J.; Anderson, D.J. Immunological microenvironments in the human vagina and cervix: Mediators of cellular immunity are concentrated in the cervical transformation zone. Biol. Reprod. 2005, 73, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Chenine, A.L.; Siddappa, N.B.; Kramer, V.G.; Sciaranghella, G.; Rasmussen, R.A.; Lee, S.J.; Santosuosso, M.; Poznansky, M.C.; Velu, V.; Amara, R.R.; et al. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J. Infect. Dis. 2010, 201, 1155–1163. [Google Scholar] [CrossRef]
- Veazey, R.S.; DeMaria, M.; Chalifoux, L.V.; Shvetz, D.E.; Pauley, D.R.; Knight, H.L.; Rosenzweig, M.; Johnson, R.P.; Desrosiers, R.C.; Lackner, A.A. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 1998, 280, 427–431. [Google Scholar] [CrossRef]
- Moretti, S.; Cafaro, A.; Tripiciano, A.; Picconi, O.; Buttò, S.; Ensoli, F.; Sgadari, C.; Monini, P.; Ensoli, B. HIV therapeutic vaccines aimed at intensifying combination antiretroviral therapy. Expert Rev. Vaccines 2020, 19, 71–84. [Google Scholar] [CrossRef]
- Qiao, X.; He, B.; Chiu, A.; Knowles, D.M.; Chadburn, A.; Cerutti, A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat. Immunol. 2006, 7, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Santini, P.A.; Sullivan, J.S.; He, B.; Shan, M.; Ball, S.C.; Dyer, W.B.; Ketas, T.J.; Chadburn, A.; Cohen-Gould, L.; et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 2009, 10, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Shacklett, B.L.; Cox, C.A.; Quigley, M.F.; Kreis, C.; Stollman, N.H.; Jacobson, M.A.; Andersson, J.; Sandberg, J.K.; Nixon, D.F. Abundant expression of granzyme, A.; but not perforin, in granules of CD8+ T cells in GALT: Implications for immune control of HIV-1 infection. J. Immunol. 2004, 173, 641–648. [Google Scholar] [CrossRef]
- Shacklett, B.L. Mucosal Immunity in HIV/SIV Infection: T Cells, B Cells and Beyond. Curr. Immunol. Rev. 2019, 15, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Kiniry, B.E.; Ganesh, A.; Critchfield, J.W.; Hunt, P.W.; Hecht, F.M.; Somsouk, M.; Deeks, S.G.; Shacklett, B.L. Predominance of weakly cytotoxic, T-betLowEomesNeg CD8+ T-cells in human gastrointestinal mucosa: Implications for HIV infection. Mucosal Immunol. 2017, 10, 1008–1020. [Google Scholar] [CrossRef]
- Baeten, J.M.; Hendrix, C.W.; Hillier, S.L. Topical Microbicides in HIV Prevention: State of the Promise. Annu. Rev. Med. 2020, 71, 361–377. [Google Scholar] [CrossRef]
- Shattock, R.J.; Rosenberg, Z. Microbicides: Topical prevention against HIV. Cold Spring Harb. Perspect. Med. 2012, 2, a007385. [Google Scholar] [CrossRef]
- Van Damme, L.; Ramjee, G.; Alary, M.; Vuylsteke, B.; Chandeying, V.; Rees, H.; Sirivongrangson, P.; Mukenge-Tshibaka, L.; Ettiègne-Traoré, V.; Uaheowitchai, C.; et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: A randomised controlled trial. Lancet 2002, 360, 971–977. [Google Scholar] [CrossRef]
- Roddy, R.E.; Zekeng, L.; Ryan, K.A.; Tamoufe, U.; Weir, S.S.; Wong, E.L. A controlled trial of nonoxynol-9 film to reduce male-to-female transmission of sexually transmitted diseases. N. Engl. J. Med. 1998, 339, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Bax, R.; Douville, K.; McCormick, D.; Rosenberg, M.; Higgins, J.; Bowden, M. Microbicides–evaluating multiple formulations of C31G. Contraception 2002, 66, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Ballagh, S.A.; Baker, J.M.; Henry, D.M.; Archer, D.F. Safety of single daily use for one week of C31G HEC gel in women. Contraception 2002, 66, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Feldblum, P.J.; Adeiga, A.; Bakare, R.; Wevill, S.; Lendvay, A.; Obadaki, F.; Olayemi, M.O.; Wang, L.; Nanda, K.; Rountree, W. SAVVY vaginal gel (C31G) for prevention of HIV infection: A randomized controlled trial in Nigeria. PLoS ONE 2008, 3, e1474. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.; Désormeaux, A.; Bergeron, M.G. Sodium lauryl sulfate, a microbicide effective against enveloped and nonenveloped viruses. Curr. Drug Targets 2002, 3, 17–30. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, T.J.; Kinchington, D.; Kangro, H.O.; Jeffries, D.J. The activity of candidate virucidal agents, low pH and genital secretions against HIV-1 in vitro. Int. J. STD AIDS 1995, 6, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ongradi, J.; Ceccherini-Nelli, L.; Pistello, M.; Specter, S.; Bendinelli, M. Acid sensitivity of cell-free and cell-associated HIV-1: Clinical implications. AIDS Res. Hum. Retroviruses 1990, 6, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- van de Wijgert, J.; Fullem, A.; Kelly, C.; Mehendale, S.; Rugpao, S.; Kumwenda, N.; Chirenje, Z.; Joshi, S.; Taha, T.; Padian, N.; et al. Phase 1 trial of the topical microbicide BufferGel: Safety results from four international sites. J. Acquir. Immune Defic. Syndr. 2001, 26, 21–27. [Google Scholar] [CrossRef] [PubMed]
- van de Wijgert, J.H.H.M.; Braunstein, S.L.; Morar, N.S.; E Jones, H.; Madurai, L.; Strickfaden, T.T.E.; Moodley, M.; Aboobaker, J.; Ndlovu, G.; Ferguson, T.M.; et al. Carraguard Vaginal Gel Safety in HIV-Positive Women and Men in South Africa. J. Acquir. Immune Defic. Syndr. 2007, 46, 538–546. [Google Scholar] [CrossRef]
- Fletcher, P.S.; Wallace, G.S.; Mesquita, P.M.; Shattock, R.J. Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology 2006, 3, 46. [Google Scholar] [CrossRef]
- Rupp, R.; Rosenthal, S.L.; Stanberry, L.R. VivaGel (SPL7013 Gel): A candidate dendrimer--microbicide for the prevention of HIV and HSV infection. Int. J. Nanomed. 2007, 2, 561–566. [Google Scholar]
- Schneider, J.; Weis, R.; Manner, C.; Kary, B.; Werner, A.; Seubert, B.J.; Riede, U.N. Inhibition of HIV-1 in cell culture by synthetic humate analogues derived from hydroquinone: Mechanism of inhibition. Virology 1996, 218, 389–395. [Google Scholar] [CrossRef]
- Joone, G.K.; Dekker, J.; van Rensburg, C.E. Investigation of the immunostimulatory properties of oxihumate. Z. Naturforsch C 2003, 58, 263–267. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Vojdani, F.; Buffa, V.; Shattock, R.J.; Montefiori, D.C.; Bakke, J.; Mirsalis, J.; d’Andrea, A.L.; Hume, S.D.; Bratcher, B.; et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. USA 2009, 106, 6099–6104. [Google Scholar] [CrossRef]
- Griffithsin-Based Rectal Microbicide for PREvention of Viral ENTry (PREVENT). Available online: https://clinicaltrials.gov/study/NCT04032717 (accessed on 14 May 2024).
- Garg, A.B.; Nuttall, J.; Romano, J. The future of HIV microbicides: Challenges and opportunities. Antivir. Chem. Chemother. 2009, 19, 143–150. [Google Scholar] [CrossRef]
- Di Fabio, S.; Van Roey, J.; Giannini, G.; van den Mooter, G.; Spada, M.; Binelli, A.; Pirillo, M.F.; Germinario, E.; Belardelli, F.; de Bethune, M.P.; et al. Inhibition of vaginal transmission of HIV-1 in hu-SCID mice by the non-nucleoside reverse transcriptase inhibitor TMC120 in a gel formulation. AIDS 2003, 17, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Abdool Karim, Q.; Abdool Karim, S.S.; Frohlich, J.A.; Grobler, A.C.; Baxter, C.; Mansoor, L.E.; Kharsany, A.B.; Sibeko, S.; Mlisana, K.P.; Omar, Z.; et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010, 329, 1168–1174. [Google Scholar] [CrossRef]
- Dabee, S.; Mudhune, V.; McLellan-Lemal, E.; Peacock, S.; O’connor, S.; Njoroge, B.; Nyagol, B.; Thurman, A.R.; Ouma, E.; Ridzon, R.; et al. Genital microbiota of women using a 90 day tenofovir or tenofovir and levonorgestrel intravaginal ring in a placebo controlled randomized safety trial in Kenya. Sci. Rep. 2022, 12, 12040. [Google Scholar] [CrossRef]
- Drug Database: Dapivirine. Available online: https://clinicalinfo.hiv.gov/en/drugs/dapivirine/patient (accessed on 14 May 2024).
- Drug Database: Tenofovir-Based Microbicides. Available online: https://clinicalinfo.hiv.gov/en/drugs/tenofovir-based-microbicides/patient (accessed on 14 May 2024).
- Thurman, A.R.; Ouattara, L.A.; Yousefieh, N.; Anderson, P.L.; Bushman, L.R.; Fang, X.; Hanif, H.; Clark, M.; Singh, O.; Doncel, G.F. A Phase I Study to Assess Safety, Pharmacokinetics, and Pharmacodynamics of a Vaginal Insert Containing Tenofovir Alafenamide and Elvitegravir. Front. Cell Infect. Microbiol. 2023, 13, 1130101. [Google Scholar] [CrossRef] [PubMed]
- Drug Database: Emtricitabine/Tenofovir Disoproxil Fumarate. Available online: https://clinicalinfo.hiv.gov/en/drugs/emtricitabine-tenofovir-disoproxil-fumarate/patient (accessed on 14 May 2024).
- Drug Database: Emtricitabine/Tenofovir Alafenamide. Available online: https://clinicalinfo.hiv.gov/en/drugs/emtricitabine-tenofovir-alafenamide/patient (accessed on 14 May 2024).
- Drug Database: Cabotegravir. Available online: https://clinicalinfo.hiv.gov/en/drugs/cabotegravir-1/patient (accessed on 14 May 2024).
- Drug Database: Lenacapavir (HIV Prevention). Available online: https://clinicalinfo.hiv.gov/en/drugs/lenacapavir-hiv-prevention (accessed on 14 May 2024).
- PK, Safety Study of 90-Day Use of Vaginal Rings Containing Dapivirine and Levonorgestrel. Available online: https://clinicaltrials.gov/study/NCT05041699 (accessed on 14 May 2024).
- Dapivirine-Contraceptive Ring. Available online: https://www.ipmglobal.org/our-work/our-products/dapivirine-contraceptive-ring (accessed on 14 May 2024).
- A Study of MK-8527 in Human Immunodeficiency Type 1 Virus (HIV-1) Infected Participants (MK-8527-002). Available online: https://clinicaltrials.gov/study/NCT03615183 (accessed on 14 May 2024).
- North, B.B.; Weitzel, M.B.; Waller, D.P.; Birch, W.X.; Feathergill, K.A.; Birch, L.A.; De Jonge, C.J.; Prins, G.S. Evaluation of the novel vaginal contraceptive agent PPCM in preclinical studies using sperm hyaluronan binding and acrosome status assays. Andrology 2022, 10, 367–376. [Google Scholar] [CrossRef]
- Cunha-Reis, C.; Machado, A.; Barreiros, L.; Araújo, F.; Nunes, R.; Seabra, V.; Ferreira, D.; Segundo, M.A.; Sarmento, B.; das Neves, J. Nanoparticles-in-film for the combined vaginal delivery of anti-HIV microbicide drugs. J. Control Release 2016, 243, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Young, I.C.; Benhabbour, S.R. Multipurpose Prevention Technologies: Oral, Parenteral, and Vaginal Dosage Forms for Prevention of HIV/STIs and Unplanned Pregnancy. Polymers 2021, 13, 2450. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Patel, M.V.; Wira, C.R. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J. Reprod. Immunol. 2013, 97, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Baxi, K.; Sawarkar, S.; Sarmento, B.; das Neves, J. Vaginal multipurpose prevention technologies: Promising approaches for enhancing women’s sexual and reproductive health. Expert. Opin. Drug Deliv. 2020, 17, 379–393. [Google Scholar] [CrossRef]
- Maier, I.; Schiestl, R.H.; Kontaxis, G. Cyanovirin-N Binds Viral Envelope Proteins at the Low-Affinity Carbohydrate Binding Site without Direct Virus Neutralization Ability. Molecules 2021, 26, 3621. [Google Scholar] [CrossRef]
- An Etonogestrel/Ethinyl Estradiol/QGriffithsin (ETG/EE/QGRFT) IVR to Prevent Pregnancy and HIV. Available online: https://reporter.nih.gov/project-details/10138979 (accessed on 14 May 2024).
- Innovative 3D Printed Intravaginal Rings: Reengineering Multipurpose Intravaginal Rings for Prevention of HIV, STIs and Unintended Pregnancy. Available online: https://reporter.nih.gov/project-details/9761451 (accessed on 14 May 2024).
- Novel Intravaginal Ring as a Non-Hormonal Contraceptive—Population Council. Available online: https://popcouncil.org/project/novel-intravaginal-ring-as-a-non-hormonal-contraceptive/ (accessed on 14 May 2024).
- Next Generation Multipurpose Intravaginal Ring Technology Using Innovative CLIP 3D Printing. Available online: https://reporter.nih.gov/search/1z4GeTVlh0mC6fnWYAk0lw/project-details/10063935 (accessed on 14 May 2024).
- Gonorrhea and HIV Prevention with Intravaginal Ring Drug Delivery. Available online: https://reporter.nih.gov/search/VIBGcRE-3kWMKyipADFpXw/project-details/10137883 (accessed on 14 May 2024).
- Next Generation Multipurpose Prevention Technology: An Intravaginal Ring for HIV Prevention and Nonhormonal Contraception. Available online: https://reporter.nih.gov/search/qZ_a5xi4M0SzX0laS07Tjw/project-details/10158504 (accessed on 14 May 2024).
- Capsule-Intravaginal Ring for Sustained Release of Antibodies for Non-Hormonal Contraception and Vaginal Protection against HIV. Available online: https://reporter.nih.gov/project-details/9799170 (accessed on 14 May 2024).
- Non-ARV/Non-Hormonal Multi-Purpose Vaginal Ring|MATRIX. Available online: https://www.matrix4prevention.org/products/non-arvnon-hormonal-multi-purpose-vaginal-ring (accessed on 14 May 2024).
- Novel pre-coital, non-hormonal multipurpose prevention technology (MPT). Available online: https://reporter.nih.gov/project-details/10395456 (accessed on 14 May 2024).
- Griffithsin Fast Dissolving Vaginal Insert|MATRIX. Available online: https://www.matrix4prevention.org/products/griffithsin-fast-dissolving-vaginal-insert (accessed on 14 May 2024).
- Long Acting Film Technology for Contraception and HIV Prevention (LATCH). Available online: https://reporter.nih.gov/project-details/10085198 (accessed on 14 May 2024).
- Extended Release Dapivirine Vaginal Film with Levonorgestrel|MATRIX. Available online: https://www.matrix4prevention.org/products/extended-release-dapivirine-vaginal-film-levonorgestrel (accessed on 14 May 2024).
- Cabotegravir Pellet Implant with Levonorgestrel. Available online: https://www.conrad.org/ (accessed on 14 May 2024).
- Long-Acting Multi Prevention Implant for 2-Year Contraception and HIV PrEP. Available online: https://reporter.nih.gov/search/t0FXMGGdiE622_eUszaw7g/project-details/10619811 (accessed on 14 May 2024).
- Ultra-Long-Acting Polymeric Injectable Multi-Purpose Prevention Technology for Contraception and HIV Prevention. Available online: https://reporter.nih.gov/search/WcUn8fWZy0KhTneo0yrZJQ/project-details/10258079 (accessed on 14 May 2024).
- Continuing Preclinical Development of PPCM Vaginal Contraceptive MPT to IND. Available online: https://reporter.nih.gov/project-details/9889971 (accessed on 14 May 2024).
- Dual Prevention Pill. Available online: https://www.prepwatch.org/products/dual-prevention-pill/ (accessed on 14 May 2024).
- Copper Intravaginal Contraception. Available online: https://reporter.nih.gov/project-details/10018526 (accessed on 14 May 2024).

| Product Name | Delivery Method | Product Developer (Location) | Active Ingredients | Hormonal Type | Useful Links | Registration Number |
|---|---|---|---|---|---|---|
| Dapivirine + Levonorgestrel IVR | Intravaginal Ring (IVR) | International Partnership for Microbicides (Silver Spring, MD, USA) | Dapivirine Levonorgestrel | Hormonal | [49,50] | NCT05041699 |
| MK-8527 | Oral tablet | Merck (Darmstadt, Germany) | MK-8527 new nucleoside reverse transcriptase translocation inhibitor | Non-hormonal | [51] | NCT05494736 NCT06045507 |
| Lenacapavir | Injectables (Subcutaneus) | Gilead (Foster City, CA, USA) | Lenacapavir in combination with other antiretrovirals | Non-hormonal | [48] | NCT04994509 NCT04925752 |
| Product Developer (Location) | Active Ingredients | Hormonal Type | Mechanism of Action (Meaning) |
|---|---|---|---|
| Population Council (New York City, NY, USA) Oak Crest Institute of Science (Monrovia, CA, USA) | Etonogestrel Ethinyl Estradiol QGRFT | Hormonal | Ovulation inhibition (pregnancy); binding to HIV envelope glycoproteins to prevent entry of HIV into target cells (QGRFT) |
| University of North Carolina (Chapel Hill, NC, USA) | Dapivirine Levonorgestrel Pritelivir | Hormonal | Antiretroviral drug + hormonal contraceptive |
| Auritec Pharmaceuticals (Pasadena, CA, USA) | Acyclovir Dolutegravir Etonogestrel Ethinyl Estradiol Rilpivirine | Hormonal | Antiretroviral drug + hormonal contraceptive |
| University of North Carolina (Chapel Hill, NC, USA) | Etonogestrel Ethinyl Estradiol Islatravir (EFdA) | Hormonal | Antiretroviral drug + hormonal contraceptive |
| MassBiologics (Boston, MA, USA) Oak Crest Institute of Science (Monrovia, CA, USA) Planet Biotechnology, Inc. (Hayward, CA, USA) University of Massachusetts (Amherst, MA, USA) | Monoclonal Antibodies Tenofovir Disoproxil Fumarate (TDF) | Non-Contraceptive | mAb 2C7 mediates complement-dependent killing of Neisseria gonorrhoeae. TDF is NRTI targeting HIV infection. |
| Oak Crest Institute of Science (Monrovia, CA, USA) University of North Carolina (Chapel Hill, NC, USA) | Monoclonal Antibodies Tenofovir Disoproxil Fumarate (TDF) | Non-Hormonal | Nucleoside analogue reverse transcriptase inhibitor (NRTI) and sperm-agglutinating antibody |
| MUCCOMMUNE LLC (Morrisville, NC, USA) | HCA Monoclonal Antibodies VRC01 + N6 | Non-Hormonal | Viral neutralisation (sustained release of antibodies for non-hormonal contraception and HIV prevention) |
| Population Council (New York City, NY, USA) | QGRFT Organic acids | Non-Hormonal | The selected organic acids will lower vaginal pH (pH = 3.5–4.5) in the presence of semen. The low pH inactivates sperm and bacteria. When inserted into the vagina, the FDI dissolves into a viscous gel delivering the active agents throughout the vaginal lumen to prevent sperm penetration. QGRFT is a potent non-antiretroviral (ARV) inhibitor of HIV. QGRFT binds to HIV envelope glycoproteins and prevents the entry of HIV into target cells. |
| Magee-Women’s Research Inst. and Fndn., University of Pittsburgh (Pittsburgh, PA, USA) | 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) 4′-Ethynyl-2-fluoro-2′-deoxyadenosine prodrug (EFdA-P) Progestin | Hormonal | Nucleoside reverse transcriptase translocation inhibitor and progestin hormonal contraceptive |
| Magee-Women’s Research Inst. and Fndn., University of Pittsburgh (Pittsburgh, PA, USA) | Dapivirine Levonorgestrel | Hormonal | Antiretroviral drug + hormonal contraceptive |
| Inst. For Research and Innovation in Health (i3S), University of Porto (Porto, Portugal) | Efavirenz (EFV) Tenofovir (TFV) | Non-Contraceptive | Short-term (on-demand) protective film that dissolves on contact with vaginal fluids to deliver a combination of antiviral compounds |
| Research Triangle International (RTI) (Research Triangle Park, NC, USA) | Unspecified | Hormonal | Nucleoside reverse transcriptase inhibitor; ovulation suppression |
| PATH (Seattle, WA, USA) Queen’s University Belfast (Belfast, Northern Ireland, UK) | Norelgestromin ARV candidates under review | Hormonal | ARV + Progestin |
| CONRAD (Norfolk, VA, USA) | Cabotegravir Levonorgestrel | Hormonal | Integrase strand-transfer inhibitor; progestin |
| University of North Carolina (Chapel Hill, NC, USA) | Hormonal Contraceptive Antiretroviral (ARV) | Hormonal | Antiretroviral drug + hormonal contraceptive |
| Yaso Therapeutics (Frisco, TX, USA) | Polyphenylene Carboxymethylene (PPCM) | Non-Hormonal | PPCM prevents viral binding and fusion to a host cell by attaching to and blocking key viral binding sites. It has been demonstrated that PPCM binds gB on HSV-2 and gp120 on HIV. PPCM has also shown promise against the Ebola virus in vitro, as well as gonorrhea in vitro and in mice. PPCM renders sperm infertile without damaging other cells. |
| Product Name | Indications | Clinical Phase | Product Developer (Location) | Active Ingredients | Hormonal Type | Useful Links |
|---|---|---|---|---|---|---|
| Intravaginal ring (IVR) | ||||||
| 90-day Pod-type Etonogestrel/Ethinyl Estradiol/QGriffithsin (EEQ) | HIV, Pregnancy | Preclinical—Early (Pre1) | Population Council (New York City, NY, USA) Oak Crest Institute of Science (Monrovia, CA, USA) | Etonogestrel Ethinyl Estradiol QGRFT | Hormonal | [58] |
| Dapivirine + Pritelivir + Levonorgestrel 3D Printed IVR | HIV, Pregnancy, HSV-2 | Preclinical—Early (Pre1) | University of North Carolina (Chapel Hill, NC, USA) | Dapivirine Levonorgestrel Pritelivir | Hormonal | [59] |
| Novel Intravaginal Ring as a Non-Hormonal Contraceptive Multipurpose Prevention Technology (MPT) | Bacterial Vaginosis (BV) Chlamydia Gonorrhea HIV HSV-2 Pregnancy | Pre-formulation, pre-Phase 1, Non-clinical | Population Council (New York City, NY, USA) Queen’s University Belfast (Belfast, Northern Ireland, UK) Weill Cornell Medical College (New York City, NY, USA) | Copper Zinc Lactide | Non-Hormonal | [60] |
| Islatravir (EFdA) + Etonogestrel/Ethinyl Estradiol 3D Printed IVR | HIV Pregnancy | Preclinical—Early (Pre1) | University of North Carolina (Chapel Hill, NC, USA) | Etonogestrel Ethinyl Estradiol Islatravir (EFdA) | Hormonal | [61] |
| IVR. mAb | ||||||
| mAb 2C7 + TDF IVR | HIV, Gonorrhea | Preclinical—Advanced (Pre2) | MassBiologics (Boston, MA, USA) Oak Crest Institute of Science (Monrovia, CA, USA) Planet Biotechnology, Inc. (Hayward, CA, USA) University of Massachusetts (Amherst, MA, USA) | Monoclonal Antibodies Tenofovir Disoproxil Fumarate (TDF) | Non-Contraceptive | [62] |
| Novel mAb contraceptive + Tenofovir Disoproxil Fumarate (TDF) IVR | HIV, Pregnancy | Preclinical—Early (Pre1) | Oak Crest Institute of Science (Monrovia, CA, USA) University of North Carolina (Chapel Hill, NC, USA) | Monoclonal Antibodies Tenofovir Disoproxil Fumarate (TDF) | Non-Hormonal | [63] |
| Human Contraceptive Antibody (HCA) + VRC01 + N6 IVR | HIV, Pregnancy | Preclinical—Advanced (Pre2) | MUCCOMMUNE LLC (Morrisville, NC, USA) | HCA Monoclonal Antibodies VRC01 + N6 | Non-Hormonal | [64] |
| Non-ARV/Non-Hormonal Multi-Purpose Vaginal Ring | HIV HPV HSV-1 HSV-2 Pregnancy | Pre-formulation | Oak Crest Institute of Science (Monrovia, CA, USA) | Unspecified | Non-Hormonal | [65] |
| Fast-dissolving inserts (FDIs) (Vaginal) | ||||||
| Non-hormonal Contraceptive Multipurpose Prevention Technology (MPT) Containing Q-Griffithsin (QGRFT) | Bacterial Vaginosis (BV) Chlamydia Gonorrhea HIV HSV-2 Pregnancy | Preclinical—Early (Pre1) | Population Council (New York City, NY, USA) | QGRFT Organic acids | Non-Hormonal | [66] |
| Griffithsin (GRFT) Fast-Dissolving Insert (FDI) | HIV HPV HSV-1 HSV-2 | Pre-formulation | Population Council (New York City, NY, USA) | Unspecified | Non-Contraceptive | [67] |
| Films (Vaginal) | ||||||
| EFdA-P + Progestin Intravaginal Film | HIV, Pregnancy | Early Preclinical | Magee-Women’s Research Inst. and Fndn., University of Pittsburgh (Pittsburgh, PA, USA) | 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) 4′-Ethynyl-2-fluoro-2′-deoxyadenosine prodrug (EFdA-P) Progestin | Hormonal | [68] |
| Dapivirine/Levonorgestrel Extended-Release Monthly Film | HIV, Pregnancy | Early Preclinical | Magee-Women’s Research Inst. and Fndn., University of Pittsburgh (Pittsburgh, PA, USA) | Dapivirine Levonorgestrel | Hormonal | [69] |
| Tenofovir (TFV)/Efavirenz (EFV) Nanoparticles-in-film | HIV, HSV-1, HSV-2 | Advanced Preclinical | Inst. For Research and Innovation in Health (i3S), University of Porto (Porto, Portugal) | Efavirenz (EFV) Tenofovir (TFV) | Non-Contraceptive | [53] |
| Implants | ||||||
| Cabotegravir Pellet Implant with Levonorgestrel | HIV, Pregnancy | Early Preclinical | CONRAD (Norfolk, VA, USA) | Cabotegravir Levonorgestrel | Hormonal | [70] |
| Long-acting refillable nanofluidic implant (NanoMPI)- SC | HIV, Pregnancy | Early Preclinical | University of Washington (Seattle, WA, USA) Houston Methodist Hospital Research Institute (HMRI) (Houston, TX, USA) | Etonogestrel Islatravir (EFdA) | Hormonal | [71] |
| Injectables (Subcutaneus) | ||||||
| Cabotegravir/Levonorgestrel Long-Acting MPT Injectable | HIV Pregnancy | Advanced Preclinical | CONRAD (Norfolk, VA, USA) | Cabotegravir Levonorgestrel | Hormonal | [70] |
| Injectable In-situ Forming Implants | ||||||
| Ultra-Long-Acting MPT In situ Forming Implant (ISFI) | HIV Pregnancy | Early Preclinical | University of North Carolina (Chapel Hill, NC, USA) | Hormonal Contraceptive Antiretroviral (ARV) | Hormonal | [72] |
| Gels (Vaginal/Rectal) | ||||||
| Yaso-GEL | HIV, Pregnancy, HSV-2, HPV, Gonorrhea | Advanced Preclinical | Yaso Therapeutics (Frisco, TX, USA) | Polyphenylene Carboxymethylene (PPCM) | Non-Hormonal | [73] |
| Oral Tablet | ||||||
| Dual Prevention Pill (DPP) Regimen | HIV Pregnancy | Preclinical—Early (Pre1) | Population Council (New York City, NY, USA) Medicines360 (San Francisco, CA, USA) | Emtricitabine (FTC) Ethinyl Estradiol (EE) Levonorgestrel Tenofovir Alafenamide (TAF) | Hormonal | [74] |
| Intravaginal Rings (Non-HIV) | ||||||
| Copper Intravaginal Ring (Cu-IVR) | Pregnancy, HSV-2, Zika virus | Early Preclinical | University of California (Davis, CA, USA) | Copper | Non-Hormonal | [75] |
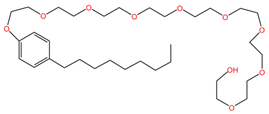 | 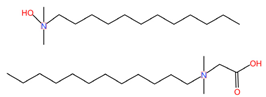 |
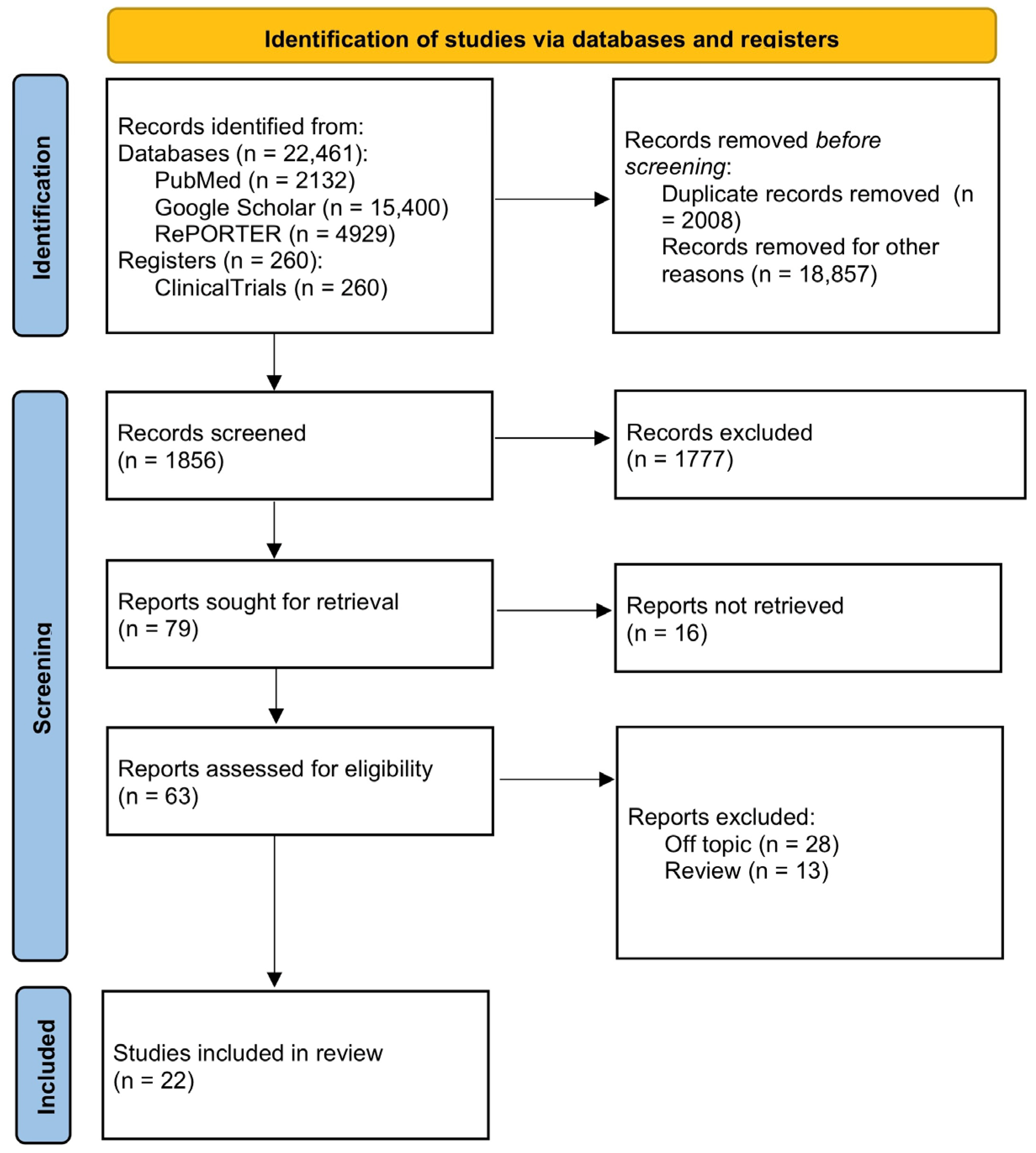
| Nonoxynol-9 (C33H60O10) | C31G (C30H64N2O3) |
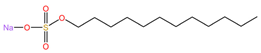 | 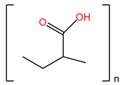 |
| Sodium lauryl sulfate (C12H25SO4Na) | Carbopol 974P ([C3H4O2]n) |
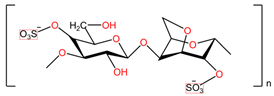 | 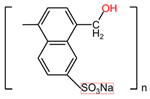 |
| Ϳ-Carrageenans ([C12H16O15S2 2-]n) | Naftalane sulfonate polymers ([C11H9NaSO4]n) |
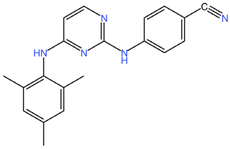 | 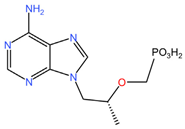 |
| Dapivirine (C20H19N5) | Tenofovir (C9H14N5O4P) |
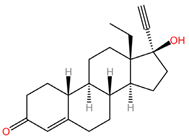 | 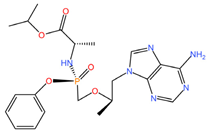 |
| Levonorgestrel (C21H28O2) | Tenofovir alafenamide (C21H29N6O5P) |
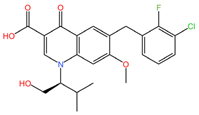 | 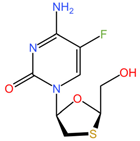 |
| Elvitegravir (C23H23ClFNO5) | Emtricibatine (C8H10FN3O3S) |
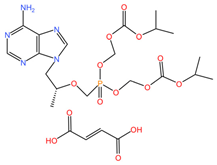 | 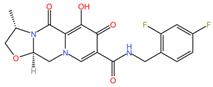 |
| Tenofovir disoproxil fumarate (C23H34N5O14P) | Cabotegravir (C19H17F2N3O5) |
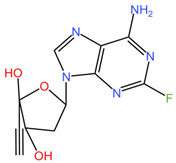 | |
| Islatravir (C12H12FN5O3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhernov, Y.V.; Petrova, V.O.; Simanduyev, M.Y.; Shcherbakov, D.V.; Polibin, R.V.; Mitrokhin, O.V.; Basov, A.A.; Zabroda, N.N.; Vysochanskaya, S.O.; Al-khaleefa, E.; et al. Microbicides for Topical HIV Immunoprophylaxis: Current Status and Future Prospects. Pharmaceuticals 2024, 17, 668. https://doi.org/10.3390/ph17060668
Zhernov YV, Petrova VO, Simanduyev MY, Shcherbakov DV, Polibin RV, Mitrokhin OV, Basov AA, Zabroda NN, Vysochanskaya SO, Al-khaleefa E, et al. Microbicides for Topical HIV Immunoprophylaxis: Current Status and Future Prospects. Pharmaceuticals. 2024; 17(6):668. https://doi.org/10.3390/ph17060668
Chicago/Turabian StyleZhernov, Yury V., Vladislava O. Petrova, Mark Y. Simanduyev, Denis V. Shcherbakov, Roman V. Polibin, Oleg V. Mitrokhin, Artem A. Basov, Nadezhda N. Zabroda, Sonya O. Vysochanskaya, Ezzulddin Al-khaleefa, and et al. 2024. "Microbicides for Topical HIV Immunoprophylaxis: Current Status and Future Prospects" Pharmaceuticals 17, no. 6: 668. https://doi.org/10.3390/ph17060668
APA StyleZhernov, Y. V., Petrova, V. O., Simanduyev, M. Y., Shcherbakov, D. V., Polibin, R. V., Mitrokhin, O. V., Basov, A. A., Zabroda, N. N., Vysochanskaya, S. O., Al-khaleefa, E., Pashayeva, K. R., & Feyziyeva, N. Y. (2024). Microbicides for Topical HIV Immunoprophylaxis: Current Status and Future Prospects. Pharmaceuticals, 17(6), 668. https://doi.org/10.3390/ph17060668








