Emerging Vistas for the Nutraceutical Withania somnifera in Inflammaging
Abstract
1. Introduction
2. Chemistry of Withania somnifera
3. Etiology of Inflammaging
4. Withania somnifera in Inflammaging
4.1. Withania somnifera in Aging
Withania somnifera in Healthy Aging
4.2. Withania somnifera in Inflammation
4.2.1. Withania somnifera in Neuroinflammation
4.2.2. Withania somnifera in Lung Inflammation
4.2.3. Withania somnifera in Kidney Inflammation
4.2.4. Withania somnifera in Liver Inflammation
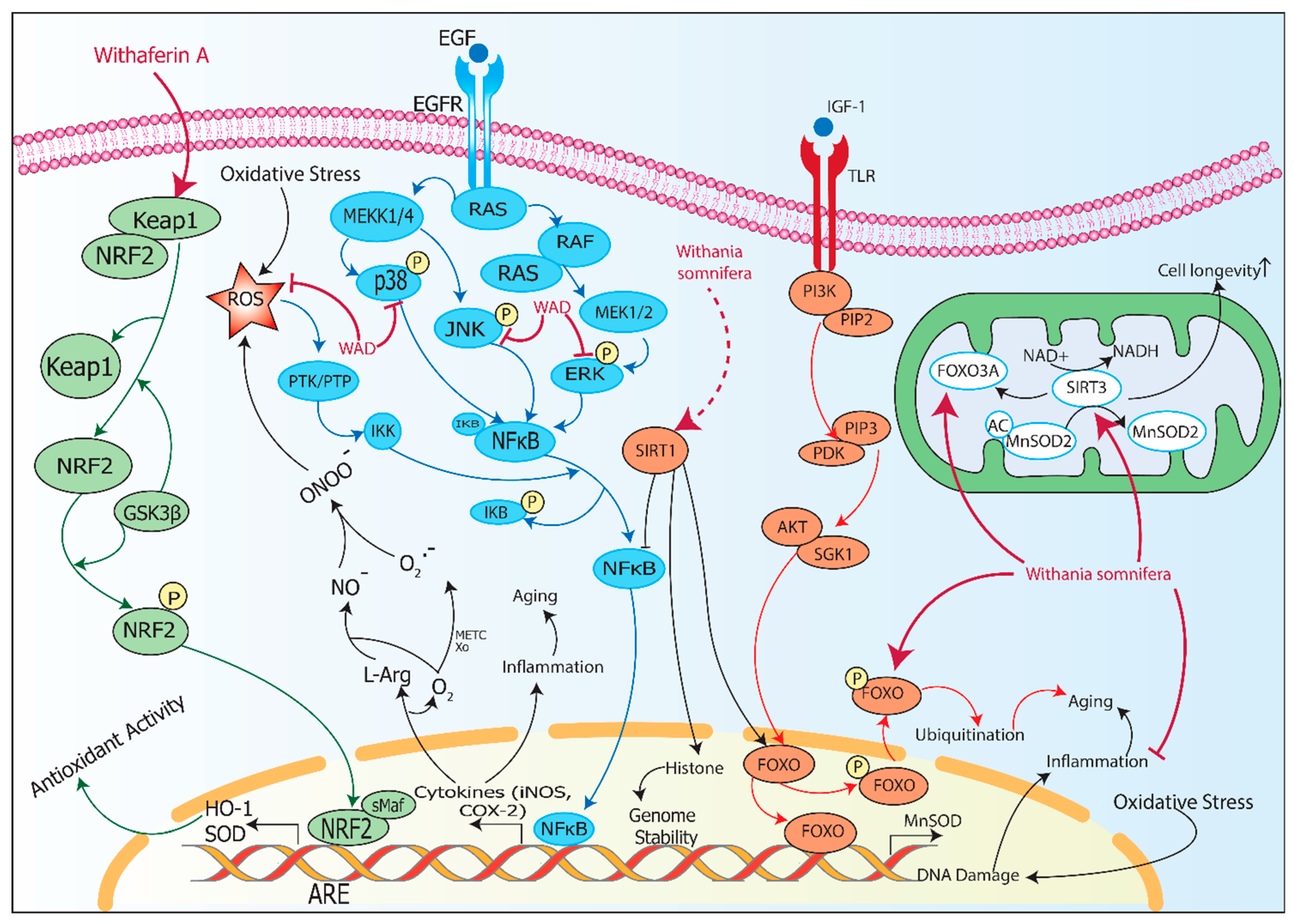
5. Inflammaging and Oxidative Stress: Protective Role of Withania somnifera
6. Inflammaging and DNA Damage: Protective Role of Withania somnifera
7. Inflammaging and Immunomodulation: Protective Role of Withania somnifera
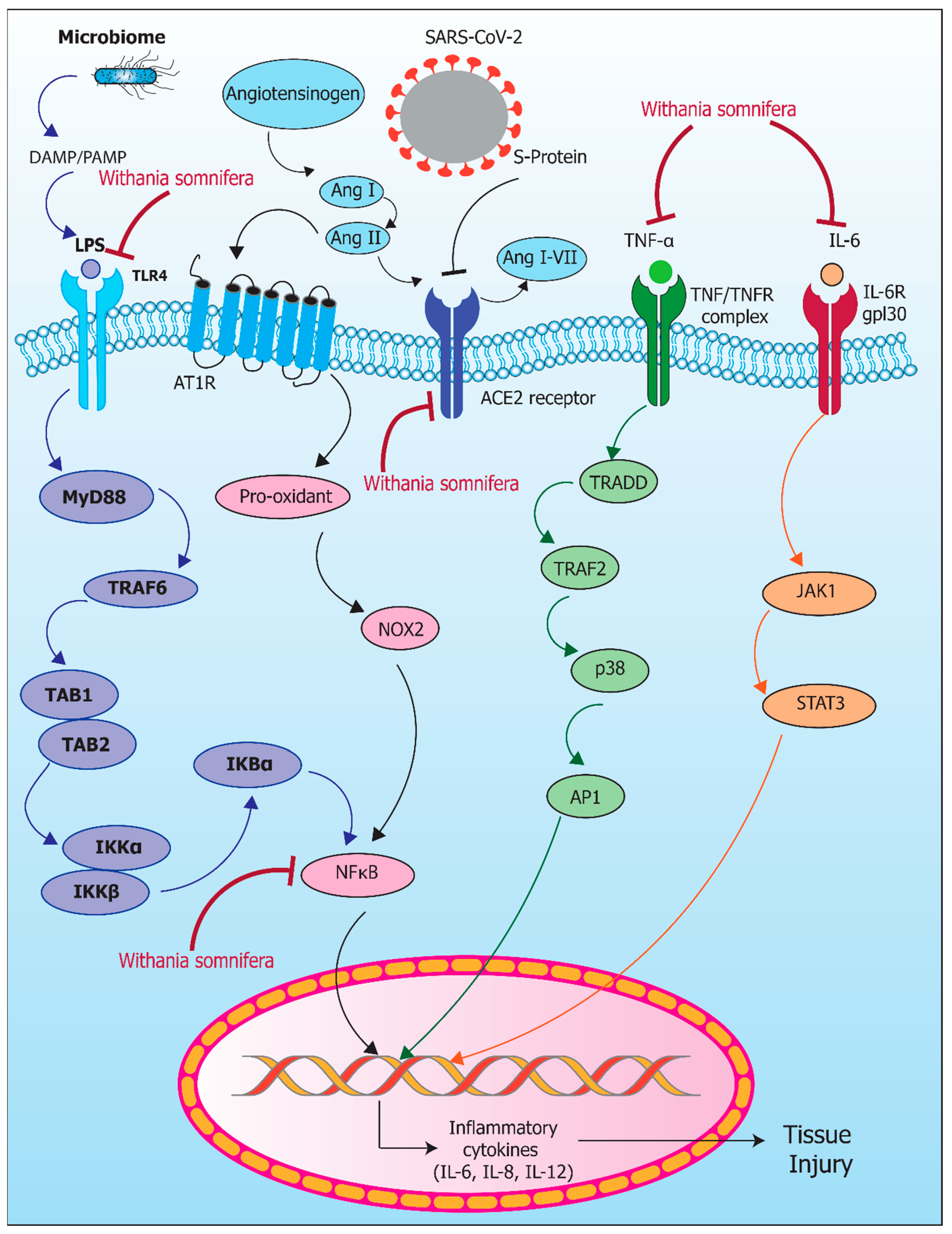
8. Inflammaging and Microbiome: Protective Role of Withania somnifera
9. Inflammaging and COVID-19: The Protective Role of Withania somnifera
10. Safety and Toxicity of Withania somnifera
11. Clinical Studies on Withania somnifera
12. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Antuña, E.; Cachán-Vega, C.; Bermejo-Millo, J.C.; Potes, Y.; Caballero, B.; Vega-Naredo, I.; Coto-Montes, A.; Garcia-Gonzalez, C. Inflammaging: Implications in Sarcopenia. Int. J. Mol. Sci. 2022, 23, 15039. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Sig. Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A New Immune–Metabolic Viewpoint for Age-Related Diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sahardi, N.F.N.; Makpol, S. Suppression of Inflamm-Aging by Moringa Oleifera and Zingiber Officinale Roscoe in the Prevention of Degenerative Diseases: A Review of Current Evidence. Molecules 2023, 28, 5867. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Salim, E.; Asri, R.M.; Hori, A.; Kuraishi, T. Neurodegenerative Disorders and Sterile Inflammation: Lessons from a Drosophila Model. J. Biochem. 2019, 166, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Basudkar, V.; Ajgaonkar, S.; Mehta, D.; Nair, S. Current Clinical Insights into circRNAs and Signal Transduction in Diabetic Nephropathy. Diabet. Nephrop. 2023, 3, 58–67. [Google Scholar] [CrossRef]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic Gut Microbiota Dysbiosis as an Inflammaging and Immunosenescence Condition That Fosters Progression of Retinopathy and Nephropathy. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Li, J.; Lv, J.; Cao, X.; Zhang, H.; Tan, Y.; Chu, T.; Zhao, L.; Liu, Z.; Ren, Y. Gut Microbiota Dysbiosis as an Inflammaging Condition That Regulates Obesity-Related Retinopathy and Nephropathy. Front. Microbiol. 2022, 13, 1040846. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Ajgaonkar, S.; Jadhav, N.; Saha, P.; Gurav, P.; Panda, S.; Mehta, D.; Nair, S. Current Insights into miRNA and lncRNA Dysregulation in Diabetes: Signal Transduction, Clinical Trials and Biomarker Discovery. Pharmaceuticals 2022, 15, 1269. [Google Scholar] [CrossRef]
- Zhang, Q.; Jazwinski, S.M. A Novel Strategy to Model Age-Related Cancer for Elucidation of the Role of Th17 Inflammaging in Cancer Progression. Cancers 2022, 14, 5185. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, D.S.; Namdeo, A.G. Pharmacological Evaluation of Ashwagandha Highlighting Its Healthcare Claims, Safety, and Toxicity Aspects. J. Diet. Suppl. 2021, 18, 183–226. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, P.; Malinowska, M.; Ignacyk, M.; Szustowski, P.; Nowak, J.; Pesta, K.; Szeląg, M.; Szklanny, D.; Judasz, E.; Kaczmarek, G.; et al. Ashwagandha (Withania somnifera)—Current Research on the Health-Promoting Activities: A Narrative Review. Pharmaceutics 2023, 15, 1057. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Malvi, H.; Kodgule, R. An Investigation into the Stress-Relieving and Pharmacological Actions of an Ashwagandha (Withania somnifera) Extract: A Randomized, Double-Blind, Placebo-Controlled Study. Medicine 2019, 98, e17186. [Google Scholar] [CrossRef]
- Speers, A.B.; Cabey, K.A.; Soumyanath, A.; Wright, K.M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress—Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Curr. Neuropharmacol. 2021, 19, 1468–1495. [Google Scholar] [CrossRef] [PubMed]
- Chincholikar, M. Concept of Rasayan and Common Rasayana. Available online: http://ccras.nic.in/content/concept-rasayan-and-common-rasayana (accessed on 5 January 2024).
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A Comprehensive Review on Ethnopharmacology, Pharmacotherapeutics, Biomedicinal and Toxicological Aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef] [PubMed]
- Akhoon, B.A.; Pandey, S.; Tiwari, S.; Pandey, R. Withanolide A Offers Neuroprotection, Ameliorates Stress Resistance and Prolongs the Life Expectancy of Caenorhabditis Elegans. Exp. Gerontol. 2016, 78, 47–56. [Google Scholar] [CrossRef]
- KrishnaRaju, A.V.; Somepalli, V.; Thanawala, S.; Shah, R. Efficacy and Anti-Inflammatory Activity of Ashwagandha Sustained-Release Formulation on Depression and Anxiety Induced by Chronic Unpredictable Stress: In Vivo and in Vitro Studies. JEP 2023, 15, 291–305. [Google Scholar] [CrossRef]
- Singh, N.; Bhalla, M.; de Jager, P.; Gilca, M. An Overview on Ashwagandha: A Rasayana (Rejuvenator) of Ayurveda. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Jayant, K.; Singh, D.; Bhutani, S.; Poddar, N.K.; Chaudhary, A.A.; Khan, S.-U.-D.; Adnan, M.; Siddiqui, A.J.; Hassan, M.I.; et al. Withania somnifera (L.) Dunal (Ashwagandha) for the Possible Therapeutics and Clinical Management of SARS-CoV-2 Infection: Plant-Based Drug Discovery and Targeted Therapy. Front. Cell. Infect. Microbiol. 2022, 12, 933824. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Mofed, D.; Zekri, A.-R.; El-Sayed, N.; Rahouma, M.; Sabet, S. Antioxidant Activity and Apoptotic Induction as Mechanisms of Action of Withania somnifera (Ashwagandha) against a Hepatocellular Carcinoma Cell Line. J. Int. Med. Res. 2018, 46, 1358–1369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Kumar, L.; Goswami, Y.; Pujani, M.; Dikshit, M.; Tandon, R. The Aqueous Root Extract of Withania somnifera Ameliorates LPS-Induced Inflammatory Changes in the in Vitro Cell-Based and Mice Models of Inflammation. Front. Pharmacol. 2023, 14, 1139654. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Gupta, S.K.; Pathak, A.K. A Double-Blind, Randomized, Placebo-Controlled Trial on the Effect of Ashwagandha (Withania somnifera Dunal.) Root Extract in Improving Cardiorespiratory Endurance and Recovery in Healthy Athletic Adults. J. Ethnopharmacol. 2021, 272, 113929. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, T.; Tohda, C.; Komatsu, K. Effects of Ashwagandha (Roots of Withania somnifera) on Neurodegenerative Diseases. Biol. Pharm. Bull. 2014, 37, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Agarwal, A.; Pogrebetskaya, M.; Roychoudhury, S.; Durairajanayagam, D.; Henkel, R. Role of Withania somnifera (Ashwagandha) in the Management of Male Infertility. Reprod. BioMedicine Online 2018, 36, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Chander, H.; Munshi, A. Mechanisms of Anti-Tumor Activity of Withania somnifera (Ashwagandha). Nutr. Cancer 2021, 73, 914–926. [Google Scholar] [CrossRef]
- Shah, D.; Gandhi, M.; Kumar, A.; Cruz-Martins, N.; Sharma, R.; Nair, S. Current Insights into Epigenetics, Noncoding RNA Interactome and Clinical Pharmacokinetics of Dietary Polyphenols in Cancer Chemoprevention. Crit. Rev. Food Sci. Nutr. 2021, 63, 1755–1791. [Google Scholar] [CrossRef]
- Sodvadiya, M.; Patel, H.; Mishra, A.; Nair, S. Emerging Insights into Anticancer Chemopreventive Activities of Nutraceutical Moringa Oleifera: Molecular Mechanisms, Signal Transduction and In Vivo Efficacy. Curr. Pharmacol. Rep. 2020, 6, 38–51. [Google Scholar] [CrossRef]
- Jain, R.; Nair, S. Sandalwood Oil for the Chemoprevention of Skin Cancer: Mechanistic Insights, Anti-Inflammatory, and In Vivo Anticancer Potential. Curr. Pharmacol. Rep. 2019, 5, 345–358. [Google Scholar] [CrossRef]
- Nair, S.; Ah-Ng Tony, K. Pharmacometrics of Nutraceutical Sulforaphane and Its Implications in Prostate Cancer Prevention. J. Chin. Phram. Sci. 2016, 25, 12–22. [Google Scholar] [CrossRef]
- Jadhav, N.; Ajgaonkar, S.; Saha, P.; Gurav, P.; Pandey, A.; Basudkar, V.; Gada, Y.; Panda, S.; Jadhav, S.; Mehta, D.; et al. Molecular Pathways and Roles for Vitamin K2-7 as a Health-Beneficial Nutraceutical: Challenges and Opportunities. Front. Pharmacol. 2022, 13, 896920. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.; Godse, C.; Jadhav, S.; Saha, P.; Ajgaonkar, S.; Pandey, A.; Gurav, P.; Jadhav, N.; Mehta, D.; Nair, S. An Intrinsic Need for Vitamin K2-7 Supplementation: A Narrative Review of K2-7 and Peripheral Neuropathy. BJSTR 2022, 42, 33679–33687. [Google Scholar] [CrossRef]
- Chen, C.; Pung, D.; Leong, V.; Hebbar, V.; Shen, G.; Nair, S.; Li, W.; Tony Kong, A.-N. Induction of Detoxifying Enzymes by Garlic Organosulfur Compounds through Transcription Factor Nrf2: Effect of Chemical Structure and Stress Signals. Free. Radic. Biol. Med. 2004, 37, 1578–1590. [Google Scholar] [CrossRef]
- Shen, G.; Xu, C.; Hu, R.; Jain, M.R.; Nair, S.; Lin, W.; Yang, C.S.; Chan, J.Y.; Kong, A.-N.T. Comparison of (−)-Epigallocatechin-3-Gallate Elicited Liver and Small Intestine Gene Expression Profiles Between C57BL/6J Mice and C57BL/6J/Nrf2 (−/−) Mice. Pharm. Res. 2005, 22, 1805–1820. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Xu, C.; Hu, R.; Jain, M.R.; Gopalkrishnan, A.; Nair, S.; Huang, M.-T.; Chan, J.Y.; Kong, A.-N.T. Modulation of Nuclear Factor E2-Related Factor 2–Mediated Gene Expression in Mice Liver and Small Intestine by Cancer Chemopreventive Agent Curcumin. Mol. Cancer Ther. 2006, 5, 39–51. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Xu, C.-J.; Nair, S.S.; Chen, C.; Hebbar, V.; Kong, A.-N.T. Modulation of Activator Protein-1 (AP-1) and MAPK Pathway by Flavonoids in Human Prostate Cancer PC3 Cells. Arch. Pharm. Res. 2006, 29, 633–644. [Google Scholar] [CrossRef]
- Barve, A.; Khor, T.O.; Nair, S.; Lin, W.; Yu, S.; Jain, M.R.; Chan, J.Y.; Kong, A.-N. Pharmacogenomic Profile of Soy Isoflavone Concentrate in the Prostate of Nrf2 Deficient and Wild-Type Mice. J. Pharm. Sci. 2008, 97, 4528–4545. [Google Scholar] [CrossRef]
- Barve, A.; Khor, T.O.; Nair, S.; Reuhl, K.; Suh, N.; Reddy, B.; Newmark, H.; Kong, A.-N. γ-Tocopherol-Enriched Mixed Tocopherol Diet Inhibits Prostate Carcinogenesis in TRAMP Mice. Int. J. Cancer 2009, 124, 1693–1699. [Google Scholar] [CrossRef]
- Nair, S.; Barve, A.; Khor, T.-O.; Shen, G.; Lin, W.; Chan, J.Y.; Cai, L.; Kong, A.-N. Regulation of Nrf2- and AP-1-Mediated Gene Expression by Epigallocatechin-3-Gallate and Sulforaphane in Prostate of Nrf2-Knockout or C57BL/6J Mice and PC-3 AP-1 Human Prostate Cancer Cells. Acta Pharmacol. Sin. 2010, 31, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Hebbar, V.; Shen, G.; Gopalakrishnan, A.; Khor, T.O.; Yu, S.; Xu, C.; Kong, A.-N. Synergistic Effects of a Combination of Dietary Factors Sulforaphane and (−) Epigallocatechin-3-Gallate in HT-29 AP-1 Human Colon Carcinoma Cells. Pharm. Res. 2008, 25, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, F.; Gomez, Y.; Paredes-Gonzalez, X.; Barve, A.; Nair, S.; Yu, S. Nrf2-Mediated Antioxidant and Detoxifying Enzyme Induction by a Combination of Curcumin and Sulforaphane. J. Chin. Pharm. Sci. 2016, 25, 559–569. [Google Scholar]
- Mishra, L.-C.; Singh, B.B.; Dagenais, S. Scientific Basis for the Therapeutic Use of Withania somnifera (Ashwagandha): A Review. Altern. Med. Rev. 2000, 5, 334–346. [Google Scholar]
- Ghosal, S.; Lal, J.; Srivastava, R.; Bhattacharya, S.K.; Upadhyay, S.N.; Jaiswal, A.K.; Chattopadhyay, U. Immunomodulatory and CNS Effects of Sitoindosides IX and X, Two New Glycowithanolides from Withania somnifera. Phytother. Res. 1989, 3, 201–206. [Google Scholar] [CrossRef]
- Singh, M.; Manoj, J.; Sahu, D.; Kumar, Y. Augmentation of Meat Quality Attributes of Broilers by Dietary Supplementation of Selenium and Ashwagandha. Int. J. Livest. Res. 2020, 1, 73–78. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The Underconsumed and Underappreciated Essential Nutrient. Nutr. Today 2018, 53, 240–253. [Google Scholar] [CrossRef]
- Ahmed, H.A.; El-Darier, S.M. Phytochemistry, Allelopathy and Anticancer Potentiality of Withania somnifera (L.) Dunal (Solanaceae). Braz. J. Biol. 2024, 84, e263815. [Google Scholar] [CrossRef] [PubMed]
- Bhople, S.; Singh, M. Effect of Iron Enrichment on Textural Properties of Rice Based Ashwagandha (Withania somnifera) Fortified Extruded Snacks. Chem. Sci. Rev. Lett. 2017, 6, 1468–1475. [Google Scholar]
- Lagoumtzi, S.M.; Chondrogianni, N. Senolytics and Senomorphics: Natural and Synthetic Therapeutics in the Treatment of Aging and Chronic Diseases. Free. Radic. Biol. Med. 2021, 171, 169–190. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [PubMed]
- Grunz-Borgmann, E.; Mossine, V.; Fritsche, K.; Parrish, A.R. Ashwagandha Attenuates TNF-α- and LPS-Induced NF-κB Activation and CCL2 and CCL5 Gene Expression in NRK-52E Cells. BMC Complement. Altern. Med. 2015, 15, 434. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, Y.J.; Lee, S.; Kang, K.S.; Jang, T.S.; Kim, K.H. Protective Effects of Withagenin A Diglucoside from Indian Ginseng (Withania somnifera) against Human Dermal Fibroblast Damaged by TNF-α Stimulation. Antioxidants 2022, 11, 2248. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Pyo, J.-S.; Cho, W.J. Roles of NF-κB Activation in Benign Prostatic Hyperplasia and Association between NF-κB and HIF-1α. Pathol. Res. Pract. 2022, 237, 154021. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Miyauchi, H.; Tateno, K.; Kunieda, T.; Komuro, I. Akt-Induced Cellular Senescence: Implication for Human Disease. Cell Cycle 2004, 3, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Zdanov, S.; Bernard, D.; Debacq-Chainiaux, F.; Martien, S.; Gosselin, K.; Vercamer, C.; Chelli, F.; Toussaint, O.; Abbadie, C. Normal or Stress-Induced Fibroblast Senescence Involves COX-2 Activity. Exp. Cell Res. 2007, 313, 3046–3056. [Google Scholar] [CrossRef]
- Babu, P.V.A.; Gokulakrishnan, A.; Dhandayuthabani, R.; Ameethkhan, D.; Kumar, C.V.P.; Ahamed, M.I.N. Protective Effect of Withania somnifera (Solanaceae) on Collagen Glycation and Cross-Linking. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 147, 308–313. [Google Scholar] [CrossRef]
- Salvadori, L.; Mandrone, M.; Manenti, T.; Ercolani, C.; Cornioli, L.; Lianza, M.; Tomasi, P.; Chiappalupi, S.; Di Filippo, E.S.; Fulle, S.; et al. Identification of Withania somnifera-Silybum Marianum-Trigonella Foenum-Graecum Formulation as a Nutritional Supplement to Contrast Muscle Atrophy and Sarcopenia. Nutrients 2020, 13, 49. [Google Scholar] [CrossRef]
- Brennan, C.M.; Emerson, C.P.; Owens, J.; Christoforou, N. P38 MAPKs—Roles in Skeletal Muscle Physiology, Disease Mechanisms, and as Potential Therapeutic Targets. JCI Insight 2021, 6, e149915. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, R.; Yadav, S.K.; Prem, N.N.; Bhagel, V.; Pathak, M.; Shekhar, S.; Gaikwad, S.; Dwivedi, S.N.; Bal, C.S.; Dey, A.B.; et al. Serum FOXO3A: A Ray of Hope for Early Diagnosis of Alzheimer’s Disease. Mech. Ageing Dev. 2020, 190, 111290. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.A.; Brunet, A. FoxO Transcription Factors in the Maintenance of Cellular Homeostasis during Aging. Curr. Opin. Cell Biol. 2008, 20, 126–136. [Google Scholar] [CrossRef]
- Pradhan, R.; Kumar, R.; Shekhar, S.; Rai, N.; Ambashtha, A.; Banerjee, J.; Pathak, M.; Dwivedi, S.N.; Dey, S.; Dey, A.B. Longevity and Healthy Ageing Genes FOXO3A and SIRT3: Serum Protein Marker and New Road Map to Burst Oxidative Stress by Withania somnifera. Exp. Gerontol. 2017, 95, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kuchewar, V.; Borkar, M.; Nisargandha, M. Evaluation of Antioxidant Potential of Rasayana Drugs in Healthy Human Volunteers. Ayu 2014, 35, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.I.; Maurya, P. Alterations in Antioxidant Enzymes During Aging in Humans. Mol. Biotechnol. 2007, 37, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Bruns, D.R.; Drake, J.C.; Biela, L.M.; Peelor, F.F.; Miller, B.F.; Hamilton, K.L. Nrf2 Signaling and the Slowed Aging Phenotype: Evidence from Long-Lived Models. Oxidative Med. Cell. Longev. 2015, 2015, 732596. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B.; Bose, S.; McCord, J.M. Phytochemical Combination PB125 Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Antioxidants 2019, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Cabey, K.; Long, D.M.; Law, A.; Gray, N.E.; McClure, C.; Caruso, M.; Lak, P.; Wright, K.M.; Stevens, J.F.; Maier, C.S.; et al. Withania somnifera and Centella Asiatica Extracts Ameliorate Behavioral Deficits in an In Vivo Drosophila Melanogaster Model of Oxidative Stress. Antioxidants 2022, 11, 121. [Google Scholar] [CrossRef]
- Holvoet, H.; Long, D.M.; Law, A.; McClure, C.; Choi, J.; Yang, L.; Marney, L.; Poeck, B.; Strauss, R.; Stevens, J.F.; et al. Withania somnifera Extracts Promote Resilience against Age-Related and Stress-Induced Behavioral Phenotypes in Drosophila Melanogaster; a Possible Role of Other Compounds besides Withanolides. Nutrients 2022, 14, 3923. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, T.; Manchanda, S.; Kaur, G. Intermittent Fasting Combined with Supplementation with Ayurvedic Herbs Reduces Anxiety in Middle Aged Female Rats by Anti-Inflammatory Pathways. Biogerontology 2017, 18, 601–614. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Murshid, A.; Prince, T. The Shock of Aging: Molecular Chaperones and the Heat Shock Response in Longevity and Aging—A Mini-Review. Gerontology 2009, 55, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-Reactive Protein, Successful Aging, and Mortality: The PolSenior Study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef]
- Kirwan, J.P.; Krishnan, R.K.; Weaver, J.A.; Del Aguila, L.F.; Evans, W.J. Human Aging Is Associated with Altered TNF-α Production during Hyperglycemia and Hyperinsulinemia. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1137–E1143. [Google Scholar] [CrossRef]
- Böni-Schnetzler, M.; Méreau, H.; Rachid, L.; Wiedemann, S.J.; Schulze, F.; Trimigliozzi, K.; Meier, D.T.; Donath, M.Y. IL-1beta Promotes the Age-Associated Decline of Beta Cell Function. iScience 2021, 24, 103250. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Mishra, R.K. Withania somnifera Ameliorates Sexual Arousal and Impotence in Stressed Sexually Sluggish Male Rats by Modulating Neurotransmitters and NO/cGMP/PDE5α Pathway. J. Ethnopharmacol. 2024, 318, 116971. [Google Scholar] [CrossRef]
- Musicki, B.; Burnett, A.L. eNOS Function and Dysfunction in the Penis. Exp. Biol. Med. 2006, 231, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, M.R.; Crump, A.; Shi-Wen, X.; Loesch, A. Identification of Neuronal Nitric Oxide Synthase (nNOS) in Human Penis: A Potential Role of Reduced Neuronally-Derived Nitric Oxide in Erectile Dysfunction. Curr. Pharm. Biotechnol. 2011, 12, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mahdi, A.A.; Shukla, K.K.; Ahmad, M.K.; Bansal, N.; Sankhwar, P.; Sankhwar, S.N. Efficacy of Withania somnifera on Seminal Plasma Metabolites of Infertile Males: A Proton NMR Study at 800MHz. J. Ethnopharmacol. 2013, 149, 208–214. [Google Scholar] [CrossRef]
- Kukkemane, K.; Jagota, A. Therapeutic Effects of Hydro-Alcoholic Leaf Extract of Withania somnifera on Age-Induced Changes in Daily Rhythms of Sirt1, Nrf2 and Rev-Erbα in the SCN of Male Wistar Rats. Biogerontology 2020, 21, 593–607. [Google Scholar] [CrossRef]
- Sikandan, A.; Shinomiya, T.; Nagahara, Y. Ashwagandha Root Extract Exerts Anti-inflammatory Effects in HaCaT Cells by Inhibiting the MAPK/NF-κB Pathways and by Regulating Cytokines. Int. J. Mol. Med. 2018, 42, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The Role of Interleukin-8 in Inflammation and Mechanisms of Regulation. J. Periodontol. 1993, 64, 456–460. [Google Scholar] [PubMed]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Kuo, P.-H.; Liu, Y.-L.; Yang, A.C.; Tsai, S.-J. Association and Interaction Effects of Interleukin-12 Related Genes and Physical Activity on Cognitive Aging in Old Adults in the Taiwanese Population. Front. Neurol. 2019, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Ineffective Levels of Transforming Growth Factors and Their Receptor Account for Old Age Being a Risk Factor for Alzheimer’s Disease. Alzheimers Dement. 2019, 5, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Andronie-Cioara, F.L.; Ardelean, A.I.; Nistor-Cseppento, C.D.; Jurcau, A.; Jurcau, M.C.; Pascalau, N.; Marcu, F. Molecular Mechanisms of Neuroinflammation in Aging and Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2023, 24, 1869. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Kaur, G. Withania somnifera as a Potential Anxiolytic and Anti-Inflammatory Candidate Against Systemic Lipopolysaccharide-Induced Neuroinflammation. Neuromol Med 2018, 20, 343–362. [Google Scholar] [CrossRef]
- Gupta, M.; Kaur, G. Aqueous Extract from the Withania somnifera Leaves as a Potential Anti-Neuroinflammatory Agent: A Mechanistic Study. J. Neuroinflamm. 2016, 13, 193. [Google Scholar] [CrossRef]
- Atluri, V.S.R.; Tiwari, S.; Rodriguez, M.; Kaushik, A.; Yndart, A.; Kolishetti, N.; Yatham, M.; Nair, M. Inhibition of Amyloid-Beta Production, Associated Neuroinflammation, and Histone Deacetylase 2-Mediated Epigenetic Modifications Prevent Neuropathology in Alzheimer’s Disease in Vitro Model. Front. Aging Neurosci. 2020, 11, 342. [Google Scholar] [CrossRef]
- Zhu, J.; Park, S.; Jeong, K.H.; Kim, W.-J. Withanolide-A Treatment Exerts a Neuroprotective Effect via Inhibiting Neuroinflammation in the Hippocampus after Pilocarpine-Induced Status Epilepticus. Epilepsy Res. 2020, 165, 106394. [Google Scholar] [CrossRef]
- Gupta, M.; Kaur, G. Withania somnifera (L.) Dunal Ameliorates Neurodegeneration and Cognitive Impairments Associated with Systemic Inflammation. BMC Complement. Altern. Med. 2019, 19, 217. [Google Scholar] [CrossRef] [PubMed]
- Costa, G.; Serra, M.; Maccioni, R.; Casu, M.A.; Kasture, S.B.; Acquas, E.; Morelli, M. Withania somnifera Influences MDMA-Induced Hyperthermic, Cognitive, Neurotoxic and Neuroinflammatory Effects in Mice. Biomed. Pharmacother. 2023, 161, 114475. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.S.; Shah, V.D.; Baker, S.L.; Vogel, J.W.; O’Neil, J.P.; Janabi, M.; Schwimmer, H.D.; Marks, S.M.; Jagust, W.J. Aging Affects Dopaminergic Neural Mechanisms of Cognitive Flexibility. J. Neurosci. 2016, 36, 12559–12569. [Google Scholar] [CrossRef] [PubMed]
- Faniyi, A.A.; Hughes, M.J.; Scott, A.; Belchamber, K.B.R.; Sapey, E. Inflammation, Ageing and Diseases of the Lung: Potential Therapeutic Strategies from Shared Biological Pathways. Br. J Pharmacol. 2022, 179, 1790–1807. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, N.; Samuel, S.S.; Bora, H.K.; Sharma, S.; Pachauri, S.D.; Dwivedi, A.K.; Siddiqui, H.H.; Hanif, K. Withania somnifera Shows a Protective Effect in Monocrotaline-Induced Pulmonary Hypertension. Pharm. Biol. 2015, 53, 147–157. [Google Scholar] [CrossRef] [PubMed]
- IL-10 Prevents Aging-associated Inflammation and Insulin Resistance in Skeletal Muscle—Dagdeviren—2017—The FASEB Journal—Wiley Online Library. Available online: https://faseb.onlinelibrary.wiley.com/doi/abs/10.1096/fj.201600832R (accessed on 25 April 2024).
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Chung, Y.-P.; Liu, C.-H.; Huang, K.-T.; Guan, S.-S.; Chiang, C.-K.; Wu, C.-T.; Liu, S.-H. Withaferin A Protects against Endoplasmic Reticulum Stress-Associated Apoptosis, Inflammation, and Fibrosis in the Kidney of a Mouse Model of Unilateral Ureteral Obstruction. Phytomedicine 2020, 79, 153352. [Google Scholar] [CrossRef] [PubMed]
- Hunt, N.J.; Kang, S.W.; Lockwood, G.P.; Le Couteur, D.G.; Cogger, V.C. Hallmarks of Aging in the Liver. Comput. Struct. Biotechnol. J. 2019, 17, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, P.; Yan, N.; Gonzalez, F.J.; Yan, T. Withaferin A Alleviates Fulminant Hepatitis by Targeting Macrophage and NLRP3. Cell Death Dis. 2021, 12, 174. [Google Scholar] [CrossRef]
- The Role of NLRP3 Inflammasome in Aging and Age-Related Diseases—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/38317229/ (accessed on 25 April 2024).
- Im, S.-S.; Osborne, T.F. Liver X Receptors in Atherosclerosis and Inflammation. Circ. Res. 2011, 108, 996–1001. [Google Scholar] [CrossRef]
- Shiragannavar, V.D.; Gowda, N.G.S.; Kumar, D.P.; Mirshahi, F.; Santhekadur, P.K. Withaferin A Acts as a Novel Regulator of Liver X Receptor-α in HCC. Front. Oncol. 2021, 10, 628506. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Chen, C.; Wang, J.; Chen, T.; Yao, W.; Yan, T.; Liu, Z. Withaferin A Exerts Preventive Effect on Liver Fibrosis through Oxidative Stress Inhibition in a Sirtuin 3-Dependent Manner. Oxidative Med. Cell. Longev. 2020, 2020, 2452848. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, S.E.; Guo, H.; Fedarko, N.; DeZern, A.; Fried, L.P.; Xue, Q.-L.; Leng, S.; Beamer, B.; Walston, J.D. Glutathione Peroxidase Enzyme Activity in Aging. J. Gerontol. Biol. Sci. Med. Sci. 2008, 63, 505–509. [Google Scholar] [CrossRef]
- Devkar, S.T.; Kandhare, A.D.; Zanwar, A.A.; Jagtap, S.D.; Katyare, S.S.; Bodhankar, S.L.; Hegde, M.V. Hepatoprotective Effect of Withanolide-Rich Fraction in Acetaminophen-Intoxicated Rat: Decisive Role of TNF-α, IL-1β, COX-II and iNOS. Pharm. Biol. 2016, 54, 2394–2403. [Google Scholar] [CrossRef] [PubMed]
- Abu Bakar, M.H.; Azmi, M.N.; Shariff, K.A.; Tan, J.S. Withaferin A Protects Against High-Fat Diet–Induced Obesity Via Attenuation of Oxidative Stress, Inflammation, and Insulin Resistance. Appl. Biochem. Biotechnol. 2019, 188, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Available online: https://www.hindawi.com/journals/omcl/2019/9613090/ (accessed on 25 April 2024).
- Weschawalit, S.; Thongthip, S.; Phutrakool, P.; Asawanonda, P. Glutathione and Its Antiaging and Antimelanogenic Effects. Clin. Cosmet. Investig. Dermatol. 2017, 10, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tiruveedi, V.L.; Bale, S.; Khurana, A.; Godugu, C. Withaferin A, a Novel Compound of Indian Ginseng (Withania somnifera), Ameliorates Cerulein-Induced Acute Pancreatitis: Possible Role of Oxidative Stress and Inflammation: Withaferin a Ameliorates Acute Pancreatitis. Phytother. Res. 2018, 32, 2586–2596. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Onder, G.; Leeuwenburgh, C.; Carter, C.; Marzetti, E.; Russo, A.; Capoluongo, E.; Pahor, M.; Bernabei, R.; Landi, F. Myeloperoxidase Levels and Mortality in Frail Community-Living Elderly Individuals. J. Gerontol. Biol. Sci. Med. Sci. 2010, 65A, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Ahmed, R.S.; Chandra, N.; Arora, V.K.; Ali, A. In Vivo, Extract from Withania somnifera Root Ameliorates Arthritis via Regulation of Key Immune Mediators of Inflammation in Experimental Model of Arthritis. AIAAMC 2019, 18, 55–70. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating In Vitro DNA Damage Using Comet Assay. JoVE 2017, 128, e56450. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA Damage, Repair, and Mutagenesis. Env. Mol Mutagen 2017, 58, 235–263. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130. [Google Scholar] [CrossRef]
- White, R.R.; Milholland, B.; de Bruin, A.; Curran, S.; Laberge, R.-M.; van Steeg, H.; Campisi, J.; Maslov, A.Y.; Vijg, J. Controlled Induction of DNA Double-Strand Breaks in the Mouse Liver Induces Features of Tissue Ageing. Nat. Commun. 2015, 6, 6790. [Google Scholar] [CrossRef] [PubMed]
- Chengappa, K.N.R.; Brar, J.S.; Gannon, J.M.; Schlicht, P.J. Adjunctive Use of a Standardized Extract of Withania somnifera (Ashwagandha) to Treat Symptom Exacerbation in Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Psychiatry 2018, 79, 17m11826. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A.; Srikanth, N.; Patwardhan, B. Withania somnifera as a Safer Option to Hydroxychloroquine in the Chemoprophylaxis of COVID-19: Results of Interim Analysis. Complement. Ther. Med. 2021, 62, 102768. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Vani, G.P.A.; Singh, C.; Purvia, R.P.; Adlakha, M. A Review Article on Aswagandha (Withania somnifera)—The Natural Immunity Booster. IRJAY 2022, 05, 104–109. [Google Scholar] [CrossRef]
- Tiwari, R.; Chakraborty, S.; Saminathan, M.; Dhama, K.; Singh, S. Ashwagandha (Withania somnifera): Role in Safegaurding Health, Immunomodulatory Effects, Combating Infections and Therapeutic Applications: A Review. J. Biol. Sci. 2014, 14, 77–94. [Google Scholar] [CrossRef]
- Tharakan, A.; Shukla, H.; Benny, I.R.; Tharakan, M.; George, L.; Koshy, S. Immunomodulatory Effect of Withania somnifera (Ashwagandha) Extract—A Randomized, Double-Blind, Placebo Controlled Trial with an Open Label Extension on Healthy Participants. JCM 2021, 10, 3644. [Google Scholar] [CrossRef]
- Challacombe, S.J.; Percival, R.S.; Marsh, P.D. Age-Related Changes in Immunoglobulin Isotypes in Whole and Parotid Saliva and Serum in Healthy Individuals. Oral Microbiol. Immunol. 1995, 10, 202–207. [Google Scholar] [CrossRef]
- Yen, C.J.; Lin, S.L.; Huang, K.T.; Lin, R.H. Age-Associated Changes in Interferon-Gamma and Interleukin-4 Secretion by Purified Human CD4+ and CD8+ T Cells. J. Biomed Sci. 2000, 7, 317–321. [Google Scholar] [CrossRef]
- Yalcin, A.D.; Gorczynski, R.M.; Kahraman, M.S.; Demirel, M.U.; Terzioglu, E. CD40, CD45 CTLA-4 Levels Are Elevated in Healthy Older Adults. Clin. Lab 2012, 58, 449–456. [Google Scholar] [PubMed]
- Priyanka, G.; Anil Kumar, B.; Lakshman, M.; Manvitha, V.; Kala Kumar, B. Adaptogenic and Immunomodulatory Activity of Ashwagandha Root Extract: An Experimental Study in an Equine Model. Front. Vet. Sci. 2020, 7, 541112. [Google Scholar] [CrossRef] [PubMed]
- Mikolai, J.; Erlandsen, A.; Murison, A.; Brown, K.A.; Gregory, W.L.; Raman-Caplan, P.; Zwickey, H.L. In Vivo Effects of Ashwagandha (Withania somnifera) Extract on the Activation of Lymphocytes. J. Altern. Complement. Med. 2009, 15, 423–430. [Google Scholar] [CrossRef]
- Ziauddin, M.; Phansalkar, N.; Patki, P.; Diwanay, S.; Patwardhan, B. Studies on the Immunomodulatory Effects of Ashwagandha. J. Ethnopharmacol. 1996, 50, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.I.; Salazar, A.; Yamada, R.; Fitz-Gibbon, S.; Morselli, M.; Alcaraz, J.; Rana, A.; Rera, M.; Pellegrini, M.; Ja, W.W.; et al. Distinct Shifts in Microbiota Composition during Drosophila Aging Impair Intestinal Function and Drive Mortality. Cell Rep. 2015, 12, 1656–1667. [Google Scholar] [CrossRef]
- Conley, M.N.; Wong, C.P.; Duyck, K.M.; Hord, N.; Ho, E.; Sharpton, T.J. Aging and Serum MCP-1 Are Associated with Gut Microbiome Composition in a Murine Model. PeerJ 2016, 4, e1854. [Google Scholar] [CrossRef]
- Purushotham, P.M.; Kim, J.; Jo, E.; Senthil, K. Withanolides against TLR4-Activated Innate Inflammatory Signalling Pathways: A Comparative Computational and Experimental Study. Phytother. Res. 2017, 31, 152–163. [Google Scholar] [CrossRef]
- Wong, M.K. COVID-19 Mortality and Progress Toward Vaccinating Older Adults—World Health Organization, Worldwide, 2020–2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Rea, I.M.; Alexander, H.D. Triple Jeopardy in Ageing: COVID-19, Co-Morbidities and Inflamm-Ageing. Ageing Res. Rev. 2022, 73, 101494. [Google Scholar] [CrossRef]
- Akbar, A.N.; Gilroy, D.W. Aging Immunity May Exacerbate COVID-19. Science 2020, 369, 256–257. [Google Scholar] [CrossRef]
- Bonafè, M.; Prattichizzo, F.; Giuliani, A.; Storci, G.; Sabbatinelli, J.; Olivieri, F. Inflamm-Aging: Why Older Men Are the Most Susceptible to SARS-CoV-2 Complicated Outcomes. Cytokine Growth Factor Rev. 2020, 53, 33–37. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Oh, J.H.; Kwon, T.K. Withaferin A Inhibits Tumor Necrosis Factor-α-Induced Expression of Cell Adhesion Molecules by Inactivation of Akt and NF-κB in Human Pulmonary Epithelial Cells. Int. Immunopharmacol. 2009, 9, 614–619. [Google Scholar] [CrossRef]
- Piao, L.; Canguo, Z.; Wenjie, L.; Xiaoli, C.; Wenli, S.; Li, L. Lipopolysaccharides-Stimulated Macrophage Products Enhance Withaferin A-Induced Apoptosis via Activation of Caspases and Inhibition of NF-κB Pathway in Human Cancer Cells. Mol. Immunol. 2017, 81, 92–101. [Google Scholar] [CrossRef]
- Davis, L.; Kuttan, G. Effect of Withania somnifera On Cytokine Production in Nol and Cyclophosphamide Treated Mice. Immunopharmacol. Immunotoxicol. 1999, 21, 695–703. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Zarandi, P.K.; Ghiasi, M.; Kooshki, H.; Mohammadi, M.; Amani, J.; Rezaei, N. Immunosenescence and Inflamm-Ageing in COVID-19. Ageing Res. Rev. 2023, 84, 101818. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Pokhrel, S.; Singh, H.; Joshi, M.; Mulay, V.P.; Haldar, S.; Varshney, A. Withanone from Withania somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model. DDDT 2021, 15, 1111–1133. [Google Scholar] [CrossRef]
- STRING: Functional Protein Association Networks. Available online: https://string-db.org/ (accessed on 25 December 2023).
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering Workflow-Based Network Analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Panda, V.; Deshmukh, A.; Hare, A.; Singh, S.; Hingorani, L.; Sudhamani, S. Effect of Withania somnifera Hydroalcoholic Extract and Other Dietary Interventions in Improving Muscle Strength in Aging Rats. J. Ayurveda Integr. Med. 2021, 12, 623–632. [Google Scholar] [CrossRef]
- Gupta, S.K.; Dua, A.; Vohra, B.P.S. Withania somnifera (Ashwagandha) Attenuates Antioxidant Defense in Aged Spinal Cord and Inhibits Copper Induced Lipid Peroxidation and Protein Oxidative Modifications. Drug Metab. Drug Interact. 2003, 19, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Sanap, A.; Chandravanshi, B.; Shah, T.; Tillu, G.; Dhanushkodi, A.; Bhonde, R.; Joshi, K. Herbal Pre-Conditioning Induces Proliferation and Delays Senescence in Wharton’s Jelly Mesenchymal Stem Cells. Biomed. Pharmacother. 2017, 93, 772–778. [Google Scholar] [CrossRef] [PubMed]
- Naß, J.; Abdelfatah, S.; Efferth, T. Induction of Stress Resistance and Extension of Lifespan in Chaenorhabditis Elegans Serotonin-Receptor Knockout Strains by Withanolide A. Phytomedicine 2021, 84, 153482. [Google Scholar] [CrossRef] [PubMed]
- Widodo, N.; Shah, N.; Priyandoko, D.; Ishii, T.; Kaul, S.C.; Wadhwa, R. Deceleration of Senescence in Normal Human Fibroblasts by Withanone Extracted From Ashwagandha Leaves. J. Gerontol. Ser. Biol. Sci. Med. Sci. 2009, 64A, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Mácsai, L.; Datki, Z.L.; Csupor, D.; Horváth, A.; Zomborszki, Z.P. Biological Activities of Four Adaptogenic Plant Extracts and Their Active Substances on a Rotifer Model. Evid. -Based Complement. Altern. Med. 2018, 2018, 3690683. [Google Scholar] [CrossRef]
- Panossian, A.; Seo, E.-J.; Efferth, T. Novel Molecular Mechanisms for the Adaptogenic Effects of Herbal Extracts on Isolated Brain Cells Using Systems Biology. Phytomedicine 2018, 50, 257–284. [Google Scholar] [CrossRef]
- Salvadori, L.; Belladonna, M.L.; Castiglioni, B.; Paiella, M.; Panfili, E.; Manenti, T.; Ercolani, C.; Cornioli, L.; Chiappalupi, S.; Gentili, G.; et al. KYMASIN UP Natural Product Inhibits Osteoclastogenesis and Improves Osteoblast Activity by Modulating Src and P38 MAPK. Nutrients 2022, 14, 3053. [Google Scholar] [CrossRef]
- De Rose, F.; Marotta, R.; Talani, G.; Catelani, T.; Solari, P.; Poddighe, S.; Borghero, G.; Marrosu, F.; Sanna, E.; Kasture, S.; et al. Differential Effects of Phytotherapic Preparations in the hSOD1 Drosophila Melanogaster Model of ALS. Sci. Rep. 2017, 7, 41059. [Google Scholar] [CrossRef]
- Akhoon, B.A.; Rathor, L.; Pandey, R. Withanolide A Extends the Lifespan in Human EGFR-Driven Cancerous Caenorhabditis Elegans. Exp. Gerontol. 2018, 104, 113–117. [Google Scholar] [CrossRef]
- Konar, A.; Gupta, R.; Shukla, R.K.; Maloney, B.; Khanna, V.K.; Wadhwa, R.; Lahiri, D.K.; Thakur, M.K. M1 Muscarinic Receptor Is a Key Target of Neuroprotection, Neuroregeneration and Memory Recovery by i-Extract from Withania somnifera. Sci. Rep. 2019, 9, 13990. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Bhattacharya, A.; Sairam, K.; Ghosal, S. Anxiolytic-Antidepressant Activity of Withania somnifera Glycowithanolides: An Experimental Study. Phytomedicine 2000, 7, 463–469. [Google Scholar] [CrossRef] [PubMed]
- De Rose, F.; Marotta, R.; Poddighe, S.; Talani, G.; Catelani, T.; Setzu, M.D.; Solla, P.; Marrosu, F.; Sanna, E.; Kasture, S.; et al. Functional and Morphological Correlates in the Drosophila LRRK2 Loss-of-Function Model of Parkinson’s Disease: Drug Effects of Withania somnifera (Dunal) Administration. PLoS ONE 2016, 11, e0146140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gupta, K.; Saharia, K.; Pradhan, D.; Subramaniam, J.R. Withania somnifera Root Extract Extends Lifespan of Caenorhabditis Elegans. Ann. Neurosci. 2013, 20, 13–16. [Google Scholar] [CrossRef]
- Raguraman, V.; Subramaniam, J.R. Withania somnifera Root Extract Enhances Telomerase Activity in the Human HeLa Cell Line. ABB 2016, 07, 199–204. [Google Scholar] [CrossRef]
- Kaur, J.; Seshadri, S.; Golla, K.H.; Sampara, P. Efficacy and Safety of Standardized Ashwagandha (Withania somnifera) Root Extract on Reducing Stress and Anxiety in Domestic Dogs: A Randomized Controlled Trial. J. Vet. Behav. 2022, 51, 8–15. [Google Scholar] [CrossRef]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and Safety of Ashwagandha Root Extract in Subclinical Hypothyroid Patients: A Double-Blind, Randomized Placebo-Controlled Trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Gupta, S.K.; Tiwari, S.; Mishra, A.K. Safety of Ashwagandha Root Extract: A Randomized, Placebo-Controlled, Study in Healthy Volunteers. Complement. Ther. Med. 2021, 57, 102642. [Google Scholar] [CrossRef]
- Langade, D.; Thakare, V.; Kanchi, S.; Kelgane, S. Clinical Evaluation of the Pharmacological Impact of Ashwagandha Root Extract on Sleep in Healthy Volunteers and Insomnia Patients: A Double-Blind, Randomized, Parallel-Group, Placebo-Controlled Study. J. Ethnopharmacol. 2021, 264, 113276. [Google Scholar] [CrossRef]
- Peñas-LLedó, E.; Terán, E.; Sosa-Macías, M.; Galaviz-Hernández, C.; Gil, J.-P.; Nair, S.; Diwakar, S.; Hernández, I.; Lara-Riegos, J.; Ramírez-Roa, R.; et al. Challenges and Opportunities for Clinical Pharmacogenetic Research Studies in Resource-Limited Settings: Conclusions From the Council for International Organizations of Medical Sciences–Ibero-American Network of Pharmacogenetics and Pharmacogenomics Meeting. Clin. Ther. 2020, 42, 1595–1610.e5. [Google Scholar] [CrossRef]
- ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ (accessed on 26 December 2023).
- ANZCTR. Available online: https://www.anzctr.org.au/ (accessed on 26 December 2023).
- International Clinical Trials Registry Platform (ICTRP). Available online: https://www.who.int/clinical-trials-registry-platform (accessed on 26 December 2023).
- Harris, M. The Impact of Ashwagandha on Stress, Sleep and Food Cravings in College Students: A Mixed Method Double-Blinded Randomized Control Trial. 2022. Available online: https://clinicaltrials.gov/study/NCT05430685?intr=ashwagandha&rank=2 (accessed on 26 December 2023).
- Behl, D. A Randomized Placebo-Controlled Trial of Ashwagandha (Withania somnifera) for Cognitive Dysfunction Associated with Cancer Chemotherapy. 2023. Available online: https://clinicaltrials.gov/study/NCT04092647?intr=ashwagandha&rank=5 (accessed on 26 December 2023).
- Kulkarni, D.N.S. Effect of Ashwagandha (Withania somnifera) as an Adjunct to Scaling and Root Planing on Salivary Antioxidant and Serum c Reactive Protein Levels in Chronic Generalized Periodontitis—A Randomized Double Blind Clinico-Biochemical Trial. 2018. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03533972 (accessed on 26 December 2023).
- NCT04092647. The Effects of Ashwagandha in Endurance Exercise Performance. 2018. Available online: https://clinicaltrials.gov/study/NCT03596307?intr=ashwagandha&rank=9 (accessed on 26 December 2023).
- Abrahao Agessandro Nuclear Factor Kappa Beta Inhibition in Patients with Amyotrophic Lateral Sclerosis: A Phase II Randomized Placebo Controlled Trial. 2022. Available online: https://clinicaltrials.gov/study/NCT05031351?intr=ashwagandha&limit=100&page=1&rank=11 (accessed on 26 December 2023).
- Chengappa, K.N.R. Sensoril® (Ashwagandha), an Immunomodulator and Anti-Inflammatory Agent for Schizophrenia: A Parallel Group, Randomized Double Blind, and Placebo Controlled Study. 2017. Available online: https://clinicaltrials.gov/study/NCT01793935?intr=ashwagandha&limit=100&page=1&rank=14 (accessed on 26 December 2023).
- Chengappa, K.N.R. Sensoril® (Ashwagandha)—A Standardized Extract From a Medicinal Plant—(Withania somnifera) for Cognitive Enhancement in Persons with Bipolar Disorder: A Parallel Group, Randomized Double Blind, and Placebo Controlled Study. 2016. Available online: https://clinicaltrials.gov/study/NCT00761761?intr=ashwagandha&limit=100&page=1&rank=16 (accessed on 26 December 2023).
- Marder, S. Adjunctive Withania somnifera (Ashwagandha) for Persistent Symptoms in People with Schizophrenia. 2023. Available online: https://clinicaltrials.gov/study/NCT03437668?intr=ashwagandha&limit=100&page=1&rank=15 (accessed on 26 December 2023).
- Natreon, Inc. A Phase II Double-Blind, Parallel Group, Randomized, Placebo Controlled Clinical Trial of Sensoril® for Patients With Generalized Anxiety Disorder. 2015. Available online: https://clinicaltrials.gov/study/NCT01311180?intr=ashwagandha&limit=100&page=1&rank=17 (accessed on 26 December 2023).
- Goldsberry Whitney Combination Therapy with Liposomal Doxorubicin and Withaferin A (Ashwagandha, ASWD) in Recurrent Ovarian Cancer. 2023. Available online: https://clinicaltrials.gov/study/NCT05610735?intr=ashwagandha&limit=100&page=1&rank=18 (accessed on 26 December 2023).
- Peters Warren Functional Assessment of Ashwagandaha Root Extract during Weight Loss. 2023. Available online: https://clinicaltrials.gov/study/NCT03112824?intr=ashwagandha&limit=100&page=1&rank=19 (accessed on 26 December 2023).
- El-Khodor Bassem A Randomized, Single-Blind, Placebo-Controlled Trial for the Role of a Dietary Supplement in Lowering S-Adenosylhomocysteine (SAH) in Healthy Adults With Elevated Plasma SAH and Normal Homocysteine Levels and Identification of Participants with Elevated Plasma SAH in the General Population Using the MethylQ Score. 2023. Available online: https://clinicaltrials.gov/study/NCT05994794?intr=ashwagandha&limit=100&page=1&rank=20 (accessed on 26 December 2023).
- University of Roma La Sapienza Effect on Weight Loss of an Oral Association of Cinnamon Bark (Cinnamomum Cassia) and Withania somnifera in Adult Patients with Overweight or Obesity: A Randomized, Prospective, Placebo-Controlled, Multicenter, Cross-Over, Pilot Study. 2022. Available online: https://clinicaltrials.gov/study/NCT05210218?intr=ashwagandha&limit=100&page=1&rank=21 (accessed on 26 December 2023).
- Vedic Lifesciences Pvt Ltd. Efficacy and Tolerability of Sensoril® in Improving Immunity and Thereby Reducing Incidence of Upper Respiratory Tract Infections; 2021. Available online: https://clinicaltrials.gov/study/NCT04733924?intr=ashwagandha&limit=100&page=1&rank=23 (accessed on 26 December 2023).
- Koch Klaus Effects of an Adaptogenic Extract on Electrical Activity of Brain in Elderly Subjects with Cognitive Impairment: A Randomized, Double Blind, Placebo-Controlled, Two Arms Cross-over Study. 2019. Available online: https://clinicaltrials.gov/study/NCT03780621?intr=ashwagandha&limit=100&page=1&rank=25 (accessed on 26 December 2023).
- Practitioners Alliance Network Treatment of Fibromyalgia and CFS With Ribose, Ashwagandha, Rhodiola, Licorice, Schisandra and Green Tea Extract. 2020. Available online: https://clinicaltrials.gov/study/NCT04598243?intr=ashwagandha&limit=100&page=1&rank=26 (accessed on 26 December 2023).
- Kulkarni Vishwesh a Community-Based Participatory Research to Assess the Feasibility of Ayurveda Intervention in Patients with Mild-to-Moderate COVID-19. 2021. Available online: https://clinicaltrials.gov/study/NCT04716647?intr=ashwagandha&limit=100&page=1&rank=27 (accessed on 26 December 2023).
- Srivastava Shalini A Randomized, Double Blind, Placebo Controlled, Parallel Group Study to Assess the Effect of Multi-Herb Formulae VL-G-A57 and an Ashwagandha Root Formula (VL-G-E12) on the Modulation of the Hypothalamic-Pituitary-Adrenal Axis (HPA Axis) and Related Symptoms. 2023. Available online: https://clinicaltrials.gov/study/NCT05602389?intr=ashwagandha&limit=100&page=1&rank=29 (accessed on 26 December 2023).
- Eckel Robert Protandim and the Metabolic Syndrome. Available online: https://clinicaltrials.gov/study/NCT01125501?intr=ashwagandha&rank=27 (accessed on 17 December 2023).
- Srivastava Shalini To Assess the Lanconone® (E-OA-07) Efficacy in Physical Activity-Related Pain-LEAP Study. 2018. Available online: https://clinicaltrials.gov/study/NCT03262805?intr=ashwagandha&limit=100&page=1&rank=32 (accessed on 26 December 2023).
- Patil Sandip Assessment of the Effect of StemAlive® Herbal Supplement on the Levels of Circulating Hematopoietic Stem Cells in Human Volunteers. 2014. Available online: https://clinicaltrials.gov/study/NCT02027467?intr=ashwagandha&limit=100&page=1&rank=33 (accessed on 26 December 2023).
- Zavorsky, G.S. The Effect of Protandim Supplementation on Oxidative Damage and Athletic Performance. 2019. Available online: https://clinicaltrials.gov/study/NCT02172625?intr=ashwagandha&limit=100&page=1&rank=35 (accessed on 26 December 2023).
- Vaisman Nachum Study Details|Study to Assess the Efficacy of Cognitex|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT00719953?intr=ashwagandha&rank=31 (accessed on 17 December 2023).
- Nutraceutical Wellness Inc. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety and Efficacy of a Nutraceutical Supplement with Standardized Botanicals in Males with Self-Perceived Thinning Hair. 2023. Available online: https://clinicaltrials.gov/study/NCT05339958?intr=ashwagandha&limit=100&page=1&rank=38 (accessed on 26 December 2023).
- Ramon Adi Randomized Double Blind Placebo-Controlled Trial to Investigate the Effect of a Botanical Formulation, LLP-01, on Proteomic Inflammatory Biomarkers and Epigenetic Changes. 2023. Available online: https://clinicaltrials.gov/study/NCT06065241?intr=ashwagandha&limit=100&page=1&rank=41 (accessed on 26 December 2023).
- Bhat Dr Shreepad Study the Result of Ayurvedic SUVED & Reimmugen (Colostrum) Treatment on Vascular Disease, CAD, CVA, DVT. (SHARP). 2017. Available online: https://clinicaltrials.gov/study/NCT02920125?intr=ashwagandha&limit=100&page=1&rank=43 (accessed on 26 December 2023).
- SF Research Institute, Inc. Effects of Ashwagandha Extract (Capsule KSM-66 300 Mg) on Sexual Health in Healthy Women: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. 2023. Available online: https://clinicaltrials.gov/study/NCT05831241?intr=ashwagandha&limit=100&page=1&rank=4 (accessed on 26 December 2023).
- SF Research Institute, Inc. Role of Ashwagandha Extract (Capsule KSM-66 300 Mg) in Improving Sexual Health in Healthy Men: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. 2023. Available online: https://clinicaltrials.gov/study/NCT05840731?intr=ashwagandha&limit=100&page=1&rank=3 (accessed on 26 December 2023).
- Steels, E. A Randomised Placebo-Controlled Trial Investigating Effectiveness of Two Herbal Combinations for Short-Term Stress Management in Healthy Adults; Australian New Zealand Clinical Trials Registry: Camperdown, NSW, Australia, 2023. [Google Scholar]
- Lauche, R. Feasibility of Herbal and Nutritional Medicines for Managing Post-Flood Stress and Anxiety: A Randomised Controlled Trial; Australian New Zealand Clinical Trials Registry: Camperdown, NSW, Australia, 2022. [Google Scholar]
- Ee, C. Effectiveness of Adjunct Naturopathy for Pregnancy Rates in Women with Diminished Ovarian Reserve Compared to Usual Care Alone: Feasibility of a Randomised Controlled Trial; Australian New Zealand Clinical Trials Registry: Camperdown, NSW, Australia, 2021. [Google Scholar]
- Lopresti, A. Examining the Efficacy and Safety of a Novel Standardised Ashwagandha (Withania somnifera) Root Extract in Overweight Middle-to-Older Age Adults Experiencing High Stress and Fatigue: A Randomised, Double-Blind, Placebo-Controlled Trial; Australian New Zealand Clinical Trials Registry: Camperdown, NSW, Australia, 2021. [Google Scholar]
- Drummond, P. Effect of Ashwagandha Supplementation on Testosterone Levels and Vitality in Healthy, Overweight Males with Mild to Moderate Symptoms of Fatigue or Reduced Vitality—A Randomised, Double-Blind, Placebo-Controlled Study; Australian New Zealand Clinical Trials Registry: Camperdown, NSW, Australia, 2017. [Google Scholar]
- Downey, L. Examining the Effects of Ionix Supreme on Stress, Mood, Energy and Anxiety in Healthy Younger Adults; Australian New Zealand Clinical Trials Registry: Camperdown, NSW, Australia, 2017. [Google Scholar]
- Rao, A. A Prospective Double Blinded, Randomised, Placebo-Controlled Study to Evaluate Safety and Efficacy of Two (2) Herbal Formulations in Reducing Menopausal Symptoms in Otherwise Healthy Women; Australian New Zealand Clinical Trials Registry: Camperdown, NSW, Australia, 2015. [Google Scholar]
- Multi-disciplinary Research Unit—GSMC A Research Study to Investigate Ashwagandha as a Supplementary Therapy for Treatment of Alcohol Use Disorder. 2022. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2022/11/047340 (accessed on 26 December 2023).
- Dr. Willmar Schwabe India Pvt. Ltd. A Clinical Trial to Study the Effects of Ashwagandha in Patients with Stress and Anxiety Having Cardiovascular Comorbidities. 2023. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/07/054711 (accessed on 26 December 2023).
- Sami Sabinsa Group Limited Ashwagandha and Selenium Combination as a Supplement in Patients with Subclinical Hypothyroidism. 2023. Available online: https://trialsearch.who.int/Trial2.aspx?TrialID=CTRI/2023/07/054940 (accessed on 26 December 2023).
- Yerram, C.; Jillella, A.; Reddy, V. Effects of Withania somnifera Root Extract Serum Application on Hair Health in Healthy Adults: A Prospective, Double-Blind, Randomized, Parallel, Placebo-Controlled Study. J. Ayurveda Integr. Med. 2023, 14, 100817. [Google Scholar] [CrossRef]
- Choudhary, B.; Shetty, A.; Langade, D. Efficacy of Ashwagandha (Withania somnifera [L.] Dunal) in Improving Cardiorespiratory Endurance in Healthy Athletic Adults. AYU 2015, 36, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, K.; Kapoor, J.; Anishetty, S. A Prospective, Randomized Double-Blind, Placebo-Controlled Study of Safety and Efficacy of a High-Concentration Full-Spectrum Extract of Ashwagandha Root in Reducing Stress and Anxiety in Adults. Indian J. Psychol. Med. 2012, 34, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Ambiye, V.R.; Langade, D.; Dongre, S.; Aptikar, P.; Kulkarni, M.; Dongre, A. Clinical Evaluation of the Spermatogenic Activity of the Root Extract of Ashwagandha (Withania somnifera) in Oligospermic Males: A Pilot Study. Evid. Based Complement. Altern. Med. 2013, 2013, 571420. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, S.; Langade, D.; Joshi, K.; Sinha, S.R.; Bhattacharyya, S. Examining the Effect of Withania somnifera Supplementation on Muscle Strength and Recovery: A Randomized Controlled Trial. J. Int. Soc. Sports Nutr. 2015, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Dongre, S.; Langade, D.; Bhattacharyya, S. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Improving Sexual Function in Women: A Pilot Study. BioMed Res. Int. 2015, 2015, 284154. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body Weight Management in Adults under Chronic Stress through Treatment with Ashwagandha Root Extract: A Double-Blind, Randomized, Placebo-Controlled Trial. J. Evid. Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and Safety of Ashwagandha (Withania somnifera (L.) Dunal) Root Extract in Improving Memory and Cognitive Functions. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Langade, D.; Kanchi, S.; Salve, J.; Debnath, K.; Ambegaokar, D. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-Blind, Randomized, Placebo-Controlled Study. Cureus 2019, 11, e5797. [Google Scholar] [CrossRef] [PubMed]
- Kelgane, S.B.; Salve, J.; Sampara, P.; Debnath, K. Efficacy and Tolerability of Ashwagandha Root Extract in the Elderly for Improvement of General Well-Being and Sleep: A Prospective, Randomized, Double-Blind, Placebo-Controlled Study. Cureus 2020, 12, e7083. [Google Scholar] [CrossRef]
- Gopal, S.; Ajgaonkar, A.; Kanchi, P.; Kaundinya, A.; Thakare, V.; Chauhan, S.; Langade, D. Effect of an Ashwagandha (Withania somnifera) Root Extract on Climacteric Symptoms in Women during Perimenopause: A Randomized, Double-Blind, Placebo-Controlled Study. J. Obstet. Gynaecol. 2021, 47, 4414–4425. [Google Scholar] [CrossRef]
- Chauhan, S.; Srivastava, M.K.; Pathak, A.K. Effect of Standardized Root Extract of Ashwagandha (Withania somnifera) on Well-being and Sexual Performance in Adult Males: A Randomized Controlled Trial. Health Sci. Rep. 2022, 5, e741. [Google Scholar] [CrossRef] [PubMed]
- Ajgaonkar, A.; Jain, M.; Debnath, K. Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract for Improvement of Sexual Health in Healthy Women: A Prospective, Randomized, Placebo-Controlled Study. Cureus 2022, 14, e30787. [Google Scholar] [CrossRef] [PubMed]
- Salve, J.; Pate, S.; Debnath, K.; Langade, D. Adaptogenic and Anxiolytic Effects of Ashwagandha Root Extract in Healthy Adults: A Double-Blind, Randomized, Placebo-Controlled Clinical Study. Cureus 2019, 11, e6466. [Google Scholar] [CrossRef] [PubMed]


| Sr No. | Withania somnifera Source | Preclinical Model | Dose | Comments | References |
|---|---|---|---|---|---|
| 1 | Withania somnifera water-soluble extract | Normal Rat kidney Epithelial cells | 1:1000 dilution of Withania somnifera capsule containing 2.5% of withanolide | Withania somnifera water-soluble extract prevented TNF-α-induced CCL5 increase, reduced CCL2 expression, and inhibited NF-κB activation; also effective against lipopolysaccharide-induced inflammation and aging-related disorders | [53] |
| 2 | Withania somnifera hydro-alcoholic extract | Male Sprague Dawley rats | 500 mg/kg orally (for 60 days) | Withania somnifera hydro-alcoholic extract decreased glucose, inflammatory markers (IL-6, TNF-α, CRP), AMPK, malondialdehyde, and Bax (downregulation); upregulated Bcl-2, enhancing muscle health in aging. | [145] |
| 3 | Withania somnifera extract | Male albino rats | 50, 100, and 150 mg/kg | Withania somnifera (150 mg/kg) enhances glutathione peroxidase, an antioxidant enzyme, showing potential in protecting against oxidative stress, especially in aging individuals, due to its antioxidant properties | [146] |
| 4 | Withania somnifera root extract | Wharton’s Jelly Mesenchymal Stem Cells | 5, 10, 20, 40, and 50 mg/mL | Withania somnifera root extracts (5 mg/mL) upregulated proliferation marker Ki-67, while downregulating senescence marker p21, enhancing Wharton’s Jelly Mesenchymal Stem Cells growth | [147] |
| 5 | Withanolide A dimethyl sulfoxide Extract | Caenorhabditis elegans | 100 µM | Withanolide A effectively reduced ROS levels, increased stress resistance, and extended lifespan in various C. elegans strains, while upregulating serotonin receptor and transporter genes, including the DA1814 strain | [148] |
| 6 | Withanone dimethyl sulfoxide Extract | Normal human fibroblasts (TIG-1, MRC5, and WI-38) cell line | 5 µg/mL | Withanone protects normal cells, downregulates aging marker p21 WAF1, extends lifespan, reduces molecular damage, and upregulates proteasomal activity, suggesting antiaging potential | [149] |
| 7 | Withania somnifera Ethanol extract | Rotifers (Phylum Rotifera) | 100 µM | Withania somnifera extract contained withaferin A, withanolide A, and withanolide B. Its effects on rotifers included changes in MCF, BSI, and toxicity | [150] |
| 8 | Withania somnifera Root Milk extract | T98G neuroglia cells | 5.0 µg/mL | Adaptogens influenced 88 genes linked to stress responses, including neuronal and melatonin pathways, consistently upregulating key genes such as UCN, GNRH1, and RORA, suggesting stress protection and aging-related disorders | [151] |
| 9 | Withania somnifera Dried root extract | Human dermal fibroblasts | 25, 50, and 100 µM | Withagenin A diglucoside reduces ROS, preserving collagen by inhibiting MMP-1 via MAPK, Akt, c-Jun, COX-2, and NF-κB pathways (downregulation). Withagenin A diglucoside also decreases IL-6 and IL-8 (downregulation), alleviating skin inflammation. These findings suggest Withagenin A diglucoside’s potential in cosmetics and pharmaceuticals for combating skin aging | [55] |
| 10 | Withania somnifera Dried leaf powder extract | Middle-aged female albino rats | 200 mg/kg | Reduced anxiety and inflammation (downregulating Iba1, TNF-α, IL-1β, IL-6) in middle-aged rats, while upregulating astrocyte marker Glial fibrillary acidic protein | [72] |
| 11 | Withania somnifera, Silybum marianum and Trigonella foenum-graecum extract | RAW 264.7, C2C12 myoblasts and Primary human Osteoblast cells | 10 µg/mL to 40 µg/mL | Dietary product of Withania somnifera, Silybum marianum, and Trigonella foenum-graecum extract downregulates TRAP and OC-related genes and inhibits osteoclast formation by downregulating RANK receptor pathways, including Src and p38 MAPK pathways, aiding osteoporosis treatment and bone mineralization | [152] |
| 12 | Withania somnifera Ethanol extract | C2C12 cells | 100 µg/mL | Withania somnifera formulation upregulates MyHC-II, promotes protein synthesis via Akt pathway, and enhances myoblast differentiation through p38 MAPK/myogenin pathway, countering muscle atrophy markers | [61] |
| 13 | Withania somnifera leaf hydro-alcoholic extract | Male Wistar rats | 10 mg/kg | Withania somnifera restores SIRT1 and NRF2 daily rhythms and phases, indicating potential circadian and antioxidant benefits in aging | [81] |
| 14 | Withania somnifera extract | Drosophila melanogaster | 0.1% w/w | Withania somnifera extract improved survival, motor function, and neuronal health, and reduced damaged mitochondria, showing potential therapeutic benefits for Amyotrophic Lateral Sclerosis-like conditions | [153] |
| 15 | Withanolides A dimethyl sulfoxide Extract | Caenorhabditis elegans | 2, 5, 25, and 50 µM | Withanolides A extended C. elegans lifespan (29%), lowered lipofuscin (aging marker), and upregulated neuroprotective factors (SGK-1, DAF-16, SKN-1, HSF-1), signifying antiaging and neuroprotective potential | [20] |
| 16 | Withania somnifera dried root ethanol extract | Tail tendons of male Wistar rats | 100 mg | Withania somnifera, especially its ethanolic extract, reduced collagen glycation, AGE formation, and cross-linking, potentially through its antioxidant properties, offering therapeutic potential against diabetes and aging | [60] |
| 17 | Withanolides A dimethyl sulfoxide (DMSO) Extract | Caenorhabditis elegans | 5, 50, 100, 250, and 500 μM | Withanolide A at 5 μM upregulated Serum and glucocorticoid regulated kinase-1 in the insulin/insulin-like growth factor-1 pathway, downregulating fat accumulation, and extended lifespan in C. elegans models, showing antiaging potential | [154] |
| 18 | Withania dimethyl sulfoxide (DMSO) Extract | Male Swiss albino strain mice | 200 mg/kg | Withania somnifera upregulated genes such as KLK8 and factors such as MAP2c, enhancing dendritic growth, memory, and neuroprotection while reducing age-related neurodegeneration | [155] |
| 19 | Withania somnifera root extract | Charles Foster strain male rats | 20 mg/kg and 50 mg/kg | The study supported Withania somnifera for mood stabilization, attributing anxiolytic and antidepressant effects to GABA-mimetic activity, aligning with clinical use in Ayurveda | [156] |
| 20 | Withania somnifera rootMethanol extract | Drosophila melanogaster males | 0.1, 1 and 10% w/w | The study employed LRRK2WD40 mutant fruit flies as a marker for Parkinson’s disease, showing motor impairments and mitochondrial dysfunction. Withania somnifera root upregulated protective factors but also downregulated toxic ones | [157] |
| 21 | Withaferin A methanol extract | SH-SY5Y cells | 50 nM to 1 µM | The study identified markers (NF-κB, inflammasome), genes (HDAC2), and factors involved in Alzheimer’s disease. NF-κB and inflammasome genes were upregulated, while HDAC2 was downregulated | [90] |
| 22 | Withania somnifera root extract | Caenorhabditis elegans | 100 ng/ml | Withania somnifera root extract extended C. elegans lifespan, particularly in acr-16 mutants | [158] |
| 23 | Withania somnifera root extract powder (KSM-66) | HeLa cell line | 10–50 µg/mL | Withania somnifera root extract enhances telomerase in HeLa cells, indicating potential antiaging effects | [159] |
| 24 | Withania somnifera root extract (KSM-66) | Dogs | 15 mg/kg | Withania somnifera root extract might alleviate stress, anxiety, pain and enhance well-being in dogs | [160] |
| Registry ID Number | Status | Phase | Number of Participants | Conditions or Disease | Objective | Dose | Reference |
|---|---|---|---|---|---|---|---|
| NCT05430685 | Completed | Phase 2 | 60 | Craving, stress, sleep, well being | Effect of Withania somnifera in sleep, stress, and food cravings of healthy college students | 350 mg twice daily for 30 days | [168] |
| NCT04092647 | Recruiting | Phase 2 | 80 | Chemo Fog | Role of Withania somnifera in cognitive dysfunction | 350 mg for 9 weeks | [169] |
| (Kulkarni, 2018) | Completed | Phase 1 & 2 | 50 | Chronic Periodontitis | Role of Withania somnifera in serum C reactive protein and salivary antioxidant in chronic generalized periodontitis | 500 mg twice daily for 1 month | [170] |
| NCT03596307 | Unknown | NA | 12 | Healthy | Role of Withania somnifera in endurance exercise performance | 180 mg for 2 days and 16 days | [171] |
| NCT05031351 | Recruiting | Phase 2 | 75 | Amyotrophic lateral Sclerosis | Safety of Withania somnifera in participants with amyotrophic lateral sclerosis | 1088 mg and 544 mg for 8 weeks | [172] |
| NCT01793935 | Completed | NA | 68 | Schizophrenia, schizoaffective disorder | To determine whether Withania somnifera can reduce psychopathology scores and stress scores in schizophrenia | 500 mg for first week followed by 1000 mg for 12 weeks | [173] |
| NCT00761761 | Completed | Phase 3 | 60 | Bipolar disorder | Effect of Withania somnifera in bipolar disorder | 250 mg for first week followed by 500 mg for 8 weeks | [174] |
| NCT03437668 | Active, not recruiting | Phase 2 & 3 | 66 | Schizophrenia | Role of Withania somnifera in persistent symptoms of schizophrenia | 500 mg for 12 weeks | [175] |
| NCT01311180 | Completed | Phase 2 | 120 | Generalized anxiety disorder | Role of Withania somnifera in generalized anxiety disorder | 250 mg in morning for 7 days and 250 mg twice a day for 7 weeks | [176] |
| NCT05610735 | Not yet recruiting | Phase 1 & 2 | 72 | Recurrent ovarian cancer | To determine the tolerance of Withania somnifera with liposomal doxorubicin | Administration of liposomal doxorubicin 40 mg/m2 on day 1 of a 28-day cycle for 4 cycles; 2 g, 4 g, and 8 g of Withania somnifera for 2 years | [177] |
| NCT03112824 | Completed | NA | 35 | Craving, obesity, sleep disturbance, stress reaction | Role of Withania somnifera in weight loss | 300 mg of Withania somnifera twice a day for 12 weeks | [178] |
| NCT05994794 | Active, not recruiting | NA | 40 | Elevated S-adenosylhomocysteine | Role of dietary supplement of normal homocysteine levels in healthy adults | Dietary supplement of Withania somnifera, creatine, and apha-GPC | [179] |
| NCT05210218 | Completed | NA | 40 | Obesity | Dietary supplement of cinnamon and Withania somnifera in weight loss | Cinnamon and Withania somnifera (300 mg and 150 mg) for 4 weeks | [180] |
| NCT04733924 | Completed | NA | 77 | Immune health | Investigate the efficacy and tolerability in improve immunity and reducing respiratory tract infection | 125 mg and 250 mg for 84 days | [181] |
| NCT03780621 | Completed | Phase 1 | 16 | Cognitive impairment | Effect of adaptogenic extract on cognitive impairment | 550 mg Andrographis and 10 mg of withanolides | [182] |
| NCT04598243 | Unknown status | Early Phase 1 | 70 | Fibromyalgia, chronic fatigue syndrome | Ribose, Withania somnifera, Rhodiola, Licorice, Schisandra, and Green Tea Extract for treating fibromyalgia and chronic fatigue syndrome | Herbal combination of Ribose, Withania somnifera, Rhodiola, liquorice, Schisandra, and Green Tea Extract | [183] |
| NCT04716647 | Completed | NA | 28 | COVID-19 | Investigate the feasibility of ayurveda in treating COVID-19 | Withania somnifera: 250 mg to 5 g; Giloy: 500 mg to 1 g; Tulsi: 500 mg−1g | [184] |
| NCT05602389 | Completed | NA | 186 | Stress | Investigate the role of multi-herb formulae and Withania somnifera root formula in stress | 700 mg daily for 60 days | [185] |
| NCT01125501 | Withdrawn | NA | 0 | Metabolic Syndrome | Evaluate the effects of Protandim on protein profile changes and markers of inflammation and oxidation in subjects (40–60 years of age) with metabolic syndrome | One capsule containing protandim (derived from the botanical extracts Bacopa monniera, Silybum marianum, Withania somnifera, Camellia sinensis, and Curcuma longa) daily for 30 days | [186] |
| NCT03262805 | Completed | NA | 73 | Knee Osteoarthritis | Evaluate the efficacy of Lanconone® (E-OA-07) in physical activity-related pain—LEAP study | 1000 mg twice daily of Lanconone (Shyonak, Withania somnifera, Shunthi, Guggul, Chopchini, Rasna, and Shallaki) for 4 weeks | [187] |
| NCT02027467 | Completed | NA | 23 | Healthy | Investigate the effect of StemAlive® supplement on the levels of stem cells in human volunteers (hematoalive) | Three capsules of StemAlive® (green tea, Withania somnifera) twice daily for 14 days | [188] |
| NCT02172625 | Completed | NA | 40 | Oxidative Stress | To examine the impact of Protandim supplementation on oxidative damage and athletic performance | One pill daily for 90 days of pritandim (675 mg) containing Bacopa extract 150 mg; milk thistle 225 mg; Withania somnifera 150 mg; green tea 75 mg; turmeric 75 mg | [189] |
| NCT00719953 | Completed | Phase 4 | 30 | Elderly memory impairment | To assess the efficacy of Cognitex | One capsule thrice a day containing 600 mg GPC, 100 mg PS-omega 3, 20 mg vinpocetine, 50 mg uridine-5′-monophosphate (disodium), 550 mg plant extracts (150 mg wild blueberry, 125 mg Withania somnifera, 150 mg grape seed, 125 mg hops, ginger and rosemary) | [190] |
| NCT05339958 | Completed | NA | 112 | Hair Thinning | To assess the effectiveness and safety of a novel dietary supplement containing botanical ingredients for hair thinning in men over the course of six months of continuous daily usage | Four capsules of Nutrafol® Men (Sensoril Withania somnifera, BCM-95 BioCurcumin, USPlus Saw Palmetto, EVNolMax 20% Tocotrienol/Tocopherol complex, Bioperine (piperine), Cynatine HNS (solubilized keratin), and Capsimax (capsaicin)) once daily for 6 months | [191] |
| NCT06065241 | Active, not recruiting | NA | 40 | Aging Inflammation | To investigate whether the botanical formulation, LLP-01, has a significant clinical effect on proteomic inflammatory biomarkers and epigenetic changes in healthy, older individuals | Two LLP-01 capsules containing 1000 mg (extracts from Withania somnifera, Rosmarinus officinalis, Curcuma longa, Cotinus coggygria, Panax ginseng, Cordyceps militaris, Camellia sinensis, Cotinus coggygria, and Piper nigrum) for 60 days | [192] |
| NCT02920125 | Completed | Phase 3 | 96 | Coronary artery disease, cerebrovascular disease, ischemic heart disease, deep vein thrombosis, peripheral arterial diseases, and vascular disease | To evaluate result of Ayurvedic SUVED & Reimmugen (Colostrum) treatment on vascular disease, CAD, CVA, and DVT | 500 mg of SUVED capsule (Terminalia Arjuna, Withania somnifera, Terminalia chebula, Cyperus rotundus, Apium graveolens, Vitis vinifera, Piper longum, Fagonia Arabica, Emblica officinalis, Terminalia belerica, Nymphaea stellate, Punica granatum, Bacopa Monnieri) for 3 months | [193] |
| NCT05831241 | Recruiting | Phase 4 | 45 | Sexual health | To determine the effect of Withania somnifera extract (Capsule KSM-66) on sexual health in healthy women | 300 mg twice daily of KSM-66 capsule for 8 weeks | [194] |
| NCT05840731 | Recruiting | Phase 4 | 45 | Sexual health | To investigate the role of Withania somnifera extract (Capsule KSM-66) in improving sexual health in healthy men | 300 mg twice daily of KSM-66 capsule for 8 weeks | [195] |
| ACTRN12623000287639p | Not yet recruiting | Phase 2 | 100 | Stress | To investigate effectiveness of herbal formulations for stress | Compare stress reduction: three tablets (Ziziphus, Passionflower, Lemon balm, Chamomile) vs. twp tablets (Withania somnifera, stigma) daily for 21 days | [196] |
| ACTRN12622001226796 | Not yet recruiting | NA | 100 | Stress, anxiety | To assess the feasibility of herbal and nutritional medicines for managing post-flood stress and anxiety: a randomized controlled trial | Evaluate post-flood stress: Noble Kava (200 mg daily, two capsules twice), Executive B stress Formula (two tablets daily), Withania somnifera complex day (four tablets daily). | [197] |
| ACTRN12621001769875 | Active, not recruiting | NA | 40 | Diminished ovarian reserve, infertility | To assess the effectiveness of adjunct naturopathy for pregnancy rates in women with diminished ovarian reserve compared to usual care alone: feasibility of a randomized controlled trial | Assessing naturopathic practice for women with diminished ovarian reserve: four consultations, five supplements (including Withania somnifera), a d personalized herbal medicine for 16 weeks. | [198] |
| ACTRN12621001551886 | Completed | NA | 120 | High stress, fatigue, inflammation | To examine the efficacy and safety of a novel standardized Withania somnifera root extract in overweight middle-to-older age adults experiencing high stress and fatigue: a randomized, double-blind, placebo-controlled trial | Withania somnifera extract (one capsule taken orally, twice daily with or without food, delivering 400 mg a day for 12 weeks) | [199] |
| ACTRN12617000971336 | Completed | NA | 50 | Energy and vitality, hormonal changes, wellbeing | To examine the impact of Withania somnifera supplementation on testosterone levels and vitality in healthy, overweight men | Two tablets daily (containing 10.5 mg of withanolide glycosides) for 8 weeks | [200] |
| ACTRN12617000698370 | Completed | NA | 78 | Stress, cognitive performance, anxiety | To examine the effects of Ionix Supreme on stress, mood, energy, and anxiety | Ionix Supreme liquid Oral liquid, 60 mL daily for 4 weeks, ingredients include Chinese Wolfberry, Withania somnifera, vitamins and minerals | [201] |
| ACTRN12615000324516 | Active, not recruiting | Phase 3/Phase 4 | 180 | Menopause | To evaluate the safety and efficacy of two herbal formulations in reducing menopausal symptoms in otherwise healthy women. | Capsule Twice daily: Tinospora, Asparagus, Withania somnifera, Commiphora. Fenugreek capsule, 300mg extract, twice daily for 3 months | [202] |
| CTRI/2022/11/047340 | Not Yet Recruiting | NA | 60 | Alcohol related disorders | To investigate Withania somnifera as a supplementary therapy for treatment of alcohol use disorder | Withania somnifera 250 mg two tablets before bedtime for 90 days | [203] |
| CTRI/2023/07/054711 | Completed | Phase 3 | 60 | Generalized anxiety disorder | To study the effects of Withania somnifera in patients with stress and anxiety having cardiovascular comorbidities | Withania somnifera: 300 mg twice a day after meals | [204] |
| CTRI/2023/07/054940 | Open to Recruitment | Phase 3/ Phase 4 | 60 | Iodine-deficiency hypothyroidism | To evaluate the safety and efficacy of Withania somnifera and selenium combination as a supplement in patients with subclinical hypothyroidism | Withania somnifera 500 mg and selenium 40 mcg: one capsule once a day after dinner for 90 days | [205] |
| CTRI/2022/11/047539 | Completed | NA | 68 | Hair health | Safety and efficacy of Withania somnifera on hair health | One to two drops of Withania somnifera topical formulation daily for 75 days | [206] |
| - | Completed | NA | 50 | Quality of life and cardiorespiratory endurance | Efficacy of Withania somnifera in improving quality of life and cardiorespiratory endurance in human athletic adults | 300 mg twice daily of KSM-66 capsule for 12 weeks | [207] |
| - | Completed | - | 64 | Stress and anxiety | To assess the safety and efficacy of a high concentration full-spectrum extract of Withania somnifera root in reducing stress and anxiety in adults | 300 mg Withania somnifera or placebo capsule twice daily for 60 days | [208] |
| - | Completed | - | 68 | Male infertility | To evaluate Withania somnifera impact on sperm production in oligospermic males through a clinical pilot study | 225 mg Withania somnifera capsule thrice daily for 12 weeks | [209] |
| - | Completed | - | 50 | Muscle strength | To examine the effect of Withania somnifera supplementation on muscle strength and recovery | 300 mg twice daily for 8 weeks | [210] |
| CTRI/2015/07/006045 | Completed | - | 50 | Female sexual dysfunction | To determine the efficacy and safety of a high concentration Withania somnifera root extract (HCARE) supplementation for improving sexual function in healthy females | 300 mg twice daily for 8 weeks | [211] |
| Completed | - | 52 | Chronic stress | To evaluate the safety and efficacy of a standardized root extract of Withania somnifera through a double-blind, randomized, placebo-controlled trial | 300 mg twice daily for 8 weeks | [212] | |
| CTRI/2019/11/021990 | Completed | - | 50 | Mild cognitive impairment | To evaluate the efficacy and safety of Withania somnifera in improving memory and cognitive functioning in adults with mild cognitive impairment | 300 mg twice daily for 8 weeks | [213] |
| CTRI/2016/05/006903 | Completed | - | 50 | Subclinical hypothyroidism | To evaluate the efficacy and safety of Withania somnifera root extract in subclinical hypothyroid patients | 300 mg twice daily for 8 weeks | [161] |
| - | Completed | - | 60 | Insomnia and anxiety | To determine the efficacy and safety of Withania somnifera root extract in patients with insomnia and anxiety | 300 mg twice daily for 10 weeks | [214] |
| - | Completed | - | 50 | Mental alertness | To assess the safety, efficacy, and tolerability of Withania somnifera root extract on the improvement of general health and sleep in elderly people | 300 mg twice daily for 12 weeks | [215] |
| CTRI/2016/04/006791 | Completed | - | 50 | Cardiorespiratory endurance | To evaluate the efficacy and safety of Withania somnifera root extract in enhancing cardiorespiratory endurance in healthy athletic adults | 300 mg twice daily for 8 weeks | [26] |
| CTRI/2019/10/021547 | Completed | - | 100 | Perimenopause | To assess the efficacy and tolerability of an Withania somnifera root extract on the climacteric symptoms, quality of life, and hormonal parameters in perimenopausal women | 300 mg twice daily for 8 weeks | [216] |
| CTRI/2016/05/006906 | Completed | - | 50 | Overall well-being | To evaluate the effect of Withania somnifera root extract on improving sexual health in adult males | 300 mg twice daily for 8 weeks | [195,217] |
| - | Completed | - | 80 | Poor sexual function | To evaluate the efficacy and safety of standardized Withania somnifera root extract in improving sexual function in healthy females | 300 mg twice daily for 8 weeks | [218] |
| - | Completed | - | 75 | Stress, anxiety, and impeded sleep | To investigate the adaptogenic and anxiolytic effects of Withania somnifera root extract in healthy adults | 250 mg/day and 600 mg/day twice daily for 8 weeks | [219] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basudkar, V.; Gujrati, G.; Ajgaonkar, S.; Gandhi, M.; Mehta, D.; Nair, S. Emerging Vistas for the Nutraceutical Withania somnifera in Inflammaging. Pharmaceuticals 2024, 17, 597. https://doi.org/10.3390/ph17050597
Basudkar V, Gujrati G, Ajgaonkar S, Gandhi M, Mehta D, Nair S. Emerging Vistas for the Nutraceutical Withania somnifera in Inflammaging. Pharmaceuticals. 2024; 17(5):597. https://doi.org/10.3390/ph17050597
Chicago/Turabian StyleBasudkar, Vivek, Gunjan Gujrati, Saiprasad Ajgaonkar, Manav Gandhi, Dilip Mehta, and Sujit Nair. 2024. "Emerging Vistas for the Nutraceutical Withania somnifera in Inflammaging" Pharmaceuticals 17, no. 5: 597. https://doi.org/10.3390/ph17050597
APA StyleBasudkar, V., Gujrati, G., Ajgaonkar, S., Gandhi, M., Mehta, D., & Nair, S. (2024). Emerging Vistas for the Nutraceutical Withania somnifera in Inflammaging. Pharmaceuticals, 17(5), 597. https://doi.org/10.3390/ph17050597






