An Effective Method to Prepare Curcumin-Loaded Soy Protein Isolate Nanoparticles Co-Stabilized by Carrageenan and Fucoidan
Abstract
1. Introduction
2. Results and Discussion
2.1. Effects of the SPI-to-Car Mass Ratio on the Properties of the Nanoparticles
2.2. Effect of pH on the Properties of the Nanoparticles
2.3. Effects of the SPI-to-Curcumin Mass Ratio on the Properties of the Nanoparticles
2.4. Effects of the SPI-to-Fuc Mass Ratio on the Properties of the Nanoparticles
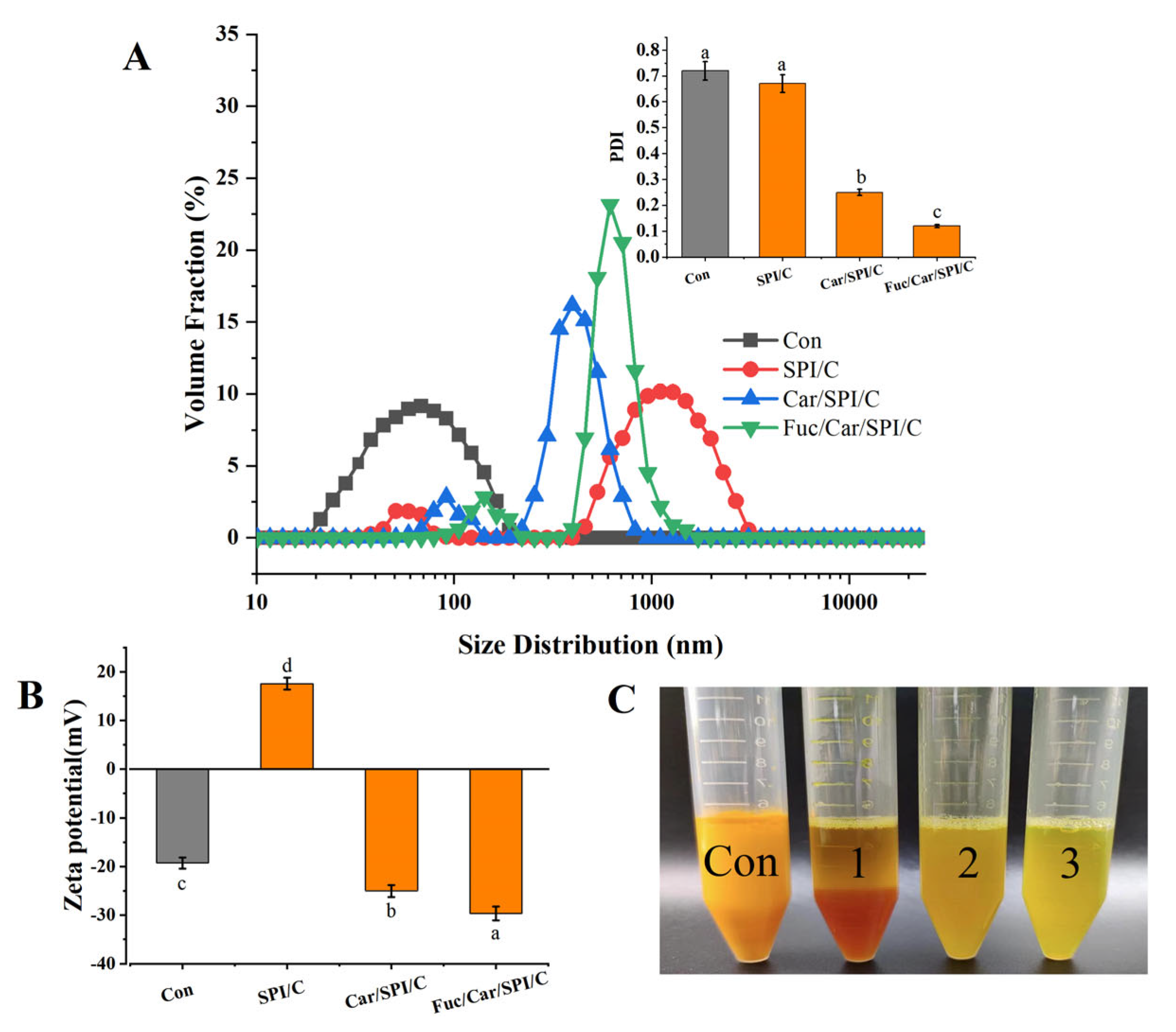
2.5. Particle Size, Zeta Potential, PDI, and Redispersibility of the Nanoparticles
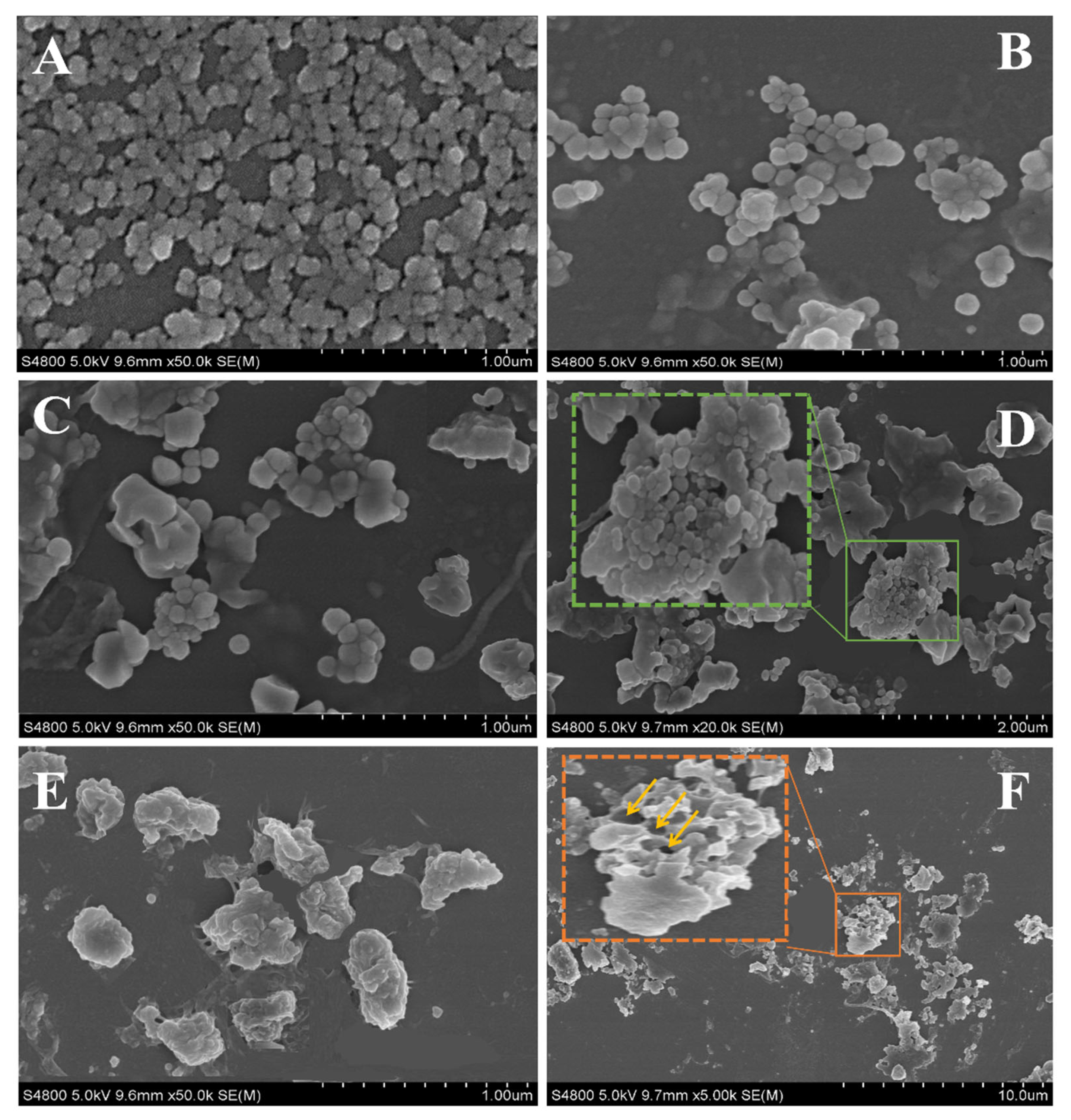
2.6. Morphology Analysis of the Nanoparticles
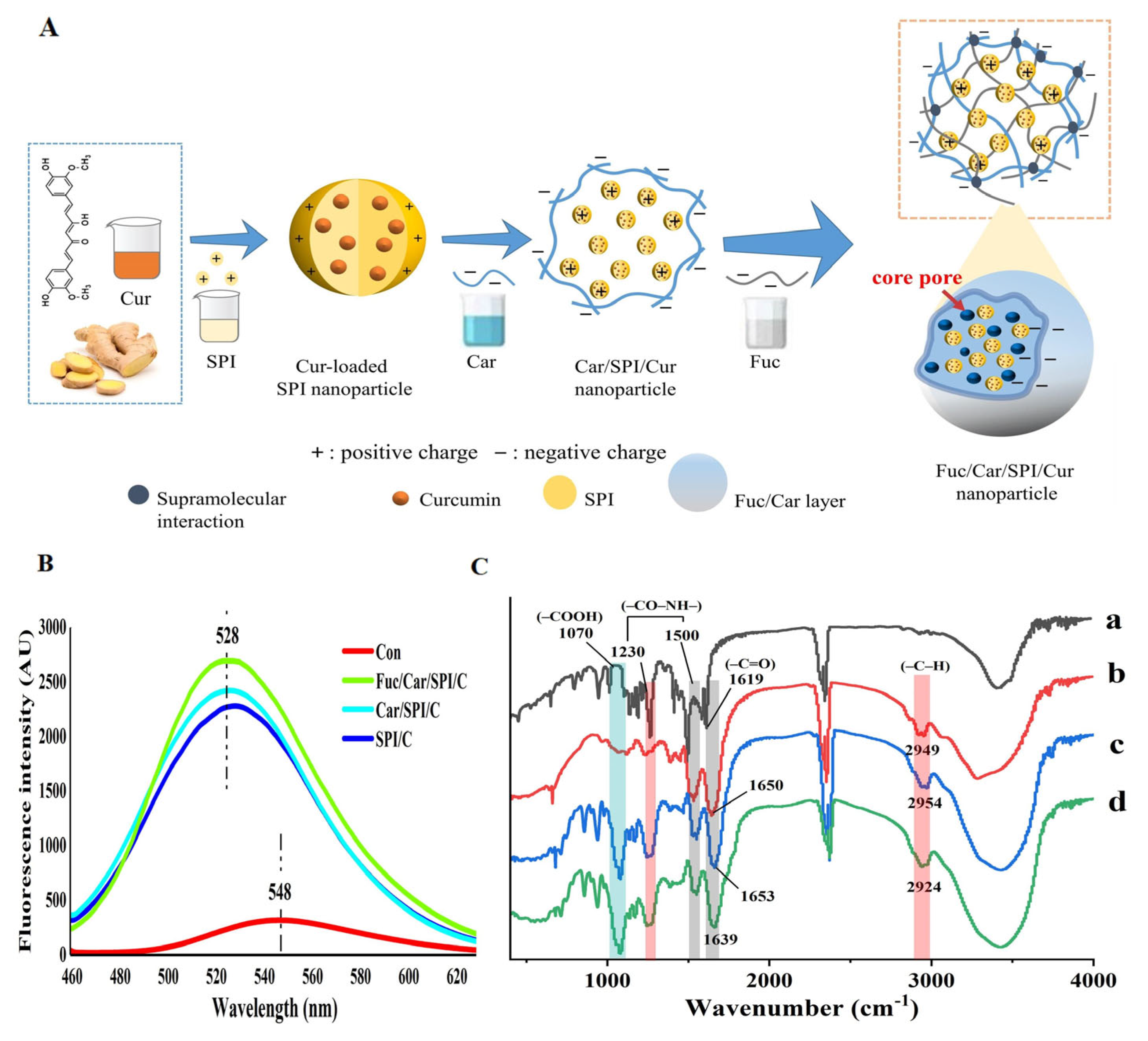
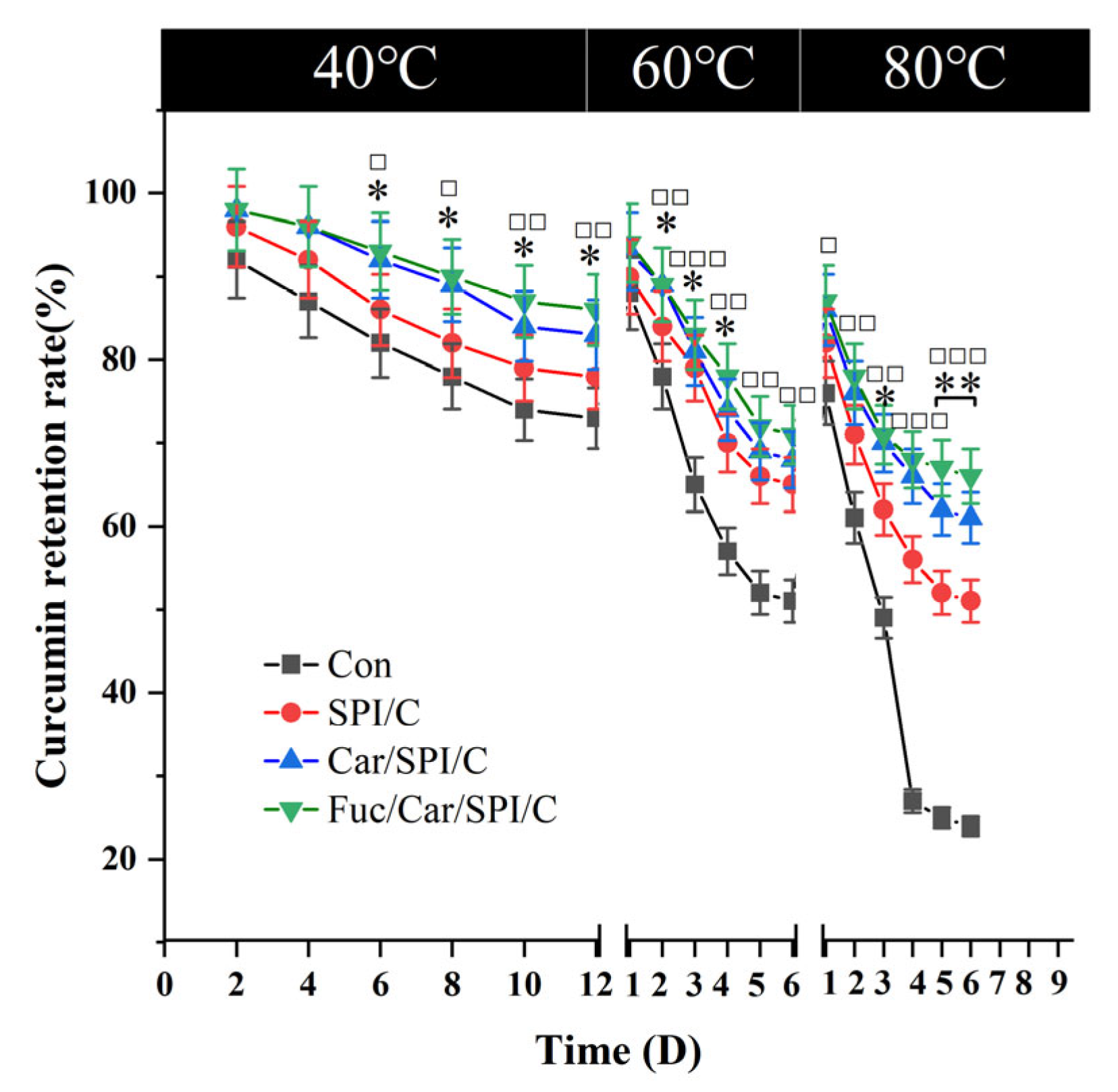
2.7. Superior Stability of the Fuc/Car/SPI/C
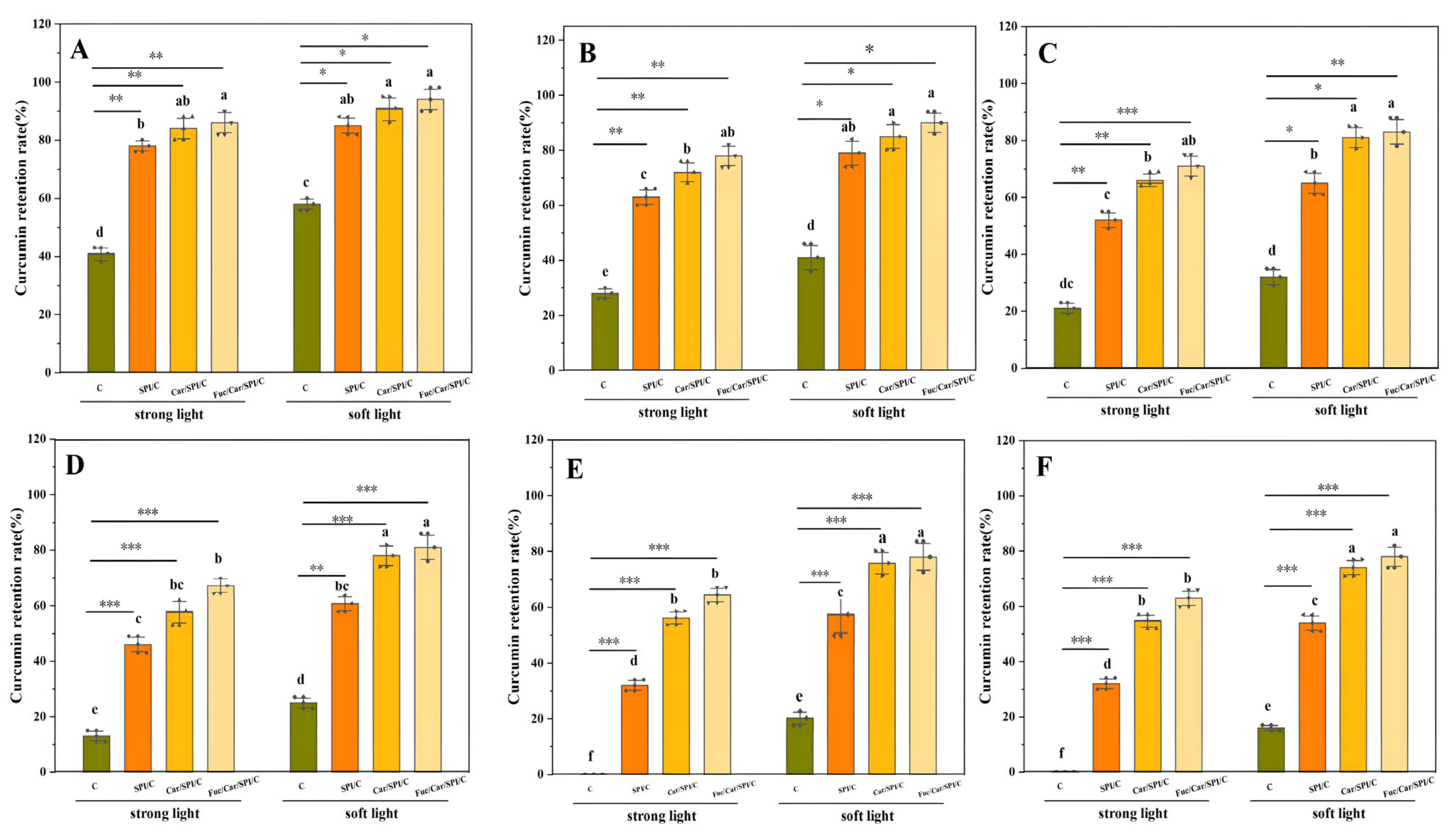
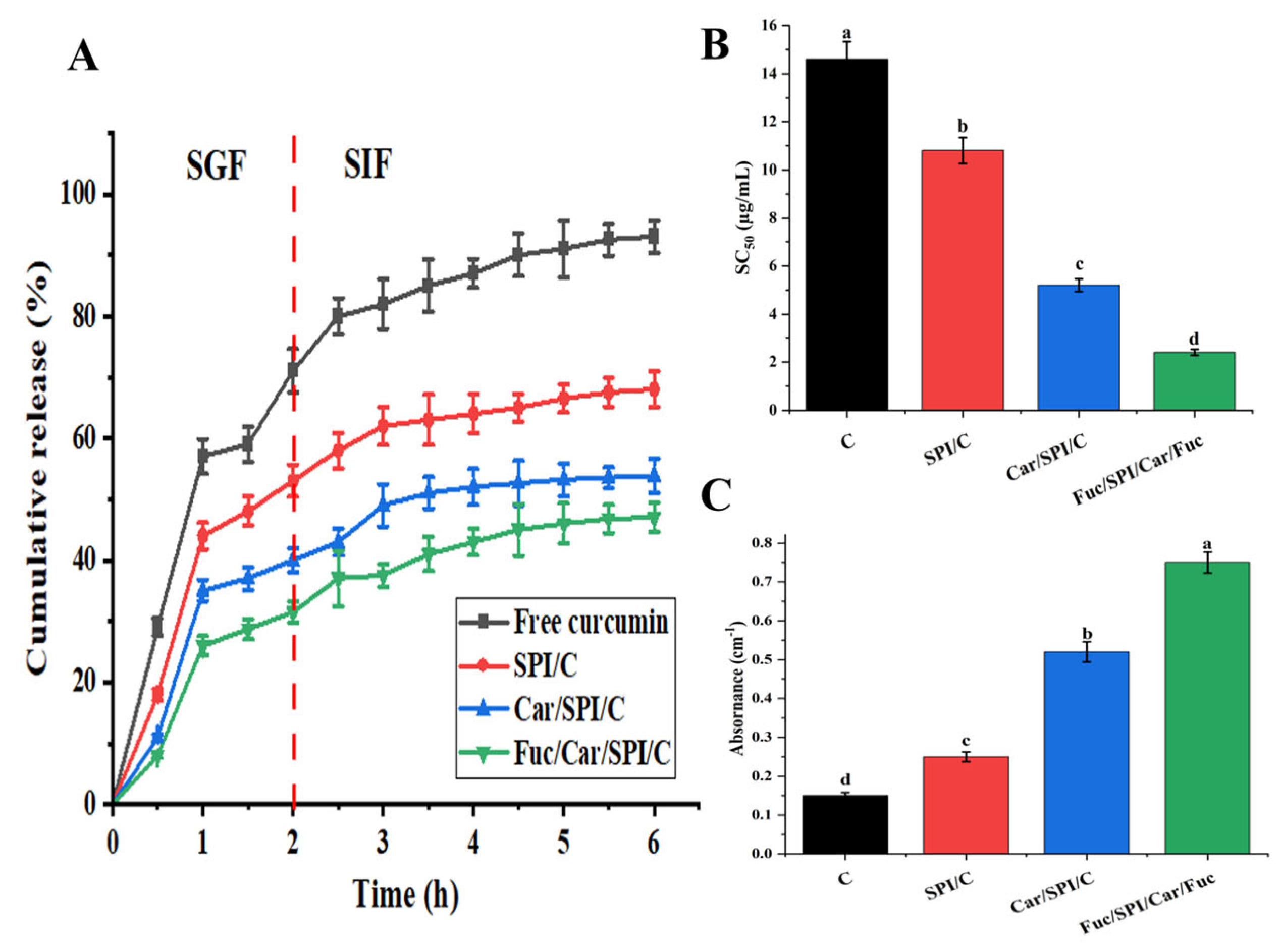
2.8. Better Controlled Release and Antioxidant Activity of Fuc/Car/SPI/C
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Nanoparticles
3.2.1. Car-Stabilized SPI Nanoparticles under Different Car/SPI Ratios
3.2.2. Car-Stabilized SPI Nanoparticles under Different pH
3.2.3. Curcumin-Loaded Car-Stabilized SPI Nanoparticles (Car/SPI/C) under Different Curcumin Content
3.2.4. Curcumin-Loaded Fuc/Car-Stabilized SPI Nanoparticles (Fuc/Car/SPI/C) under Different Car/SPI Ratios
3.3. Determination of Particle Size, Zeta Potential, and PDI
3.4. Determination of Turbidity
3.5. Calculation of the Encapsulation Efficiency and Loading Capacity
3.6. Scanning Electron Microscopy (SEM)
3.7. Fluorescence Spectroscopy and FTIR
3.8. Photochemical Stability
3.9. Thermal Stability
3.10. Controlled Release of Curcumin under Simulated Gastrointestinal Fluid
3.11. Antioxidant Properties of Curcumin-Loaded Nanoparticles
3.11.1. DPPH Radical Scavenging Activity
3.11.2. Reducing Power Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Wang, X. Binding, Stability, and Antioxidant Activity of Curcumin with Self-Assembled Casein–Dextran Conjugate Micelles. Int. J. Food Prop. 2017, 20, 3295–3307. [Google Scholar] [CrossRef]
- Jeenger, M.K.; Shrivastava, S.; Yerra, V.G.; Naidu, V.G.M.; Ramakrishna, S.; Kumar, A. Curcumin: A Pleiotropic Phytonutrient in Diabetic Complications. Nutrition 2015, 31, 276–282. [Google Scholar] [CrossRef]
- Arshad, L.; Haque, M.A.; Abbas Bukhari, S.N.; Jantan, I. An Overview of Structure–Activity Relationship Studies of Curcumin Analogs as Antioxidant and Anti-Inflammatory Agents. Future Med. Chem. 2017, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, F.; Chen, J.; Xie, X.; Xu, H.; Bai, C.; Qiao, W.; Shen, W. Antitumor Effect of Curcumin Liposome after Transcatheter Arterial Embolization in VX2 Rabbits. Cancer Biol. Ther. 2019, 20, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Kok-Khiang, P. Antibacterial Activity of Curcumin and Solubility-Enhanced Curcumin in Microemulsion: A Comparative Study. Lat. Am. J. Pharm. 2018, 37, 1468–1477. [Google Scholar]
- Wang, K.; Zhang, T.; Liu, L.; Wang, X.; Wu, P.; Chen, Z.; Ni, C.; Zhang, J.; Hu, F.; Huang, J. Novel Micelle Formulation of Curcumin for Enhancing Antitumor Activity and Inhibiting Colorectal Cancer Stem Cells. Int. J. Nanomed. 2012, 7, 4487–4497. [Google Scholar] [CrossRef]
- Rai, M.; Pandit, R.; Gaikwad, S.; Yadav, A.; Gade, A. Potential Applications of Curcumin and Curcumin Nanoparticles: From Traditional Therapeutics to Modern Nanomedicine. Nanotechnol. Rev. 2015, 4, 161–172. [Google Scholar] [CrossRef]
- Tang, C.-H. Nanocomplexation of Proteins with Curcumin: From Interaction to Nanoencapsulation (A Review). Food Hydrocoll. 2020, 109, 106106. [Google Scholar] [CrossRef]
- Akman, P.K.; Bozkurt, F.; Tornuk, F. Fabrication and Characterization of Curcumin Loaded Ovalbumin Nanocarriers and Bioactive Properties. Food Sci. Technol. 2022, 42, e38421. [Google Scholar] [CrossRef]
- Chen, F.-P.; Li, B.-S.; Tang, C.-H. Nanocomplexation between Curcumin and Soy Protein Isolate: Influence on Curcumin Stability/Bioaccessibility and in Vitro Protein Digestibility. J. Agric. Food Chem. 2015, 63, 3559–3569. [Google Scholar] [CrossRef]
- Tapal, A.; Tiku, P.K. Complexation of Curcumin with Soy Protein Isolate and Its Implications on Solubility and Stability of Curcumin. Food Chem. 2012, 130, 960–965. [Google Scholar] [CrossRef]
- Dickinson, E. Interfacial Structure and Stability of Food Emulsions as Affected by Protein–Polysaccharide Interactions. Soft Matter 2008, 4, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Su, Z.; Meng, X.; Zhang, X.; Kennedy, J.F.; Liu, B. Fabrication and Characterization of Pickering Emulsion Stabilized by Soy Protein Isolate-Chitosan Nanoparticles. Carbohydr. Polym. 2020, 247, 116712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, F.; Fang, Y.; Wang, S. Alginate-Shelled SPI Nanoparticle for Encapsulation of Resveratrol with Enhanced Colloidal and Chemical Stability. Food Hydrocoll. 2019, 90, 313–320. [Google Scholar] [CrossRef]
- Rojas-Nery, E.; Güemes-Vera, N.; Meza-Marquez, O.G.; Totosaus, A. Carrageenan Type Effect on Soybean Oil/Soy Protein Isolate Emulsion Employed as Fat Replacer in Panela-Type Cheese. Grasas Y Aceites 2015, 66, e097. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Ouyang, X.; Ling, J. Development and Characterization of Soybean Protein Isolate and Fucoidan Nanoparticles for Curcumin Encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, I.; Patel, A.R.; Van der Meeren, P.; Dewettinck, K. Emulsion-Templated Liquid Oil Structuring with Soy Protein and Soy Protein: κ-Carrageenan Complexes. Food Hydrocoll. 2017, 65, 107–120. [Google Scholar] [CrossRef]
- Luo, W.; Huang, H.; Zhang, Y.; Wang, F.; Yu, J.; Liu, Y.; Li, X. Complex Coacervation Behavior and the Mechanism between Rice Glutelin and Gum Arabic at pH 3.0 Studied by Turbidity, Light Scattering, Fluorescence Spectra and Molecular Docking. LWT 2021, 150, 112084. [Google Scholar] [CrossRef]
- Stone, A.K.; Nickerson, M.T. Formation and Functionality of Whey Protein Isolate–(Kappa-, Iota-, and Lambda-Type) Carrageenan Electrostatic Complexes. Food Hydrocoll. 2012, 27, 271–277. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, Y.; Chen, J.; Jiang, B.; Zhang, T. Fabrication, Characterization, Physicochemical Stability and Simulated Gastrointestinal Digestion of Pterostilbene Loaded Zein-Sodium Caseinate-Fucoidan Nanoparticles Using pH-Driven Method. Food Hydrocoll. 2021, 119, 106851. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and Ionic Strength on the Physical and Rheological Properties and Stability of Whey Protein Stabilized o/w Emulsions Containing Xanthan Gum. J. Food Eng. 2019, 242, 141–152. [Google Scholar] [CrossRef]
- Amin, M.L.; Mawad, D.; Dokos, S.; Koshy, P.; Martens, P.J.; Sorrell, C.C. Fucoidan- and Carrageenan-Based Biosynthetic Poly(Vinyl Alcohol) Hydrogels for Controlled Permeation. Mater. Sci. Eng. C 2021, 121, 111821. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Takai, C.; Fuji, M.; Shirai, T. Formation of Nanoparticle Added Functional Polymer Network Membrane via Micro-Phase Separation Process. Adv. Powder Technol. 2016, 27, 871–876. [Google Scholar] [CrossRef]
- Li, Y.; Jin, B.; Wang, K.; Song, L.; Ren, L.; Hou, Y.; Gao, X.; Zhan, X.; Zhang, Q. Coordinatively-Intertwined Dual Anionic Polysaccharides as Binder with 3D Network Conducive for Stable SEI Formation in Advanced Silicon-Based Anodes. Chem. Eng. J. 2022, 429, 132235. [Google Scholar] [CrossRef]
- Liu, Q.; Jing, Y.; Han, C.; Zhang, H.; Tian, Y. Encapsulation of Curcumin in Zein/ Caseinate/Sodium Alginate Nanoparticles with Improved Physicochemical and Controlled Release Properties. Food Hydrocoll. 2019, 93, 432–442. [Google Scholar] [CrossRef]
- Ghanta, K.P.; Mondal, S.; Mondal, S.; Bandyopadhyay, S. Contrasting Effects of Ionic Liquids of Varying Degree of Hydrophilicity on the Conformational and Interfacial Properties of a Globular Protein. J. Phys. Chem. B 2021, 125, 9441–9453. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hou, F.; Zhao, H.; Wang, D.; Chen, W.; Miao, S.; Liu, D. Conjugation of Soy Protein Isolate (SPI) with Pectin by Ultrasound Treatment. Food Hydrocoll. 2020, 108, 106056. [Google Scholar] [CrossRef]
- Li, D.; Wei, Z.; Sun, J.; Xue, C. Tremella Polysaccharides-Coated Zein Nanoparticles for Enhancing Stability and Bioaccessibility of Curcumin. Curr. Res. Food Sci. 2022, 5, 611–618. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Development of Protein-Polysaccharide-Surfactant Ternary Complex Particles as Delivery Vehicles for Curcumin. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Gupta, S.; Ghoshal, G. Plant Protein Hydrogel as a Delivery System of Curcumin: Characterization and in Vitro Release Kinetics. Food Bioprod. Process. 2024, 143, 66–79. [Google Scholar] [CrossRef]
- Wang, N.; Tian, J.; Wang, L.; Song, S.; Ai, C.; Janaswamy, S.; Wen, C. Fucoidan Hydrogels Induced by κ-Carrageenan: Rheological, Thermal and Structural Characterization. Int. J. Biol. Macromol. 2021, 191, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hung, Y.-C. Effect of Water Compounds on Photo-Disinfection Efficacy of TiO2 NP-Embedded Cellulose Acetate Film in Natural Water. Water Supply 2020, 21, 2825–2836. [Google Scholar] [CrossRef]
- Tiwari, P.; Ali, R.; Ishrat, R.; Arfin, N. Study of Interaction between Zein and Curcumin Using Spectroscopic and in Silico Techniques. J. Mol. Struct. 2021, 1230, 129637. [Google Scholar] [CrossRef]
- Zheng, X.; Li, Y.; Yang, J.; Cui, S. Z-Scheme Heterojunction Ag/NH2-MIL-125(Ti)/CdS with Enhanced Photocatalytic Activity for Ketoprofen Degradation: Mechanism and Intermediates. Chem. Eng. J. 2021, 422, 130105. [Google Scholar] [CrossRef]
- Li, S.; Mu, B.; Yan, P.; Kang, Y.; Wang, Q.; Wang, A. Incorporation of Different Metal Ion for Tuning Color and Enhancing Antioxidant Activity of Curcumin/Palygorskite Hybrid Materials. Front. Chem. 2021, 9, 760941. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle Encapsulation Improves Oral Bioavailability of Curcumin by at Least 9-Fold When Compared to Curcumin Administered with Piperine as Absorption Enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Huang, X.; Huang, X.; Gong, Y.; Xiao, H.; McClements, D.J.; Hu, K. Enhancement of Curcumin Water Dispersibility and Antioxidant Activity Using Core-Shell Protein-Polysaccharide Nanoparticles. Food Res. Int. 2016, 87, 1–9. [Google Scholar] [CrossRef]
| SPI:Car (w/w) | Particle Size (nm) | Zeta Potential (mV) | Turbidity |
|---|---|---|---|
| 1:0 | 1069.49 ± 1.57 a | 17.58 ± 0.81 a | 1.75 ± 0.01 e |
| 1:0.25 | 344.04 ± 9.26 c | −18.17 ± 0.84 d | 1.85 ± 0.04 d |
| 1:0.5 | 343.14 ± 7.63 c | −22.23 ± 1.02 bc | 2.43 ± 0.05 b |
| 1:1 | 329.82 ± 6.58 e | −24.12 ± 1.14 b | 2.58 ± 0.01 a |
| 1:1.25 | 336.97 ± 8.47 d | −21.76 ± 0.98 bc | 2.34 ± 0.04 c |
| 1:1.5 | 355.03 ± 9.83 b | −21.24 ± 0.74 c | 2.31 ± 0.04 c |
| pH | Particle Size (nm) | Zeta Potential (mV) | Turbidity |
|---|---|---|---|
| 4.5 | 356.94 ± 1.07 a | −15.24 ± 0.82 d | 2.45 ± 0.02 b |
| 4.0 | 334.76 ± 2.80 c | −21.71 ± 0.88 b | 3.14 ± 0.03 a |
| 3.5 | 320.15 ± 1.53 e | −24.78 ± 1.21 a | 3.21 ± 0.02 a |
| 3.0 | 324.42 ± 1.09 d | −23.56 ± 1.10 ab | 3.12 ± 0.02 a |
| 2.5 | 344.97 ± 3.10 b | −18.42 ± 0.82 c | 2.46 ± 0.01 b |
| SPI:Curcumin (w/w) | Particle Size (nm) | Zeta Potential (mV) | Turbidity | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| 50:1 | 420.41 ± 0.04 a | −23.71 ± 1.23 ab | 4.17 ± 0.03 c | 87.55 ± 0.20 a |
| 25:1 | 419.23 ± 0.85 ab | −24.26 ± 1.17 a | 4.19 ± 0.05 bc | 86.60 ± 0.31 b |
| 10:1 | 419.11 ± 0.33 ab | −25.07 ± 1.31 a | 4.23 ± 0.02 bc | 86.51 ± 0.17 b |
| 5:1 | 417.88 ± 0.52 b | −22.72 ± 1.02 ab | 4.28 ± 0.06 b | 85.58 ± 0.23 c |
| 3:1 | 418.10 ± 0.61 b | −21.56 ± 0.98 b | 5.04 ± 0.08 a | 78.88 ± 0.30 d |
| SPI:Fuc (w/w) | Zeta Potential (mV) | Turbidity | Loading Capacity (%) |
|---|---|---|---|
| 1:0 | −25.07 ± 1.31 c | 4.23 ± 0.02 d | 5.01 ± 0.09 a |
| 1:0.1 | −27.24 ± 1.01 abc | 6.05 ± 0.10 c | 4.73 ± 0.09 b |
| 1:0.2 | −27.83 ± 1.28 ab | 6.16 ± 0.01 bc | 4.56 ± 0.06 c |
| 1:0.3 | −28.31 ± 1.43 ab | 6.23 ± 0.01 b | 4.34 ± 0.05 d |
| 1:0.4 | −29.67 ± 1.38 a | 6.30 ± 0.01 ab | 4.16 ± 0.04 e |
| 1:0.5 | −26.02 ± 1.15 bc | 6.43 ± 0.01 a | 4.12 ± 0.07 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cai, S.; He, N.; Huang, X.; Hong, Z.; He, J.; Chen, H.; Zhang, Y. An Effective Method to Prepare Curcumin-Loaded Soy Protein Isolate Nanoparticles Co-Stabilized by Carrageenan and Fucoidan. Pharmaceuticals 2024, 17, 534. https://doi.org/10.3390/ph17040534
Chen Y, Cai S, He N, Huang X, Hong Z, He J, Chen H, Zhang Y. An Effective Method to Prepare Curcumin-Loaded Soy Protein Isolate Nanoparticles Co-Stabilized by Carrageenan and Fucoidan. Pharmaceuticals. 2024; 17(4):534. https://doi.org/10.3390/ph17040534
Chicago/Turabian StyleChen, Yaxin, Shuyun Cai, Niaoniao He, Xiaomei Huang, Zhuan Hong, Jianlin He, Hui Chen, and Yiping Zhang. 2024. "An Effective Method to Prepare Curcumin-Loaded Soy Protein Isolate Nanoparticles Co-Stabilized by Carrageenan and Fucoidan" Pharmaceuticals 17, no. 4: 534. https://doi.org/10.3390/ph17040534
APA StyleChen, Y., Cai, S., He, N., Huang, X., Hong, Z., He, J., Chen, H., & Zhang, Y. (2024). An Effective Method to Prepare Curcumin-Loaded Soy Protein Isolate Nanoparticles Co-Stabilized by Carrageenan and Fucoidan. Pharmaceuticals, 17(4), 534. https://doi.org/10.3390/ph17040534







