Abstract
The increasing use of immune checkpoint inhibitors (ICI) in cancer therapy has brought attention to their associated neurotoxicities, termed neurological immune-related adverse events (n-irAEs). Despite their relatively rare incidence, n-irAEs pose a significant risk, potentially leading to severe, long-lasting disabilities or even fatal outcomes. This narrative review aims to provide a comprehensive overview of n-irAEs, focusing on their recognition and management. The review addresses a spectrum of n-irAEs, encompassing myositis, myasthenia gravis, various neuropathies, and central nervous system complications, such as encephalitis, meningitis, and demyelinating diseases. The key features of n-irAEs are emphasized in this review, including their early onset after initiation of ICIs, potential association with non-neurological irAEs and/or concurrent oncological response, the significance of ruling out other etiologies, and the expected improvement upon discontinuation of ICIs and/or immunosuppression. Furthermore, this review delves into considerations for ICI re-challenge and the intricate nature of n-irAEs within the context of pre-existing autoimmune and paraneoplastic syndromes. It underscores the importance of a multidisciplinary approach to diagnosis and treatment, highlighting the pivotal role of severity grading in guiding treatment decisions.
1. Introduction
The immune system detects and eradicates cancer cells by recognizing tumor-associated antigens (neoantigens) captured by antigen-presenting cells (APCs) and presented to T cells via the major histocompatibility complex (MHC) I. Positive regulatory signals for T-cell activation include the binding of CD80 on APCs to CD28 on T cells, activating various signaling cascades, such as the phosphoinositol-3-kinase (PI3K)—protein B (AKT)—mammalian target of rapamycin (mTOR) pathway, the mitogen-activated pathway kinase (MAPK), and the Janus kinase pathway, ultimately leading to NF-kβ activation and IL-2 production. However, cancers often evade immune detection through immune checkpoints, such as the cytotoxic T lymphocyte-associated protein 4 (CTLA-4), the programmed cell death protein-1 (PD-1), and the programmed cell death ligand-1 (PD-L1), which inhibit the physiological activation of T cells. CTLA-4 competes with CD28 and binds to B7 on APCs, impeding T-cell activation in the priming phase. Similarly, PD-1 on T cells suppresses their activation in the effector phase by downregulating T-cell receptor surface expression and hindering MHC recognition. Moreover, PD-1 inhibits T-cell proliferation by blocking the PI3K-AKT-mTOR cascade and suppressing cyclin-dependent kinases (CDKs) [1,2,3]. Figure 1 describes the action mechanisms of ICIs.
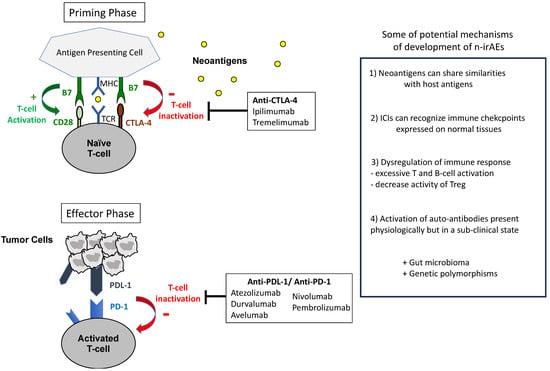
Figure 1.
Mechanisms of action of ICIs and hypotheses for the development of (neurological) irAEs.
Immune checkpoint inhibitors (ICIs) disrupt inhibitory signals, reactivating the immune system. Notably, ipilimumab and tremelimumab target CTLA-4, while atezolizumab, durvalumab, and avelumab target PD-L1, and nivolumab and pembrolizumab target PD-1 [4]. In current clinical practice, ICIs are used across various cancer types employing different strategies and durations. Initially evaluated in the metastatic setting, they can be used for an indefinite period as monotherapy, in combination with chemotherapy, or with tyrosine kinase inhibitors. Furthermore, they have shown efficacy in the perioperative setting with a limited duration period. A combination therapy involving anti-CTLA-4 and anti-PD-1 inhibitors is also proposed in metastatic cancer, with dosage and duration varying depending on the cancer type [4,5]. A large meta-analysis demonstrated that most patients treated with ICIs experience immune-related adverse events (irAEs); this irAEs rate can reach 83% with anti-CTLA-4, 72% with anti-PD-1, and 59% with anti-PD-L1 therapy [6]. Neurotoxicities associated with ICIs, referred to as neurological immune-related adverse events (n-irAEs), are relatively rare compared to other irAEs, such as skin or gastrointestinal toxicities. A review of 59 studies, encompassing a total of 9,208 patients, revealed an incidence of n-irAEs reaching 3.8% with anti-CTLA4 therapy, 6.1% with anti-PD1 therapy, and 12% with combination therapy (i.e., anti-CTLA4 plus anti-PD1) [7]. However, this incidence is probably over- or under-estimated, as 55% of these n-irAEs consisted in non-specific symptoms, such as asthenia or headache and confusion, which are not included in a recent classification of n-irAEs [8,9,10]. Grade ≥ 3 n-irAEs represented less than 2% of these cases [11].
The early recognition of n-irAEs is crucial, as their potential severity is significant. With a mortality rate that can reach 24%, these toxicities generate the most frequent fatalities related to ICIs, alongside myocarditis [12,13]. This is particularly true for conditions such as encephalitis, Guillain–Barré syndrome (GBS), and myasthenia gravis (MG), which lead to rapid deterioration and have notably high mortality rates [10,12,13]. A multidisciplinary approach is thus essential to ensure a rapid diagnosis and optimal management.
The aim of this narrative review is to provide a comprehensive overview and update on these conditions, which often represent a challenge for clinicians. This review draws mainly upon the latest guidelines from the European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO), and the Society for Immunotherapy of Cancer (SITC) [14,15,16].
2. General Management
2.1. Classification
Neurological disorders are divided in two categories: central and peripheral disorders. Central nervous system (CNS) disorders, representing 17–25% of n-irAEs, include encephalitis/encephalopathy, meningitis and demyelinating conditions such as multiple sclerosis and transverse myelitis [8,10,13].
Peripheral nervous system disorders account for 75–83% of cases, include neuropathies, polyradiculoneuropathies (such as GBS and its variants), neuromuscular junction dysfunctions (such as MG) and myopathy/myositis [8,10,13].
2.2. Pathogenesis
Numerous mechanisms may be advanced for irAE development; yet, the pathogenesis remains largely unknown. The intricacies of the immune system, coupled with the low incidence of n-irAEs and limited access to histological samples (particularly CNS tissue), hinder our understanding of these autoimmune toxicities.
The mechanism of action of ICIs is not specific to tumoral tissue, targeting molecules such as CTLA-4 and PD-1 expressed on various immune cells beyond T cells, including regulatory T cells (Tregs) and B cells. Tregs play protective roles, preventing excessive immune activation and impairing autoimmune neurological disorders, while B cells produce antibodies after immune stimulation. ICI therapy disrupts these immune properties, leading to a disequilibrium in immune self-modulation. This may result in decreased peripheral self-tolerance due to Treg depletion and excessive autoantibody production through B-cell activation.
Another potential mechanism underlying irAEs is molecular mimicry, where the similarities between host antigens and tumor antigens induce a cross-reactive autoimmune response. For example, in melanoma patients developing ICI-related demyelinating polyneuropathy, molecular mimicry between melanocytes and Schwann cells may occur due to shared epitopes for immune responses. This phenomenon is akin to paraneoplastic syndromes (PNS), where ICIs may promote immune-mediated PNS via cross-reactivity against self-antigens expressed on both neural cells and tumors [17].
Additionally, ICIs can affect non-hematopoietic cell lines expressing the target immune checkpoint ligands. For instance, the high rate of immune-related hypophysitis observed during anti-CTLA4 therapy may be attributed to CTLA-4 protein expression on pituitary endocrine cells [18]. Moreover, PD-1 protein expression has been observed in the cortex and basal ganglia, with RNA expression of PD-1, PD-2, and CTLA-4 verified throughout the CNS [17].
ICIs may exacerbate pre-existing immune responses against the nervous system, such as multiple sclerosis, auto-immune encephalitis, inflammatory demyelinating neuropathies, and MG. This escalation of local inflammation or unveiling of latent central or peripheral inflammation is challenging to confirm, as clinical trials involving ICIs typically exclude patients with autoimmune diseases due to their potential for serious irAEs, making robust clinical responses difficult to establish.
The reasons why some patients develop irAEs while others do not, remain unclear. Genetic factors, such as CTLA-4 polymorphisms, have been associated with increased auto-immune disease risk, but studies have not consistently linked specific genotypes to irAE susceptibility [19]. Similarly, the role of microbiome composition in irAE development is uncertain, although some evidences suggest correlations between certain gut bacteria and resistance to immune-related colitis during ipilimumab treatment for melanoma. However, further research—particularly prospective studies—is necessary to elucidate the relationships between genetic predisposition, microbiome composition, and irAE susceptibility, especially in the context of n-irAEs [20,21].
While n-irAEs share similar features with their traditional counterparts, it is important to be aware of significant differences, which contribute to accurate diagnosis and enable tailored treatments. For instance, ICI-induced neuropathies may behave similarly to classical GBS but are more likely to respond to corticosteroids and often present with lymphocytic pleocytosis in cerebro-spinal fluid (CSF) [7,10]. Figure 1 describes some hypotheses for the development of (neurological) irAEs.
2.3. Approaching the Diagnosis
A multidisciplinary approach is of importance, involving neurologists but also other specialists (ophthalmologists, rheumatologists, radiologists, cardiologists…) in order to provide the most appropriate diagnosis and treatment.
The diagnosis of n-irAEs first highlights the importance of systematically excluding other potential causes before attributing symptoms to n-irAEs. These include a wide range of conditions, such as infections (viral or bacterial), cancer progression (including leptomeningeal carcinomatosis), metabolic issues (hypothyroidism, hypercalcemia), nutritional deficiencies, neurotoxicity from other treatments (e.g., platinum-induced sensory neuronopathy and cognitive side effects from radiotherapy), epilepsy, and vascular causes. Other features can help to suspect irAEs:
- (a)
- Timing is crucial for identifying n-irAEs, as they usually emerge within 3–6 months after initiating ICI treatment, with a median onset of 4 weeks (ranging from 1 to 85 weeks) [9,22,23,24,25]. Neurological symptoms emerging beyond 6 months after the last ICI dose are less likely to be secondary to an irAE, although it cannot be entirely ruled out solely on this basis [8];
- (b)
- Clinical and/or radiological evidence of cancer control coinciding with the onset of neurological symptoms supports n-irAE diagnosis rather than attributing the symptoms to cancer progression [22];
- (c)
- The concurrent occurrence of neurological symptoms with other irAEs increases the likelihood of a n-irAE [11,22];
- (d)
- A positive response to ICI interruption and/or immunosuppression also suggests a n-irAE, although it is not a definitive indicator [8].
2.4. Diagnosis Tools
The optimal diagnostic evaluation for patients with suspected n-irAEs should be tailored based on their clinical presentation and may encompass different tools, including brain and/or spinal magnetic resonance imaging (MRI), lumbar puncture for CSF analysis, electroneuromyography (EMG), electroencephalography (EEG). Brain or meningeal biopsy, although rarely necessary, has to sometimes be considered to exclude alternative diagnosis (e.g., chronic pachymeningitis or persistent suspicion of leptomeningeal carcinomatosis despite negative lumbar puncture). Auto-immune antibodies belong to the common tools in the diagnosis of n-irAEs. Most are assessed in serum, whereas antibodies specifically targeting membrane components often require a CSF analysis (e.g., NMDAR, LGI1, CASPR2). Some, referred to as syndrome-associated autoantibodies, support the diagnosis of specific syndromes [8,26]. However, as many antibodies lack specificity, it is essential to correlate the clinical presentation with established antibody-associated syndromes before making a diagnosis [8]. Furthermore, given the prevalence of false positives, the results should be validated using confirmatory tests [22,27]. Antinuclear antibody (ANA) testing is non-specific but sometimes advised, as it can suggest an autoimmune tendency [8,14,15,16].
The diagnosis of a n-irAE also requires a broader evaluation; myositis/myopathy or MG should lead to investigations of myocarditis with EKG, troponin levels, and consideration of additional tests, such as brain natriuretic peptide (BNP), CK-MB, cardiac ultrasonography (US), and cardiac MRI [8]. Moreover, pulmonary function tests and video-fluoroscopic swallow studies can help in assessing restrictive syndromes and dysphagia secondary to MG, myositis, or GBS.
3. Importance of Rapid Diagnosis and Severity Score
A multidisciplinary approach is required, involving neurologists and other specialists, including ophthalmologists, cardiologist, pneumologist and rheumatologists in suspected ocular or muscular involvement cases [28]. This approach, linked to improved outcomes, helps locate the neurological lesion, rule out other causes, and guide both diagnostic evaluation and therapeutic management [29]. Therefore, consulting organ specialists is recommended for all severity levels [14,16]. Guidon et al. proposed a set of criteria with three levels of diagnostic certainty—definite, probable, and possible—based on clinical signs, radiological, electrophysiological, and biological findings, as well as on the evolution under immunomodulatory agents [8].
Severity grading is crucial for determining the appropriate therapeutic approach and level of care. The severity of n-irAEs has been categorized based on the Common Terminology Criteria for Adverse Events v5.0 (CTCAE) system, originally designed for chemotherapy-related toxicities, and it may not fully capture the severity of irAEs [14]. Guidon et al. introduced a novel severity-based grading system, essential for guiding treatment decisions. Table 1 summarizes the severity score of n-irAEs based on CTCAE v5.0 and Guidon et al.’s recommendations.

Table 1.
Global severity score of n-irAEs [8,14].
It is important to note that for conditions such as MG, GBS, and encephalitis, Grade 1 may not be applicable due to the potential for rapid deterioration and the importance of early management. These patients should be admitted into units capable of rapid transfer to intensive care level monitoring, with regular assessment of bulbar, autonomic, or respiratory symptoms. A rapid and accurate diagnosis can only be achieved through collaboration with neurological teams experienced in managing n-irAEs. Physicians should also be vigilant about the potential impacts of ICIs on other organs, such as the cardiac system. Table 2 summarizes the severity score and related treatment for key n-irAE entities, including irMyopathy/Myositis (irM/M), irNeuromuscular Junction Disorders (irNMJD), irNeuropathy, irMeningitis, irEncephalitis, and irDemyelinating Diseases (irDDs).

Table 2.
Severity score for key n-irAEs and their respective management.
4. Recognizing the Key Neurological Immune-Related Adverse Events
4.1. irMyopathy/Myositis (irM/M)
4.1.1. General Overview
Immune-related M/M can either emerge de novo or as a reactivation of pre-existing paraneoplastic polymyositis or dermatomyositis [15]. It is the most frequent n-irAE, accounting for approximately 32% of cases [10,30]. Typically, the onset occurs within 2 months following ICI therapy [24,31], with a median of 5–6 weeks [24,30,31,32]. Anti-PD(L)-1 therapy is more likely associated with irM/M than anti-CTLA4 therapy [33,34].
Up to 70–80% of patients improve after discontinuing ICI therapy and starting immunomodulatory treatment [10]. However, half of patients experience sustained effects after 5 years, and the mortality rate can reach 17%, resulting mainly from bulbar involvement, respiratory muscle failure, and concurrent myocarditis [10,30].
The “triple M syndrome” describes the frequent overlap of myositis, myocarditis, and MG in ICI-treated patients, and it has been reported in up to 53% of myositis cases [35]. This syndrome is associated with poor prognosis, with the mortality rate reaching 60% [36]. Therefore, routine screening for myocarditis and MG is mandatory in all patients presenting with irM/M.
4.1.2. Clinical Presentation
irM/M presents with a fixed—sometimes focal—muscular weakness [8]:
- -
- Proximal limb muscles are more affected than distal ones, leading to limitations in ambulating, raising, and lifting arms;
- -
- Axial weakness, particularly in the cervical region, results in difficulties in neck extension and flexion;
- -
- Oculo-bulbar involvement, characterized by ptosis, diplopia, dysphagia, and dysarthria, is a distinct and prominent feature of irM/M, being the primary or sole manifestation in 42% of patients, contrasting with those unexposed to ICI [37].
Muscular pain generally occurs only in severe myositis cases and sometimes precedes muscular weakness. Cutaneous manifestations associated with dermatomyositis (malar rash, heliotrope rash, Gottron papules, and shawl sign) are rare in de novo irM/M.
4.1.3. Diagnostic Tools
The following tests should be conducted:
- -
- Creatine kinase (CK) levels, while often elevated, do not consistently correlate with the severity of myopathy and can be normal in up to 30% of cases [37]. Aldolase, another marker of muscle breakdown, may be high even in the absence of CK elevation, providing additional diagnostic insight [37];
- -
- EMG to detect a myopathic pattern (although it presents as normal in up to 20% of cases) and to guide muscle biopsy [10,35,37];
- -
- EKG and troponin levels;
- -
- Assessment of MG-specific antibodies (AChR, MuSK, LRP4) can also help to evaluate the presence of co-occurring myasthenia, especially when ocular or bulbar symptoms are present [35,37]. While myositis-specific antibodies (e.g., ANA, Jo-1, PL-7, PL-12, EJ) may be considered for pretherapeutic muscular manifestations or suspected underlying dermatomyositis, they often yield negative results in ir-M/M [31,37,38];
- -
- Limb MRI with contrast typically reveals STIR hyperintensity or contrast enhancement in affected muscles. While these findings are non-specific and can be observed in denervated muscles, they provide diagnostic support and assist in directing biopsy [8].
- -
- Muscle biopsy is only recommended in cases of doubtful diagnosis [31,37];
- -
- Pulmonary function tests and video-fluoroscopic swallow studies to assess restrictive syndromes and dysphagia, respectively.
4.2. irNeuromuscular Junction Disorders (irNMJD), Including Mysthenia Gravis (MG)
4.2.1. General Overview
Immune-related MG can present either as new-onset MG or as exacerbations of a pre-existing condition [39,40]. MG tends to develop particularly early, with the median onset of 4–6 weeks after starting ICI, and in some cases, as early as 6 days [24,32]. It is more likely to develop in patients receiving anti-PD-(L)1 therapy compared to those treated with anti-CTLA-4 therapy [24]. While most patients improve, the mortality rate can reach up to 28–30%, primarily due to complications, such as bulbar involvement, respiratory muscle failure, and concurrent myocarditis [10,41]. Long-term immunosuppression is often necessary, except in a few patients who experience minor manifestations confined to ocular or facial muscles [14]. Distinguishing between MG and myositis can be challenging, as myositis can mimic MG, leading to a clinical presentation referred to as “pseudo-myasthenia” [8,42,43]. In such cases, myopathy can display weakness patterns in the oculo-bulbar, respiratory, and axial muscles. Furthermore, AChR antibodies can be non-specifically present in patients with myopathy, but they often do not show clinical or electrophysiological fluctuations characteristic of neuromuscular junction dysfunction [37]. Instead, these patients frequently exhibit varying degrees of myopathy, ranging from elevated CK levels to necrotizing myopathy. About 80% of patients also experience non-neurological irAEs, such as myocarditis [32].
4.2.2. Clinical Presentation
Neuromuscular junction disorder should be suspected if a patient exhibits fluctuating, exercise-dependent muscle weakness, which usually involves
- -
- Proximal and axial cervical muscles (neck and shoulder weakness) [16];
- -
- Ocular muscles (diplopia, asymmetrical ptosis, and/or fatigability);
- -
- Bulbar muscles (dysphagia, dysphonia, and dysarthria);
- -
- Respiratory muscles, affecting over 50% of cases, more frequently than in idiopathic MG [10,32].
4.2.3. Diagnostic Tools
The ice pack test is a simple yet helpful diagnostic tool [44]: placing an ice pack on the ptotic eyelid for two minutes should relieve symptoms in the case of MG, likely due to the slowing of cholinesterase activity.
The recommended assessments include
- -
- Electrodiagnostic studies to detect a decremental pattern (reduction in the amplitude of action potentials with repeated nerve stimulation), a hallmark of MG [45];
- -
- AChR antibodies, detected in 59–66.7% of patients [10,41];
- -
- Consideration of MuSK and LRP4 antibodies, especially in those with pre-existing NMJ disorders, due to their rare documentation [41];
- -
- Myocarditis and myositis evaluation via EKG, troponin, CK, or aldolase, given their frequent association with irNMJD;
- -
- MRI of the brain, spinal cord, or orbit to exclude cancer involvement of CNS.
- -
- Pulmonary function tests and fluoroscopic swallow evaluation for patients with respiratory symptoms or dysphagia;
- -
- TSH levels, given the potential impact of thyroid disorders on neuromuscular function.
4.3. irNeuropathy, Including Guillain–Barré Syndrome
4.3.1. General Overview
Neuropathies following ICI therapy encompass a broad spectrum of phenotypes, including various forms of polyradiculoneuropathies, such as GBS and rarer variants (acute inflammatory demyelinating polyneuropathy (AIDP), Miller Fisher syndrome (MFS), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN), chronic inflammatory demyelinating polyneuropathy (CIDP)) [8,10,46]. The incidence of irGBS is less than 0.5%, typically occuring within 3–4 months of therapy initiation, although delayed onset is possible [14,24,47]. irGBS is likely related to anti-CTLA-4 therapy and melanoma [10,24]. The mortality rate was reported to reach 11% [10]. Compared with idiopathic GBS, irGBS rarely occurs after an infectious episode, may present with CSF pleocytosis, and may have a better response to corticosteroids [10,14,15]. ICI-induced neuropathies may behave similarly to classical GBS but are more likely to respond to corticosteroids and often present with lymphocytic pleocytosis in CSF.
4.3.2. Clinical Presentation
The diagnosis of irGBS is primarily clinical. The key features include rapidly progressive, symmetrical, ascending weakness associated with hyporeflexia and muscle atrophy. The weakness may range from mild walking difficulties to near-complete paralysis of limb, facial, respiratory, and bulbar muscles. Sensory disturbances, such as paresthesias and neuropathic pain (usually in the lumbar and/or crural regions), along with autonomic dysfunction (blood pressure and heart rate fluctuations, urinary retention, ileus), are possible. Atypical symptoms, such as cranial nerve involvement, bulbar symptoms, and dyspnea may also be observed.
4.3.3. Diagnostic Tools
The recommended studies include
- -
- Electrodiagnostic studies, which should demonstrate a demyelinating pattern indicative of acquired polyradiculoneuropathy, occasionally with secondary axonal loss [10,47,48]. Concomitant myopathy is rare;
- -
- CSF analysis, usually showing elevated protein levels and pleocytosis [10,47,48];
- -
- Anti-ganglioside and neuronal antibodies; while typically negative in ICI-induced cases, can help identify GBS variants (anti-GQ1b for MFS or BBE) and paraneoplastic neuropathies (anti-Hu, anti-amphiphysin), respectively [10,47];
- -
- A large infectious screening, as many infections can cause idiopathic GBS: CMV, mycoplasma, HIV, campylobacter, syphilis, Lyme…;
- -
- Brain and spine MRI, especially when bulbar weakness or quadriparesis are present, to rule out a compressive lesion and other causes. Root nerve thickening and enhancement can be present in ICI-related GBS [10,23,48].
4.4. irMeningitis
4.4.1. General Overview
ICI-related meningitis has an incidence of less than 0.5% and usually occurs within 3–4 months of ICI initiation, after a median of 2 cycles (ranging from 1 to 14) [24,49,50]. Anti-CTLA-4 therapies are more likely to induce irMeningitis than anti-PD(L)-1 therapies [10,24,51]. Most cases fully recover without long-term consequences [49].
4.4.2. Clinical Presentation
The symptoms may be aspecific, ranging from headache only to meningeal syndrome (headache, neck stiffness, photophobia, vomiting, with or without fever) [10]. The signs and symptoms of encephalitis (confusion, behavioral changes, aphasia, seizure-like activity, short-term memory disturbances, or other focal neurological deficits) are associated with a worse prognosis [14,43].
4.4.3. Diagnostic Tools
The recommended diagnostic modalities include
- -
- Blood and CSF samples to exclude bacterial and viral meningitis. CSF analysis usually reveals a lymphocytic pleocytosis and hyperproteinorrachia [50];
- -
- Brain MRI, which shows meningeal enhancement in half of patients [50].
4.5. irEncephalitis
4.5.1. General Overview
irEncephalitis represents the most frequent CNS condition among n-irAEs, with an incidence of 0.5–1% [24,49]. It typically occurs within 3–4 months of ICI initiation, with a median onset of 61 days (ranging from 18 to 153 days), and it is more likely to manifest with anti-PD(L)-1 therapy and lung cancer [24]. Despite favorable outcomes in most cases, rapid deterioration may occur, leading to epilepsy, altered mental status, respiratory failure, and occasionally death [52,53].
4.5.2. Clinical Presentation
irEncephalitis often presents with aspecific symptoms [43]. Two main presentation types have been described [42,43,52]:
- -
- Diffuse, meningoencephalitis-like picture, with general symptoms (fever, headache, altered consciousness, and/or seizures) and without focal signs;
- -
- Focal presentations, including limbic encephalitis (neuropsychiatric disturbances, memory issues, behavioral changes, delusions, hallucinations, temporal epilepsy), cerebellitis (ataxia, dysmetria), brainstem encephalitis (altered vigilance, cranial nerve paresis, vertigo, bulbar syndromes), basal ganglia encephalitis (extrapyramidal syndrome with abnormal movements), dysphasia, pyramidal syndrome, sensory disturbances, and dysautonomia.
4.5.3. Diagnostic Tools
The following workup should be conducted:
- -
- Brain MRI with contrast. The most frequent findings are hyperintensities in the mesial temporal regions on T2/FLAIR sequences for limbic encephalitis and meningeal enhancement/thickening for meningoencephalitis [51]. MRI can be repeated, as radiological abnormalities are sometimes delayed [10,42,52,53,54,55];
- -
- Lumbar puncture often reveals lymphocytic pleocytosis and hyperproteinorrachia (77–98%), oligoclonal bands (38–53%), and normal or slightly raised glycorrhachia [52,53,54];
- -
- Neuronal antibody testing in blood and CSF. The seropositivity rate ranges from 6% to 54% [52,54]. Specifically, onco-neuronal antibodies targeting intracellular antigens are more common [52]. There have also been reports of other antibodies, including anti-GFAP in the context of meningoencephalitis, as well as anti-Ri, anti-GAD, anti-NMDAR, anti-CASPR2, anti-CRMP5, and anti-SOX1 [42,43]. The presence of these antibodies is often linked to an unfavorable prognosis;
- -
- EEG, which can indicate focal or diffuse slowing, subclinical seizures, or status epilepticus;
- -
- Additional assessments may involve spinal MRI, further infectious workup, and—in rare cases—brain biopsy to rule out oncological progression.
4.6. irDemyelinating Diseases (irDDs)
4.6.1. General Overview
Demyelinating diseases represent a heterogeneous group of conditions affecting the myelin sheath of nerve fibers in the brain, spinal cord, and optic nerve. These include multiple sclerosis (MS), acute disseminated encephalomyelitis (ADEM), neuromyelitis optica spectrum disorder (NMOSD), optic neuritis (ON), and transverse myelitis (TM), among others [8,56]. The incidence of irDDs is less than 0.5% [24,49]. Manifestations typically appear at a median time of 6.5 weeks after ICI initiation (ranging from 1 to 43 weeks) [56]. Most cases resolve at least partially after ICI interruption [24,49].
4.6.2. Clinical Presentation
The symptoms vary depending on the demyelinating lesions [8]:
- -
- Involvement of cerebral hemispheres can lead to muscle weakness with pyramidal signs, sensory disturbances, and mental status changes;
- -
- Posterior fossa localization is associated with diplopia, ophthalmoplegia, nystagmus, ataxia, dysmetria, dysarthria, and dysphagia;
- -
- Optic neuropathy results in reduced visual acuity, visual field loss, dyschromatopsia, afferent pupillary defect, and optic disc edema;
- -
- Transverse myelitis manifests as sensory disturbances with a sensory level, pyramidal weakness, and ataxia.
Other symptoms include numbness, paresthesia, and autonomic dysfunction (urinary and fecal incontinence).
4.6.3. Diagnostic Tools
The recommended workup includes
- -
- Brain, orbit, and/or spinal MRI with contrast, which typically shows enhancements and/or hyperintense T2/FLAIR lesions, although no definitive imaging feature has been identified [43];
- -
- Lumbar puncture, usually revealing lymphocytic pleocytosis and elevated protein levels [56];
- -
- Autoantibody testing, including demyelinating antibodies (i.e., AQP4 and MOG) in serum and spinal fluid, although the impact on sensitivity is limited compared to serum analysis alone. While most patients test negative for these antibodies, AQP4, CRMP5, Hu, or other neural antibodies can be present in some patients [10,43,56,57];
- -
- Other diagnostic tools include EEG, neuro-ophthalmologic evaluation and evoked potentials;
- -
- Brain biopsy—exceptionally—to allow definitive proof of CNS demyelination.
5. Treatment
5.1. General Management
ICIs have become indispensable in cancer treatment, significantly enhancing the response rates, progression-free survival, and overall survival. In some cancers, ICIs represent the cornerstone of therapy, and in certain cases, the only viable option. It is crucial to acknowledge the absence of prospective, randomized studies guiding therapy in this context. The current recommendations are based on retrospective data and expert opinions alone, emphasizing the need to view the proposed regimens as suggestive rather than definitive [14].
According to certain guidelines, Grade 1 n-irAEs (characterized by mild symptoms, which do not disrupt daily activities) may permit the continuation of ICI treatment in select cases. However, it is essential to exercise caution, as a Grade 1 event has the potential to escalate to a Grade 2 severity level rapidly. Delaying administration may also be considered in order to confirm the diagnosis and ensure there is no deterioration. Of course, this has to be discussed with the patient, based on the oncological situation.
To minimize the risk of exacerbating an infectious cause while addressing the potential delay in ruling out infectious meningitis, two strategies can be considered:
- (a)
- Prioritize excluding bacterial infections and—if feasible—viral infections before initiating immunosuppression;
- (b)
- Administer antimicrobials concurrently while awaiting negative PCR and culture results, particularly in severe cases.
5.2. Steroids, IVIG, and Plasmapheresis
Table 2 summarizes the recommended management of key n-irAEs.
As with most immune-induced toxicities, the initial treatment step often involves suspending the ICI and starting corticosteroid therapy [14,15]. A suitable dosage regimen is prednisolone (0.5–1 mg/kg) for Grade 2 symptoms and high-dose oral or intravenous prednisolone (1–2 mg/kg) for significant neurological toxicity (Grades 3 and 4). If no improvement occurs after the initial dosing, steroid doses can be escalated up to 2 mg/kg/day. However, for conditions such as MG, GBS, or encephalitis, it is prudent to consider higher doses, such as IV methylprednisolone at 1–4 mg/kg/day. Upon improvement, transitioning to oral steroids with a prolonged tapering over 4–8 weeks is suggested, with consideration of steroid-related adverse events, such as gastritis and osteoporosis. Re-assessment is warranted after 3–5 days, and pulse dose methylprednisolone (500–1000 mg/day for 3–5 days) has to be considered in case of deterioration.
Intravenous immunoglobulins (IVIG; 2 mg/kg/day over 3–5 days) or plasmapheresis (or plasma exchange; 5–7 sessions) should be considered in case of corticosteroid resistance, higher severity at onset, concurrent myocarditis, all cases of GBS, and the majority of MG cases, particularly in the presence of bulbar and/or respiratory involvement. The choice between IVIG and plasmapheresis should be individualized: IVIG are more readily available and require less monitoring compared to plasmapheresis, which should be favored in more acute situations due to their faster onset of action, possibly linked to the rapid clearance of autoantibodies, cytokines, and the ICI itself [58]. Contra-indications for plasmapheresis include renal failure, hypercoagulable states, sepsis, and hemodynamic instability, whereas IVIG should not be administered to individuals with a high thromboembolic risk or severe hyponatremia.
In refractory or recurrent cases, escalation of immunosuppression can include options such as abatacept, mycophenolate mofetil, tacrolimus, azathioprine, cyclophosphamide, Rituximab, anti-TNFα, and anti-IL6 therapies. Natalizumab—a monoclonal antibody, which inhibits leucocyte migration through the blood–brain barrier by blocking α4-integrin—has exhibited benefits in a case of steroid-refractory ICI-related limbic encephalitis [59].
5.3. ICI Re-Challenge
As with other irAEs, ICI re-challenge may lead to a recurrence of symptoms. Retrospective data show a 12–29% recurrence rate after re-exposure to ICI [60,61,62,63]; however, drawing conclusions is challenging, as clinical outcomes are not always reported.
Globally, ICI re-challenge in n-irAEs should be avoided; however, in cases where there are limited alternative therapies and where ICI remains crucial for oncological management, a re-challenge may need to be considered on a patient-specific basis.
Based on the general considerations for irAEs, we can suggest that
- -
- ICI re-challenge or continuation should always be discussed with the patient, and the risk/benefit balance should always be weighted. A close collaboration with neurologists specialized in n-irAEs is highly recommended;
- -
- Since irAEs may correlate with an oncologic response, assessing disease control before deciding to re-challenge or continue ICI therapy is crucial in evaluating the real benefit for the patient. Retrospective analysis of a cohort of 937 patients with melanoma treated with ICIs showed an association between the development of n-irAEs (n = 76, 8%) and longer survival (HR = 0.4, 95% CI 0.32–0.77) [63];
- -
- Reinitiating ICI can be considered if irAE severity did not exceed Grade 1 or 2, the patient’s symptoms have resolved or at least stabilized, and corticosteroids have been reduced to a dose of less than 10 mg/day of prednisone [16]. However, particularly in Grade 2, the oncological situation should be re-evaluated in order to confirm the need to reinitiate ICI.
- -
- However, for severe symptoms (Grades 3 and 4) or conditions such as MG, GBS, or transverse myelitis, permanent ICI discontinuation is advised [16].
5.4. Pre-Existing Autoimmune and Paraneoplastic Neurological Syndromes (PNSs)
ICI may also function as a trigger of both autoimmune and paraneoplastic neurological syndromes. Retrospective studies suggest that ICI may exacerbate pre-existing autoimmune neurological conditions, such as myositis, MG, and multiple sclerosis (MS), with the observed degradation rates shown to reach 71% and 60%, respectively [64,65,66]. Consequently, a risk/benefit assessment for patients with pre-existing immune-related neurological disorders is warranted before starting ICI. The use of some specific questionnaire (for instance, the one devised by Aoun et al.) and close collaboration with a neurological team dedicated to ICI complications are essential [66].
The impact of ICI on PNS is less clear. Retrospective studies indicate an increased risk of both exacerbation and de novo emergence in patients undergoing ICI treatment [67,68,69]. In practice, given the frequent overlaps in diagnostic and therapeutic features, it is recommended to classify PNSs as Grade 3–4 n-irAEs and manage them accordingly [69]; however, PNSs associated with antibodies targeting intracellular neuronal antigens tend to have a poorer response to immunosuppressive therapies compared to n-irAEs.
6. Conclusions
Recognition of n-irAEs is not easy, as they encompass a broad spectrum of intricate neurological syndromes, often with non-specific heterogeneous presentations and incomplete overlap relative to their traditional counterparts. This complexity is further amplified in the context of pre-existing autoimmune and paraneoplastic syndromes. Rapid recognition is the key, as n-irAEs carry a significant risk of lasting disability and potentially fatal outcomes compared to other irAEs.
The key indicators of n-irAE diagnosis include a close temporal relation with ICI initiation, concurrent irAEs, a simultaneous oncological response, and a clinical improvement after ICI discontinuation and/or introduction of immunosuppressive therapy.
Early recognition is of importance in this field, as n-irAEs can carry a significant risk of lasting disability and potentially fatal outcomes compared to other irAEs. The primary therapeutic strategy remains the suspension of ICI therapy and the use of corticosteroids, possibly supplemented with other immunomodulatory agents. More severe conditions, such as encephalitis, MG, and GBS, warrant more aggressive interventions.
Understanding the consequences of ICI re-challenge after an n-irAE is an area, which still requires further investigations, since prospective data are lacking.
Naturally, these considerations are challenging due to the scarcity of prospective evidence and definite consensus among the guidelines available to clinicians.
Author Contributions
Conceptualization, F.Z. and E.S.; writing—original draft preparation, F.Z. and E.S.; writing—review and editing, F.Z. and E.S.; supervision, E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iranzo, P.; Callejo, A.; Assaf, J.D.; Molina, G.; Lopez, D.E.; Garcia-Illescas, D.; Pardo, N.; Navarro, A.; Martinez-Marti, A.; Cedres, S.; et al. Overview of Checkpoint Inhibitors Mechanism of Action: Role of Immune-Related Adverse Events and Their Treatment on Progression of Underlying Cancer. Front. Med. 2022, 9, 875974. [Google Scholar] [CrossRef]
- Riha, P.; Rudd, C.E. CD28 Co-Signaling in the Adaptive Immune Response. Self Nonself 2010, 1, 231. [Google Scholar] [CrossRef]
- Patsoukis, N.; Brown, J.; Petkova, V.; Liu, F.; Li, L.; Boussiotis, V.A. Selective Effects of PD-1 on Akt and Ras Pathways Regulate Molecular Components of the Cell Cycle and Inhibit T Cell Proliferation. Sci. Signal. 2012, 5, ra46. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044. [Google Scholar] [CrossRef]
- Haslam, A.; Gill, J.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw. Open 2020, 3, e200423. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, D.; Cui, X.; Zhang, L. Meta-Analysis of Immune-Related Adverse Events of Immune Checkpoint Inhibitor Therapy in Cancer Patients. Thorac. Cancer 2020, 11, 2406–2430. [Google Scholar] [CrossRef] [PubMed]
- Cuzzubbo, S.; Javeri, F.; Tissier, M.; Roumi, A.; Barlog, C.; Doridam, J.; Lebbe, C.; Belin, C.; Ursu, R.; Carpentier, A.F. Neurological Adverse Events Associated with Immune Checkpoint Inhibitors: Review of the Literature. Eur. J. Cancer 2017, 73, 1–8. [Google Scholar] [CrossRef]
- Guidon, A.C.; Burton, L.B.; Chwalisz, B.K.; Hillis, J.; Schaller, T.H.; Amato, A.A.; Betof Warner, A.; Brastianos, P.K.; Cho, T.A.; Clardy, S.L.; et al. Consensus Disease Definitions for Neurologic Immune-Related Adverse Events of Immune Checkpoint Inhibitors. J. Immunother. Cancer 2021, 9, e002890. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.P.; Sharda, E.; Kolli, A.; Mora-Rodriguez, K.; Boldig, C.; Gatewood, T.; Shah, A.; Swank, J.; Pina, Y.; Peguero, E.; et al. Neurological Complications of Immune Checkpoint Inhibitors (P12-13.004). Neurology 2023, 100, 2188. [Google Scholar] [CrossRef]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muñiz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic Adverse Events of Immune Checkpoint Inhibitors: A Systematic Review. Neurology 2021, 96, 754–766. [Google Scholar] [CrossRef]
- Dubey, D.; David, W.S.; Reynolds, K.L.; Chute, D.F.; Clement, N.F.; Cohen, J.V.; Lawrence, D.P.; Mooradian, M.J.; Sullivan, R.J.; Guidon, A.C. Severe Neurological Toxicity of Immune Checkpoint Inhibitors: Growing Spectrum. Ann. Neurol. 2020, 87, 659–669. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Khan, E.; Shrestha, A.K.; Elkhooly, M.; Wilson, H.; Ebbert, M.; Srivastava, S.; Wen, S.; Rollins, S.; Sriwastava, S. CNS and PNS Manifestation in Immune Checkpoint Inhibitors: A Systematic Review. J. Neurol. Sci. 2022, 432, 120089. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.R.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of Toxicities from Immunotherapy: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated with Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) Clinical Practice Guideline on Immune Checkpoint Inhibitor-Related Adverse Events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef]
- Vilariño, N.; Bruna, J.; Kalofonou, F.; Anastopoulou, G.G.; Argyriou, A.A. Immune-Driven Pathogenesis of Neurotoxicity after Exposure of Cancer Patients to Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2020, 21, 5774. [Google Scholar] [CrossRef]
- Menotti, S.; Giampietro, A.; Raia, S.; Veleno, M.; Angelini, F.; Tartaglione, T.; Gaudino, S.; Doglietto, F.; De Marinis, L.; Pontecorvi, A.; et al. Unveiling the Etiopathogenic Spectrum of Hypophysitis: A Narrative Review. J. Pers. Med. 2023, 13, 1210. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-Related Adverse Events of Checkpoint Inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Zou, H.; Han, Z.; Xie, T.; Zhang, B.; Dai, D.; Yin, X.; Liang, Y.; Kou, Y.; et al. Correlation of the Gut Microbiome and Immune-Related Adverse Events in Gastrointestinal Cancer Patients Treated with Immune Checkpoint Inhibitors. Front. Cell. Infect. Microbiol. 2023, 13, 1099063. [Google Scholar] [CrossRef]
- Chaput, N.; Lepage, P.; Coutzac, C.; Soularue, E.; Le Roux, K.; Monot, C.; Boselli, L.; Routier, E.; Cassard, L.; Collins, M.; et al. Baseline Gut Microbiota Predicts Clinical Response and Colitis in Metastatic Melanoma Patients Treated with Ipilimumab. Ann. Oncol. 2017, 28, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Muñiz-Castrillo, S.; Farina, A.; Honnorat, J.; Joubert, B. How to Diagnose and Manage Neurological Toxicities of Immune Checkpoint Inhibitors: An Update. J. Neurol. 2022, 269, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; David, W.; Reynolds, K.; Chute, D.; Clement, N.F.; Cohen, J.V.; Lawrence, D.P.; Mooradian, M.J.; Sullivan, R.J.; Guidon, A. Immune Checkpoint Inhibitor Related Neurologic Adverse Events: Clinical Spectrum, Management and Outcomes (S21.003). Neurology 2019, 92, 15. [Google Scholar] [CrossRef]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.E. Neurologic Toxicity Associated with Immune Checkpoint Inhibitors: A Pharmacovigilance Study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Q.; Tang, L.L.; Mao, Y.P.; Li, W.F.; Chen, L.; Zhang, Y.; Guo, Y.; Liu, Q.; Sun, Y.; Xu, C.; et al. The Pattern of Time to Onset and Resolution of Immune-Related Adverse Events Caused by Immune Checkpoint Inhibitors in Cancer: A Pooled Analysis of 23 Clinical Trials and 8,436 Patients. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2021, 53, 339. [Google Scholar] [CrossRef]
- Seki, M.; Kitano, S.; Suzuki, S. Neurological Disorders Associated with Immune Checkpoint Inhibitors: An Association with Autoantibodies. Cancer Immunol. Immunother. 2022, 71, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Déchelotte, B.; Muñiz-Castrillo, S.; Joubert, B.; Vogrig, A.; Picard, G.; Rogemond, V.; Pinto, A.L.; Lombard, C.; Desestret, V.; Fabien, N.; et al. Diagnostic Yield of Commercial Immunodots to Diagnose Paraneoplastic Neurologic Syndromes. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e701. [Google Scholar] [CrossRef]
- Belgian Multidisciplinary Immunotoxicity Board (BITOX)—BSMO. Available online: https://www.bsmo.be/immunomanager/start/ (accessed on 27 November 2023).
- Zubiri, L.; Molina, G.E.; Mooradian, M.J.; Cohen, J.; Durbin, S.M.; Petrillo, L.; Boland, G.M.; Juric, D.; Dougan, M.; Thomas, M.F.; et al. Effect of a Multidisciplinary Severe Immunotherapy Complications Service on Outcomes for Patients Receiving Immune Checkpoint Inhibitor Therapy for Cancer. J. Immunother. Cancer 2021, 9, e002886. [Google Scholar] [CrossRef]
- Moreira, A.; Loquai, C.; Pföhler, C.; Kähler, K.C.; Knauss, S.; Heppt, M.V.; Gutzmer, R.; Dimitriou, F.; Meier, F.; Mitzel-Rink, H.; et al. Myositis and Neuromuscular Side-Effects Induced by Immune Checkpoint Inhibitors. Eur. J. Cancer 2019, 106, 12–23. [Google Scholar] [CrossRef]
- Touat, M.; Maisonobe, T.; Knauss, S.; Ben Hadj Salem, O.; Hervier, B.; Auré, K.; Szwebel, T.A.; Kramkimel, N.; Lethrosne, C.; Bruch, J.F.; et al. Immune Checkpoint Inhibitor-Related Myositis and Myocarditis in Patients with Cancer. Neurology 2018, 91, e985–e994. [Google Scholar] [CrossRef]
- Rossi, S.; Gelsomino, F.; Rinaldi, R.; Muccioli, L.; Comito, F.; Di Federico, A.; De Giglio, A.; Lamberti, G.; Andrini, E.; Mollica, V.; et al. Peripheral Nervous System Adverse Events Associated with Immune Checkpoint Inhibitors. J. Neurol. 2023, 270, 2975–2986. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Gutierrez, A.K.; Bingham, C.O.; Shah, A.A. Rheumatic and Musculoskeletal Immune-Related Adverse Events Due to Immune Checkpoint Inhibitors: A Systematic Review of the Literature. Arthritis Care Res. 2017, 69, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Tayar, J.H.; Abdel-Wahab, N.; Suarez-Almazor, M.E. Myositis as an Adverse Event of Immune Checkpoint Blockade for Cancer Therapy. Semin. Arthritis Rheum. 2019, 48, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.; Pundole, X.; Tummala, S.; Palaskas, N.; Andersen, C.R.; Shoukier, M.; Abdel-Wahab, N.; Deswal, A.; Suarez-Almazor, M.E. Inflammatory Myositis in Cancer Patients Receiving Immune Checkpoint Inhibitors. Arthritis Rheumatol. 2021, 73, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Katel, A.; Massarelli, E.; Villaflor, V.M.; Sun, V.; Salgia, R. Immune Checkpoint Inhibitor–Induced Myocarditis with Myositis/Myasthenia Gravis Overlap Syndrome: A Systematic Review of Cases. Oncologist 2021, 26, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Shelly, S.; Triplett, J.D.; Pinto, M.V.; Milone, M.; Diehn, F.E.; Zekeridou, A.; Liewluck, T. Immune Checkpoint Inhibitor-Associated Myopathy: A Clinicoseropathologically Distinct Myopathy. Brain Commun. 2020, 2, fcaa181. [Google Scholar] [CrossRef] [PubMed]
- Sechi, E.; Markovic, S.N.; McKeon, A.; Dubey, D.; Liewluck, T.; Lennon, V.A.; Lopez-Chiriboga, A.S.; Klein, C.J.; Mauermann, M.; Pittock, S.J.; et al. Neurologic Autoimmunity and Immune Checkpoint Inhibitors. Neurology 2020, 95, e2442–e2452. [Google Scholar] [CrossRef] [PubMed]
- Safa, H.; Johnson, D.H.; Trinh, V.A.; Rodgers, T.E.; Lin, H.; Suarez-Almazor, M.E.; Fa’Ak, F.; Saberian, C.; Yee, C.; Davies, M.A.; et al. Immune Checkpoint Inhibitor Related Myasthenia Gravis: Single Center Experience and Systematic Review of the Literature. J. Immunother. Cancer 2019, 7, 319. [Google Scholar] [CrossRef] [PubMed]
- Guidon, A.C. Lambert-Eaton Myasthenic Syndrome, Botulism, and Immune Checkpoint Inhibitor-Related Myasthenia Gravis. Continuum 2019, 25, 1785–1806. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chen, Y.P.; Lin, W.C.; Su, W.C.; Sun, Y.T. Immune Checkpoint Inhibitor-Induced Myasthenia Gravis. Front. Neurol. 2020, 11, 528324. [Google Scholar] [CrossRef]
- Farina, A.; Villagrán-García, M.; Honnorat, J. Neurological Adverse Events of Immune Checkpoint Inhibitors: An Update of Clinical Presentations, Diagnosis, and Management. Rev. Neurol. 2023, 179, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Alsalem, A.N.; Scarffe, L.A.; Briemberg, H.R.; Aaroe, A.E.; Harrison, R.A. Neurologic Complications of Cancer Immunotherapy. Curr. Oncol. 2023, 30, 5876–5897. [Google Scholar] [CrossRef] [PubMed]
- Giannoccaro, M.P.; Paolucci, M.; Zenesini, C.; Di Stasi, V.; Donadio, V.; Avoni, P.; Liguori, R. Comparison of Ice Pack Test and Single-Fiber EMG Diagnostic Accuracy in Patients Referred for Myasthenic Ptosis. Neurology 2020, 95, E1800–E1806. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Haggiagi, A.; Tzatha, E.; DeAngelis, L.M.; Santomasso, B. Electrophysiological Findings in Immune Checkpoint Inhibitor-Related Peripheral Neuropathy. Clin. Neurophysiol. 2019, 130, 1440–1445. [Google Scholar] [CrossRef] [PubMed]
- Dubey, D.; David, W.S.; Amato, A.A.; Reynolds, K.L.; Clement, N.F.; Chute, D.F.; Cohen, J.V.; Lawrence, D.P.; Mooradian, M.J.; Sullivan, R.J.; et al. Varied Phenotypes and Management of Immune Checkpoint Inhibitor-Associated Neuropathies. Neurology 2019, 93, e1093–e1103. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhao, C. Guillain-Barré Syndrome-like Polyneuropathy Associated with Immune Checkpoint Inhibitors: A Systematic Review of 33 Cases. Biomed. Res. Int. 2021, 2021, 9800488. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, L.; Picca, A.; Bini, P.; Gastaldi, M.; Alfonsi, E.; Pichiecchio, A.; Rota, E.; Rudà, R.; Bruno, F.; Villani, V.; et al. Characterization and Management of Neurological Adverse Events during Immune-Checkpoint Inhibitors Treatment: An Italian Multicentric Experience. Neurol. Sci. 2022, 43, 2031–2041. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Liaw, B.; Asada, M.; Niimura, T.; Zamami, Y.; Green-LaRoche, D.; Pai, L.; Levy, M.; Jeyapalan, S. Neuroimmunological Adverse Events Associated with Immune Checkpoint Inhibitor: A Retrospective, Pharmacovigilance Study Using FAERS Database. J. Neurooncol. 2021, 152, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Nannini, S.; Koshenkova, L.; Baloglu, S.; Chaussemy, D.; Noël, G.; Schott, R. Immune-Related Aseptic Meningitis and Strategies to Manage Immune Checkpoint Inhibitor Therapy: A Systematic Review. J. Neurooncol 2022, 157, 533–550. [Google Scholar] [CrossRef]
- Sato, K.; Mano, T.; Iwata, A.; Toda, T. Neurological and Related Adverse Events in Immune Checkpoint Inhibitors: A Pharmacovigilance Study from the Japanese Adverse Drug Event Report Database. J. Neurooncol 2019, 145, 1–9. [Google Scholar] [CrossRef]
- Velasco, R.; Villagrán, M.; Jové, M.; Simó, M.; Vilariño, N.; Alemany, M.; Palmero, R.; Martínez-Villacampa, M.M.; Nadal, E.; Bruna, J. Encephalitis Induced by Immune Checkpoint Inhibitors: A Systematic Review. JAMA Neurol. 2021, 78, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Nersesjan, V.; McWilliam, O.; Krarup, L.H.; Kondziella, D. Autoimmune Encephalitis Related to Cancer Treatment with Immune Checkpoint Inhibitors. Neurology 2021, 97, e191–e202. [Google Scholar] [CrossRef] [PubMed]
- Müller-Jensen, L.; Zierold, S.; Versluis, J.M.; Boehmerle, W.; Huehnchen, P.; Endres, M.; Mohr, R.; Compter, A.; Blank, C.U.; Hagenacker, T.; et al. Characteristics of Immune Checkpoint Inhibitor-Induced Encephalitis and Comparison with HSV-1 and Anti-LGI1 Encephalitis: A Retrospective Multicentre Cohort Study. Eur. J. Cancer 2022, 175, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Muñiz-Castrillo, S.; Joubert, B.; Picard, G.; Rogemond, V.; Marchal, C.; Chiappa, A.M.; Chanson, E.; Skowron, F.; Leblanc, A.; et al. Central Nervous System Complications Associated with Immune Checkpoint Inhibitors. J. Neurol. Neurosurg. Psychiatry 2020, 91, 772–778. [Google Scholar] [CrossRef]
- Oliveira, M.C.B.; de Brito, M.H.; Simabukuro, M.M. Central Nervous System Demyelination Associated with Immune Checkpoint Inhibitors: Review of the Literature. Front. Neurol. 2020, 11, 538695. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Berzero, G.; Bihan, K.; Jachiet, V.; Januel, E.; Coustans, M.; Cauquil, C.; Perrin, J.; Berlanga, P.; Kramkimel, N.; et al. Longitudinally Extensive Myelitis Associated with Immune Checkpoint Inhibitors. Neurol. Neuroimmunol. Neuroinflammation 2021, 8, e967. [Google Scholar] [CrossRef] [PubMed]
- Katsumoto, T.R.; Wilson, K.L.; Giri, V.K.; Zhu, H.; Anand, S.; Ramchandran, K.J.; Martin, B.A.; Yunce, M.; Muppidi, S. Plasma Exchange for Severe Immune-Related Adverse Events from Checkpoint Inhibitors: An Early Window of Opportunity? Immunother. Adv. 2022, 2, ltac012. [Google Scholar] [CrossRef] [PubMed]
- Hottinger, A.F.; De Micheli, R.; Guido, V.; Karampera, A.; Hagmann, P.; Du Pasquier, R. Natalizumab May Control Immune Checkpoint Inhibitor–Induced Limbic Encephalitis. Neurol. Neuroimmunol. Neuroinflammation 2018, 5, 439. [Google Scholar] [CrossRef] [PubMed]
- Eskian, M.; Singh, B.; Zubiri, L.; Burton, L.B.; Philpotts, L.; Reynolds, K.; Guidon, A. Immune Checkpoint Inhibitor Rechallenge Following Neurologic Immune-Related Adverse Events in Cancer Patients: A Systematic Review (P8-1.002). Neurology 2022, 98, 18. [Google Scholar] [CrossRef]
- Farina, A.; Birzu, C.; Elsensohn, M.H.; Picca, A.; Muniz-Castrillo, S.; Vogrig, A.; Villagran-Garcia, M.; Ciano-Petersen, N.L.; Massacesi, L.; Hervier, B.; et al. Neurological Outcomes in Immune Checkpoint Inhibitor-Related Neurotoxicity. Brain Commun. 2023, 5, 20. [Google Scholar] [CrossRef]
- Pepys, J.; Stoff, R.; Ramon-Gonen, R.; Ben-Betzalel, G.; Grynberg, S.; Frommer, R.S.; Schachter, J.; Asher, N.; Taliansky, A.; Nikitin, V.; et al. Incidence and Outcome of Neurologic Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors in Patients with Melanoma. Neurology 2023, 101, E2472–E2482. [Google Scholar] [CrossRef] [PubMed]
- Dolladille, C.; Ederhy, S.; Sassier, M.; Cautela, J.; Thuny, F.; Cohen, A.A.; Fedrizzi, S.; Chrétien, B.; Da-Silva, A.; Plane, A.F.; et al. Immune Checkpoint Inhibitor Rechallenge After Immune-Related Adverse Events in Patients with Cancer. JAMA Oncol. 2020, 6, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ishikawa, N.; Konoeda, F.; Seki, N.; Fukushima, S.; Takahashi, K.; Uhara, H.; Hasegawa, Y.; Inomata, S.; Otani, Y.; et al. Nivolumab-Related Myasthenia Gravis with Myositis and Myocarditis in Japan. Neurology 2017, 89, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Jayswal, R.; Adams, V.; Anthony, L.B.; Villano, J.L. Multiple Sclerosis Outcomes after Cancer Immunotherapy. Clin. Transl. Oncol. 2019, 21, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Aoun, R.; Gratch, D.; Kaminetzky, D.; Kister, I. Immune Checkpoint Inhibitors in Patients with Pre-Existing Neurologic Autoimmune Disorders. Curr. Neurol. Neurosci. Rep. 2023, 23, 735–750. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Fouret, M.; Joubert, B.; Picard, G.; Rogemond, V.; Pinto, A.L.; Muñiz-Castrillo, S.; Roger, M.; Raimbourg, J.; Dayen, C.; et al. Increased Frequency of Anti-Ma2 Encephalitis Associated with Immune Checkpoint Inhibitors. Neurol. Neuroimmunol. Neuroinflammation 2019, 6, e604. [Google Scholar] [CrossRef] [PubMed]
- Farina, A.; Villagrán-García, M.; Ciano-Petersen, N.L.; Vogrig, A.; Muñiz-Castrillo, S.; Taillandier, L.; Michaud, M.; Lefilliatre, M.; Wang, A.; Lepine, Z.; et al. Anti-Hu Antibodies in Patients with Neurologic Side Effects of Immune Checkpoint Inhibitors. Neurol. Neuroimmunol. Neuroinflammation 2023, 10, e200058. [Google Scholar] [CrossRef]
- Graus, F.; Dalmau, J. Paraneoplastic Neurological Syndromes in the Era of Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2019, 16, 535–548. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).