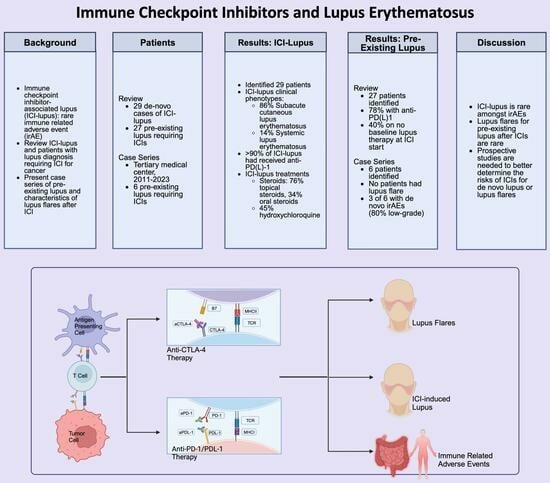
Immune Checkpoint Inhibitors and Lupus Erythematosus
Abstract
1. Introduction
2. Role of Checkpoints in the Pathogenesis of Lupus Erythematosus
2.1. PD-1 and PDL-1 and Lupus Erythematosus
2.2. CTLA-4 and Lupus Erythematosus
3. Immune Checkpoint Inhibitor-Associated Lupus Erythematosus
3.1. ICI-CLE
3.2. ICI-SLE
3.3. Management of ICI-LE
4. Pre-Existing Lupus and Treatment with Immune Checkpoint Inhibitors
Case Series of Patients with Lupus Receiving Immunotherapy
5. Review of Pre-Existing Lupus and ICI Treatment
6. Review of Any Pre-Existing Autoimmune Disease and ICI Treatment
7. Clinical Trial Studying ICI for Patients with pAIDs
8. Discussion
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The CRI Timeline. 2023. Available online: https://www.cancerresearch.org/timeline (accessed on 18 June 2023).
- Sadeghi Rad, H.; Monkman, J.; Warkiani, M.E.; Ladwa, R.; O’Byrne, K.; Rezaei, N.; Kulasinghe, A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021, 41, 1474–1498. [Google Scholar] [CrossRef]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf (accessed on 23 July 2023).
- Albandar, H.J.; Fuqua, J.; Albandar, J.M.; Safi, S.; Merrill, S.A.; Ma, P.C. Immune-related adverse events (Irae) in cancer immune checkpoint inhibitors (ici) and survival outcomes correlation: To rechallenge or not? Cancers 2021, 13, 989. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Ennis, D.; Hudson, M.; Ye, C.; Saltman, A.; Himmel, M.; Rottapel, R.; Pope, J.; Hoa, S.; Tisseverasinghe, A.; et al. Rheumatic immune-related adverse events associated with cancer immunotherapy: A nationwide multi-center cohort. Autoimmun. Rev. 2020, 19, 102595. [Google Scholar] [CrossRef]
- Dema, B.; Charles, N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies 2016, 5, 2. [Google Scholar] [CrossRef]
- Lee, Y.H.; Woo, J.H.; Choi, S.J.; Ji, J.D.; Song, G.G. Association of programmed cell death 1 polymorphisms and systemic lupus erythematosus: A meta-analysis. Lupus 2009, 18, 9–15. [Google Scholar] [CrossRef]
- Curran, C.S.; Gupta, S.; Sanz, I.; Sharon, E. PD-1 immunobiology in systemic lupus erythematosus. J. Autoimmun. 2019, 97, 1–9. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef]
- Tong, M.; Fang, X.; Yang, J.; Wu, P.; Guo, Y.; Sun, J. Abnormal membrane-bound and soluble programmed death ligand 2 (PD-L2) expression in systemic lupus erythematosus is associated with disease activity. Immunol. Lett. 2020, 227, 96–101. [Google Scholar] [CrossRef]
- Nishimura, H.; Nose, M.; Hiai, H.; Minato, N.; Honjo, T. Development of Lupus-like Autoimmune Diseases by Disruption of the PD-1 Gene Encoding an ITIM Motif-Carrying Immunoreceptor. Immunity 1999, 11, 141–151. [Google Scholar] [CrossRef]
- Liao, W.; Zheng, H.; Wu, S.; Zhang, Y.; Wang, W.; Zhang, Z.; Zhou, C.; Wu, H.; Min, J. The Systemic Activation of Programmed Death 1-PD-L1 Axis Protects Systemic Lupus Erythematosus Model from Nephritis. Am. J. Nephrol. 2017, 46, 371–379. [Google Scholar] [CrossRef]
- Eissa, E.; Kandi, E.; El-Ghobashy, N.; Abdelfattah, W.; Hammouda, Z.; Gadelsayed, S.; Bayoumi, F. Association of Disease Activity with Programmed Cell Death 1 and its Ligand Programmed Cell Death Ligand 1 Expressions in Lupus Patients. Indian, J. Rheumatol. 2022, 17, 347–352. [Google Scholar] [CrossRef]
- Liu, M.F.; Weng, C.T.; Weng, M.Y. Variable Increased Expression of Program Death-1 and Program Death-1 Ligands on Peripheral Mononuclear Cells Is Not Impaired in Patients with Systemic Lupus Erythematosus. J. Biomed. Biotechnol. 2009, 2009, 406136. [Google Scholar] [CrossRef]
- Shi, H.; Ye, J.; Teng, J.; Yin, Y.; Hu, Q.; Wu, X.; Liu, H.; Cheng, X.; Su, Y.; Liu, M.; et al. Elevated serum autoantibodies against co-inhibitory PD-1 facilitate T cell proliferation and correlate with disease activity in new-onset systemic lupus erythematosus patients. Arthritis Res. Ther. 2017, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Gotot, J.; Gottschalk, C.; Leopold, S.; Knolle, P.A.; Yagita, H.; Kurts, C.; Ludwig-Portugall, I. Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 10468–10473. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Chen, Y.J.; Ou, T.T.; Wu, C.C.; Tsai, W.C.; Liu, H.W.; Yen, J.H. Programmed death-1 gene polymorphisms in patients with systemic lupus erythematosus in Taiwan. J. Clin. Immunol. 2006, 26, 506–511. [Google Scholar] [CrossRef]
- Celhar, T.; Fairhurst, A.M. Toll-like receptors in systemic lupus erythematosus: Potential for personalized treatment. Front. Pharmacol. 2014, 5, 265. [Google Scholar] [CrossRef]
- Crow, M.K. Type I interferon in the pathogenesis of lupus. J. Immunol. 2014, 192, 5459–5468. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression [published correction appears in Cell Rep. 2019 Dec 10, 29, 3766]. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Uematsu, S.; Akira, S. Toll-like receptors and Type I interferons. J. Biol. Chem. 2007, 282, 15319–15323. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Chikuma, S. CTLA-4, an Essential Immune-Checkpoint for T-Cell Activation. Curr. Top. Microbiol. Immunol. 2017, 410, 99–126. [Google Scholar] [CrossRef]
- Gaffney, P.M.; Kearns, G.M.; Shark, K.B.; Ortmann, W.A.; Selby, S.A.; Malmgren, M.L.; Rohlf, K.E.; Ockenden, T.C.; Messner, R.P.; King, R.A.; et al. A genome-wide search for susceptibility genes in human systemic lupus erythematosus sib-pair families. Proc. Natl. Acad. Sci. USA 1998, 95, 14875–14879. [Google Scholar] [CrossRef]
- Ahmed, S.; Ihara, K.; Kanemitsu, S.; Nakashima, H.; Otsuka, T.; Tsuzaka, K.; Takeuchi, T.; Hara, T. Association of CTLA-4 but not CD28 gene polymorphisms with systemic lupus erythematosus in the Japanese population. Rheumatology 2001, 40, 662–667. [Google Scholar] [CrossRef]
- Lee, Y.; Harley, J.; Nath, S. CTLA-4 polymorphisms and systemic lupus erythematosus (SLE): A meta-analysis. Hum Genet 2005, 116, 361–367. [Google Scholar] [CrossRef]
- Stohl, W.; Jacob, N.; Quinn, W.J.; Cancro, M.P.; Gao, H.; Putterman, C.; Gao, X.; Pricop, L.; Koss, M.N. Global T cell dysregulation in non-autoimmune-prone mice promotes rapid development of BAFF-independent, systemic lupus erythematosus-like autoimmunity. J. Immunol. 2008, 181, 833–841. [Google Scholar] [CrossRef]
- Laurent, L.; Le Fur, A.; Le Bloas, R.; Néel, M.; Mary, C.; Moreau, A.; Poirier, N.; Vanhove, B.; Fakhouri, F. Prevention of lupus nephritis development in NZB/NZW mice by selective blockade of CD28. Eur. J. Immunol. 2017, 47, 1368–1376. [Google Scholar] [CrossRef]
- Walker, L.S. Treg and CTLA-4: Two intertwining pathways to immune tolerance. J. Autoimmun. 2013, 45, 49–57. [Google Scholar] [CrossRef]
- Tai, X.; Van Laethem, F.; Pobezinsky, L.; Guinter, T.; Sharrow, S.O.; Adams, A.; Granger, L.; Kruhlak, M.; Lindsten, T.; Thompson, C.B.; et al. Basis of CTLA-4 function in regulatory and conventional CD4(+) T cells. Blood 2012, 119, 5155–5163. [Google Scholar] [CrossRef] [PubMed]
- Jury, E.C.; Flores-Borja, F.; Kalsi, H.S.; Lazarus, M.; Isenberg, D.A.; Mauri, C.; Ehrenstein, M.R. Abnormal CTLA-4 function in T cells from patients with systemic lupus erythematosus. Eur. J. Immunol. 2010, 40, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Merrill, J.T.; Burgos-Vargas, R.; Westhovens, R.; Chalmers, A.; D’Cruz, D.; Wallace, D.J.; Bae, S.C.; Sigal, L.; Becker, J.C.; Kelly, S.; et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: Results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010, 62, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Richter, M.D.; Crowson, C.; Kottschade, L.A.; Finnes, H.D.; Markovic, S.N.; Thanarajasingam, U. Rheumatic Syndromes Associated with Immune Checkpoint Inhibitors: A Single-Center Cohort of Sixty-One Patients. Arthritis Rheumatol. 2019, 71, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, N.; Suarez-Almazor, M.E. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology 2019, 58 (Suppl. 7), vii40–vii48. [Google Scholar] [CrossRef] [PubMed]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bähr, O.; Eigentler, T.K.; Grimm, M.O.; Grünwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, L.C.; Shah, A.A.; Bingham, C.O., 3rd. Immune-Related Adverse Effects of Cancer Immunotherapy—Implications for Rheumatology. Rheum. Dis. Clin. N. Am. 2017, 43, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Fusellier, M.; Champiat, S.; Velter, C.; Baldini, C.; Voisin, A.L.; Danlos, F.X.; El Dakdouki, Y.; Annereau, M.; Mariette, X.; et al. Drug-induced lupus erythematosus following immunotherapy with anti-programmed death-(ligand) 1. Ann. Rheum. Dis. 2019, 78, e67. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Nakano, K.; Udagawa, S.; Fukuda, N.; Nishizawa, A.; Ono, M.; Urasaki, T.; Tomomatsu, J.; Mochizuki, T.; Shiga, T.; et al. Chilblain lupus-like cutaneous reaction associated with systemic lupus erythematosus induced by immune checkpoint inhibitor. Rheumatology 2021, 61, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Manjunath, J.; Mochel, M.; Nutan, F. Nivolumab-Induced de novo Discoid Lupus Erythematosus. Case Rep. Dermatol. 2022, 14, 88–92. [Google Scholar] [CrossRef]
- Fadel, F.; El Karoui, K.; Knebelmann, B. Anti-CTLA4 antibody-induced lupus nephritis. N. Engl. J. Med. 2009, 361, 211–212. [Google Scholar] [CrossRef]
- Kosche, C.; Owen, J.L.; Choi, J.N. Widespread subacute cutaneous lupus erythematosus in a patient receiving checkpoint inhibitor immunotherapy with ipilimumab and nivolumab. Dermatol. Online J. 2019, 25, e51–e53. [Google Scholar] [CrossRef]
- Marano, A.L.; Clarke, J.M.; Morse, M.A.; Shah, A.; Barrow, W.; Selim, M.A.; Hall, R.P., III; Cardones, A.R. Subacute cutaneous lupus erythematosus and dermatomyositis associated with anti-programmed cell death 1 therapy. Br. J. Dermatol. 2018, 181, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; McGettigan, S.; Elenitsas, R.; Chu, E.Y. Lupus-like cutaneous reaction following pembrolizumab: An immune-related adverse event associated with anti-PD-1 therapy. J. Cutan. Pathol. 2017, 45, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Pratumchart, N.; Chanprapaph, K.; Topibulpong, N.; Tankunakorn, J. Subacute Cutaneous Lupus Erythematosus-Like Eruption Induced by Durvalumab: A Case Report and Literature Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.C.; Sebaratnam, D.F.; Jackett, L.; Kao, S.; Lowe, P.M. Subacute cutaneous lupus erythematosus induced by nivolumab. Australas J. Dermatol. 2018, 59, e152–e154. [Google Scholar] [CrossRef] [PubMed]
- Andersson, N.W.; Zachariae, C.; Simonsen, A.B. Late onset of subacute cutaneous lupus erythematosus following pembrolizumab therapy. Eur. J. Cancer. 2021, 145, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Ogawa-Momohara, M.; Muro, Y.; Goto, K.; Obuse, C.; Satoh, M.; Kono, M.; Akiyama, M. Subacute cutaneous lupus erythematosus with melanocyte elimination induced by pembrolizumab. J. Dermatol. 2020, 47, e217–e219. [Google Scholar] [CrossRef] [PubMed]
- Bui, A.N.; Hirner, J.; Singer, S.B.; Eberly-Puleo, A.; Larocca, C.; Lian, C.G.; LeBoeuf, N.R. De novo subacute cutaneous lupus erythematosus-like eruptions in the setting of programmed death-1 or programmed death ligand-1 inhibitor therapy: Clinicopathological correlation. Clin. Exp. Dermatol. 2021, 46, 328–337. [Google Scholar] [CrossRef]
- Diago, A.; Hueso, L.; Ara-Martín, M.; Abadías-Granado, I. Subacute cutaneous lupus erythematosus induced by PD-1 Inhibitor therapy: Two case reports and literature review. Australas J. Dermatol. 2021, 62, e347–e349. [Google Scholar] [CrossRef]
- Gambichler, T.; Doerler, M.; Scheel, C.H. Onset of subacute cutaneous lupus erythematosus after the initiation of immune checkpoint inhibitor therapy of cancer. Lupus 2021, 30, 531–533. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Mancuso, S.; Lucchetti, R.; Conti, F. Systemic lupus erythematosus onset in patient receiving anti-PD1 treatment with pembrolizumab: A case report. Rheumatology 2021, 60, e39–e40. [Google Scholar] [CrossRef]
- Blakeway, E.A.; Elshimy, N.; Muinonen-Martin, A.; Marples, M.; Mathew, B.; Mitra, A. Cutaneous lupus associated with pembrolizumab therapy for advanced melanoma: A report of three cases. Melanoma Res. 2019, 29, 338–341. [Google Scholar] [CrossRef]
- Zitouni, N.B.; Arnault, J.P.; Dadban, A.; Attencourt, C.; Lok, C.C.; Chaby, G. Subacute cutaneous lupus erythematosus induced by nivolumab: Two case reports and a literature review. Melanoma Res. 2019, 29, 212–215. [Google Scholar] [CrossRef]
- Rekvig, O.P. SLE classification criteria: Science-based icons or algorithmic distractions—An intellectually demanding dilemma. Front. Immunol. 2022, 13, 1011591. [Google Scholar] [CrossRef]
- Spagnoletti, A.; Platania, M.; Brambilla, M.; Occhipinti, M.; Canziani, L.; Cabras, A.; Provenzano, L.; Leone, A.G.; Ambrosini, P.; Prelaj, A. Systemic lupus erythematosus reactivation after chemoimmunotherapy in preexisting autoimmune disease. Tumori J. 2022, 108, 609–614. [Google Scholar] [CrossRef]
- Zakharian, L.; Lee, L. Successful Use of Immunotherapy in a Patient with Metastatic Squamous Cell Lung Cancer and Underlying Autoimmune Disease. Cureus 2021, 13, e15918. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, G.C.; Gainor, J.F.; Altan, M.; Kravets, S.; Dahlberg, S.E.; Gedmintas, L.; Azimi, R.; Rizvi, H.; Riess, J.W.; Hellmann, M.D.; et al. Safety of Programmed Death-1 Pathway Inhibitors Among Patients with Non-Small-Cell Lung Cancer and Preexisting Autoimmune Disorders. J. Clin. Oncol. 2018, 36, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Issa, M.; Dzimitrowicz, H.; Tripathi, A.; Beuselinck, B.; Lam, E.; Zakharia, Y.; Mckay, R.; Shah, S.; Mortazavi, A.; et al. Safety and efficacy of immune checkpoint inhibitors in advanced urological cancers with pre-existing autoimmune disorders: A retrospective international multicenter study. J. Immunother. Cancer 2020, 8, e000538. [Google Scholar] [CrossRef]
- Tison, A.; Quéré, G.; Misery, L.; Funck-Brentano, E.; Danlos, F.X.; Routier, E.; Robert, C.; Loriot, Y.; Lambotte, O.; Bonniaud, B.; et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients with Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019, 71, 2100–2111. [Google Scholar] [CrossRef]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab Therapy in Patients with Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016, 2, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Doberstein, T.; Amberker, R.R.; Garje, R.; Field, E.H.; Singh, N. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: A single-center experience. Medicine 2019, 98, e17348. [Google Scholar] [CrossRef] [PubMed]
- Danlos, F.X.; Voisin, A.L.; Dyevre, V.; Michot, J.M.; Routier, E.; Taillade, L.; Champiat, S.; Aspeslagh, S.; Haroche, J.; Albiges, L.; et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur. J. Cancer 2018, 91, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Buti, S.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Bersanelli, M.; Michiara, M.; Grassadonia, A.; Brocco, D.; et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist 2019, 24, e327–e337. [Google Scholar] [CrossRef] [PubMed]
- Haanen, J.; Ernstoff, M.S.; Wang, Y.; Menzies, A.M.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.A.; Obeid, M. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 2020, 31, 724–744. [Google Scholar] [CrossRef]
- Franks, A.L.; Slansky, J.E. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012, 32, 1119–1136. [Google Scholar]
- Kehl, K.L.; Yang, S.; Awad, M.M.; Palmer, N.; Kohane, I.S.; Schrag, D. Pre-existing autoimmune disease and the risk of immune-related adverse events among patients receiving checkpoint inhibitors for cancer. Cancer Immunol. Immunother. 2019, 68, 917–926. [Google Scholar] [CrossRef]
| Patient | Age | Sex | History of Autoimmune Disease | Prior irAE | Malignancy | ICI | Time to Lupus Manifestation Onset (Months) | Diagnosis | Systemic Lupus Erythematosus Criteria Met [55] | Positive Serologies | Negative Serologies | Histopathology | Treatment | Outcomes of Lupus Manifestations | Time to Lupus Symptom Resolution | Resumption of ICI? | Tumor Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 74 | M | None | None | SCLC | Durvalumab | 2 | SCLE | None | ANA, SSA, SSB | Anti-dsDNA, Anti-Sm | H&E: Superficial perivascular infiltrate, epidermal atrophy with marked interface change, thin and necrosed epidermis with dysmaturation of atypical basal keratinocyes | HCQ, prednisolone, topical corticosteroid | Resolved | 1 month | No | Progression | Pratumchart et al., 2022 [45] |
| 2 | 58 | F | AIHA | None | NSCLC | Nivolumab | 5 | SCLE | None | SSA, anti-cardiolipin | NR | H&E: Epidermal atrophy, an interface dermatitis composed of a lymphocytic and histiocytic infiltrate and moderate basal vacuolar damage with the presence of colloid bodies | HCQ, prednisolone, topical corticosteroid | Resolved | NR | Yes | NR | Liu et al., 2018 [46] |
| 3 | 62 | F | None | None | NSCLC | Pembrolizumab | 47 | SCLE | None | ANA, SSA, SSB | NR | H&E: Interface dermatitis with Civatte bodies | HCQ, prednisolone, topical corticosteroid | Resolved | 3 months | NR | NR | Andersson et al., 2021 [47] |
| 4 | 80 | M | None | None | Melanoma | Pembrolizumab | 6.75 | SCLE | None | SSA, anti-cardiolipin | Anti-dsDNA | H&E: Infiltration of lymphocytes in the basement membrane zone, the superficial dermis, and perivascular regions | Prednisolone, topical corticosteroid | Resolved | 3 months | No | NR | Ogawa et al., 2020 [48] |
| 5 | 54 | M | None | None | Melanoma | Pembrolizumab | 7 | SCLE | None | NR | NR | H&E: Interface dermatitis, perivascular and perifollicular lymphocytic infiltrate, occasional dyskeratotic keratinocytes | None | Resolved | 1 month | No | NR | Shao et al., 2017 [44] |
| 6 | 75 | F | None | None | Serous Ovarian carcinoma | Ipilimumab + Nivolumab | 1.5 | SCLE | None | ANA, SSA | Anti-dsDNA, Anti-Sm, SSB | H&E: Interface lymphocytic infiltrate and focal basal vacuolar change | HCQ, quinacrine, prednisone, topical corticosteroid | Resolved | 2 months | Switched to Pembrolizumab | NR | Kosche et al., 2019 [42] |
| 7 | 60 | M | None | None | SCLC | Nivolumab | 0.5 | SCLE | None | SSA | NR | H&E: Interface dermatitis | HCQ, prednisone, topical corticosteroid | Resolved | 2 months | No | Progression | Marano et al., 2019 [43] |
| 8 | 60 | F | None | None | NSCLC | Pembrolizumab | 0.5 | SCLE | None | ANA, SSA, SSB, anti-histone | NR | H&E: Interface dermatitis with adnexal involvement and increased dermal mucin | Prednisone, infliximab, topical corticosteroid | Resolved | 1 month | No | No Response | Marano et al., 2019 [43] |
| 9 | 54 | F | None | Psoriasis | SCLC | Nivolumab | 20 | SCLE | None | ANA, SSA, SSB | Anti-dsDNA | H&E: Focal interface dermatitis, focal lichenoid dermal lymphocytes infiltrate, and mild dermal mucin deposition | HCQ, topical corticosteroid | Resolved | 6 months | Continued (no interruption) | NR | Bui et al., 2021 [49] |
| 10 | 54 | F | None | None | Ovarian carcinoma | Pembrolizumab | 4 | SCLE | None | NR | ANA, SSA, SSB, Anti-dsDNA | H&E: Interface dermatitis, epidermal spongiosis, superficial dermal perivascular lymphocytes infiltrate with rare eosinophils, follicular plugging and subtle dermal mucin deposition | Topical corticosteroid | Resolved | 2 months | Yes | NR | Bui et al., 2021 [49] |
| 11 | 57 | F | None | Sjogren’s, Colitis | Breast adenocarcinoma | Atezolizumab | 11.5 | SCLE | None | ANA, SSA | SSB, Anti-dsDNA | H&E: Interface dermatitis, focal lichenoid infiltrate, superficial to mid-dermal perivascular lymphocytic infiltrate, perifollicular plugging and increased dermal mucin deposition | Topical corticosteroid | Resolved | 1 month | No | Progression | Bui et al., 2021 [49] |
| 12 | 65 | M | None | None | SCLC | Pembrolizumab | 3 | SCLE | None | ANA, SSA | SSB, Anti-dsDNA | H&E: Prominent interface dermatitis, focal vesicle formation, lichenoid infiltrate, prominent dyskeratotic keratinocytes with epidermal necrosis, and superficial to mid-dermal perivascular, periadnexal lymphocytic infiltrate and follicular plugging | HCQ, topical corticosteroid | Resolved | 2 months | No | Progression | Bui et al., 2021 [49] |
| 13 | 60 | M | None | None | Melanoma | Nivolumab | 0.5 | SCLE | None | ANA, SSA | SSB | H&E: Prominent interface dermatitis, lichenoid infiltrate, clefting, prominent superficial to deep dermal perivascular, periadnexal lymphocytic infiltrate and increased dermal mucin deposition | Topical corticosteroid | Resolved | 2 months | Continued (no interruption) | NR | Bui et al., 2021 [49] |
| 14 | 75 | M | None | None | NSCLC | Nivolumab | 3.75 | SCLE | None | ANA, SSA | NR | H&E: Lymphoid inflammatory infiltrate in the upper dermis with moderate basal vacuolar damage and an appreciable dermal mucin deposit with thickening of basement membrane | Prednisone | Resolved | NR | Yes | NR | Diago et al., 2021 [50] |
| 15 | 66 | F | None | None | NSCLC | Nivolumab | 6.75 | SCLE | None | ANA, SSA | NR | H&E: Lymphoid inflammatory infiltrate in the upper dermis with moderate basal vacuolar damage, and an appreciable dermal mucin deposit with thickening of basement membrane | Prednisone | Refractory | NA | No | NR | Diago et al., 2021 [50] |
| 16 | 49 | FF | None | None | Oropharyngeal SCC | Pembrolizumab | 0.5 | Chilblain CLE, SLE | SLICC: chronic cutaneous lupus (chilblain lupus), lymphopenia, positive anti-nuclear antibody, positive anti-Smith antibody and low C3 | ANA, SSA, Anti-Sm | SSB, Anti-dsDNA, Anti-phospholipid, ANCA, Cryglobulins | NA | HCQ, prednisolone | Resolved | NR | NR | NR | Takeda et al., 2021 [39] |
| 17 | 52 | M | None | None | NSCLC | Pembrolizumab | 1.5 | SCLE | None | SSA | NR | H&E: Focal vaculoar interface dermatiti, perivascular lymphocytic infiltrate | Prednisolone | Resolved | NR | NR | NR | Gambicher et al., 2021 [51] |
| 18 | 48 | F | None | None | Breast adenocarcinoma | Atezolizumab | 1.5 | SCLE | None | NR | ANA, anti-dsDNA, ENA | H&E: Inflammatory monomorphic lymphocyte infiltrate in perivascular and periadnexal sites throughout the dermis | Topical corticosteroid | Resolved | 0.5 months | Continued (no interruption) | Partial Response | Michot et al., 2019 [38] |
| 19 | 80 | F | None | None | DLBCL | Nivolumab | 3.5 | SCLE | None | NR | ANA, anti-dsDNA, ENA | H&E: Inflammatory perivascular lymphocytic infiltrate of the upper and middle dermis | Topical corticosteroid | Resolved | 0.75 months | No | Progression | Michot et al., 2019 [38] |
| 20 | 66 | F | None | None | Epidermoid carcinoma | Nivolumab | 1 | SCLE | None | ANA, SSA, ENA (SSA) | Anti-dsDNA | H&E: Perivascular lymphocytic infiltrate of the upper dermis with discrete vacuolization of the epidermal basal layer | Topical corticosteroid | Resolved | 0.5 months | Yes | Progression-free survival | Michot et al., 2019 [38] |
| 21 | 63 | M | None | None | Melanoma | Pembrolizumab | 5.5 | SCLE, SLE | SLICC: SCLE, arthralgia, positive antibodies | ANA, SSA, SSB, ENA (SSA, SSB) | Anti-dsDNA | H&E: Lichenoid dermatosis with staged apoptotic bodies in the epidermis. Peripheral inflammatory mononuclear infiltrate in the upper dermis | HCQ, topical corticosteroid | Resolved | 1 month | No | CR | Michot et al., 2019 [38] |
| 22 | 48 | M | None | None | Melanoma | Pembrolizumab | 2.5 | Chilblain CLE | None | NR | ANA, anti-dsDNA | NA | Topical corticosteroid | Resolved | 0.5 months | No | Partial Response | Michot et al., 2019 [38] |
| 23 | 61 | M | None | None | HCC | Nivolumab | 21 | DLE | None | ANA 1:80 | Unreported, 2 months following treatment SSA and anti-histone negative | H&E: Lichenoid interface inflammation with numerous dyskeratotic keratinocytes, pigment incontinence, parakeratosis, follicular plugging, and a dermal perivascular lymphocytic infiltrate | HCQ, topical corticosteroid | Resolved | 2 months | Yes | Progression-free survival | Marjunath et al., 2022 [40] |
| 24 | 64 | M | None | None | Melanoma | Ipilimumab | 1.5 | Lupus nephritis | EULAR 2019: lupus nephritis, antibodies | ANA, anti-dsDNA (regressed following ipilimumab halt) | NR | Kidney bx: Hypertrophy of podocytes and extramembranous deposits. An immunofluorescence study revealed extramembranous and mesangial deposits of IgG, IgM, C3, and C1q. Electron microscopy confirmed the presence of granular, electron-dense extramembranous deposits. | Prednisone | Resolved | 12 months | No | CR | Fadel et al., 2009 [41] |
| 25 | 52 | F | None | None | NSCLC | Pembrolizumab | 0.5 | SLE, SCLE | EULAR 1997: SCLE, arthritis, antibodies | ANA | NR | H&E: Epidermal mild atrophy, vacuolization of epidermal keratinocytes, perivascular inflammatory cell infiltration and epidermis leukocytes exocytosis | HCQ, prednisone | Refractory | NA | No | NR | Ceccarelli et al., 2021 [52] |
| 26 | 79 | F | None | None | Melanoma | Pembrolizumab | 2.5 | SCLE | None | None | ANA | H&E: Vacuolar interface dermatitis with colloid bodies and dermal perivascular lymphocytic infiltrate | Topical corticosteroid | Resolved | 0.75 months | Yes | Partial Response | Blakeway et al., 2019 [53] |
| 27 | 75 | M | None | None | Melanoma | Pembrolizumab | 4.5 | SCLE | None | None | ANA | H&E: Vacuolar interface dermatitis with colloid bodies and dermal perivascular lymphocytic infiltrate | Topical corticosteroid | Resolved | 0.75 months | Yes | Partial Response | Blakeway et al., 2019 [53] |
| 28 | 72 | F | None | Hepatitis | Melanoma | Nivolumab | 11 | SCLE | None | ANA, SSA, SSB | dsDNA | H&E: Lymphoid inflammatory infiltrates predominantly in perivascular areas, with focal lesions of the dermis and epidermis | HCQ, topical corticosteroid | Resolved | 3 months | No | CR | Zitouni et al., 2019 [54] |
| 29 | 43 | M | None | None | NSCLC | Nivolumab | 1.5 | SCLE | None | ANA, SSA | NR | H&E: Lymphoid perivascular inflammatory infiltrates | HCQ, prednisone, topical corticosteroid | Resolved | 0.5 months | No | Progression | Zitouni et al., 2019 [54] |
| Variable | Value |
|---|---|
| Mean Age (Years) | 62.28 |
| Sex | 15/29 (51.7%) F, 14/29 (48.3%) M |
| History of Autoimmune Disease | 1/29 (3.4%) |
| Prior irAE | 3/29 (10.3%) |
| Malignancy | 9/29 (31%) Melanoma, 7/29 (24.1%) NSCLC, 13/29 (44.8%) Other |
| ICI | 27/29 (93.2%) aPD-1/aPDL-1, 1/29 (3.4%) anti-CTLA-4, 1/29 (3.4%) Combination |
| Mean time to lupus manifestation onset (months) | 6.14 |
| Diagnosis | 4/29 (13.7%) SLE diagnosis, 25/29 (86.2%) SCLE diagnosis, 2/29 (8.6%) Chilblain diagnosis, 1/29 (3.4%) DLE diagnosis |
| Systemic Lupus Erythematosus criteria met [55] | 4/29 (13.7%) met criteria |
| Outcomes of lupus manifestations | 27/29 (93.2%) with resolution, 2/29 (6.8%) with refractory manifestations |
| Time to lupus symptom resolution | 2.1 |
| Resumption of ICI? | 7/29 (24.1%) resumed ICI, 3/29 (10.3%) continued ICI through manifestations, 3/29 (10.3%) did not have data reported, 15/29 (51.7%) halted ICI permanently, 1/29 (3.4%) switched ICIs |
| Pt ID | Sex | Age at Lupus Diagnosis | Lupus Type | Therapy (Prior to ICI) | Age at Cancer Diagnosis | Malignancy | Prior LE Manifestations | Antibodies | ICI Regimen | ICI Start Date | Concurrent Chemotherapy | Changes to LE Tx at ICI Start | LE Tx after ICI Start | Toxicity Type | CTCAE | Toxicity Tx | Adverse Effects of Toxicity Tx | Toxicity Outcome | Cancer Progression after ICI? | ICI Tx Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | NR | SLE + CLE | HCQ | 69 | NSCLC | Sicca symptoms, photosensitive rash, Raynaud’s | dsDNA+, RNP+ | Durvalumab; Atezolizumab | 5/12/2023, then 6/21/23 | Cisplatin + Pemetrexed; Abraxane | None | HCQ | NA | NA | NA | NA | NA | PD | Ongoing |
| 2 | F | 43 | SLE + CLE | HCQ | 65 | Neuroendocrine tumor | Arthralgias, rash | dsDNA+, SSA+ | Atezolizumab | 12/24/2019 | Carboplatin + Etoposide | None | HCQ | Vitiligo, Thrombocytopenia | Grade 1; Grade 1 | Desonide | None | Ongoing; Resolved | Stable | Discontinued |
| 3 | M | 63 | DLE | None | 62 | NSCLC | Rash | ANA+ | Durvalumab | 11/2/2022 | Cisplatin + Pemetrexed | None | Desonide cream | NA | NA | NA | NA | NA | Stable | Ongoing |
| 4 | M | 42 | SLE | Lose dose prednisone, HCQ | 59 | NCSLC | Inflammatory arthritis, arthralgias and fatigue, lupus nephritis (Class V) | ANA+, dsDNA+, RNP+, Sm+, SSB+ | Pembrolizumab | 11/17/2020 | Cisplatin + Pemetrexed | None | HCQ | Myocarditis | Grade 3 | IV solumedrol, prednisone | NR | Resolved | PD (deceased) | Discontinued |
| 5 | F | 50 | CLE + SLE | Hydroxychloroquine | 55 | NSCLC | Arthralgias, oral ulcers, photosensitivity | ANA+, dsDNA+ | Pembrolizumab | 2/19/2019 | Cisplatin + Pemetrexed | None | Hydroxychloroquine | Colitis, OA =/− IA | Grade 2; Grade 2 | Prednisone, Mesalamine, Vedolizumab, Leflunomide | None | Resolved; Ongoing | CR | Held, resumed, completed |
| 6 | F | NR | SLE | HCQ | 55 | Nasopharyngeal SCC | NR | NR | Pembrolizumab | 10/7/2022 | Carboplatin + Gemcitabine | None | None | NA | NA | NA | NA | NA | PR | Ongoing |
| Patient Information | |
|---|---|
| Variable | n (%) of Total Six Patients |
| Median Age LE Diagnosis in years (IQR) | 60.5 (56, 64.3) |
| Sex n (%) | M = 2 (33.3%) F = 4 (66.6%) |
| Malignancy Type | |
| NSCLC | 4 (66.6%) |
| SCC | 1 (16.6%) |
| Neuroendocrine | 1 (16.6%) |
| Median Age Malignancy Diagnosis in years (IQR) | 46.5 (42.8, 53.3) |
| Lupus Type | |
| SLE | 2 (33.3%) |
| DLE | 1 (16.6%) |
| SLE + CLE | 3 (50%) |
| Immunotherapy Type | |
| Anti- PD-1/PDL-1 | 6 (100%) |
| Concurrent Chemotherapy | 6 (100%) |
| Patients with irAEs | 3 (50%) |
| # Lupus Flares | 0 |
| IrAE Information | |
| Total # irAEs | n (%) of Total Five irAEs |
| CTCAE grading | |
| Grade 1–2 irAEs | 4 (80%) |
| Grade 3–4 irAEs | 1 (20%) |
| irAE Outcomes | |
| Resolved/Improved | 3 (60%) |
| Ongoing | 2 (40%) |
| ICI and Cancer Outcomes of Note for Six Total Patients | |
| Permanent Discontinuation of ICI | 2 (33%) |
| Malignancy Outcome | |
| Progression | 2 (33%) |
| Stable | 2 (33%) |
| Response | 2 (33%) |
| Patient | Age | Sex | Malignancy | ICI | Lupus Type | Prior Lupus Manifestations | irAE Type | Lupus Tx at Time of ICI Start | Lupus Activity at Time of ICI Start (SLEDAI if Reported for SLE Patients) | Time to Flare/irAE (Months) | CTCAE Grade (Lupus Flare) | CTCAE Grade (irAE) | Tx of Flare/irAE | Outcomes of Lupus Flare/irAE | Time to Lupus Flare/irAE Resolution (Months) | ICI Resumption? | Tumor Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 53 | F | Melanoma | Pembrolizumab | DLE | Discoid rash | NA | HCQ | NR | 0.75 | Grade 1–2 | NA | Systemic corticosteroids | NR | NR | No | Progression | Blakeway et al., [53] |
| 2 | 66 | F | NSCLC | Pembrolizumab | SLE | Inflammatory arthritis, thrombocytopenia, AKI, Libman–Sacks endocarditis, and generalized seizures | ITP | Low dose prednisone | Active, mild (SLEDAI 4) | 0.5 | Grade 3 | Grade 4 | Methylprednisolone, HCQ, IVIG | Resolved | NR | No | Stable | Spagnoletti et al., [56] |
| 3 | NR | NR | Melanoma | Anti-PD-1 | SLE | NR | Unknown | HCQ | Active, unspecified | NR | Grade 1–2 | Grade 1–2 | None | NR | NR | Continued | NR | Tison et al., [60] |
| 4 | NR | NR | Melanoma | Anti-PD-1 | SLE | NR | NA | HCQ | Active, unspecified | NA | NA | NA | NA | NA | NA | NA | NR | Tison et al., [60] |
| 5 | NR | NR | Melanoma | Ipilimumab | SLE | NR | NA | None | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Tison et al., [60] |
| 6 | NR | NR | Melanoma | Anti-PD-1 | SLE | NR | NA | None | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Tison et al., [60] |
| 7 | NR | NR | NSCLC | Anti-PD-1 | SLE | NR | NA | None | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Tison et al., [60] |
| 8 | NR | NR | NSCLC | Anti-PD-1 | SLE | NR | Vitiligo | None | Inactive | NR | Grade 1–2 | Grade 1–2 | None | NA | NA | NA | NR | Tison et al., [60] |
| 9 | NR | NR | NSCLC | Anti-PD-1 | SLE | NR | Vitiligo | None | Inactive | NR | Grade 1–2 | Grade 1–2 | None | NA | NA | NA | NR | Tison et al., [60] |
| 10 | 68 | M | NSCLC | Pembrolizumab | SLE | Discoid rash | NA | NR | NR | NR | NA | Grade 3 | Topical corticosteroids | Resolved | NR | Continued | Partial response | Zakharian et al., [57] |
| 11 | NR | NR | NR | Anti-PD-1 | SLE | NR | NA | NR | Inactive | 8.5 | Grade 1–2 | NA | Topical corticosteroids, prednisone | Improved | 1 | Yes | Stable | Leonardi et al., [58] |
| 12 | NR | NR | NR | Anti-PD-1 | SLE | NR | Central DI | NR | Inactive | 2.5 | NA | NA | Desmopressin | Resolved | NR | Yes | NR | Leonardi et al., [58] |
| 13 | NR | NR | UC | Anti-PD-1 | SLE | NR | NA | None | Inactive | 5 | NA | NA | Systemic corticosteroids | Improved | NR | Yes | NR | Martinez Chanza et al., [59] |
| 14 | NR | NR | UC | Anti-PD-1 | SLE | NR | NA | None | Active, mild | NA | NA | NA | NA | NA | NA | NA | NR | Martinez Chanza et al., [59] |
| 15 | NR | NR | UC | Anti-PD-1 | SLE | NR | NA | None | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Martinez Chanza et al., [59] |
| 16 | NR | NR | UC | Anti-PD-1 | SLE | NR | NA | None | Active, mild | NA | NA | NA | NA | NA | NA | NA | NR | Martinez Chanza et al., [59] |
| 17 | NR | NR | UC | NR | DLE | NR | NA | NR | NR | NR | Grade 1–2 | NA | NR | NR | NR | NR | NR | Martinez Chanza et al., [59] |
| 18 | NR | NR | UC | NR | DLE | NR | NA | NR | NR | NA | NA | NA | NA | NA | NA | NA | NR | Martinez Chanza et al., [59] |
| 19 | NR | NR | RCC | NR | DLE | NR | NA | NR | NR | NA | NA | NA | NA | NA | NA | NA | NR | Martinez Chanza et al., [59] |
| 20 | NR | NR | Melanoma | Ipilimumab | SLE | Arthralgias | NA | Prednisone, HCQ | NR | NA | NA | NA | NA | NA | NA | NA | NR | Johnson et al., [62] |
| 21 | NR | NR | Melanoma | Ipilimumab | SLE | NR | NA | HCQ | NR | NA | NA | NA | NA | NA | NA | NA | NR | Johnson et al., [62] |
| 22 | NR | NR | Melanoma | Anti-PD-1 | SLE | NR | NA | NR | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Menzies et al., [61] |
| 23 | NR | NR | Melanoma | Anti-PD-1 | SLE | NR | NA | NR | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Menzies et al., [61] |
| 24 | NR | NR | NR | Anti-PD-1 | CLE | NR | NA | NR | NR | NA | NA | NA | NA | NA | NA | NA | NR | Danlos et al., [64] |
| 25 | NR | NR | NR | Anti-PD-1 | DLE | NR | NA | NR | NR | NA | NA | NA | NA | NA | NA | NA | NR | Kaur et al., [63] |
| 26 | NR | NR | NR | Anti-PD-1 | SLE | NR | NA | None | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Cortellini et al., [65] |
| 27 | NR | NR | NR | Anti-PD-1 | SLE | NR | NA | None | Inactive | NA | NA | NA | NA | NA | NA | NA | NR | Cortellini et al., [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitzthum von Eckstaedt, H.; Singh, A.; Reid, P.; Trotter, K. Immune Checkpoint Inhibitors and Lupus Erythematosus. Pharmaceuticals 2024, 17, 252. https://doi.org/10.3390/ph17020252
Vitzthum von Eckstaedt H, Singh A, Reid P, Trotter K. Immune Checkpoint Inhibitors and Lupus Erythematosus. Pharmaceuticals. 2024; 17(2):252. https://doi.org/10.3390/ph17020252
Chicago/Turabian StyleVitzthum von Eckstaedt, Hans, Arohi Singh, Pankti Reid, and Kimberly Trotter. 2024. "Immune Checkpoint Inhibitors and Lupus Erythematosus" Pharmaceuticals 17, no. 2: 252. https://doi.org/10.3390/ph17020252
APA StyleVitzthum von Eckstaedt, H., Singh, A., Reid, P., & Trotter, K. (2024). Immune Checkpoint Inhibitors and Lupus Erythematosus. Pharmaceuticals, 17(2), 252. https://doi.org/10.3390/ph17020252