Effects of Administration and Intensity of Statins on Mortality in Patients Undergoing Hemodialysis
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics
2.2. Survival Analyses
2.3. Analyses Using the Balanced Cohort
3. Discussion
4. Materials and Methods
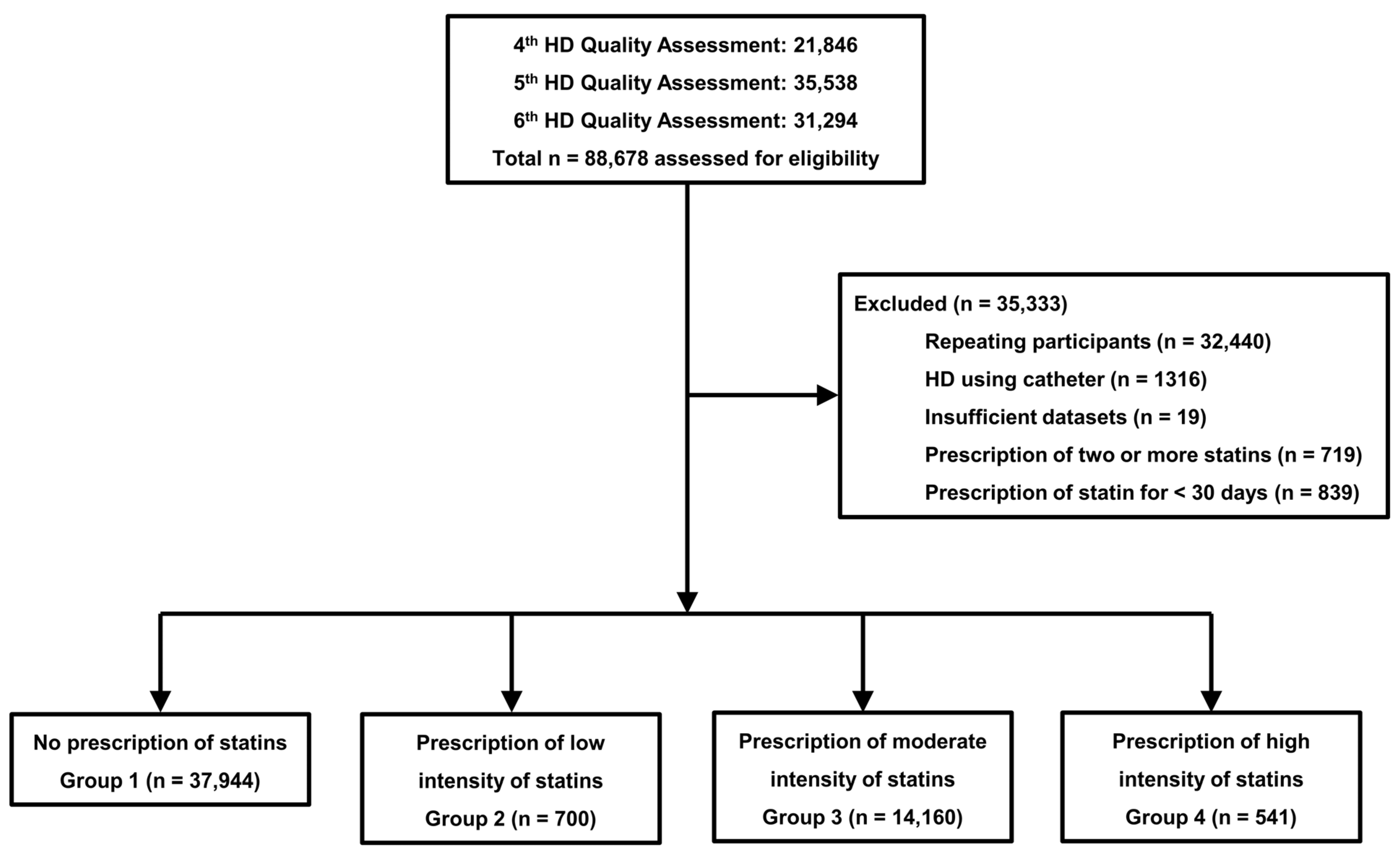
4.1. Data Source and Study Population
4.2. Variables
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ESRD Registry Committee: Korean Society of Nephrology [Internet]. Current Renal Replacement Therapy in Korea, 2021. 2021. Available online: https://ksn.or.kr/bbs/index.php?code=report (accessed on 12 March 2024).
- US Renal Data System. USRDS 2020 Annual Data Report: Atlas of Chronic Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020. Available online: https://adr.usrds.org/2020 (accessed on 12 March 2024).
- Choi, H.; Kim, M.; Kim, H.; Lee, J.P.; Lee, J.; Park, J.T.; Kim, K.H.; Ahn, H.S.; Hann, H.J.; Ryu, D.-R. Excess mortality among patients on dialysis: Comparison with the general population in Korea. Kidney Res. Clin. Pract. 2014, 33, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. The challenge of discovering patient-level cardiovascular risk factors in chronic kidney disease. Kidney Int. 2008, 73, 1340–1342. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mason, N.A.; Bailie, G.R.; Satayathum, S.; Bragg-Gresham, J.L.; Akiba, T.; Akizawa, T.; Combe, C.; Rayner, H.C.; Saito, A.; Gillespie, B.W.; et al. HMG-coenzyme a reductase inhibitor use is associated with mortality reduction in hemodialysis patients. Am. J. Kidney Dis. 2005, 45, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Seliger, S.L.; Weiss, N.S.; Gillen, D.L.; Kestenbaum, B.; Ball, A.; Sherrard, D.J.; Stehman-Breen, C.O. HMG-CoA reductase inhibitors are associated with reduced mortality in ESRD patients. Kidney Int. 2002, 61, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Krane, V.; März, W.; Olschewski, M.; Mann, J.F.E.; Ruf, G.; Ritz, E.; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005, 353, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.-W.; Chevaile, A.; Cobbe, S.M.; Grönhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qureshi, A.R.; Parini, P.; Hurt-Camejo, E.; Ripsweden, J.; Brismar, T.B.; Barany, P.; Jaminon, A.M.; Schurgers, J.J.; Heimbürger, O.; et al. Does statins promote vascular calcification in chronic kidney disease? Eur. J. Clin. Investig. 2017, 47, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Kupcsik, L.; Meurya, T.; Flury, M.; Stoddart, M.; Alini, M. Statin-induced calcification in human mesenchymal stem cells is cell death related. J. Cell. Mol. Med. 2009, 13, 4465–4473. [Google Scholar] [CrossRef]
- Wang, S.W.; Li, L.C.; Su, C.H.; Yang, Y.H.; Hsu, T.W.; Hsu, C.N. Association of Statin and Its Lipophilicity With Cardiovascular Events in Patients Receiving Chronic Dialysis. Clin. Pharmacol. Ther. 2020, 107, 1312–1324. [Google Scholar] [CrossRef]
- Bielecka-Dabrowa, A.; Bytyçi, I.; Von Haehling, S.; Bajraktari, G.; Mikhailidis, D.P.; Banach, M. Association of statin use and clinical outcomes in heart failure patients: A systematic review and meta-analysis. Lipids Health Dis. 2019, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zou, L.; Chen, M.; Liu, B. Meta-analysis of statin therapy in maintenance dialysis patients. Rent. Fail. 2015, 37, 1149–1156. [Google Scholar] [CrossRef][Green Version]
- Kassimatis, T.I.; Goldsmith, D.J. Statins in chronic kidney disease and kidney transplantation. Pharmacol. Res. 2014, 88, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ku, H.; Zhao, L.; Wheeler, D.C.; Li, L.-C.; Li, Q.; Varghese, Z.; Moorhead, J.F.; Powis, S.H.; Huang, A.; et al. Inflammatory stress induces statin resistance by disrupting 3-hydroxy-3-methylglutaryl-CoA reductase feedback regulation. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Haim-Pinhas, H.; Yoskovitz, G.; Lishner, M.; Pereg, D.; Kitay-Cohen, Y.; Topaz, G.; Sela, Y.; Wand, O.; Rozenberg, I.; Benchetrit, S.; et al. Effect of aspirin on primary prevention of cardiovascular disease and mortality among patients with chronic kidney disease. Sci. Rep. 2022, 12, 17788. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W.; Wu, C.K.; Yang, Y.H.; Huang, J.-W.; Wu, V.-C.; Lee, J.-K.; Chen, P.-C.; Lin, Y.-H.; Lin, L.-Y. Efficacy of Antiplatelet Agent Usage for Primary and Secondary Prevention in Dialysis Patients: A Nationwide Data Survey and Propensity Analysis. Cardiovasc. Drugs Ther. 2019, 33, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.M.; Larkina, M.; Thumma, J.R.; Tentori, F.; Gillespie, B.W.; Fukuhara, S.; Mendelssohn, D.C.; Chan, K.; de Sequera, P.; Komenda, P.; et al. Major bleeding events and risk stratification of antithrombotic agents in hemodialysis: Results from the DOPPS. Kidney Int. 2013, 84, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Palmer, S.C.; Di Micco, L.; Razavian, M.; Craig, J.C.; Jardine, M.J.; Webster, A.C.; Strippoli, G.F. Antiplatelet agents for chronic kidney disease. Cochrane Database Syst. Rev. 2013, 28, CD008834. [Google Scholar]
- Levin, A.; Ahmed, S.B.; Carrero, J.J.; Foster, B.; Francis, A.; Hall, R.K.; Herrington, W.G.; Hill, G.; Inker, L.A.; Kazancıoğlu, R.; et al. Executive summary of the KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: Known knowns and known unknowns. Kidney Int. 2024, 105, 684–701. [Google Scholar] [CrossRef]
- Cortese, F.; Gesualdo, M.; Cortese, A.; Carbonara, S.; Devito, F.; Zito, A.; Ricci, G.; Scicchitano, P.; Ciccone, M.M. Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol. Res. 2016, 107, 1–18. [Google Scholar] [CrossRef]
- Holdaas, H.; Holme, I.; Schmieder, R.E.; Jardine, A.G.; Zannad, F.; Norby, G.E.; Fellström, B.C.; AURORA study group. Rosuvastatin in diabetic hemodialysis patients. J. Am. Soc. Nephrol. 2011, 22, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- März, W.; Genser, B.; Drechsler, C.; Krane, V.; Grammer, T.B.; Ritz, E.; Stojakovic, T.; Scharnagl, H.; Winkler, K.; Holme, I.; et al. German Diabetes and Dialysis Study Investigators. Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2011, 6, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, S.; Paoletti, R.; Corsini, A. Safety of statins: Focus on clinical pharmacokinetics and drug interactions. Circulation 2004, 109 (Suppl. S1), III50–III57. [Google Scholar] [CrossRef] [PubMed]
- Lins, R.L.; Matthys, K.E.; Verpooten, G.A.; Peeters, P.C.; Dratwa, M.; Stolear, J.-C.; Lameire, N.H. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.K.; Ho, T.I.; Hsu, S.P.; Peng, Y.-S.; Pai, M.-F.; Yang, S.-Y.; Hung, K.-Y.; Tsai, T.-J. Low-density lipoprotein cholesterol: Association with mortality and hospitalization in hemodialysis patients. Blood Purif. 2005, 23, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhou, L.; Zhan, X.; Wen, Y.; Wang, X.; Feng, X.; Wang, N.; Peng, F.; Wu, J. Low-Density Lipoprotein Cholesterol and Mortality in Peritoneal Dialysis. Front. Nutr. 2022, 9, 910348. [Google Scholar] [CrossRef]
- Qunibi, W.Y. Dyslipidemia in Dialysis Patients. Semin. Dial. 2015, 28, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Kim, B.Y.; Son, E.J.; Kim, G.O.; Do, J.Y. Comparison of Patient Survival According to Erythropoiesis-Stimulating Agent Type of Treatment in Maintenance Hemodialysis Patients. J. Clin. Med. 2023, 12, 625. [Google Scholar] [CrossRef]
- Health Insurance Review & Assessment Service. 6th Hemodialysis Quality Assessment Program. Available online: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=6619#none (accessed on 12 March 2024).
- Daugirdas, J.T. Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J. Am. Soc. Nephrol. 1993, 4, 1205–1213. [Google Scholar] [CrossRef]
- Stone, N.J.; Robinson, J.G.; Lichtenstein, A.H.; Bairey Merz, C.N.; Blum, C.B.; Eckel, R.H.; Goldberg, A.C.; Gordon, D.; Levy, D.; Lloyd-Jones, D.M.; et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S1–S45. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]


| Group 1 (n = 37,944) | Group 2 (n = 700) | Group 3 (n = 14,160) | Group 4 (n = 541) | p | |
|---|---|---|---|---|---|
| Age (years) | 59.4 ± 13.3 | 62.8 ± 12.4 a | 62.1 ± 12.0 a | 62.1 ± 11.6 a | <0.001 |
| Sex (male, %) | 23,450 (61.8%) | 380 (54.3%) | 7777 (54.9%) | 304 (56.2%) | <0.001 |
| Hemodialysis vintage (months) | 56.4 ± 59.0 | 41.9 ± 43.1 a | 41.9 ± 47.4 a | 41.2 ± 46.6 a | <0.001 |
| Body mass index (kg/m2) | 22.1 ± 3.3 | 23.4 ± 3.6 a | 23.3 ± 3.6 a | 23.5 ± 3.6 a | <0.001 |
| Underlying causes of ESRD | <0.001 | ||||
| Diabetes mellitus | 14,642 (38.6%) | 373 (53.3%) | 7904 (55.8%) | 340 (62.8%) | |
| Hypertension | 10,707 (28.2%) | 159 (22.7%) | 3077 (21.7%) | 116 (21.4%) | |
| Glomerulonephritis | 4453 (11.7%) | 58 (8.3%) | 1150 (8.1%) | 30 (5.5%) | |
| Others | 3538 (9.3%) | 50 (7.1%) | 892 (6.3%) | 25 (4.6%) | |
| Unknown | 4604 (12.1%) | 60 (8.6%) | 1137 (8.0%) | 30 (5.5%) | |
| CCI score | 7.2 ± 2.9 | 8.1 ± 2.9 a | 8.2 ± 2.7 a | 9.1 ± 2.6 abc | <0.001 |
| Follow-up duration (months) | 62.2 ± 29.2 | 59.2 ± 29.1 a | 58.5 ± 26.7 a | 55.9 ± 24.9 a | <0.001 |
| Type of vascular access | <0.001 | ||||
| Arteriovenous fistula | 32,538 (85.8%) | 607 (86.7%) | 11,921 (84.2%) | 434 (80.2%) | |
| Arteriovenous graft | 5406 (14.2%) | 93 (13.3%) | 2239 (15.8%) | 107 (19.8%) | |
| Kt/Vurea | 1.53 ± 0.27 | 1.53 ± 0.27 | 1.54 ± 0.27 a | 1.54 ± 0.26 | 0.013 |
| Ultrafiltration volume (L/session) | 2.29 ± 0.96 | 2.20 ± 0.94 | 2.23 ± 0.94 a | 2.35 ± 0.91 bc | <0.001 |
| Hemoglobin (g/dL) | 10.6 ± 0.8 | 10.7 ± 0.8 a | 10.7 ± 0.7 a | 10.7 ± 0.7 | <0.001 |
| Serum albumin (g/dL) | 3.99 ± 0.34 | 3.95 ± 0.33 a | 3.99 ± 0.34 b | 3.94 ± 0.33 ac | <0.001 |
| Serum phosphorus (mg/dL) | 5.0 ± 1.4 | 4.8 ± 1.3 a | 4.9 ± 1.3 a | 4.7 ± 1.4 a | <0.001 |
| Serum calcium (mg/dL) | 8.92 ± 0.85 | 8.84 ± 0.75 | 8.86 ± 0.76 a | 8.80 ± 0.76 a | <0.001 |
| Systolic blood pressure (mmHg) | 141 ± 16 | 141 ± 16 | 141 ± 15 | 142 ± 17 | 0.714 |
| Diastolic blood pressure (mmHg) | 79 ± 9 | 77 ± 9 a | 77 ± 10 a | 74 ± 11 abc | <0.001 |
| Serum creatinine (mg/dL) | 9.7 ± 2.8 | 9.0 ± 2.6 a | 9.1 ± 2.6 a | 8.8 ± 2.7 a | <0.001 |
| Use of RASB | 11,161 (29.4%) | 261 (37.3%) | 4656 (32.9%) | 187 (34.6%) | <0.001 |
| Use of aspirin | 13,898 (36.6%) | 386 (55.1%) | 8006 (56.5%) | 345 (63.8%) | <0.001 |
| Use of clopidogrel | 4268 (11.2%) | 174 (24.9%) | 3923 (27.7%) | 224 (41.4%) | <0.001 |
| MI or CHF | 15,945 (42.0%) | 352 (50.3%) | 7421 (52.4%) | 357 (66.0%) | <0.001 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Group | ||||
| Ref: Group 1 | ||||
| Group 2 | 1.14 (1.02–1.28) | 0.002 | 0.98 (0.85–1.12) | 0.760 |
| Group 3 | 1.08 (1.05–1.12) | <0.001 | 0.94 (0.91–0.98) | 0.003 |
| Group 4 | 1.25 (1.10–1.43) | <0.001 | 1.03 (0.87–1.22) | 0.724 |
| Ref: Group 2 | ||||
| Group 3 | 0.95 (0.84–1.07) | 0.370 | 0.96 (0.84–1.11) | 0.597 |
| Group 4 | 1.10 (0.92–1.30) | 0.295 | 1.05 (0.85–1.30) | 0.636 |
| Ref: Group 3 | ||||
| Group 4 | 1.16 (1.01–1.32) | 0.030 | 1.09 (0.93–1.29) | 0.294 |
| Age (increase per 1 year) | 1.06 (1.06–1.06) | <0.001 | 1.06 (1.06–1.06) | <0.001 |
| Sex (ref: male) | 0.87 (0.84–0.89) | <0.001 | 0.87 (0.84–0.89) | <0.001 |
| Body mass index (increase in 1 kg/m2) | 0.98 (0.97–0.98) | <0.001 | 0.97 (0.97–0.98) | <0.001 |
| Underlying cause of ESRD (ref: DM) | 0.81 (0.80–0.82) | <0.001 | 0.89 (0.88–0.91) | <0.001 |
| Vascular access (ref: arteriovenous fistula) | 1.51 (1.46–1.56) | <0.001 | 1.18 (1.13–1.24) | <0.001 |
| Hemodialysis vintage (increase per 1 month) | 1.00 (1.00–1.00) | 0.100 | 1.00 (1.00–1.00) | <0.001 |
| CCI score (increase per 1 score) | 1.14 (1.13–1.14) | <0.001 | 1.06 (1.06–1.07) | <0.001 |
| UFV (increase per 1 kg/session) | 0.92 (0.90–0.93) | <0.001 | 1.08 (1.06–1.11) | <0.001 |
| KtVurea (increase per 1 unit) | 0.91 (0.86–0.96) | <0.001 | 0.71 (0.66–0.77) | <0.001 |
| Hemoglobin (increase per 1 g/dL) | 0.87 (0.85–0.88) | <0.001 | 0.91 (0.89–0.93) | <0.001 |
| Serum albumin (increase per 1 g/dL) | 0.37 (0.36–0.39) | <0.001 | 0.63 (0.60–0.67) | <0.001 |
| Serum creatinine (increase per 1 mg/dL) | 0.87 (0.86–0.87) | <0.001 | 0.94 (0.93–0.95) | <0.001 |
| Serum phosphorus (increase per 1 mg/dL) | 0.85 (0.84–0.86) | <0.001 | 1.04 (1.03–1.06) | <0.001 |
| Serum calcium (increase per 1 mg/dL) | 0.93 (0.92–0.95) | <0.001 | 1.06 (1.04–1.09) | <0.001 |
| Systolic blood pressure (increase per 1 mmHg) | 1.01 (1.01–1.01) | <0.001 | 1.01 (1.00–1.01) | <0.001 |
| Diastolic blood pressure (increase per 1 mmHg) | 0.98 (0.98–0.99) | <0.001 | 1.00 (0.99–1.00) | 0.066 |
| Use of renin angiotensin system blocker | 1.15 (1.12–1.18) | <0.001 | 1.00 (0.96–1.03) | 0.952 |
| Use of clopidogrel | 1.53 (1.48–1.59) | <0.001 | 1.15 (1.10–1.20) | <0.001 |
| Use of aspirin | 1.16 (1.13–1.19) | <0.001 | 0.97 (0.93–1.00) | 0.055 |
| MI or CHF | 1.49 (1.45–1.53) | <0.001 | 1.05 (1.01–1.09) | 0.003 |
| Univariate | Multivariable | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Patients with MI | ||||
| Ref: Group 1 | ||||
| Group 2 | 1.08 (0.79–1.48) | 0.619 | 1.23 (0.85–1.79) | 0.268 |
| Group 3 | 0.96 (0.88–1.04) | 0.320 | 0.94 (0.84–1.06) | 0.331 |
| Group 4 | 0.92 (0.71–1.20) | 0.559 | 1.11 (0.79–1.56) | 0.541 |
| Ref: Group 2 | ||||
| Group 3 | 0.89 (0.65–1.21) | 0.447 | 0.76 (0.53–1.11) | 0.156 |
| Group 4 | 0.85 (0.57–1.28) | 0.441 | 0.90 (0.55–1.47) | 0.675 |
| Ref: Group 3 | ||||
| Group 4 | 0.96 (0.74–1.26) | 0.791 | 1.18 (0.84–1.65) | 0.334 |
| Patients with CVAs | ||||
| Ref: Group 1 | ||||
| Group 2 | 1.12 (0.95–1.31) | 0.186 | 1.07 (0.88–1.30) | 0.505 |
| Group 3 | 0.94 (0.90–0.99) | 0.019 | 0.92 (0.86–0.98) | 0.007 |
| Group 4 | 0.95 (0.79–1.14) | 0.600 | 0.97 (0.77–1.22) | 0.797 |
| Ref: Group 2 | ||||
| Group 3 | 0.85 (0.72–0.99) | 0.048 | 0.86 (0.70–1.05) | 0.133 |
| Group 4 | 0.85 (0.67–1.09) | 0.199 | 0.91 (0.67–1.22) | 0.521 |
| Ref: Group 3 | ||||
| Group 4 | 1.01 (0.84–1.22) | 0.930 | 1.06 (0.84–1.33) | 0.633 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lho, Y.; Kim, G.O.; Kim, B.Y.; Son, E.J.; Kang, S.H. Effects of Administration and Intensity of Statins on Mortality in Patients Undergoing Hemodialysis. Pharmaceuticals 2024, 17, 498. https://doi.org/10.3390/ph17040498
Lho Y, Kim GO, Kim BY, Son EJ, Kang SH. Effects of Administration and Intensity of Statins on Mortality in Patients Undergoing Hemodialysis. Pharmaceuticals. 2024; 17(4):498. https://doi.org/10.3390/ph17040498
Chicago/Turabian StyleLho, Yunmee, Gui Ok Kim, Bo Yeon Kim, Eun Jung Son, and Seok Hui Kang. 2024. "Effects of Administration and Intensity of Statins on Mortality in Patients Undergoing Hemodialysis" Pharmaceuticals 17, no. 4: 498. https://doi.org/10.3390/ph17040498
APA StyleLho, Y., Kim, G. O., Kim, B. Y., Son, E. J., & Kang, S. H. (2024). Effects of Administration and Intensity of Statins on Mortality in Patients Undergoing Hemodialysis. Pharmaceuticals, 17(4), 498. https://doi.org/10.3390/ph17040498






