Bioactive Properties of Venoms Isolated from Whiptail Stingrays and the Search for Molecular Mechanisms and Targets
Abstract
1. Introduction
2. Results
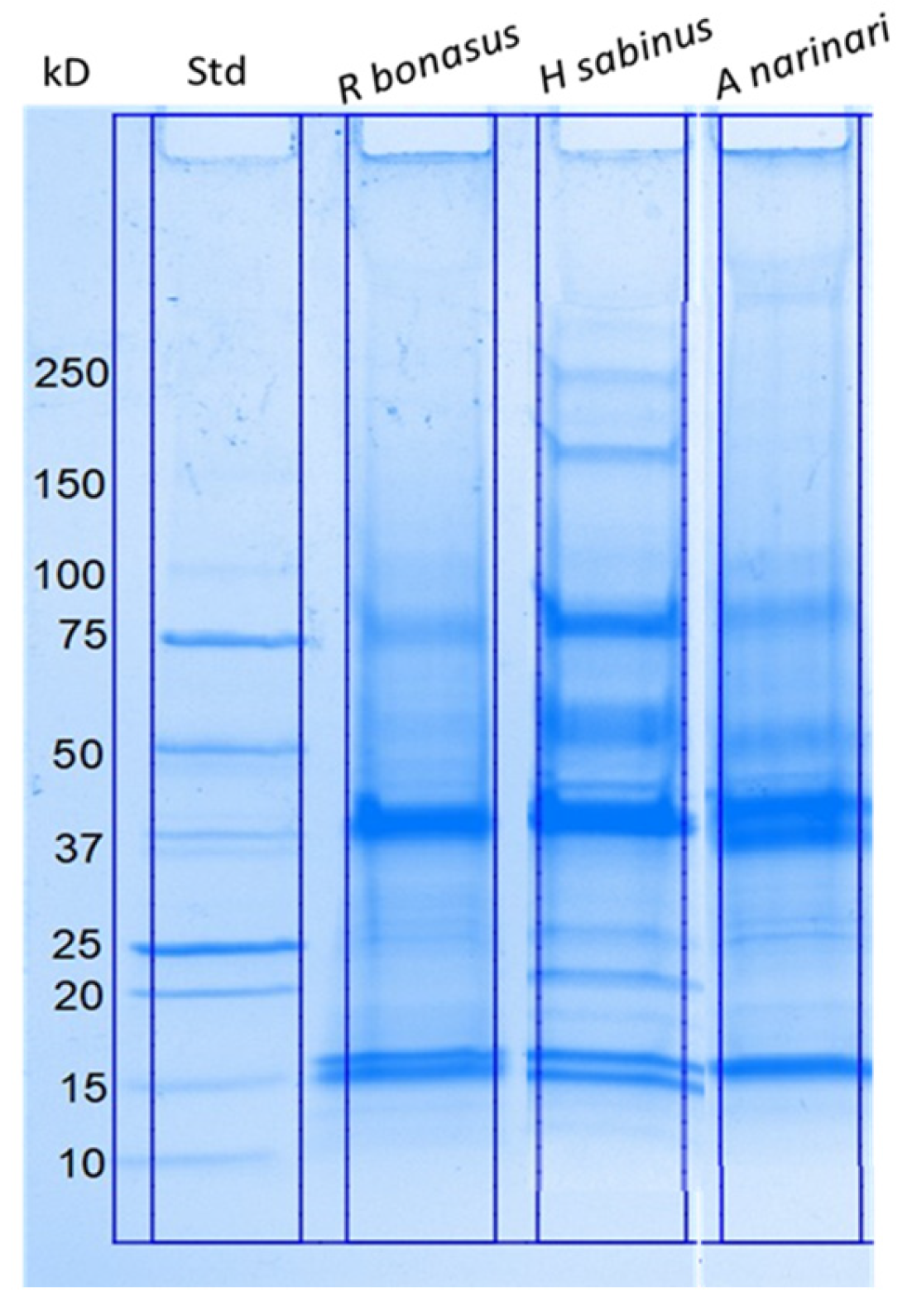
2.1. Biochemical Characterization of Isolated SRV Proteins
2.2. Bioactive Properties of SRVs on Cultured HDFa and SH-SY5Y Cells
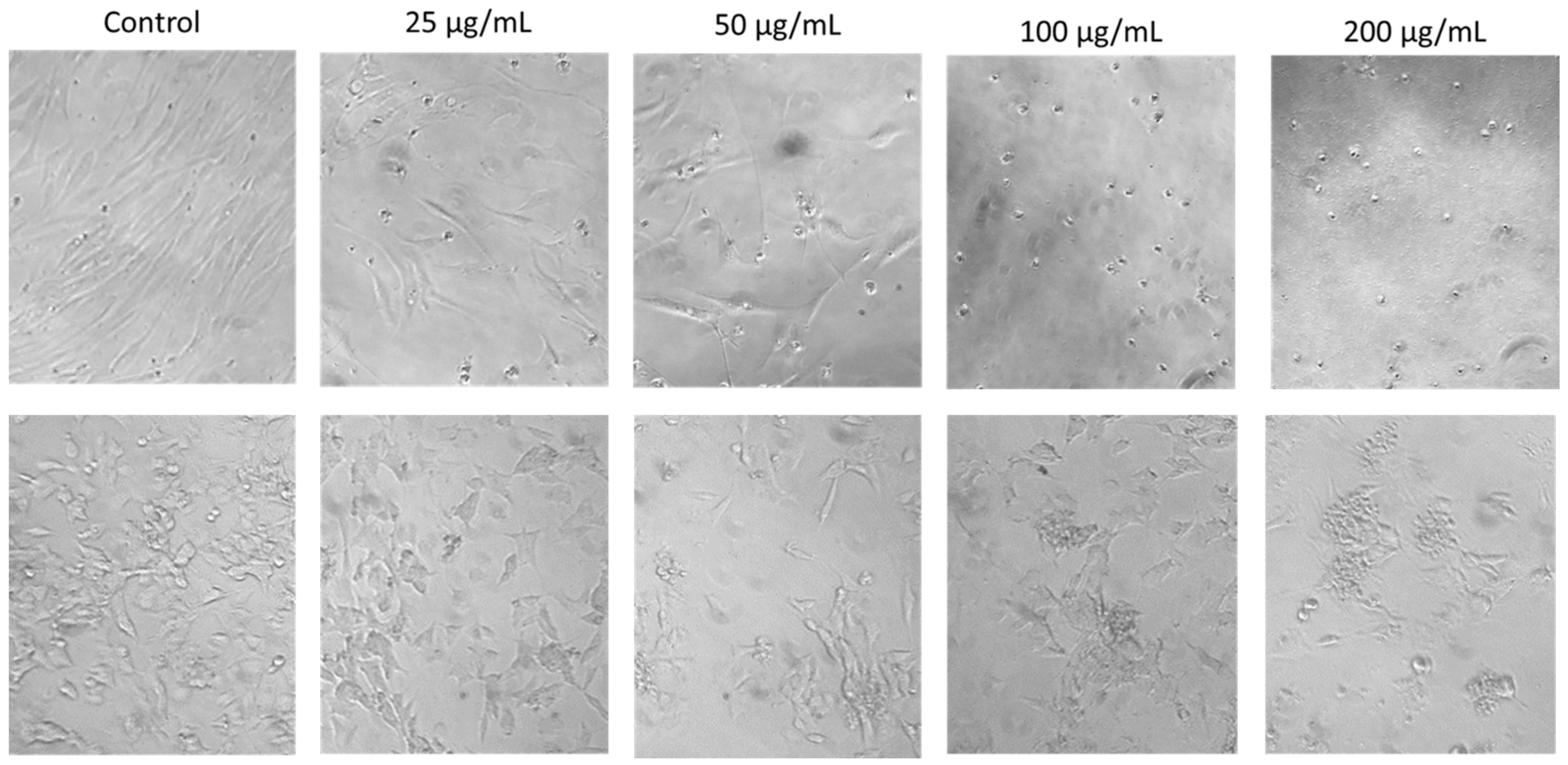
2.3. Concentration-Dependent Effects of SRV on Cell Morphology
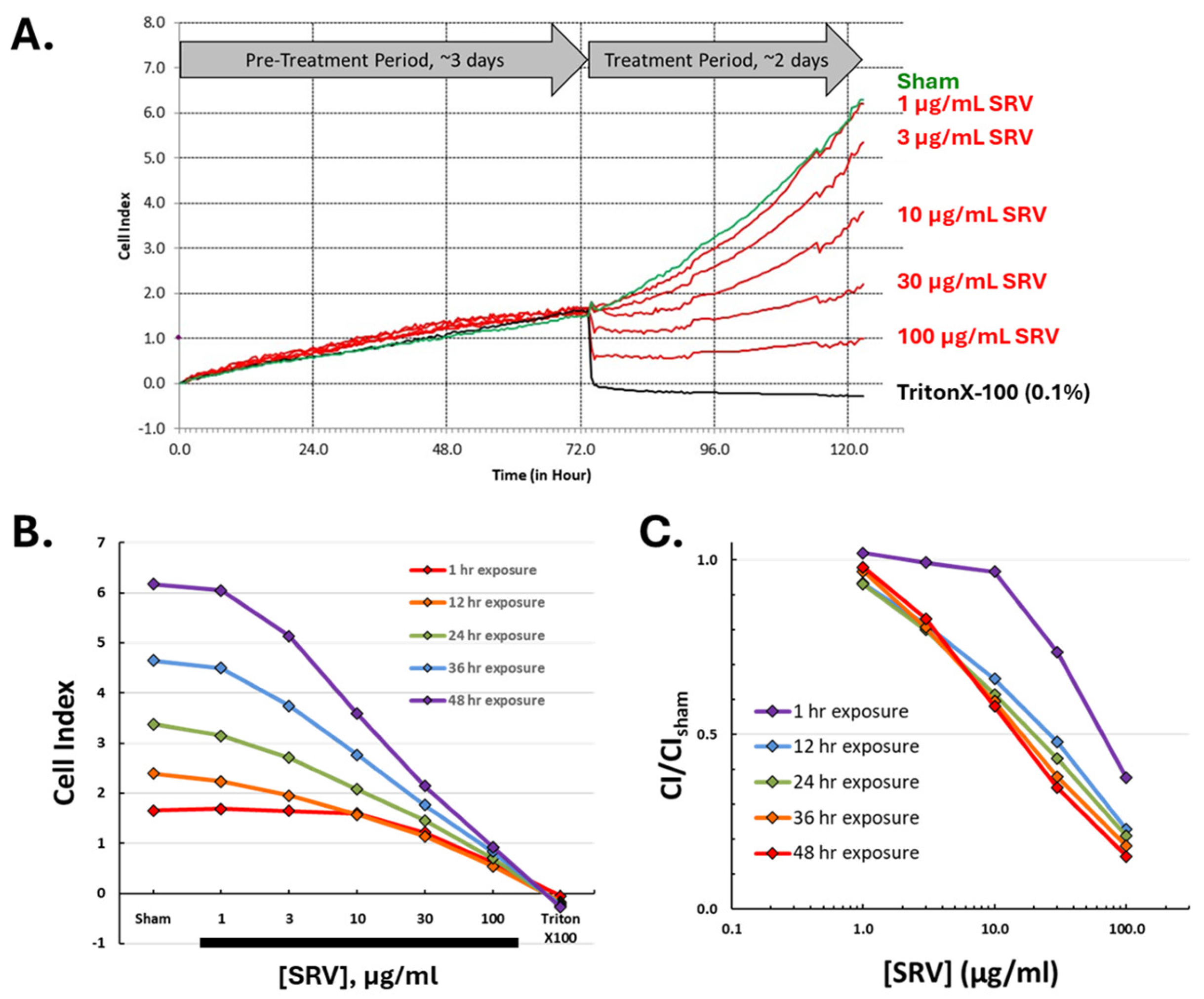
2.4. Time Course for SRV-Mediated Effects on HDFa Cell Cultures
2.5. Bioactive Effects of SRVs on Additional Tumor Cell Types
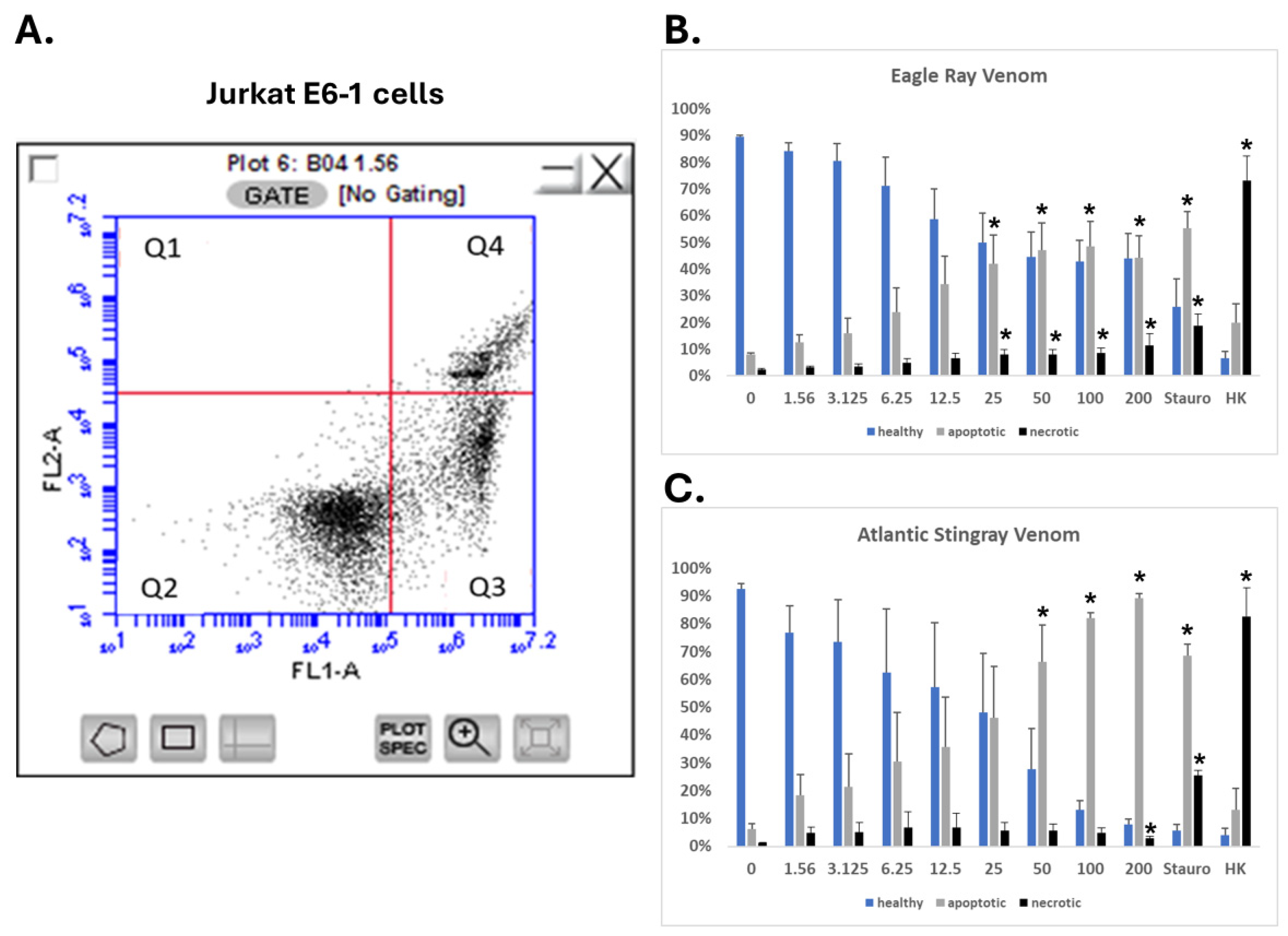
2.6. SRVs Cause Apoptosis of Jurkat E6-1 Cells
3. Discussion
3.1. The Evolution of SRVs as a Well-Conserved Defense Response
3.2. The Bioactivity of Isolated SRVs Is Well Conserved across Diverse Species
3.3. SRV Proteins Identified by Recent Venomic Studies
3.4. Mining SRV Proteins for Molecular Targets and Biomedical Applications
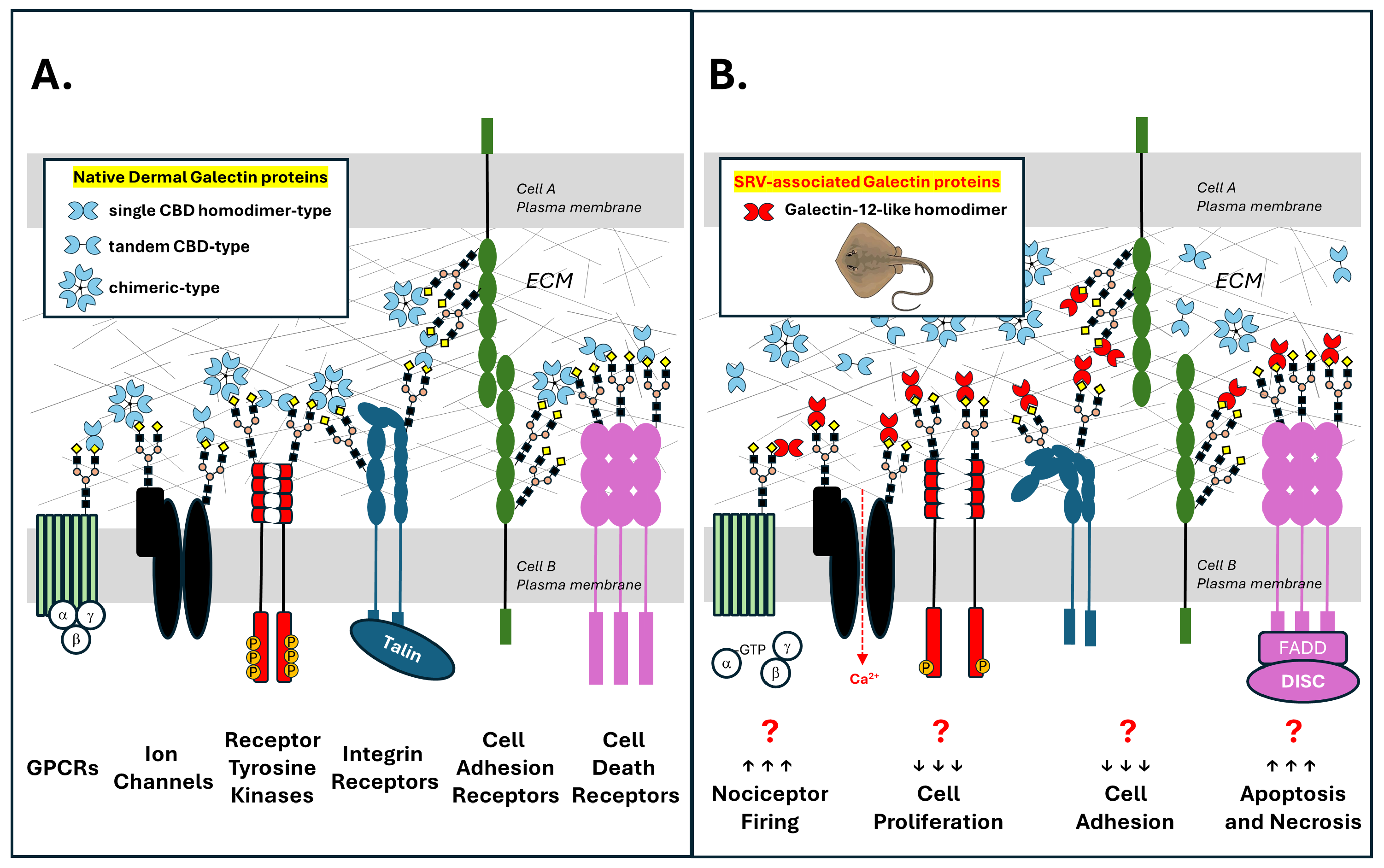
3.5. An SRV Galectin Toxin Hypothesis
- Galectin-like proteins expressed in venom gland cells of marine stingrays are released locally into target tissues during barb-mediated envenomation.
- SRV galectin-like proteins, via their carbohydrate-binding domains (CBDs), recognize and bind to β-galactoside glycan structures present on multiple N-linked and O-linked glycosylated membrane protein targets that are present on resident cells within the affected tissue (e.g., skin fibroblasts, neurons, and blood cells).
- Binding of dimerized SRV galectin-like proteins to glycosylated target cell proteins promotes aberrant signaling by
- Collectively, these SRV galectin-mediated events either cause or contribute to the hallmark features of localized stingray injury, i.e., severe pain and dermal tissue necrosis (Figure 8).
- We further hypothesize that the SRV-resistant characteristics of WEHI-164 cells are due to a distinct glycocalyx where either SRV galectin-like proteins do not recognize glycan targets on WEHI-164 cells, and/or the recognized glycan targets on HDFa, SH-SY5Y, and Jurkat-E6-1 cells are not expressed and presented by WEHI-164 cells. Abnormal glycosylation patterns are a common feature of malignant cells.
3.6. SRV Galectins
3.7. Galectin Inhibitors in Cancer Therapeutics
3.8. Venoms as a Source of Potential Anticancer Agents
4. Materials and Methods
4.1. Capture and Housing of Local Stingrays
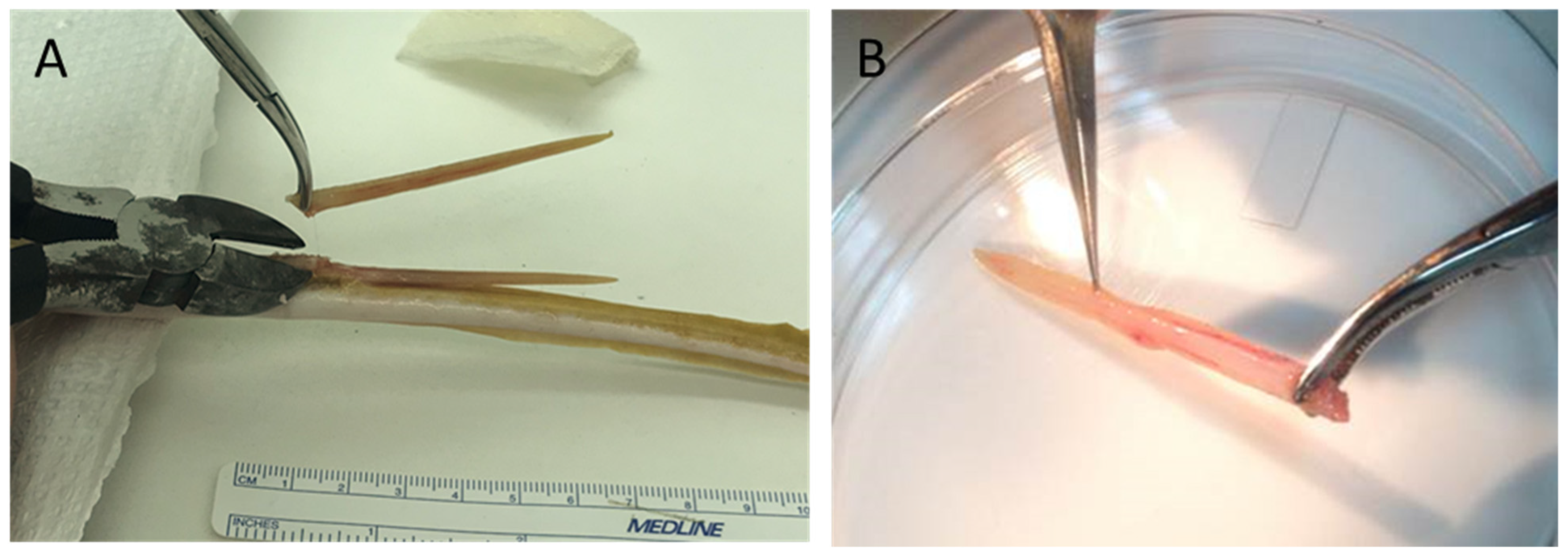
4.2. Spine/Barb Removal Procedures
4.3. Collection and Preparation of Crude SRV
4.4. SDS–Polyacrylamide Gel Electrophoresis (PAGE)
4.5. Cell Culture
4.6. Cell Growth Assays
4.7. Biosensor-Based Real-Time Cell Impedance Recordings
4.8. Flow Cytometry Assay
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet 2009, 10, 483–511. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wuster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef] [PubMed]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Why do we study animal toxins? Dongwuxue Yanjiu 2015, 36, 183–222. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Junior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, R.R.; Halstead, B.W.; Modglin, F.R. The microscopic anatomy of the caudal appendage of the spotted eagleray, Aetobatus narinari (Euphrasen), with special reference to the venom apparatus. Anat. Rec. 1953, 115, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Halstead, B.W.; Ocampo, R.R.; Modglin, F.R. A study on the comparative anatomy of the venom apparatus of certain north American stingrays. J. Morphol. 1955, 97, 1–21. [Google Scholar] [CrossRef]
- Smith, D.S.; Cayer, M.L.; Russell, F.E. Membrane-limited microtubular aggregates in the venom secreting cells of a stingray. Toxicon 1974, 12, 331–335. [Google Scholar] [CrossRef]
- Brighton Ndandala, C.; Mustapha, U.F.; Wang, Y.; Assan, D.; Zhao, G.; Huang, C.; Mkuye, R.; Huang, H.; Li, G.; Chen, H. The perspective of fish venom: An overview of the physiology, evolution, molecular and genetics. Front. Mar. Sci. 2023, 10, 1085669. [Google Scholar] [CrossRef]
- Harris, R.J. The piscine arsenal: An updated review of venomous fishes. Rev. Fish Biol. Fish. 2023, 1–36. [Google Scholar] [CrossRef]
- Pedroso, C.M.; Jared, C.; Charvet-Almeida, P.; Almeida, M.P.; Garrone Neto, D.; Lira, M.S.; Haddad, V., Jr.; Barbaro, K.C.; Antoniazzi, M.M. Morphological characterization of the venom secretory epidermal cells in the stinger of marine and freshwater stingrays. Toxicon 2007, 50, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Sajjadi, M.M.; Parto, P.; Rajaian, H.; Mokhlesi, A. Histological characterization of the special venom secretory cells in the stinger of rays in the northern waters of Persian Gulf and Oman Sea. Toxicon 2010, 55, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Casewell, N.R.; Ali, S.A.; Jackson, T.N.; Vetter, I.; Dobson, J.S.; Cutmore, S.C.; Nouwens, A.; Lavergne, V.; Fry, B.G. A ray of venom: Combined proteomic and transcriptomic investigation of fish venom composition using barb tissue from the blue-spotted stingray (Neotrygon kuhlii). J. Proteom. 2014, 109, 188–198. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Junior, N.G.; Fernandes Gda, R.; Cardoso, M.H.; Costa, F.F.; Candido Ede, S.; Garrone Neto, D.; Mortari, M.R.; Schwartz, E.F.; Franco, O.L.; de Alencar, S.A. Venom gland transcriptome analyses of two freshwater stingrays (Myliobatiformes: Potamotrygonidae) from Brazil. Sci. Rep. 2016, 6, 21935. [Google Scholar] [CrossRef]
- Silva, F.; Huang, Y.; Yang, V.; Mu, X.; Shi, Q.; Antunes, A. Transcriptomic Characterization of the South American Freshwater Stingray Potamotrygon motoro Venom Apparatus. Toxins 2018, 10, 544. [Google Scholar] [CrossRef]
- Kirchhoff, K.N.; Billion, A.; Voolstra, C.R.; Kremb, S.; Wilke, T.; Vilcinskas, A. Stingray Venom Proteins: Mechanisms of Action Revealed Using a Novel Network Pharmacology Approach. Mar. Drugs 2022, 20, 27. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest Inc. IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 19 March 2024).
- Russell, F.E. Stingray injuries: A review and discussion of their treatment. Am. J. Med. Sci. 1953, 226, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.H. The epidemiology, evaluation, and management of stingray injuries. J. La. State Med. Soc. 2007, 159, 198–204, 230. [Google Scholar] [PubMed]
- White, J. Venomous animals: Clinical toxinology. In Molecular, Clinical and Environmental Toxicology: Volume 2: Clinical Toxicology; Luch, A., Ed.; Birkhäuser Basel: Basel, Switzerland, 2010; pp. 233–291. [Google Scholar]
- Koopman, G.; Reutelingsperger, C.P.; Kuijten, G.A.; Keehnen, R.M.; Pals, S.T.; van Oers, M.H. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 1994, 84, 1415–1420. [Google Scholar] [CrossRef] [PubMed]
- Marmi, J.; Vila, B.; Oms, O.; Galobart, À.; Cappetta, H. Oldest records of stingray spines (Chondrichthyes, Myliobatiformes). J. Vertebr. Paleontol. 2010, 30, 970–974. [Google Scholar] [CrossRef]
- Shea-Vantine, C.S.; Galloway, K.A.; Ingle, D.N.; Porter, M.E.; Kajiura, S.M. Caudal Spine Morphology and Puncture Performance of Two Coastal Stingrays. Integr. Comp. Biol. 2021, 61, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.E.; Panos, T.C.; Kang, L.W.; Warner, A.M.; Colket, T.C., 3rd. Studies on the mechanism of death from stingray venom; a report of two fatal cases. Am. J. Med. Sci. 1958, 235, 566–584. [Google Scholar] [CrossRef]
- Last, P.R.; Naylor, G.J.; Manjaji-Matsumoto, B.M. A revised classification of the family Dasyatidae (Chondrichthyes: Myliobatiformes) based on new morphological and molecular insights. Zootaxa 2016, 4139, 345–368. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Tang, N.; Wan, R.; Qi, Y.; Lin, X.; Lin, J. Recombinant snake venom cystatin inhibits the growth, invasion and metastasis of B16F10 cells and MHCC97H cells in vitro and in vivo. Toxicon 2011, 57, 704–711. [Google Scholar] [CrossRef]
- Barbaro, K.C.; Lira, M.S.; Malta, M.B.; Soares, S.L.; Garrone Neto, D.; Cardoso, J.L.; Santoro, M.L.; Haddad Junior, V. Comparative study on extracts from the tissue covering the stingers of freshwater (Potamotrygon falkneri) and marine (Dasyatis guttata) stingrays. Toxicon 2007, 50, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Verkerke, H.; Dias-Baruffi, M.; Cummings, R.D.; Arthur, C.M.; Stowell, S.R. Galectins: An Ancient Family of Carbohydrate Binding Proteins with Modern Functions. Methods Mol. Biol. 2022, 2442, 1–40. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Elola, M.T.; Blidner, A.G.; Sarrias, L.; Espelt, M.V.; Rabinovich, G.A. The universe of galectin-binding partners and their functions in health and disease. J. Biol. Chem. 2023, 299, 105400. [Google Scholar] [CrossRef] [PubMed]
- Sartim, M.A.; Sampaio, S.V. Snake venom galactoside-binding lectins: A structural and functional overview. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A.; Jackson, S.S.; Vasta, G.R. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007, 17, 513–520. [Google Scholar] [CrossRef]
- Cha, S.K.; Ortega, B.; Kurosu, H.; Rosenblatt, K.P.; Kuro, O.M.; Huang, C.L. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc. Natl. Acad. Sci. USA 2008, 105, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Kai, W.; Kikuchi, K.; Tohari, S.; Chew, A.K.; Tay, A.; Fujiwara, A.; Hosoya, S.; Suetake, H.; Naruse, K.; Brenner, S.; et al. Integration of the genetic map and genome assembly of fugu facilitates insights into distinct features of genome evolution in teleosts and mammals. Genome Biol. Evol. 2011, 3, 424–442. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Marino, K.V.; Cagnoni, A.J.; Croci, D.O.; Rabinovich, G.A. Targeting galectin-driven regulatory circuits in cancer and fibrosis. Nat. Rev. Drug Discov. 2023, 22, 295–316. [Google Scholar] [CrossRef]
- Bertuzzi, S.; Quintana, J.I.; Arda, A.; Gimeno, A.; Jimenez-Barbero, J. Targeting Galectins With Glycomimetics. Front. Chem. 2020, 8, 593. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.K.; Brahmbhatt, K.; Bhatt, H.; Parmar, U. Therapeutic potential of snake venom in cancer therapy: Current perspectives. Asian Pac. J. Trop. Biomed. 2013, 3, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.; Zuliani, J.P.; et al. Antitumoral activity of snake venom proteins: New trends in cancer therapy. Biomed. Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, V.K.L. Applications of snake venoms in treatment of cancer. Asian Pac. J. Trop. Biomed. 2015, 5, 275–276. [Google Scholar] [CrossRef]
- Offor, B.C.; Piater, L.A. Snake venom toxins: Potential anticancer therapeutics. J. Appl. Toxicol. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kerkkamp, H.; Bagowski, C.; Kool, J.; van Soolingen, B.; Vonk, F.J.; Vlecken, D. Whole snake venoms: Cytotoxic, anti-metastatic and antiangiogenic properties. Toxicon 2018, 150, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, K.; Vatanpour, H.; Zare, A.; Shirazi, F.H.; Nakhjavani, M. Anticancer Activity a of Caspian Cobra (Naja naja oxiana) snake Venom in Human Cancer Cell Lines Via Induction of Apoptosis. Iran. J. Pharm. Res. 2016, 15, 101–112. [Google Scholar] [PubMed]
- Debnath, A.; Chatterjee, U.; Das, M.; Vedasiromoni, J.R.; Gomes, A. Venom of Indian monocellate cobra and Russell’s viper show anticancer activity in experimental models. J. Ethnopharmacol. 2007, 111, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Al-Asmari, A.K.; Riyasdeen, A.; Al-Shahrani, M.H.; Islam, M. Snake venom causes apoptosis by increasing the reactive oxygen species in colorectal and breast cancer cell lines. Onco. Targets Ther. 2016, 9, 6485–6498. [Google Scholar] [CrossRef]
- Yalcin, H.T.; Ozen, M.O.; Gocmen, B.; Nalbantsoy, A. Effect of Ottoman Viper (Montivipera xanthina (Gray, 1849)) Venom on Various Cancer Cells and on Microorganisms. Cytotechnology 2014, 66, 87–94. [Google Scholar] [CrossRef]
- Al-Sadoon, M.K.; Abdel-Maksoud, M.A.; Rabah, D.M.; Badr, G. Induction of apoptosis and growth arrest in human breast carcinoma cells by a snake (Walterinnesia aegyptia) venom combined with silica nanoparticles: Crosstalk between Bcl2 and caspase 3. Cell. Physiol. Biochem. 2012, 30, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Malekara, E.; Pazhouhi, M.; Rashidi, I.; Jalili, C. Anti-proliferative and cytotoxic effect of Iranian snake (Vipera raddei kurdistanica) venom on human breast cancer cells via reactive oxygen species-mediated apoptosis. Res. Pharm. Sci. 2020, 15, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Ji, Y.H. Scorpion venom induces glioma cell apoptosis in vivo and inhibits glioma tumor growth in vitro. J. Neurooncol. 2005, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mikaelian, A.G.; Traboulay, E.; Zhang, X.M.; Yeritsyan, E.; Pedersen, P.L.; Ko, Y.H.; Matalka, K.Z. Pleiotropic Anticancer Properties of Scorpion Venom Peptides: Rhopalurus princeps Venom as an Anticancer Agent. Drug Des. Devel. Ther. 2020, 14, 881–893. [Google Scholar] [CrossRef]
- Ip, S.W.; Liao, S.S.; Lin, S.Y.; Lin, J.P.; Yang, J.S.; Lin, M.L.; Chen, G.W.; Lu, H.F.; Lin, M.W.; Han, S.M.; et al. The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. In Vivo 2008, 22, 237–245. [Google Scholar] [PubMed]
- Kwon, N.Y.; Sung, S.H.; Sung, H.K.; Park, J.K. Anticancer Activity of Bee Venom Components against Breast Cancer. Toxins 2022, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Park, M.H.; Kollipara, P.S.; An, B.J.; Song, H.S.; Han, S.B.; Kim, J.H.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom toxin and melittin in ovarian cancer cells through induction of death receptors and inhibition of JAK2/STAT3 pathway. Toxicol. Appl. Pharmacol. 2012, 258, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Amini, E.; Baharara, J.; Nikdel, N.; Salek Abdollahi, F. Cytotoxic and Pro-Apoptotic Effects of Honey Bee Venom and Chrysin on Human Ovarian Cancer Cells. Asia Pac. J. Med. Toxicol. 2015, 4, 68–73. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvatescu, C.A.; Ifteni, P.; Ples, L. Anticancer Activity of Toxins from Bee and Snake Venom-An Overview on Ovarian Cancer. Molecules 2018, 23, 692. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, R.K.; Vennila, R.; Karthikeyan, S.; Prasad, N.R.; Arumugam, M.; Velpandian, T.; Balasubramaniam, T. Antiproliferative activity of marine stingray Dasyatis sephen venom on human cervical carcinoma cell line. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 41. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.R.; Vennila, R.; Kanchana, S.; Arumugam, M.; Balasubramaniam, T. Fibrinogenolytic and anticoagulant activities in the tissue covering the stingers of marine stingrays Dasyatis sephen and Aetobatis narinari. J. Thromb. Thrombolysis 2011, 31, 464–471. [Google Scholar] [CrossRef] [PubMed]





| Cell Line | ATCC # | Origin | Culture Media | Sub-Culture |
|---|---|---|---|---|
| HDFa | PCS-201-012 | Human, primary dermal fibroblast, normal | Fibroblast Basal Medium (ATCC PCS-201-030); Fibroblast Growth Kit-Low Serum (ATCC PCS-201-041) | At 80–100% confluence |
| SH-SY5Y | CRL-2266 | Human, neuroblastoma | 1:1 Eagle’s Minimum Essential Medium/F12 Medium; 10% FBS | 4–7 days |
| Jurkat E6-1 | TIB-152 | Human, acute T-cell leukemia | RPMI + 10% FBS | 2–3 days |
| WEHI 164 | CRL-1751 | Mouse, fibrosarcoma | RPMI + 10% FBS | 2–3 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doupnik, C.A.; Luer, C.A.; Walsh, C.J.; Restivo, J.; Brick, J.X. Bioactive Properties of Venoms Isolated from Whiptail Stingrays and the Search for Molecular Mechanisms and Targets. Pharmaceuticals 2024, 17, 488. https://doi.org/10.3390/ph17040488
Doupnik CA, Luer CA, Walsh CJ, Restivo J, Brick JX. Bioactive Properties of Venoms Isolated from Whiptail Stingrays and the Search for Molecular Mechanisms and Targets. Pharmaceuticals. 2024; 17(4):488. https://doi.org/10.3390/ph17040488
Chicago/Turabian StyleDoupnik, Craig A., Carl A. Luer, Catherine J. Walsh, Jessica Restivo, and Jacqueline Xinlan Brick. 2024. "Bioactive Properties of Venoms Isolated from Whiptail Stingrays and the Search for Molecular Mechanisms and Targets" Pharmaceuticals 17, no. 4: 488. https://doi.org/10.3390/ph17040488
APA StyleDoupnik, C. A., Luer, C. A., Walsh, C. J., Restivo, J., & Brick, J. X. (2024). Bioactive Properties of Venoms Isolated from Whiptail Stingrays and the Search for Molecular Mechanisms and Targets. Pharmaceuticals, 17(4), 488. https://doi.org/10.3390/ph17040488









