Comparative Assessment of Lignan Profiling and Biological Activities of Schisandra henryi Leaf and In Vitro PlantForm Bioreactor-Grown Culture Extracts
Abstract
1. Introduction
2. Results
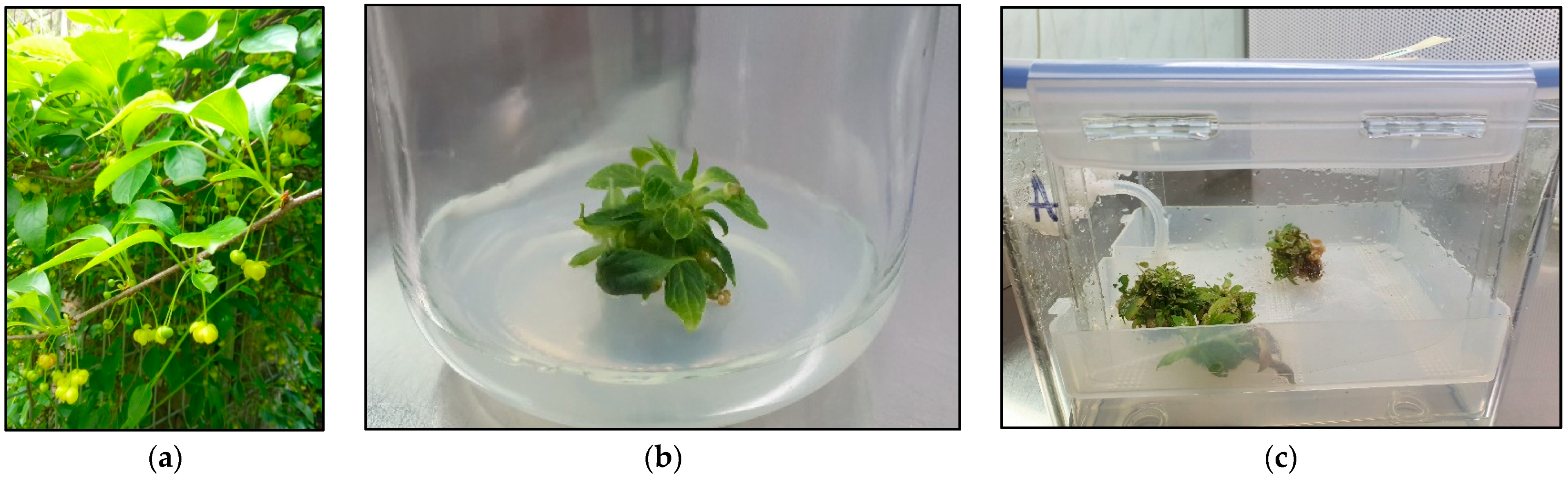
2.1. The Microshoot Cultures Grown in PlanForm Bioreactors
2.2. Lignan Profiling
2.3. Anti-Inflammatory Activity
2.4. Antioxidant Potential
2.5. Antiproliferative and Cytotoxic Activities
2.6. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Parent Plant Material
4.2. In Vitro Microshoot Cultures
4.3. Experimental Microshoot Cultures Grown in PlantForm Bioreactors
4.4. Extraction
4.5. Chromatographic Separation with CPC and Identification with HPLC-DAD and UHPLC-MS/MS
4.6. Chromatographic Profiling with UHPLC-MS/MS
4.7. Anti-Inflammatory Activity
4.8. Antioxidant Potential and Assay of Total Phenolic Content
4.9. Antiproliferative Activitiy
4.10. Antimicrobal Activity
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruňáková, K.; Babincová, Z.; Čellárová, E. Production of Taxanes in Callus and Suspension Cultures of Taxus baccata L. In Liquid Culture Systems for In Vitro Plant Propagation; Springer: Berlin/Heidelberg, Germany, 2005; Volume 9781402031, ISBN 9781402032004. [Google Scholar]
- Seem, K.; Kaur, S. Potential Plant Bioreactors. In Plants as Bioreactors for Industrial Molecules; Wiley Online Library: Hoboken, NJ, USA, 2023; pp. 427–456. [Google Scholar] [CrossRef]
- Taghizadeh, S.; Özzambak, M.E.; Zeybekoglu, E.; Salehian, H.; Azadi, P. In Vitro Micropropagation and Hairy Root Induction for Astragaloside IV Production in Astragalus membranaceus Var. Mongholicus. Plant Cell Tissue Organ. Cult. 2023. under review. [Google Scholar] [CrossRef]
- Erol, M.H.; Dönmez, D.; Biçen, B.; Şimşek, Ö.; Kaçar, Y.A. Modern Approaches to In Vitro Clonal Banana Production: Next-Generation Tissue Culture Systems. Horticulturae 2023, 9, 1154. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Szopa, A.; Ekiert, H.M.; Luczkiewicz, M. Bioreactor-Grown Shoot Cultures for the Secondary Metabolite Production. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Springer: Berlin/Heidelberg, Germany, 2021; pp. 187–247. [Google Scholar] [CrossRef]
- Mordocco, A.M.; Brumbley, J.A.; Lakshmanan, P. Development of a Temporary Immersion System (RITA®) for Mass Production of Sugarcane (Saccharum Spp. Interspecific Hybrids). Vitr. Cell. Dev. Biol.-Plant 2009, 45, 450–457. [Google Scholar] [CrossRef]
- Souza, D.M.S.C.; Avelar, M.L.M.; Fernandes, S.B.; Silva, E.O.; Duarte, V.P.; Molinari, L.V.; Brondani, G.E. Spectral Quality and Temporary Immersion Bioreactor for in Vitro Multiplication of Eucalytpus grandis × Eucalyptus urophylla. 3 Biotech 2020, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Babaei, A.; Shokri, S.; Hessami, S. Micropropagation of Carnation (Dianthus caryophyllus L.) in Liquid Medium by Temporary Immersion Bioreactor in Comparison with Solid Culture. J. Genet. Eng. Biotechnol. 2017, 15, 309–315. [Google Scholar] [CrossRef]
- Rico, S.; Garrido, J.; Sánchez, C.; Ferreiro-Vera, C.; Codesido, V.; Vidal, N. A Temporary Immersion System to Improve Cannabis sativa Micropropagation. Front. Plant Sci. 2022, 13, 895971. [Google Scholar] [CrossRef]
- Zhang, B.; Niu, Z.; Li, C.; Hou, Z.; Xue, Q.; Liu, W.; Ding, X. Improving Large-Scale Biomass and Total Alkaloid Production of Dendrobium nobile Lindl. Using a Temporary Immersion Bioreactor System and MeJA Elicitation. Plant Methods 2022, 18, 10. [Google Scholar] [CrossRef]
- López, C.Q.; Corral, P.; Lorrain-Lorrette, B.; Martinez-Swatson, K.; Michoux, F.; Simonsen, H.T. Use of a Temporary Immersion Bioreactor System for the Sustainable Production of Thapsigargin in Shoot Cultures of Thapsia garganica. Plant Methods 2018, 14, 79. [Google Scholar] [CrossRef]
- Www.Plantform.Se: Home. Available online: https://www.plantform.se/pub/default.aspx?p=100 (accessed on 5 December 2023).
- Szopa, A.; Kokotkiewicz, A.; Bednarz, M.; Jafernik, K.; Łuczkiewicz, M.; Ekiert, H. Bioreactor type affects the accumulation of phenolic acids and flavonoids in microshoot cultures of Schisandra chinensis (Turcz.) Baill. Plant Cell Tissue Organ. Cult. 2019, 139, 199–206. [Google Scholar] [CrossRef]
- Szopa, A.; Klimek-Szczykutowicz, M.; Kokotkiewicz, A.; Dziurka Michałand Luczkiewicz, M.; Ekiert, H. Phenolic Acid and Flavonoid Production in Agar, Agitated and Bioreactor-Grown Microshoot Cultures of Schisandra chinensis Cv. Sadova No. 1—A Valuable Medicinal Plant. J. Biotechnol. 2019, 305, 61–70. [Google Scholar] [CrossRef] [PubMed]
- González, E.J. Mass Propagation of Tropical Crops in Temporary Immersion Systems. In Liquid Culture Systems for In Vitro Plant Propagation; Springer: Berlin/Heidelberg, Germany, 2005; pp. 197–211. [Google Scholar]
- Carvalho, L.S.O.; Ozudogru, E.A.; Lambardi, M.; Paiva, L.V. Temporary Immersion System for Micropropagation of Tree Species: A Bibliographic and Systematic Review. Not. Bot. Horti Agrobot. Cluj. Napoca 2019, 47, 269–277. [Google Scholar] [CrossRef]
- García-Ramírez, Y. Temporary Immersion System for In Vitro Propagation via Organogenesis of Forest Plant Species. Trees-Struct. Funct. 2023, 37, 611–626. [Google Scholar] [CrossRef]
- Watt, M.P. The Status of Temporary Immersion System (TIS) Technology for Plant Micropropagation. Afr. J. Biotechnol. 2012, 11, 14025–14035. [Google Scholar] [CrossRef]
- De Carlo, A.; Tarraf, W.; Lambardi, M.; Benelli, C.; Carlo, D.; Tarraf, A.; Lambardi, W.; Benelli, M.; Temporary, C. Temporary Immersion System for Production of Biomass and Bioactive Compounds from Medicinal Plants. Agronomy 2021, 11, 2414. [Google Scholar] [CrossRef]
- Mirzabe, A.H.; Hajiahmad, A.; Fadavi, A.; Rafiee, S. Temporary Immersion Systems (TISs): A Comprehensive Review. J. Biotechnol. 2022, 357, 56–83. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Naser, S.; Abdulhussein, M.A.A. Effect of Sucrose in Strawberry Micropropagation Using Platform Bioreactor under Temporary Immersion System. Bionatura Issue 2023, 4, 1–7. [Google Scholar] [CrossRef]
- Jia-Sen, L.; Huang, T.Y.; Mei-Fen, H. Studies on the Constituents of Schisandra henryi V. The Structures of Wulignan Al, A2, Epiwulignan Al and Epischisandrone). Acta Chim. Sin. 1988, 46, 483–488. [Google Scholar]
- Chen, Y.-G.; Wu, Z.-C.; Lv, Y.-P.; Gui, S.-H.; Wen, J.; Liao, X.-R.; Yuan, L.-M.; Halaweish, F. Triterpenoids from Schisandra henryi with Cytotoxic Effect on Leukemia and Hela Cells In Vitro. Arch. Pharm. Res. 2003, 26, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Jia-Sen, L.; Mei-Fen, H.; Yao-Liang, G. Studies on the Constituents of Schisandra henryi Clarke. H. The Structures of Schisanhenrin and Schisanhenric Acid. Acta Chim. Sin. 1980, 38, 363–370. [Google Scholar]
- Iu, H.L.; Li-jia, X.U.; Eng, Y.P.; Ang, X.Y.; Iao, P.X. Two New Lignans from Schisandra henryi. Chem. Pharm. Bull. 2009, 57, 405–407. [Google Scholar] [CrossRef]
- Jafernik, K.; Szopa, A.; Barnaś, M.; Dziurka, M.; Ekiert, H. Schisandra henryi C. B. Clarke in Vitro Cultures: A Promising Tool for the Production of Lignans and Phenolic Compounds. Plant Cell Tissue Organ. Cult. 2020, 143, 45–60. [Google Scholar] [CrossRef]
- Szopa, A.; Barnaś, M.; Ekiert, H. Phytochemical Studies and Biological Activity of Three Chinese Schisandra Species (Schisandra sphenanthera, Schisandra henryi and Schisandra rubriflora): Current Findings and Future Applications. Phytochem. Rev. 2019, 18, 109–128. [Google Scholar] [CrossRef]
- Szopa, A.; Klimek-Szczykutowicz, M.; Kokotkiewicz, A.; Maślanka, A.; Król, A.; Luczkiewicz, M.; Ekiert, H. Phytochemical and Biotechnological Studies on Schisandra chinensis Cultivar Sadova No. 1—A High Utility Medicinal Plant. Appl. Microbiol. Biotechnol. 2018, 102, 5105–5120. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Motyka, S.; Calina, D.; Sharifi-Rad, J.; Szopa, A. Comprehensive Review of Dibenzocyclooctadiene Lignans from the Schisandra Genus: Anticancer Potential, Mechanistic Insights and Future Prospects in Oncology. Chin. Med. 2024, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Jafernik, K.; Ekiert, H.; Szopa, A. Schisandra chinensis and Schisandra sphenanthera—From Traditional Far Eastern Medicine to International Utilization. In Medicinal Plants; Springer: Cham, Switzerland, 2021; pp. 179–227. ISBN 978-3-030-74779-4. [Google Scholar]
- European Directorate for the Quality of Medicine. European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2010. [Google Scholar]
- Choi, Y.-W.; Takamatsu, S.; Khan, S.I.; Srinivas, P.V.; Ferreira, D.; Zhao, J.; Khan, I.A. Schisandrene, a Dibenzocyclooctadiene Lignan from Schisandra chinensis: Structure−antioxidant Activity Relationships of Didbenzocyclooctadiene Lignans. J. Nat. Prod. 2006, 69, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, J.; Jeong, K.; Han, S.; Jeong, T.; Yum, M. Antioxidant Activity and Inhibition of MMP-1 Expression of Schizandrae fructus (Schizandra chinensis) Extract. Korean J. Pharmacogn. 2013, 1, 47–52. [Google Scholar]
- Ko, K.M.; Mak, D.H.; Li, P.C.; Poon, M.K.; Ip, S.P. Enhancement of Hepatic Glutathione Regeneration Capacity by a Lignan-Enriched Extract of Fructus schisandrae in Rats. Jpn. J. Pharmacol. 1995, 69, 439–442. [Google Scholar] [CrossRef]
- Ip, S.P.; Mak, D.H.F.; Li, P.C.; Poon, M.K.T.; Ko, K.M. Effect of a Lignan-Enriched Extract of Schisandra chinensis on Aflatoxin B1 and Cadmium Chloride-Induced Hepatotoxicity in Rats. Pharmacol. Toxicol. 1996, 78, 413–416. [Google Scholar] [CrossRef]
- Chiu, P.Y.; Mak, D.H.F.; Poon, M.K.T.; Ko, K.M.; Ko, R. In Vivo Antioxidant Action of a Lignan-Enriched Extract of Schisandra Fruit and an Anthraquinone-Containing Extract of Polygonum Root in Comparison with Schisandrin B and Emodin. Planta Med. 2002, 68, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Opletal, L.; Sovová, H.; Bártlová, M. Dibenzo[a,c]Cyclooctadiene Lignans of the Genus Schisandra: Importance, Isolation and Determination. J. Chromatogr. B 2004, 812, 357–371. [Google Scholar] [CrossRef]
- Lu, H.; Liu, G.T. Anti-Oxidant Activity of Dibenzocyclooctene Lignans Isolated from Schisandraceae. Planta Med. 1992, 58, 311–313. [Google Scholar] [CrossRef]
- Lu, H.; Liu, G.T. Effect of Dibenzo[a,c]Cyclooctene Lignans Isolated from Fructus Schizandrae on Lipid Peroxidation and Anti-Oxidative Enzyme Activity. Chem. Biol. Interact. 1991, 78, 77–84. [Google Scholar] [CrossRef]
- Hu, D.; Yang, Z.; Yao, X.; Al, E. Dibenzocyclooktadiene Lignans from Schisandra chinensis and Their Inhibitory Activity on NO Production in Lipopolysacharide-Actiavted Microglia Cells. Phytochemistry 2014, 104, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Ci, X.X.; Li, H.Z.; Li, H.M.; Luo, G.J.; Li, R.T.; Deng, X.M. New Dibenzocyclooctadiene Lignans from Schisandra sphenanthera and Their Proinflammatory Cytokine Inhibitory Activities. Z. Fur Naturforschung—Sect. B J. Chem. Sci. 2010, 65, 211–218. [Google Scholar] [CrossRef]
- Jiang, Y.; Fan, X.; Wang, Y.; Tan, H.; Chen, P.; Zeng, H.; Huang, M.; Bi, H. Hepato-Protective Effects of Six Schisandra Lignans on Acetaminophen-Induced Liver Injury Are Partially Associated with the Inhibition of CYP-Mediated Bioactivation. Chem. Biol. Interact. 2015, 231, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.I.; Do, G.M.; Lee, H.M.; Ok, H.M.; Shin, J.H.; Kwon, O. Schisandra chinensis Baillon Regulates the Gene Expression of Phase Ii Antioxidant/Detoxifying Enzymes in Hepatic Damage Induced Rats. Nutr. Res. Pract. 2014, 8, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Casarin, E.; Dall’Acqua, S.; Smejkal, K.; Al, E. Molecular Mechanisms of Antiproliferative Effects Induced by Schisandra-Derived Dibenzocyclooctadiene Lignanas (+)-Deoxyschisandrin And (−)-Gomisin N in Human Tumour Cell Lines. Fitoterapia 2014, 98, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Dziurka, M.; Warzecha, A.; Kubica, P.; Klimek-Szczykutowicz, M.; Ekiert, H. Targeted Lignan Profiling and Anti-Inflammatory Properties of Schisandra rubriflora and Schisandra chinensis Extracts. Molecules 2018, 23, 3103. [Google Scholar] [CrossRef]
- Li, R.; Shen, Y.; Xiang, W.; Sun, H. Four Novel Nortriterpenoids Isolated from Schisandra henryi Var. Yunnanensis. Eur. J. Org. Chem. 2004, 2004, 807–811. [Google Scholar] [CrossRef]
- KNApSAcK Core System. Available online: http://www.knapsackfamily.com/knapsack_core/top.php (accessed on 17 January 2024).
- LOTUS: Natural Products Online. Available online: https://lotus.naturalproducts.net/ (accessed on 17 January 2024).
- Rutz, A.; Sorokina, M.; Galgonek, J.; Mietchen, D.; Willighagen, E.; Gaudry, A.; Graham, J.G.; Stephan, R.; Page, R.; Vondrášek, J.; et al. The LOTUS Initiative for Open Knowledge Management in Natural Products Research. eLife 2022, 11, e70780. [Google Scholar] [CrossRef]
- Feng, W.; Zhou, L.Y.; Mu, R.F.; Gao, L.; Xu, B.Y.; Liu, M.L.; Niu, L.Y.; Wang, X.G. Screening and Identification of the Main Metabolites of Schisantherin a In Vivo and In Vitro by Using UHPLC-Q-TOF-MS/MS. Molecules 2020, 25, 258. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Liu, Y.; Xue, Y.; Wang, J. A New Triterpenoid Acid from Schisandra henryi. Chem. Nat. Compd. 2010, 46, 480–481. [Google Scholar] [CrossRef]
- He, T.B.; Yan, B.C.; Hu, K.; Li, X.N.; Sun, H.D.; Puno, P.T. Neuroprotective Schinortriterpenoids with Diverse Scaffolds from Schisandra henryi. Bioorg Chem. 2020, 105, 104353. [Google Scholar] [CrossRef] [PubMed]
- Sermukhamedova, O.; Wojtanowski, K.K.; Widelski, J.; Korona-Głowniak, I.; Elansary, H.O.; Sakipova, Z.; Malm, A.; Głowniak, K.; Skalicka-Wozniak, K. Metabolic Profile of and Antimicrobial Activity in the Aerial Part of Leonurus Turkestanicus V.I. Krecz. et Kuprian. from Kazakhstan. J. AOAC Int. 2017, 100, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Kokotkiewicz, A.; Luczkiewicz, M.; Ekiert, H. Schisandra Lignans Production Regulated by Different Bioreactor Type. J. Biotechnol. 2017, 247, 11–17. [Google Scholar] [CrossRef]
- Szopa, A.; Dziurka, M.; Kubica, P.; Jafernik, K.; Siomak, O.; Ekiert, H. Stimulation of Lignan Production in Schisandra rubriflora In Vitro Cultures by Elicitation. Molecules 2022, 27, 6681. [Google Scholar] [CrossRef]
- Szopa, A.; Dziurka, M.; Granica, S.; Klimek-Szczykutowicz, M.; Kubica, P.; Warzecha, A.; Jafernik, K.; Ekiert, H. Schisandra rubriflora Plant Material and in Vitro Microshoot Cultures as Rich Sources of Natural Phenolic Antioxidants. Antioxidants 2020, 9, 488. [Google Scholar] [CrossRef]
- Mantovska, D.I.; Zhiponova, M.K.; Petrova, D.; Alipieva, K.; Bonchev, G.; Boycheva, I.; Evstatieva, Y.; Nikolova, D.; Tsacheva, I.; Simova, S.; et al. Exploring the Phytochemical Composition and Biological Potential of Balkan Endemic Species Stachys Scardica Griseb. Plants 2023, 13, 30. [Google Scholar] [CrossRef]
- Boylan, F.; Jakabfi-Csepregi, R.; Alberti, Á.; Felegyi-Tóth, C.A.; Czigle, S.; Papp, N. A Comprehensive Study on Lathyrus tuberosus L.: Insights into Phytochemical Composition, Antimicrobial Activity, Antioxidant Capacity, Cytotoxic, and Cell Migration Effects. Plants 2024, 13, 232. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, T.; Sikora, J.; Merecz-Sadowska, A.; Kukula-Koch, W.; Synowiec, E.; Majda, A.; Juda, D.; Śliwiński, T.; Sitarek, P. Biological Properties of Extracts Obtained from In Vitro Culture of Plectranthus Scutellarioides in a Cell Model. Int. J. Mol. Sci. 2024, 25, 1043. [Google Scholar] [CrossRef]
- Available online: https://www.Clematis.Com.Pl/ (accessed on 5 December 2023).
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Kokotkiewicz, A.; Bucinski, A.; Luczkiewicz, M. Xanthone, Benzophenone and Bioflavonoid Accumulation in Cyclopia genistoides (L.) Vent. (Honeybush) Shoot Cultures Grown on Membrane Rafts and in a Temporary Immersion System. Plant Cell Tissue Organ. Cult. 2015, 120, 373–378. [Google Scholar] [CrossRef]
- Friesen, J.B.; Pauli, G.F. G.U.E.S.S.—A Generally Useful Estimate of Solvent Systems for CCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2777–2806. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Bektaşoǧlu, B.; Apak, R. Spectrophotometric Determination of Ascorbic Acid by the Modified CUPRAC Method with Extractive Separation of Flavonoids–La(III) Complexes. Anal. Chim. Acta 2007, 588, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Biesaga-Kościelniak, J.; Dziurka, M.; Ostrowska, A.; Mirek, M.; Koscielniak, J.; Janeczko, A. Brassinosteroid Improves Content of Antioxidants in Seeds of Selected Leguminous Plants. Aust. J. Crop Sci. 2014, 8, 378–388. [Google Scholar]
- Dziurka, M.; Kubica, P.; Kwiecień, I.; Biesaga-Kościelniak, J.; Ekiert, H.; Abdelmohsen, S.A.M.; Al-Harbi, F.F.; Elansary, D.O.; Elansary, H.O.; Szopa, A. In Vitro Cultures of Some Medicinal Plant Species (Cistus × incanus, Verbena officinalis, Scutellaria lateriflora, and Scutellaria baicalensis) as a Rich Potential Source of Antioxidants—Evaluation by CUPRAC and QUENCHER-CUPRAC Assays. Plants 2021, 10, 454. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Methods in Enzymology—Oxidants and Antioxidants Part A. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bach, A.; Kapczyńska, A.; Dziurka, K.; Dziurka, M. Phenolic Compounds and Carbohydrates in Relation to Bulb Formation in Lachenalia ‘Ronina’ and ‘Rupert’ in Vitro Cultures under Different Lighting Environments. Sci. Hortic. 2015, 188, 23–29. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; Ali Zin El-Abedin, T.K.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidate, and Magnolia acuminata Have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, M.A.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus Spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef] [PubMed]
- Yessoufou, K.; Elansary, H.O.; Mahmoud, E.A.; Skalicka-Woźniak, K. Antifungal, Antibacterial and Anticancer Activities of Ficus drupacea L. Stem Bark Extract and Biologically Active Isolated Compounds. Ind. Crops Prod. 2015, 74, 752–758. [Google Scholar] [CrossRef]
- Elansary, H.O.; Abdelgaleil, S.A.M.; Mahmoud, E.A.; Yessoufou, K.; Elhindi, K.; El-Hendawy, S. Effective Antioxidant, Antimicrobial and Anticancer Activities of Essential Oils of Horticultural Aromatic Crops in Northern Egypt. BMC Complement. Altern. Med. 2018, 18, 214. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. Available online: https://www.eucast.org/ (accessed on 9 January 2024).
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The In Vitro Activity of Essential Oils against Helicobacter pylori Growth and Urease Activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef]


| No. | Proposed Identification | Chemical Structure | Exp. (m/z) | Calc. (m/z) | RT [min] | MF | MS/MS (+) | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Henridilactone C |  | 527.3455 [M + H]+ | 527.22756 | 17.20 | C29H34O9 | 275.1773 | [46] |
| 2 | Henridilactone C | 527.3426 [M + H]+ | 527.22756 | 17.28 | C29H34O9 | 275.1745 | [46] | |
| 3 | Unidentified | 309.2130 | 30.6 | 291.2030, 273.1955, 251.3999, 275.1822 | ||||
| Pregomisin | 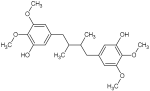 | 391.2208 [M + H]+ | 391.21206 | 32.2 | C22H30O6 | 359.1998, 337.9215, 237.1592, 205.1227, 167.0729 | [47] | |
| 4 | 4-Hydroxy-2-[hydroxy(3 hydroxy-4,5 dimethoxyphenyl)methyl]-3 (hydroxymethyl)butanoic acid | 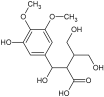 | 317.2567 [M + H]+ | 317.24806 | 30.9 | C21H32O2 | 300.2302, 299.2393, 219.1318 | [48,49] |
| Schisantherin G |  | 559.2032 [M + H]+ | 559.21794 | 36.2 | C29H34O11 | 438.1609, 416.2209, 372.1746, 237.8928 | [47] | |
| Unidentified | 611.4284 | 39.2 | 550.5979, 508.2968, 317.2182 | |||||
| 5 | Schisantherin B | 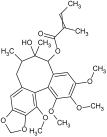 | 537.2235 [M + Na]+ | 537.2095 | 35.9 | C28H34O9 | 438.1570, 416.1903, 372.1536, 342.1123 | [47,50] |
| 6 | Schisantherin B | 537.2245 [M + Na]+ | 537.2095 | 36.0 | C28H34O9 | 438.1664, 416.1982, 372.1624 | [47,50] | |
| 7 | Schisantherin B | 537.2195 [M + Na]+ | 537.2095 | 36.0 | C28H34O9 | [47,50] | ||
| 8 | Schisantherin B | 537.2255 [M + Na]+ | 537.2095 | 36.1 | C28H34O9 | 438.1748, 416.1893, 372.1726 | [47,50] |
| Lignans | Leaves of Parent Plant | Microshoot In Vitro Culture Grown in PlantForm Bioreactors |
|---|---|---|
| Wulignan A1 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Rubrisandrin A | 0.08 * ± 0.00 | 0.04 * ± 0.00 |
| Rubriflorin A | 0.02 * ± 0.00 | nd *‡ |
| Schisandrin | nd * | 0.35 * ± 0.20 |
| Gomisin D | 0.85 * ± 0.12 | 1.15 * ± 0.31 |
| Gomisin J | 0.40 * ± 0.09 | 0.23 * ± 0.19 |
| Gomisin A | 0.02 * ± 0.00 | 0.20 * ± 0.17 |
| Gomisin G | 5.62 * ± 1.98 | 1.90 * ± 0.12 |
| Licarin B | 8.40 * ± 2.56 | 5.25 * ± 0.89 |
| Epigomisin O | 1.97 ± 1.05 | 1.21 ± 0.15 |
| Gomisin O | 1.76 ± 1.00 | 2.54 ± 0.21 |
| Schisantherin A | 61.65 * ± 4.05 | 73.16 * ± 4.31 |
| Schisantherin B | 361.24 * ± 13.06 | 390.16 * ± 12.61 |
| Licarin A | 0.06 * ± 0.00 | 0.92 * ± 0.14 |
| Schisanhenol | 0.02 ± 0.00 | 0.03 ± 0.00 |
| Deoxyschisandrin | 0.03 * ± 0.00 | 3.02 * ± 0.23 |
| Gomisin N | 0.17 * ± 0.05 | 0.62 * ± 0.16 |
| 6-O-Benzylgomisin O | 68.53 ± 3.17 | 63.17 ± 3.12 |
| Schisandrin C | 0.01 ± 0.00 | 0.03 ± 0.00 |
| Total content | 510.84 * ± 27.13 | 543.99 * ± 22.81 |
| Extracts | In Vitro Enzymes Inhibition | ||||
|---|---|---|---|---|---|
| 15-LOX | COX-1 | COX-2 | sPLA2 | ||
| Concentration μg/mL | % Inh ± SD | ||||
| Leaves of parent plant | 175.0 | 9 ± 0.6 | 70 ± 7.7 | 33 ± 3.7 | 19 * ± 0.8 |
| 17.5 | 27 ± 1.9 | 31 * ± 3.4 | 34 * ± 3.8 | 16 ± 0.6 | |
| Microshoot in vitro culture grown in PlantForm bioreactors | 175.0 | 6 ± 0.4 | 76 ± 8.4 | 66 ± 7.3 | 14 * ± 0.6 |
| 17.5 | 26 ± 1.8 | 41 * ± 4.5 | 48 * ± 5.3 | 17 ± 0.7 | |
| Schisantherin A and schisantherin B equimolar mixture | 2.2 | 31 ± 1.6 | 38 ± 5.8 | 48 ± 5.3 | 8 ± 0.3 |
| 0.2 | 55 ± 3.8 | 74 ± 8.1 | 31 ± 3.4 | 1 ± 0.04 | |
| Nordihydroguaiaretic acid (control) | 30.2 | 23 ± 2.0 | - | - | - |
| Ibuprofen (control) | 2.1 | - | 23 ± 2.5 | 21 ± 2.0 | - |
| Thioetheramide-PC (control) | 73.6 | - | - | - | 91 ± 4.0 |
| Extracts | Assays | |||
|---|---|---|---|---|
| F-C | FRAP | DPPH | CUPRAC | |
| Leaves of parent plant | 9.0 * ± 0.4 | 2.4 * ± 0.04 | 5.9 * ± 1.1 | 6.7 * ± 0.2. |
| Microshoot in vitro culture grown in PlantForm bioreactors | 37.0 * ± 0.2 | 13.5 * ± 0.2 | 19.5 * ± 2.1 | 25.9 * ± 0.7 |
| Schisantherin A and schisantherin B equimolar mixture | 24.5 ± 2.2 | 13.5 ± 0.5 | 101.3 ± 21.8 | 6.3 ± 2.1 |
| Extracts | Cancer Cells | Normal Cells | |||
|---|---|---|---|---|---|
| HeLa | HT-29 | MCF-7 | Jurkat | HEK-293 | |
| Leaves of parent plant | 65.2 * ± 3.3 | 43.57 * ± 2.1 | 73.63 * ± 3.2 | 85.48 * ± 2.7 | >400 |
| Microshoot in vitro culture grown in PlantForm bioreactors | 78.57 * ± 2.5 | 57.64 * ± 1.9 | 81.53 * ± 2.7 | 94.21 * ± 3.2 | >400 |
| Schisantherin A and schisantherin B equimolar mixture | 35.63 ± 1.2 | 24.35 ± 0.8 | 26.34 ± 1.3 | 44.62 ± 0.8 | >400 |
| Vinblastine sulfate (control) | 2.2 ± 0.05 | 17.63 ± 0.5 | - | 0.1 ± 0.02 | 45.1 ± 0.5 |
| Taxol (control) | - | - | 0.06 ± 0.005 | - | - |
| Microorganisms | Leaves of Parent Plant | Microshoot In Vitro Culture Grown in PlantForm Bioreactors | Standard Drugs (Control) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | MBC or MFC (mg/mL) | MBC/MIC or MFC/MIC Ratio | MIC (mg/mL) | MBC or MFC (mg/mL) | MBC/MIC or MFC/MIC Ratio | MIC (mg/L) | MBC or MFC (mg/L) | ||
| Vancomycin | |||||||||
| Gram-positive bacteria | S. aureus ATCC25923 | 1.25 * | 10 | 8 * | 5 * | 10 | 2 * | 0.98 | 0.98 |
| S. aureus ATCC43300 | 1.25 * | 5 | 4 * | 5 * | 10 | 2 * | 0.98 | 0.98 | |
| S. epidermidis ATCC12228 | 1.25 * | 2.5 | 2 * | 5 * | 5 * | 1 * | 0.98 | 0.98 | |
| Ciplofloxacin | |||||||||
| Gram-negative bacteria | E. coli ATCC25922 | 10 | 10 | 1 | 10 | 10 | 1 | 0.015 | 0.08 |
| P. aeruginosa ATCC9027 | 10 | 10 | 1 | 10 | 10 | 1 | 0.488 | 0.98 | |
| H. pylori ATCC 43504 | 0.625 | 0.625 | 1 | 0.625 | 0.625 | 1 | 0.98 † | 0.98 † | |
| Nystatin | |||||||||
| Fungi | C. albicans ATCC 102231 | 5 * | 10 * | 2 | 10 * | 20 * | 2 | 0.24 | 0.48 |
| C. parapsilosis ATCC 22019 | 5 * | 20 | 4 * | 10 * | 20 | 2 * | 0.24 | 0.48 | |
| C. glabrata ATCC 90030 | 10 | 10 * | 1 * | 10 | 20 * | 2 * | 0.48 | 0.48 | |
| A. niger ATCC 16404 | 10 | nd | nd | 10 | nd | nd | 0.5 ‡ | nd | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafernik, K.; Kubica, P.; Dziurka, M.; Kulinowski, Ł.; Korona-Głowniak, I.; Elansary, H.O.; Waligórski, P.; Skalicka-Woźniak, K.; Szopa, A. Comparative Assessment of Lignan Profiling and Biological Activities of Schisandra henryi Leaf and In Vitro PlantForm Bioreactor-Grown Culture Extracts. Pharmaceuticals 2024, 17, 442. https://doi.org/10.3390/ph17040442
Jafernik K, Kubica P, Dziurka M, Kulinowski Ł, Korona-Głowniak I, Elansary HO, Waligórski P, Skalicka-Woźniak K, Szopa A. Comparative Assessment of Lignan Profiling and Biological Activities of Schisandra henryi Leaf and In Vitro PlantForm Bioreactor-Grown Culture Extracts. Pharmaceuticals. 2024; 17(4):442. https://doi.org/10.3390/ph17040442
Chicago/Turabian StyleJafernik, Karolina, Paweł Kubica, Michał Dziurka, Łukasz Kulinowski, Izabela Korona-Głowniak, Hosam O. Elansary, Piotr Waligórski, Krystyna Skalicka-Woźniak, and Agnieszka Szopa. 2024. "Comparative Assessment of Lignan Profiling and Biological Activities of Schisandra henryi Leaf and In Vitro PlantForm Bioreactor-Grown Culture Extracts" Pharmaceuticals 17, no. 4: 442. https://doi.org/10.3390/ph17040442
APA StyleJafernik, K., Kubica, P., Dziurka, M., Kulinowski, Ł., Korona-Głowniak, I., Elansary, H. O., Waligórski, P., Skalicka-Woźniak, K., & Szopa, A. (2024). Comparative Assessment of Lignan Profiling and Biological Activities of Schisandra henryi Leaf and In Vitro PlantForm Bioreactor-Grown Culture Extracts. Pharmaceuticals, 17(4), 442. https://doi.org/10.3390/ph17040442











