Acute Kidney Injury and Electrolyte Imbalances Caused by Dapagliflozin Short-Term Use
Abstract
1. Introduction
2. Results
2.1. Sample Characterization
2.2. Effects of Dapagliflozin on Renal Function
2.3. Effects of Dapagliflozin on Electrolyte Balance
2.4. Comparisons with Adverse Drug Reaction Profiles Reported in the Eudravigilance Database
3. Discussion
4. Materials and Methods
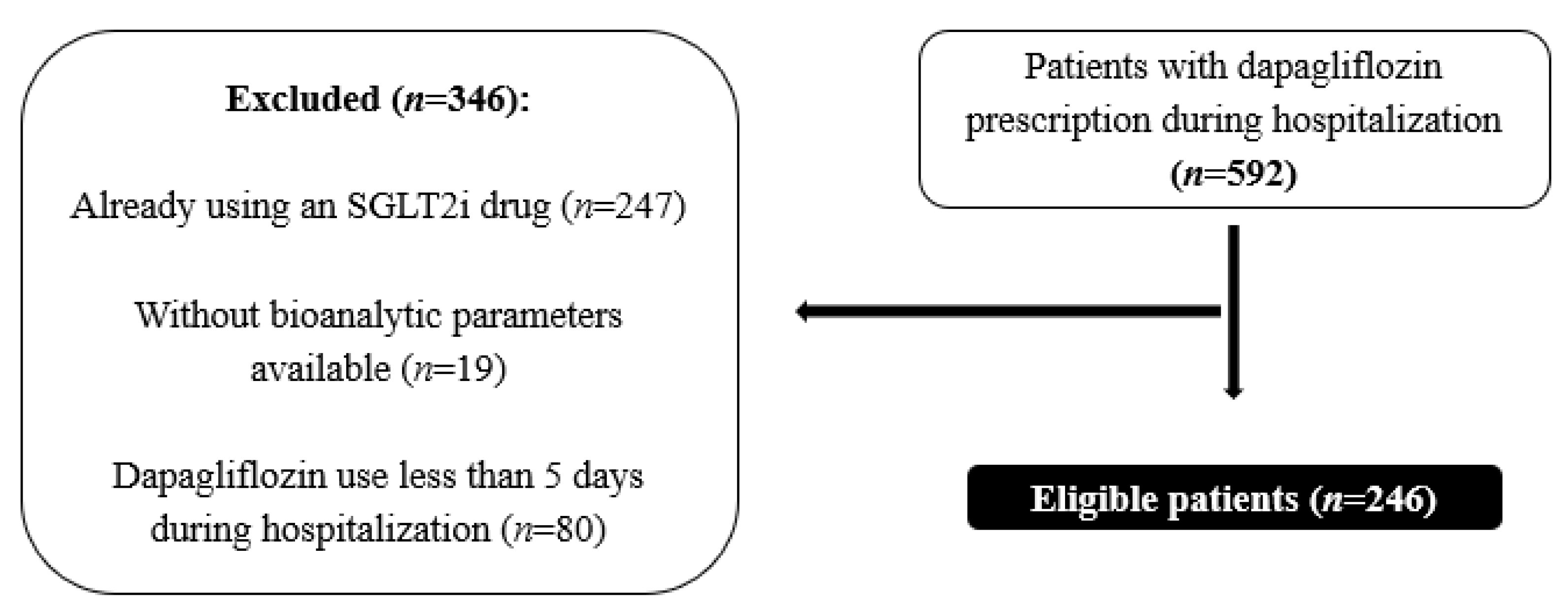
4.1. Study Design and Sampling
4.2. Data Collection
- (a)
- Age and gender.
- (b)
- Medical history: T2DM, high blood pressure (HBP), heart failure (HF), and chronic kidney disease (CKD).
- (c)
- Blood glycemia, creatinine, sodium, and potassium levels [both before dapagliflozin prescription (baseline) and 5 to 8 days after prescription (endpoint)]. The exact endpoint day (between day 5 and day 8 after dapagliflozin prescription) was determined following the KDIGO guidelines, which define AKI as an abrupt decrease in kidney function occurring within 7 days [62]. Due to limitations in the data availability, most of the hospitalized patients did not have analytical parameters available daily or on weekends.
- (d)
- Individual Cases Safety Reports (ICSRs) data were obtained from the European spontaneous reporting system EV database, accessed at www.adrreports.eu (accessed on 7 February 2024). The EV, funded by the European Medicines Agency EMA, manages and analyses the ICSRs for suspected ADRs [63,64].
- I.
- Qualitative and quantitative analyses of the main outcomes of the ICSRs were conducted from 1 January to 31 December, 2023.
- II.
- The information collected included sex, age group, outcomes, number of AKIs, hyponatremia, and hypokalemia events per ICSR, as well as reported concomitant medications (furosemide, ACEi, ARB, or spironolactone).
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Furosemide | p-Value | ||
|---|---|---|---|
| No | Yes | ||
| Without AKI at endpoint | 64 (33.5%) | 127 (66.5%) | 0.006 1 |
| AKI at endpoint | 8 (14.5%) | 47 (85.5%) | |
| Chronic Kidney Disease | p-Value | ||
|---|---|---|---|
| No | Yes | ||
| Hyponatremia | 34 (66.7%) | 17 (33.3%) | 0.181 1 |
| Hyperkaliemia | 28 (68.3%) | 13 (31.7%) | 0.363 1 |
References
- Hsia, D.S.; Grove, O.; Cefalu, W.T. An update on sodium-glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.; Fernando, K.; Milne, N.; Evans, M.; Ali, A.; Bain, S.; Hicks, D.; James, J.; Newland-Jones, P.; Patel, D.; et al. SGLT2 Inhibitors in Type 2 Diabetes Management: Key Evidence and Implications for Clinical Practice. Diabetes Ther. 2018, 9, 1757–1773. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Pharmacodynamics, Efficacy and Safety of Sodium–Glucose Co-Transporter Type 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Clar, C.; Gill, J.A.; Court, R.; Waugh, N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012, 2, e001007. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.; Kesavadev, J.; Chadha, M.; Kumar, G. Sodium-glucose cotransporter-2 inhibitors in combination with other glucose-lowering agents for the treatment of type 2 diabetes mellitus. Indian J. Endocr. Metab. 2018, 22, 827. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Correa, J.I.; Correa-Rotter, R. Sodium-Glucose Cotransporter 2 Inhibitors Mechanisms of Action: A Review. Front. Med. 2021, 8, 777861. [Google Scholar] [CrossRef] [PubMed]
- Plosker, G.L. Dapagliflozin: A Review of Its Use in Patients with Type 2 Diabetes. Drugs 2014, 74, 2191–2209. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, M.K.; Ghazal Asswad, R.; Wilding, J.P. Dapagliflozin for the treatment of type 2 diabetes mellitus—An update. Expert. Opin. Pharmacother. 2021, 22, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Ghaleb, R.; Mansour, H.; Hanafy, A.; Mahmoud, N.M.; Abdelfatah Elsharef, M.; Kamal Salama, M.; Elsaughier, S.M.; Abdel-Wahid, L.; Embarek Mohamed, M.; et al. Safety and Efficacy of Adding Dapagliflozin to Furosemide in Type 2 Diabetic Patients with Decompensated Heart Failure and Reduced Ejection Fraction. Front. Cardiovasc. Med. 2020, 7, 602251. [Google Scholar] [CrossRef]
- Cianciolo, G.; De Pascalis, A.; Gasperoni, L.; Tondolo, F.; Zappulo, F.; Capelli, I.; Cappuccilli, M.; La Manna, G. The Off-Target Effects, Electrolyte and Mineral Disorders of SGLT2i. Molecules 2020, 25, 2757. [Google Scholar] [CrossRef] [PubMed]
- Ptaszynska, A.; Johnsson, K.M.; Parikh, S.J.; De Bruin, T.W.A.; Apanovitch, A.M.; List, J.F. Safety Profile of Dapagliflozin for Type 2 Diabetes: Pooled Analysis of Clinical Studies for Overall Safety and Rare Events. Drug Saf. 2014, 37, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Menne, J.; Dumann, E.; Haller, H.; Schmidt, B.M.W. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: A systematic review and meta-analysis. PLoS Med. 2019, 16, e1002983. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Januzzi, J.L.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.; Morris, P.B.; Neumiller, J.J.; et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with Type 2 Diabetes. J. Am. Coll. Cardiol. 2020, 76, 1117–1145. [Google Scholar] [CrossRef] [PubMed]
- Kidokoro, K.; Cherney, D.Z.I.; Bozovic, A.; Nagasu, H.; Satoh, M.; Kanda, E.; Sasaki, T.; Kashihara, N. Evaluation of Glomerular Hemodynamic Function by Empagliflozin in Diabetic Mice Using In Vivo Imaging. Circulation 2019, 140, 303–315. [Google Scholar] [CrossRef]
- Poursharif, S.; Hamza, S.; Braam, B. Changes in Proximal Tubular Reabsorption Modulate Microvascular Regulation via the TGF System. Int. J. Mol. Sci. 2022, 23, 11203. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef] [PubMed]
- Kurata, Y.; Nangaku, M. Dapagliflozin for the treatment of chronic kidney disease. Expert. Rev. Endocrinol. Metab. 2022, 17, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ye, L.; Yan, Q.; Zhang, X.; Wang, L. Effects of Sodium-Glucose Cotransporter 2 Inhibitors on Water and Sodium Metabolism. Front. Pharmacol. 2022, 13, 800490. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yuan, C.; Chen, G.; Zhang, C.; Wu, X. SGLT2i: Beyond the glucose-lowering effect. Cardiovasc. Diabetol. 2020, 19, 98. [Google Scholar] [CrossRef]
- Vallon, V.; Thomson, S.C. Renal Function in Diabetic Disease Models: The Tubular System in the Pathophysiology of the Diabetic Kidney. Annu. Rev. Physiol. 2012, 74, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Scholtes, R.A.; Muskiet, M.H.A.; van Baar, M.J.B.; Hesp, A.C.; Greasley, P.J.; Karlsson, C.; Hammarstedt, A.; Arya, N.; van Raalte, D.H.; Heerspink, H.J.L. Natriuretic Effect of Two Weeks of Dapagliflozin Treatment in Patients with Type 2 Diabetes and Preserved Kidney Function During Standardized Sodium Intake: Results of the DAPASALT Trial. Diabetes Care 2021, 44, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Takano, K.; Iijima, H.; Kubo, H.; Maruyama, N.; Hashimoto, T.; Arakawa, K.; Togo, M.; Inagaki, N.; Kaku, K. Factors Affecting Canagliflozin-Induced Transient Urine Volume Increase in Patients with Type 2 Diabetes Mellitus. Adv. Ther. 2017, 34, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Velat, I.; Bušić, Ž.; Jurić Paić, M.; Čulić, V. Furosemide and spironolactone doses and hyponatremia in patients with heart failure. BMC Pharmacol. Toxicol. 2020, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Yavin, Y.; Mansfield, T.A.; Ptaszynska, A.; Johnsson, K.; Parikh, S.; Johnsson, E. Effect of the SGLT2 Inhibitor Dapagliflozin on Potassium Levels in Patients with Type 2 Diabetes Mellitus: A Pooled Analysis. Diabetes Ther. 2016, 7, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Oishi, M.; Aso, H.; Arai, K.; Sasaki, Y.; Tochikura, N.; Ootsuka, S.; Fukuoka, N.; Ooba, N.; Kikuchi, N. Effects of angiotensin II receptor blockers on serum potassium level and hyperkalemia risk: Retrospective single-centre analysis. Eur. J. Hosp. Pharm. 2023, 30, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Raebel, M.A. Hyperkalemia Associated with Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers. Cardiovasc. Ther. 2012, 30, e156–e166. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, W.; Ren, H.; Chen, X.; Xie, J.; Chen, N. Diuretics associated acute kidney injury: Clinical and pathological analysis. Ren. Fail. 2014, 36, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.M.; Power, B.M. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010, 65, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Patschan, D.; Patschan, S.; Buschmann, I.; Ritter, O. Loop Diuretics in Acute Kidney Injury Prevention, Therapy, and Risk Stratification. Kidney Blood Press Res. 2019, 44, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.; Berl, T. Dysnatremias in Patients with Kidney Disease. Am. J. Kidney Dis. 2014, 63, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Shavit, L.; Merin, O.; Grenader, T.; Jacobson, E.; Waldenberg, C.; Bitran, D.; Fink, D.; Silberman, S. Hyponatremia Predicts Poor Outcomes in Patients with Chronic Kidney Disease Undergoing Heart Operation. Ann. Thorac. Surg. 2018, 106, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R. Hyperkalemia in chronic kidney disease. Rev. Assoc. Med. Bras. 2020, 66, s31–s36. [Google Scholar] [CrossRef] [PubMed]
- Sarnowski, A.; Gama, R.M.; Dawson, A.; Mason, H.; Banerjee, D. Hyperkalemia in Chronic Kidney Disease: Links, Risks and Management. Int. J. Nov. Res. Dev. 2022, 15, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, L.M.; Zhan, M.; Hsu, V.D.; Walker, L.D.; Moen, M.F.; Seliger, S.L.; Weir, M.R.; Fink, J.C. The Frequency of Hyperkalemia and Its Significance in Chronic Kidney Disease. Arch. Intern. Med. 2009, 169, 1156. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Sang, Y.; Leddy, J.; Yahya, T.; Kirchner, H.L.; Inker, L.A.; Matsushita, K.; Ballew, S.H.; Coresh, J.; Grams, M.E. Antihypertensive Medications and the Prevalence of Hyperkalemia in a Large Health System. Hypertension 2016, 67, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, W.J.; Van Den Born, B.H.; Van Munster, B.C.; Korevaar, J.C.; Levi, M.; De Rooij, S.E. The association between serum sodium levels at time of admission and mortality and morbidity in acutely admitted elderly patients: A prospective cohort study. J. Am. Geriatr. Soc. 2010, 58, 2227–2228. [Google Scholar] [CrossRef] [PubMed]
- Dhondup, T.; Qian, Q. Electrolyte and Acid-Base Disorders in Chronic Kidney Disease and End-Stage Kidney Failure. Blood Purif. 2017, 43, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Filippatos, T.D.; Makri, A.; Elisaf, M.S.; Liamis, G. Hyponatremia in the elderly: Challenges and solutions. Clin. Interv. Aging 2017, 12, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- NHS. Dapagliflozin. Available online: https://www.nhs.uk/medicines/dapagliflozin/ (accessed on 18 February 2024).
- Delanaye, P.; Scheen, A.J. Epidemiology of acute kidney injury adverse events with SGLT2 inhibitors: A meta-analysis of observational cohort studies. Diabetes Epidemiol. Manag. 2021, 3, 100021. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C.; Bellary, S. Renal Protection with SGLT2 Inhibitors: Effects in Acute and Chronic Kidney Disease. Curr. Diab Rep. 2022, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Yau, K.; Dharia, A.; Alrowiyti, I.; Cherney, D.Z.I. Prescribing SGLT2 Inhibitors in Patients with CKD: Expanding Indications and Practical Considerations. Kidney Int. Rep. 2022, 7, 1463–1476. [Google Scholar] [CrossRef]
- Adamson, C.; Docherty, K.F.; Heerspink, H.J.L.; De Boer, R.A.; Damman, K.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Petrie, M.C.; et al. Initial Decline (Dip) in Estimated Glomerular Filtration Rate After Initiation of Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction: Insights From DAPA-HF. Circulation 2022, 146, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Muñoz, A.Y.; Weinstein, J.; Wald, R. eGFR Decline after SGLT2 Inhibitor Initiation: The Tortoise and the Hare Reimagined. Kidney 2021, 360, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Antihypertensive and Renal Mechanisms of SGLT2 (Sodium-Glucose Linked Transporter 2) Inhibitors. Hypertension 2020, 75, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Schork, A.; Saynisch, J.; Vosseler, A.; Jaghutriz, B.A.; Heyne, N.; Peter, A.; Häring, H.-U.; Stefan, N.; Fritsche, A.; Artunc, F. Effect of SGLT2 inhibitors on body composition, fluid status and renin–angiotensin–aldosterone system in type 2 diabetes: A prospective study using bioimpedance spectroscopy. Cardiovasc. Diabetol. 2019, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Masuda, T.; Morinari, M.; Okada, M.; Miki, A.; Nakagawa, S.; Murakami, T.; Oka, K.; Asakura, M.; Miyazawa, Y.; et al. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol. Metab. Syndr. 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Gankam Kengne, F.; Decaux, G. Hyponatremia and the Brain. Kidney Int. Rep. 2018, 3, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, K.; Smith, M. Disorders of sodium balance after brain injury. Contin. Educ. Anaesth. Crit. Care Pain 2008, 8, 129–133. [Google Scholar] [CrossRef]
- Chuang, C.; Guo, Y.-W.; Chen, H.-S. Corrected sodium levels for hyperglycemia is a better predictor than measured sodium levels for clinical outcomes among patients with extreme hyperglycemia. J. Chin. Med. Assoc. 2020, 83, 845–851. [Google Scholar] [CrossRef]
- Tzamaloukas, A.H.; Khitan, Z.J.; Glew, R.H.; Roumelioti, M.; Rondon-Berrios, H.; Elisaf, M.S.; Raj, D.S.; Owen, J.; Sun, Y.; Siamopoulos, K.C.; et al. Serum Sodium Concentration and Tonicity in Hyperglycemic Crises: Major Influences and Treatment Implications. J. Am. Heart Assoc. 2019, 8, e011786. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Oshima, M.; Agarwal, R.; Arnott, C.; Cherney, D.Z.; Edwards, R.; Langkilde, A.M.; Mahaffey, K.W.; McGuire, D.K.; Neal, B.; et al. Sodium-Glucose Cotransporter 2 Inhibitors and Risk of Hyperkalemia in People with Type 2 Diabetes: A Meta-Analysis of Individual Participant Data From Randomized, Controlled Trials. Circulation 2022, 145, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Serenelli, M.; Böhm, M.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Solomon, S.D.; DeMets, D.L.; et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur. Heart J. 2020, 41, 3402–3418. [Google Scholar] [CrossRef] [PubMed]
- Beusekamp, J.C.; Tromp, J.; Boorsma, E.M.; Heerspink, H.J.L.; Damman, K.; Voors, A.A.; Meer, P. Effects of sodium–glucose co-transporter 2 inhibition with empagliflozin on potassium handling in patients with acute heart failure. Eur. J. Heart Fail. 2021, 23, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Jongs, N.; Vart, P.; Stefánsson, B.V.; Chertow, G.M.; Langkilde, A.M.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Sjöström, C.D.; et al. The Kidney Protective Effects of the Sodium–Glucose Cotransporter-2 Inhibitor, Dapagliflozin, Are Present in Patients with CKD Treated with Mineralocorticoid Receptor Antagonists. Kidney Int. Rep. 2022, 7, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, S.; Zhang, M.; Cui, L. Hyponatremia in patients with chronic kidney disease: Hyponatremia and chronic kidney disease. Hemodial. Int. 2017, 21, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z.I. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus: Cardiovascular and Kidney Effects, Potential Mechanisms, and Clinical Applications. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Eckardt, K.-U.; Tsukamoto, Y.; Levin, A.; Coresh, J.; Rossert, J.; Zeeuw, D.D.E.; Hostetter, T.H.; Lameire, N.; Eknoyan, G. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2005, 67, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Genov, G.; Spooner, A.; Raine, J.; Arlett, P. Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works. Drug Saf. 2017, 40, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Ferrajolo, C.; Sullo, M.G.; Gaio, M.; Zinzi, A.; Scavone, C.; Gargano, F.; Coscioni, E.; Rossi, F.; Capuano, A. Cardiac Events Potentially Associated to Remdesivir: An Analysis from the European Spontaneous Adverse Event Reporting System. Pharmaceuticals 2021, 14, 611. [Google Scholar] [CrossRef] [PubMed]
| Age (Mean ± Std) | 78.70 ± 10.72 |
| Comorbidities | |
| Type 2 diabetes mellitus | 149 (60.6%) |
| High blood pressure | 185 (75.2%) |
| Heart failure | 114 (46.3%) |
| Chronic kidney disease | 64 (26.0%) |
| Concomitant medication | |
| Furosemide | 174 (70.7%) |
| Spironolactone | 94 (38.2%) |
| Angiotensin-converting enzyme inhibitors | 88 (35.8%) |
| Angiotensin receptor blockers | 86 (35.0%) |
| Potassium supplementation | 77 (31.7%) |
| Blood Creatinine (mg/dL) | Mean ± Std | Min–Max | p-Value |
|---|---|---|---|
| Baseline | 1.26 ± 0.59 | 0.49–4.18 | 0.0001 1 |
| Endpoint | 1.39 ± 0.77 | 0.39–6.55 | |
| Creatinine increase ≥ 0.3 mg/dL (n = 48) | |||
| Gender | Male | 28 (50.9%) | 0.878 2 |
| Female | 27 (49.1%) | ||
| Age (years) | <65 | 4 (7.3%) | 0.232 2 |
| 65–75 | 8 (14.5%) | ||
| 76–85 | 22 (40.0%) | ||
| >85 | 21 (38.2%) | ||
| Blood Sodium (mEq/L) | Mean ± Std | Min–Max | p-Value | |||
|---|---|---|---|---|---|---|
| Baseline | 139.05 ± 4.52 | 119–158 | 0.0009 1 | |||
| Endpoint | 138.48 ± 5.36 | 115–161 | ||||
| Baseline (n; %) | T2DM (n; %) | HBP (n; %) | HF (n; %) | CKD (n; %) | ||
| <135 | 25 (10.2%) | 12 (4.9%) | 20 (8.1%) | 9 (3.7%) | 11 (4.5%) | |
| 135–145 | 213 (86.6%) | 133 (54.1%) | 161 (65.4%) | 102 (41.5%) | 51 (20.7) | |
| >145 | 8 (3.3%) | 4 (1.6%) | 4 (1.6%) | 3 (1.2%) | 2 (0.8%) | |
| Endpoint (n; %) | 0.0146 2 | |||||
| <135 | 43 (17.5%) | 26 (10.6%) | 32 (13.0%) | 26 (10.6%) | 14 (5.7%) | |
| 135–145 | 188 (76.4%) | 112 (45.5%) | 140 (56.9%) | 83 (37.3%) | 47 (19.1%) | |
| >145 | 15 (6.1%) | 11 (4.5%) | 13 (5.3%) | 5 (2.0%) | 3 (1.2%) | |
| Blood Potassium (mmol/L) | Mean ± Std | Min–Max | p-Value | |||
|---|---|---|---|---|---|---|
| Baseline | 4.19 ± 0.60 | 2.5–6.2 | <0.0001 1 | |||
| Endpoint | 4.44 ± 0.62 | 2.8–6.4 | ||||
| Baseline (n; %) | T2DM (n; %) | HBP (n; %) | HF (n; %) | CKD (n; %) | ||
| <3.5 | 28 (11.4%) | 13 (5.3%) | 24 (9.8%) | 14 (5.7%) | 4 (1.6%) | 0.0002 2 |
| 3.5–5.0 | 196 (79.7%) | 125 (50.8%) | 144 (58.5%) | 92 (37.4%) | 55 (22.4%) | |
| >5.0 | 22 (8.9%) | 11 (4.5%) | 17 (6.9%) | 8 (3.3%) | 5 (2.0%) | |
| Endpoint (n; %) | ||||||
| <3.5 | 8 (3.3%) | 4 (1.6%) | 5 (2.0%) | 3 (1.2%) | 1 (0.4%) | |
| 3.5–5.0 | 197 (80.1%) | 16 (51.2%) | 149 (60.6%) | 96 (39.0%) | 50 (20.3%) | |
| >5.0 | 41 (16.7%) | 19 (7.7%) | 31 (12.6%) | 15 (6.1%) | 13 (5.3%) | |
| Blood potassium at the endpoint | ≤5 mmol/L (n; %) | >5 mmol/L (n; %) | p-value | |||
| Potassium supplementation | 61 (79.2%) | 16 (20.8%) | 0.243 2 | |||
| ACEi | 78 (88.6%) | 10 (11.4%) | 0.096 2 | |||
| ARB | 65 (75.6%) | 21 (24.4%) | 0.017 2 | |||
| Spironolactone | 74 (78.7%) | 20 (21.3%) | 0.149 2 | |||
| Individual Cases Safety Reports | 2666 (100%) | |||
| Sex | ||||
| Male | 1517 (56.9%) | |||
| Female | 1033 (38.7%) | |||
| Not Specified | 116 (4.4%) | |||
| Age group | ||||
| 16–64 | 612 (23.0%) | |||
| 65–85 | 1017 (38.1%) | |||
| >85 | 199 (7.5%) | |||
| Not specified | 833 (31.2%) | |||
| Use | ||||
| Individual cases reported by system organ classes | Total | Chronic Kidney Disease 237 (8.9%) | Diabetes Mellitus 1425 (53.5%) | Heart Failure 502 (18.9%) |
| Renal and urinary disorders | 394 (14.8%) | 53 (13.5%) | 226 (57.4%) | 113 (28.7%) |
| Cardiac disorders | 126 (4.7%) | 6 (4.8%) | 41 (32.5%) | 46 (36.5%) |
| Gastrointestinal disorders | 314 (11.8%) | 25 (8.0%) | 137 (43.6%) | 56 (17.8%) |
| Infections | 595 (22.3%) | 33 (5.5%) | 303 (50.9%) | 122 (20.5%) |
| Concomitant Drug/Adverse Drug Reaction | ||||
| Acute Kidney Injury (n = 98) | p-value | Hyponatremia (n = 21) | p-value | |
| Furosemide (n = 120) | 5 (4.2%) | 0.770 1 | 3 (2.5%) | 0.066 2 |
| Hyperkaliemia (n = 17) | p-value | |||
| Spironolactone (n = 89) | 4 (4.5%) | 0.002 2 | ||
| ACEi (n = 186) | 2 (1.1%) | 0.335 2 | ||
| ARA (n = 364) | 2 (0.5%) | 1.000 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.C.; Lourenço, O.; Morgado, S.; Gaspar, A.; Freire, I.; Eusébio, I.; Ribeiro, J.; Silva, M.; Mendes, M.; Fonseca, O.; et al. Acute Kidney Injury and Electrolyte Imbalances Caused by Dapagliflozin Short-Term Use. Pharmaceuticals 2024, 17, 420. https://doi.org/10.3390/ph17040420
Lopes AC, Lourenço O, Morgado S, Gaspar A, Freire I, Eusébio I, Ribeiro J, Silva M, Mendes M, Fonseca O, et al. Acute Kidney Injury and Electrolyte Imbalances Caused by Dapagliflozin Short-Term Use. Pharmaceuticals. 2024; 17(4):420. https://doi.org/10.3390/ph17040420
Chicago/Turabian StyleLopes, António Cabral, Olga Lourenço, Sandra Morgado, Andreia Gaspar, Idalina Freire, Inês Eusébio, João Ribeiro, Mafalda Silva, Marta Mendes, Olímpia Fonseca, and et al. 2024. "Acute Kidney Injury and Electrolyte Imbalances Caused by Dapagliflozin Short-Term Use" Pharmaceuticals 17, no. 4: 420. https://doi.org/10.3390/ph17040420
APA StyleLopes, A. C., Lourenço, O., Morgado, S., Gaspar, A., Freire, I., Eusébio, I., Ribeiro, J., Silva, M., Mendes, M., Fonseca, O., Duarte, R., & Morgado, M. (2024). Acute Kidney Injury and Electrolyte Imbalances Caused by Dapagliflozin Short-Term Use. Pharmaceuticals, 17(4), 420. https://doi.org/10.3390/ph17040420






