Abstract
Interleukin-7 (IL-7) is a versatile cytokine that plays a crucial role in regulating the immune system’s homeostasis. It is involved in the development, proliferation, and differentiation of B and T cells, as well as being essential for the differentiation and survival of naïve T cells and the production and maintenance of memory T cells. Given its potent biological functions, IL-7 is considered to have the potential to be widely used in the field of anti-tumour immunotherapy. Notably, IL-7 can improve the tumour microenvironment by promoting the development of Th17 cells, which can in turn promote the recruitment of effector T cells and NK cells. In addition, IL-7 can also down-regulate the expression of tumour growth factor-β and inhibit immunosuppression to promote anti-tumour efficacy, suggesting potential clinical applications for anti-tumour immunotherapy. This review aims to discuss the origin of IL-7 and its receptor IL-7R, its anti-tumour mechanism, and the recent advances in the application of IL-7 in tumour therapy.
1. Introduction
1.1. IL-7
IL-7, as a cellular inflammatory factor of the chemokine family, was first identified by Hunt et al. in 1987 while studying the potential role of bone marrow stromal cells in the development of pre-B cell subpopulations [1]. The human IL-7 gene, which is located on chromosome 8, spans 534 bp and comprises six exons and five introns. It encodes a protein consisting of 177 amino acids with a molecular weight of about 20 kDa [2]. The molecule contains three glycosylation sites, and depending on the degree of glycosylation, three protein bands may appear at approximately 20, 25 and 28 kDa when protein levels are assayed in vitro. The active form of IL-7 encodes a 25 kDa single-chain glycoprotein that forms a structure containing four alpha helices with a hydrophobic core [3]. IL-7 is mainly secreted by the bone marrow, thymus, and lymph nodes. Additionally, it is detected in sublymphatic organs such as the spleen, lymph nodes, and tonsils, as well as in non-lymphatic sites including the intestines, kidneys, lungs, skin, liver, brain, ovaries, and prostate [4,5]. This wide distribution indicates the significance of IL-7 in various physiological processes. These include its important role in B cell development, memory T cell development, the proliferation and survival of naïve T cells, and T cell development in the thymus [6]. Overall, IL-7 has a pervasive presence and diverse functions within the body.
1.2. IL-7R
The IL-7 receptor (IL-7R) is a heterodimeric complex located on chromosome 5 [4]. It is 1380 bp long, containing eight exons and seven introns, and encodes 459 amino acids with a molecular weight of approximately 49.5 kDa [7]. IL-7Rα is expressed in hematopoietic cells, particularly lymphoid lineages such as foetal NKs, dendritic precursors, mature T cells, and macrophages, as well as in developing T cells and B cells. There are two forms of IL-7R, membrane-bound and soluble IL-7R, each with distinct biological functions [8]. Membrane-bound IL-7R mediates IL-7 signalling, while soluble IL-7R provides regulatory control, amplifying IL-7 signalling and enhancing autoimmunity. IL-7 has been reported to play a critical role in lymphocyte maturation and differentiation through its interaction with its receptor IL-7R.
This paper reviews the origin of IL-7 and its receptor IL-7R, as well as their anti-tumour mechanisms in immunity and oncology. Additionally, recent advances in IL-7 in cancer immunotherapy and its potential for therapeutic applications are summarised and discussed.
1.3. Methods
1.3.1. Search Strategy
Our search method was designed according to the Preferred Reporting Items for Systematic Evaluation and Meta-Analyses 2020 (PRISMA 2020) guidelines [9]. The search method retrieved all studies published between 1990 and 2024. The search strategy included (but was not limited to) the following terms: cytokines, IL-7, cancer therapy, tumours, malignant stroma, clinical data, and combination therapy. The initial search was conducted using the following tools: PubMed and Web of Science.
1.3.2. Inclusion and Exclusion Criteria
Initially, 1051 relevant studies were retrieved from the databases, excluding duplicated data and irrelevant articles and accepting articles that included biological properties of IL-7 and articles related to cancer.
1.3.3. Study Selection and Quality Assessment
The titles of these publications were first reviewed to exclude articles with high expressions of IL-7 in tumours and the promotion of tumour growth. Then, the abstracts of the articles were used to identify suitable articles to exclude data on the high expression of IL-7 during treatment with other drugs. Subsequently, the selected manuscripts were proofread. Finally, conference abstracts, articles with no significant effect of treatment, and articles that were not studies from the last five years were excluded (Figure 1).
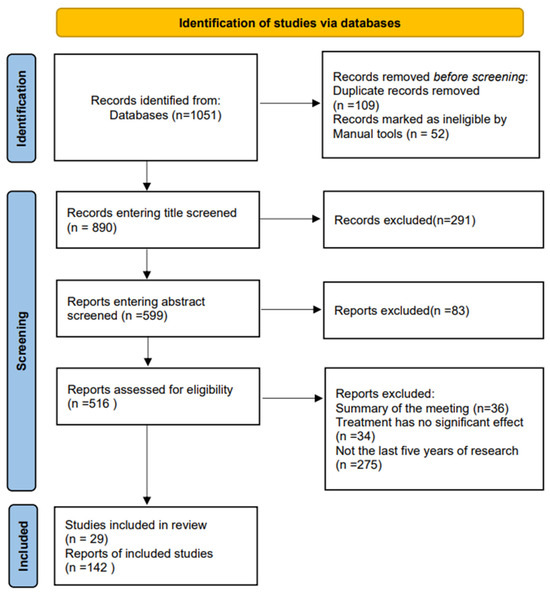
Figure 1.
Literature screening flow chart. Initially, a total of 1051 relevant studies were retrieved from the database, 109 duplicates and 52 irrelevant articles were excluded, and 890 articles were accepted, including those on the biological properties of IL-7 and its relevance to cancer. The titles were reviewed, 291 articles were excluded, 83 articles were then excluded from the data based on the abstracts, and finally, 345 articles were excluded based on content, therapeutic efficacy, and year of publication. This process resulted in a total of 29 eligible reviews and 142 studies with their data screened.
1.3.4. Data Extraction and Analysis
All relevant information was obtained from eligible studies. Statistical analyses were not performed due to the great heterogeneity of the included studies in terms of study design and data reporting. Therefore, the findings were presented, highlighting the similarities and differences, as well as the strengths and weaknesses, of the existing literature. Finally, this systematic evaluation did not require ethical approval.
2. Biological Functions of IL-7
IL-7 is a crucial regulatory factor in the development of the entire immune system, serving as a mitogen and trophic, survival, and differentiation factor for various immune cells (Figure 2), especially lymphocytes, where IL-7 is essential for B cell and T cell development, proliferation, and survival. The biological activities of IL-7 in lymphocytes are summarised in Table 1.
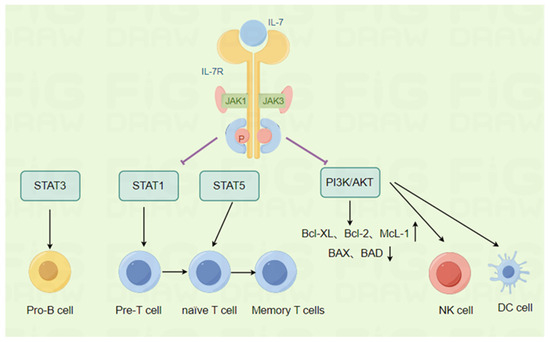
Figure 2.
Biological functions of IL-7. The associated image materials were prepared by Figdraw 2.0. IL-7 promotes the proliferation and survival of B cell precursors by activating STAT3. Similarly, IL-7 plays an important role in the proliferation, differentiation, and survival of T cells by mediating the activation of STAT1 and STAT5. In addition to lymphocytes, IL-7 also plays an important regulatory role in the immune response of natural killer (NK) cells and dendritic cells (DC). Thus, IL-7 has a significant impact on various immune cell types.
2.1. Promotion of Pre-B Cell Growth
When binding to its receptor, IL-7 activates intracytoplasmic JAK1 and JAK3 to phosphorylate STAT proteins, and the type of STAT protein determines the specificity of its response. IL-7 promotes B cell progenitor cell proliferation and survival by activating STAT3 [10]. IL-7 fails to promote the growth of mature B cells in the spleen and lymph nodes, suggesting that IL-7 can promote pre-B cell growth and has no effect on mature B cells [11]. In addition, human pre-B cells require IL-7 for direct contact with bone marrow stromal cells, which in turn activate STAT-5 for proliferation through IL-7 [12], and IL-7 is required for adult bone marrow to produce B cells [13].
2.2. Promote T cell Growth and Development
IL-7 plays an important role in T cell proliferation [14], differentiation, and survival by activating STAT1 and STAT5 [15].
2.2.1. Promotion of Pre-T Cell Growth and Development
T cell development in the thymus begins at the double-negative (DN; CD4(-)/CD8(-)) stage and comprises four subpopulations (DN1–4) [16]. IL-7 promotes the growth of both foetal and adult thymic double-negative (CD4(-)/CD8(-)) immature T cells [17], suggesting that IL-7 may act directly as a growth factor for this cell population [18]. Furthermore, IL-7 stimulates the Bcl-2-mediated survival of DN2 and DN3 thymocytes and facilitates the differentiation of DN3 and DN4 thymocytes [5,19]. IL-7 signalling is also required for the transition from the DN to the DP phase [20]. When thymocytes reach the DP phase, they differentiate into single CD4+ or CD8+ T cells, at which point IL-7 is also required for cell survival [21].
2.2.2. Promotion of T Cell Proliferation
IL-7 plays a crucial role in thymocyte development and also serves as a vital cytokine in T cell development and proliferation, maintaining immune homeostasis, and ensuring normal T cell function. JAK3 can induce p85 phosphorylation and activate the PI3K/Akt pathway [22]. When IL-7/IL-7R stimulates JAK1/3, the p85 subunit of PI3K becomes phosphorylated, leading to Akt kinase activation and the subsequent stimulation of the PI3K/Akt pathway. This activation results in the upregulation of anti-apoptotic proteins Bcl-XL, Bcl-2, and Mcl-1 of the Bcl-2 protein family, along with the downregulation of pro-apoptotic proteins BAX and BAD, ultimately promoting cell survival, proliferation, and growth [23]. In mice, IL-7 mediates T cell proliferation and activation, a process that is subdued by PI3K inhibitors [24]. This implies that the PI3K/AKT pathway plays a crucial role in IL-7 signalling in the cell cycle. Research has shown that using IL-7 in mice results in a preferential increase in the number of CD8+ cells over CD4+ cells and enhances the proliferation of both CD4+ and CD8+ T cells [25]. Moreover, IL-7 fosters the survival and homeostatic proliferation of both CD4+ and CD8+ T cells after peripheral blood withdrawal [26]. IL-7 also promotes the proliferation of activated CD4+ and CD8+ peripheral blood T cells [27]. IL-7 can help T cells restore homeostasis through the signal transducer suppressors of the cytokine signaling proteins (SOCS) pathway and STAT5, a cytokine signalling inhibitor [28]. In addition, the JAK/STAT pathway is not only activated but can also be inhibited by the family of SOCS, creating a negative feedback loop [29].
The PI3K pathway plays a critical role in promoting cell viability and the cell cycle by phosphorylating AKT. This process also phosphorylates downstream targets including GSK-3, FOXO-1, and FOXO-3a, subsequently upregulating the expression of BCL-2 and downregulating the cell cycle inhibitor p27kip1. Multiple pieces of evidence support the idea that PI3K/AKT is a significant signalling pathway for IL-7R activation, which ultimately leads to the upregulation of the GLUT-1 transporter and glucose uptake. This, in turn, enhances peripheral T cell metabolism, homeostasis, and survival.
2.2.3. Maintenance of Memory T Cells
IL-7 is critical not only for T cell development but also for the survival of naïve T cells and the formation and maintenance of memory T cells [29]. In addition, IL-7 signaling helps to maintain CD8+ memory T cells by activating STAT5 and STAT3 [30]. IL-7 also plays a role in regulating the number of memory CD8+ T cells produced [31] and in guiding naïve CD8+ cells to transition into memory CD8+ T cells [32], which promotes the proliferation [33] and activity of effector T cells. Furthermore, IL-7 has a significant impact on the transition from effector to memory T cells [34]. In CD8+ memory T cells, IL-7 can act both directly [33] and indirectly by stimulating IL-2 production and IL-2 receptor expressions [35]. Importantly, in the presence of lymphopenia, the survival and proliferation of naïve T cells are reliant on IL-7, as indicated by the inability of these cells to survive in hosts lacking IL-7 [36]. Likewise, in cases of lymphopenia, the survival and maintenance of CD4+ memory T cells depend on IL-7 and the TCR pathway [37]. Likewise, a study on a mouse model of airway inflammation shows that allergen-specific memory CD4+ T cells rely on IL-7 for their survival in the lungs and airways, as well as for their homeostatic proliferation in lymph nodes [38]. Moreover, reducing IL-7 by injecting IL-7 antibodies in normal mice leads to a significant decrease in the number of naïve T cells [39]. Additionally, IL-7 also affects the cytotoxicity and drug resistance of T cells [40].
2.2.4. Treg Cell Development
IL-7 is essential for the development, homeostasis, and function of regulatory T cells (Tregs) [41]. However, there have been conflicting results regarding the role of IL-7/IL-7R in Treg development. While IL-2 is known to play a crucial role in Treg homeostasis [42], it has been discovered that CD25-deficient Tregs rely on IL-7 for survival in the absence of IL-2 [43]. On the other hand, some studies have indicated that IL-7Rα alone is not enough to promote mature Treg development [44]. Although necessary for Treg development [45], IL-7Rα has a notably low expression in mature Tregs [45]. Consequently, further research is needed to fully understand the role of IL-7 in Treg development.

Table 1.
Biological activities of IL-7 in lymphoid cells. Reproduced with permission from Ref. [5]. Copyright 2022, Published by Elsevier Ltd.
Table 1.
Biological activities of IL-7 in lymphoid cells. Reproduced with permission from Ref. [5]. Copyright 2022, Published by Elsevier Ltd.
| Function | Cell Types | Activities |
|---|---|---|
| Development | CLP | “Transcription of Genes Related to the B Cell Lineage” [46] |
| Thymocytes | Recombination at the TCR γ,β loci for VDJ [47] | |
| Thymocytes | Preventing premature TCR α chain recombination during the selection of the β chain [48] | |
| Double positive thymocytes | Silencing CD4 transcription and activating CD8 transcription leads to the conversion of cells to a single positive state [49] | |
| B cell progenitors | Recombination of V segments in heavy-chain locus [7] | |
| B cell progenitors | Downregulation of IL-7 receptor expression and completion of Ig light gene rearrangement [50] | |
| Survival | Thymocytes (DN cells) | Stimulation of Bcl2 expression [51] |
| B cell progenitors | Activation of Jak3/Stat5-dependent pathways [52] | |
| Naïve T cells | Expression of pro-survival Bcl2 family | |
| Memory T cells | members in mitochondria [53] | |
| NK cells | Increased expression of Bcl2 [54] | |
| Proliferation | B cell progenitors | Jak3/STAT5 [55] |
| Thymocytes | Cooperation between IL-7 and Notch signals [56] | |
| Activated T cells | Increase in the production of IL-2 and expression of IL-2 receptors [57] | |
| Activation | CD8 Effector cells | Expansion of CD8+ cells and increased cytolytic activity [58] |
| Memory T cells | Cytokine production [59] | |
| Differentiation | Transition of mature effector T cells to memory cells | The hypothesis of a combination of survival signals with epigenetic modifications has not yet been fully defined [60] |
| Homeostatic proliferation | Naïve T cells | The downregulation of p27 cyclin-dependent kinase inhibitor activity leads to increased mTor phosphorylation [61] |
| NKT cells | Still undefined | |
| Memory T cells | Still undefined |
2.3. Promotion of Lymphangiogenesis
IL-7 plays a crucial role in the lymphocytes and also has a significant impact on the lymphovascular system. IL-7 interfering with the activation of GSK3 through AKT causes the upregulation of NFAT2 activity [62]. Another pathway involving the JAK1/STAT5 pathway or the phosphorylation of JAK3 can activate NFAT2, which targets a wide range of cytokines and growth factors with pro-inflammatory and angiogenic activities [29]. IL-7 is also responsible for activating lymphatic endothelial cells (LECs), a process that promotes cutaneous lymphatic drainage [63]. IL-7 also promotes lymphatic vessel dilatation during inflammation or post-immunisation lymphatic dilatation [64]. IL-7 mediates the formation of lymphoid organs by increasing the number of lymphoid tissue inducer (LTi) cells, a process known as lymphangiogenesis [65]. S. Chappaz et al. [66] showed that lymph node development was defective in IL-7-deficient mice and that the IL-7 expression increased the number of LTi cells and restored lymph node development.
2.4. Other Effects
IL-7 has been reported to play an important regulatory role in the immune response of natural killer (NK) cells and dendritic cells (DC). Furthermore, IL-7 preferentially induces the JAK-mediated activation of STAT5 [10], which is involved in the transduction of the pro-survival, mitogenic, trophic, and differentiation signals of immune cells [29]. Additionally, IL-7 maintains the homeostasis and normal function of mature T cells and promotes the survival of mature NK cells [67]. Consequently, IL-7 is essential for the development of NK cells.
3. Anti-Tumour Mechanism
Due to its crucial role in the growth and development of various immune cells, IL-7 holds great promise for cancer therapy in a clinical setting. The specific anti-tumour mechanism of IL-7 is illustrated in Figure 3.
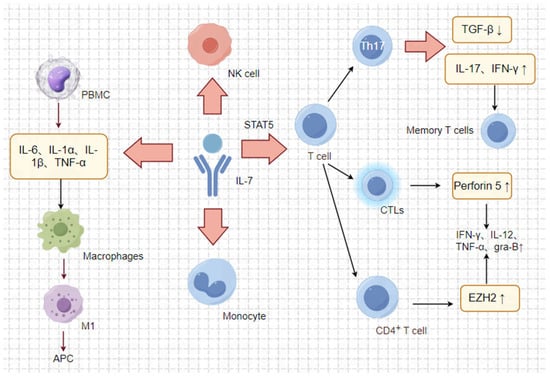
Figure 3.
Anti-tumour mechanism of IL-7. The associated image materials were prepared using Figdraw 2.0. IL-7 plays a critical role in the tumour microenvironment by attracting T lymphocytes and promoting their infiltration into tumours, thus exerting an anti-tumour effect. Moreover, IL-7 also enhances the cytotoxicity of cytotoxic T lymphocytes (CTLs) and induces a degree of lymphokine-activated killer (LAK) activity. Additionally, IL-7 acts as a cytotoxic agent by modulating the release of cytokines, such as IFN-γ, IL-1β, IL-1α, and TNF-α, by immune cells. This modulation promotes macrophage polarisation toward the M1 phenotype and the expression of a pro-inflammatory milieu, which, in turn, promotes the differentiation, maturation, and effective activation of antigen-presenting cells (APCs). Overall, IL-7 serves as a key regulator in the immune response within the tumour microenvironment, influencing various immune cell activities and further promoting the anti-tumour environment.
3.1. Enhancing Adaptive Immunity
IL-7 plays a crucial and multifaceted role in the tumour microenvironment, selectively influencing the development of naïve and memory T cells while avoiding the stimulation of immunosuppressive Tregs. Moreover, the cytokine IL-7 promotes the differentiation of CD4+ T cells into Th9 cells with enhanced anti-tumour activity. Specifically, the complex formed by IL-7 with IL-7R-Fc has been shown to induce an anti-tumour response by increasing the infiltration of T cells into tumours through the CXCR3 chemokine signalling pathway [68].
IL-7 promotes the development of helper T17 (Th17) cells. In the tumour microenvironment, Th17 cells enter and expand through antigen-presenting cells. Furthermore, they promote the operation and maintenance of effector T cells and NK cells by inducing primary tumour cells to produce CXCL9 and CXCL10. As a result, Th17 cells can facilitate protective anti-tumour immunity by recruiting pro-inflammatory immune effector cells. Studies have demonstrated that IL-7 not only promotes Th17 cell proliferation to enhance anti-tumour efficacy in a mouse B16F10 tumour model [69] but also is necessary for developing effector Th17 cells into resident memory T cells [70,71]. Some of these resident memory cells express IL-17, while others express IFN-γ. Thus, IL-7 is vital for the development of Th17. In a mouse glioblastoma model, Shireman JM et al. [72] demonstrated that IL-7-stimulated T cell translocation to the brain facilitates the formation of Th17 cells and generates persistent long-term anti-tumour immunity.
3.2. Reversal of Immunosuppression
IL-7 also enhances anti-tumour immune responses by counteracting immunosuppressive mechanisms. IL-7 promotes the differentiation of Th17 cells by inducing the expression of SMAD ubiquitination regulatory factor 2, which inhibits the TGF-β signalling pathway and deregulates the inhibition of effector T cells by Tregs. IL-7 and TGF-β act antagonistically, and the inhibition of TGF-β enhances the anti-tumour immune response, thereby inhibiting tumour growth. Tumour-infiltrating suppressor cells often express adenosine, which accumulates extracellularly and suppresses CD8+ T cells. Findings from a single study indicate that IL-7 signaling is crucial in protecting CD8+ T cells from this suppression and in preventing immunosuppression. These results may have implications for immunotherapies that target the adenosine pathway [58].
3.3. Enhancing Immune Cell Recruitment
IL-7 has been reported to also upregulate the expression of the adhesion molecule V-CAM on endothelial cells. This upregulation promotes the recruitment of circulating immune cells. YW Choi showed that IL-7-Fc induced cervicovaginal epithelial cells to stimulate endothelial cell V-CAM expression, resulting in the inhibition of cervicovaginal carcinoma growth in mice [68].
3.4. Enhancing the Expressions of Inflammatory Factors
IL-7 enhances the cytotoxicity of cytotoxic T lymphocytes (CTLs), as well as monocytes, NKT, NK, and LAK cells. Additionally, IL-7 induces a degree of lymphokine-activated killer (LAK) activity. It also promotes the secretion of perforin 5 from CTLs in a STAT5-dependent manner and stimulates the expressions of IFN-γ [58], IL-12, and MIG [58]. Furthermore, in the presence of lymphopenia, IL-7 not only leads to the IL-7-dependent activation of STAT1 and STAT5 but also enhances the T cell response to IFN by regulating STAT1 protein expression levels [73]. In response to IL-7 stimulation, human peripheral blood mononuclear cells release a variety of cytokines, such as IL-6, IL-1α, IL-1β, and TNF-α. Additionally, in the presence of IFN-γ and TNF-α, it promotes macrophage polarisation toward the M1 phenotype and creates a pro-inflammatory environment [74]. Finally, it can also confer stat5-dependent multifunctionality on CD4+ T cells, resulting in an increased expression of the histone methyltransferase EZH2, which in turn promotes the expressions of IFN-γ, IL-2, TNF-α, and granzyme B.
A pro-inflammatory environment is crucial for the differentiation, maturation, and efficient activation of antigen-presenting cells (APCs). These cells can then provide co-stimulatory signals to cytotoxic T cells. Host APCs are necessary for successful antigen presentation, even in highly immunogenic tumours. In a study on gastric cancer, IL-7 was found to be closely associated with regulatory T cells. They may influence gastric carcinogenesis and progression by regulating immune infiltration and inflammation [75].
4. IL-7 in Tumour Therapy
Based on the powerful immunomodulatory effects of IL-7, which can act directly or indirectly on tumour cells, IL-7 can effectively reduce the tumour load in tumour therapy [76] and exert anti-tumour effects by enhancing eradication or adaptive immunity. Table 2 provides a summary of examples of IL-7 that are currently undergoing clinical trials.

Table 2.
Current clinical trials are studying the use of IL-7 in cancer immunotherapy.
4.1. Targeted Therapies
It is fascinating to learn about the potential of IL-7 in immune reconstitution and its anti-tumour activity based on preclinical studies. The fact that IL-7 has been well tolerated in early clinical trials and that there have been no reports related to greater irritation responses is promising. The development of drugs targeting the IL-7 gene for autoimmune diseases and cancer seems very promising, particularly the use of recombinant IL-7 proteins, IL-7 gene vaccines, IL-7 receptor antagonists, and IL-7 signalling pathway blockers.
4.1.1. IL-7 Recombinant Protein
Previous studies have shown that IL-7 has a short half-life in vivo and can act only in an autocrine or paracrine manner over short distances. Scientists have therefore extended its half-life by genetically engineering it to fuse with the c-terminal end of hIgG1 Fc to enhance IL-7 activity to increase the immune effect of IL-7 in tumour therapy. Previous clinical trials have shown that IL-7 fusion proteins are stable [77], well tolerated, have a prolonged half-life [78], and have good prospects for clinical application. YW Choi et al. [68] showed that IL-7 Fc successfully inhibited the growth of cervicovaginal tumours in mice. Clinical trials of immunotherapy with human IL-7-Fc are also underway. rhIL-7 (also known as rhIL-7-hyfc) has been developed by Genexine with an extended half-life and reduced off-target immunogenicity. In a lymphocytopenic mouse model of melanoma, IL-7-Fc treatment inhibited tumour growth by increasing the number of overtly metastatic CD8+ T cells in tumour tissue and tumour-draining lymph nodes [79]. A clinical result for the treatment of recurrent glioblastoma (GBM) showed that administering different doses of rhIL-7-hyFc to patients separately significantly increased TLC in all but one patient in a dose-dependent manner and did not result in severe toxicity [80].
NT-I7, a longer-acting IL-7 Fc, was tested for its ability to prevent systemic lymphopenia and improve survival in a GBM mouse model, and it was demonstrated that in a mouse model of glioma in situ, NT-I7 increased central and effector memory CD8+ T cells in both lymphoid organs and tumours [81]. In addition, it significantly inhibited the growth of glioma in situ and improved the survival rate in mice compared to the control group [81]. A phase I/II trial evaluating NT-I7 in patients with high-grade glioma (NCT03687957) is also ongoing [81].
4.1.2. IL-7 Gene Vaccine
The IL-7 gene vaccine is a gene therapy that enhances anti-tumour immune responses by introducing the IL-7 gene into tumour cells or immune cells to cause them to produce the IL-7 protein, thereby increasing IL-7 levels. A notable clinical trial of IL-7 gene therapy in cancer treatment is the safety and feasibility in patients with relapsed or refractory haematological malignancies. Early results from this trial suggest that IL-7 gene therapy may enhance anti-tumour immune responses and improve clinical outcomes in such patients [82]. In conclusion, preclinical and early clinical data suggest the potential to enhance anti-tumour immune responses [83]. However, further studies are needed to fully understand its safety and efficacy in the treatment of cancer.
4.1.3. IL-7 Receptor Antagonists and IL-7 Signalling Pathway Blockers
IL-7 plays a complex role in cancer pathology, and there has been increasing interest in its potential role in cancer cachexia. Cachexia is a state in which cancer patients suffer from systemic dysfunctions triggered by the disease or its treatment, resulting in malnutrition, reduced immune function, and a decline in quality of life [84]. Studies have shown that the effects of IL-7 on immune cell function and metabolism may contribute to cancer cachexia. Specifically, IL-7 levels are significantly elevated in cancer patients with cachexia compared to those without cachexia [85], and the promotion of differentiation and activation of T cells by IL-7 may lead to muscle wasting and weight loss. Additionally, IL-7 has been associated with dysregulated energy metabolism, leading to malnutrition in cancer patients [86]. There is also evidence that IL-7 may affect the mental state of cancer patients by influencing neurotransmitter levels, leading to psychological problems such as anxiety and depression, further reducing the patient’s quality of life [87].
The association between IL-7 and cancer cachexia highlights the potential for targeted therapies to mitigate the effects of cachexia in cancer patients. Strategies targeting IL-7, such as designing IL-7 receptor antagonists or blockers of the IL-7 signalling pathway, are being investigated to mitigate cancer cachexia. Researchers have engineered an alternative antagonist to the anti-IL-7R antibody, a 64-amino acid-less peptide based on the structure of the IL-7R antibody, which is hyperstable and blocks the IL-7-induced phosphorylation of Stat5 in cell lines [88]. This antagonist could be clinically tested in patients with relapsed paediatric acute lymphoblastic leukaemia (ALL). Clinical trials of IL-7 receptor agonists have investigated their safety, tolerability, and efficacy in a variety of cancers to identify the optimal dosage, treatment schedule, and patient population for IL-7 receptor agonist therapy [89].
IL-7-targeted therapies have made breakthroughs in several cancer types, including non-small cell lung cancer (NSCLC), lymphoma, colorectal cancer, and melanoma. Studies have shown that IL-7 can increase the number and function of tumour-infiltrating lymphocytes and improve the efficacy of immune checkpoint inhibitors such as PD-1 and PD-L1 antibodies. A clinical trial found that combination therapy with IL-7 and immune checkpoint inhibitors significantly improved survival and response rates in NSCLC patients compared to using immune checkpoint inhibitors alone [90]. IL-7 has been extensively studied in the treatment of lymphoma. One study found that IL-7 was able to increase the number of CD4+ and CD8+ T cells and improve the function of these cells in lymphoma patients. The combination of IL-7 with chemotherapy or immunotherapy has been suggested as a potential therapeutic strategy for improving the treatment response and survival in lymphoma patients [91]. IL-7 has also shown potential in the treatment of colorectal cancer. Studies have shown that IL-7 can increase the number of tumour-infiltrating lymphocytes in colorectal cancer patients and improve the activity of these cells. A clinical trial found that combining IL-7 with chemotherapy significantly improved the survival rate of patients with advanced colorectal cancer [92]. IL-7 has also shown potential efficacy in the treatment of melanoma. Studies have shown that IL-7 can increase the number of anti-tumour immune cells in melanoma patients and improve the activity of these cells. A clinical trial found that combining IL-7 with immune checkpoint inhibitors significantly improved treatment response rates and survival in patients with advanced melanoma [93].
4.2. Combination Therapy
4.2.1. Combination with Other Cytokines
IL-7, which has demonstrated strong effectiveness in fighting tumours when used alone, can also be utilised in combination with other cytokines such as IL-12, IL-15, and GM-CSF.
IL-12 is a widely recognised pro-inflammatory cytokine that plays a crucial role in activating both NK cells and T cells, creating a connection between innate and adaptive immunity. This ultimately results in a more effective enhancement of anti-tumour immunity. Tasaki M [94] showed that in a murine lung cancer (LLC) model, combined treatment with IL-7 and IL-12 promoted the activation of CD8+ T cells, which had a better effect on CD8+ T cell activation than the control group expressing IL-12 alone. Shireman JM et al. constructed a fusion of IL-7 and GM-CSF, i.e., GIFT-7, which led to tumour regression after three weeks of administration in a mouse glioma model [72].
IL-15 is also an important immunomodulator in cancer therapy. In a recent study, the structural domains of other cytokines were genetically engineered to bind to IL-7 to form a bifunctional protein fusion. Song et al. constructed a dual cytokine: a hybrid protein of IL-7 and IL-15 and injected different doses of rIL7/IL15 at the tumour site every 2 days in a mouse CT26 tumour model. The results of the study showed that rIL7/IL15 inhibited tumour growth in a dose-dependent manner by approximately 60% at the 2.5 μg level and >80% at the 20 μg level, which are both superior to cytokine monotherapy in terms of anti-tumour effect [95]. Recently, an injectable hydrogel microsphere integrated training field (MS-ITC) containing anti-CD3 and anti-CD28 antibodies and bovine serum albumin nanoparticles encapsulated with IL-7 and IL-15 was newly developed [96]. The MS-ITC were injected into osteosarcoma tumour tissues of homozygous mice. The results of the study showed that combined treatment with IL-7 and IL-15 induced the recruitment and activation of TIL-Ts, which disrupted tumour cell growth and ultimately led to the regression of the primary osteosarcoma [96].
4.2.2. Combined Anti-Cancer Drug Therapy
IL-7 has shown strong anti-tumour activity as a monotherapy and it can also be combined with other drugs to enhance anti-tumour activity.
Cisplatin (DDP) is a therapeutic agent used to treat non-small cell lung cancer (NSCLC) but has the disadvantage of making cancer cells resistant to the drug. The combination of IL-7 with DDP induces NSCLC tumour regression while reducing ABCG2 levels in tumour tissue. This result suggests that IL-7 can also overcome the multidrug resistance of DDP and restore its chemosensitivity [97]. Currently, sip-T is the only immunotherapy approved by the US Food and Drug Administration (FDA) for the treatment of metastatic desmoplasia-resistant prostate cancer (mCRPC). The results from a preclinical study showed that the combination of IL-7 and sip-T significantly inhibited tumour growth in a mouse model of prostate cancer, with potential clinical efficacy that should be further evaluated in clinical trials [98]. The results of a phase II clinical trial (NCT01881867) showed that the combination of IL-7 and sip-T in mCRPC patients resulted in a significant increase in antigen-specific humoral IgG and T cells, and increased expressions of activation markers and beneficial cytokines such as IL-2 and IFN-γ were observed. In comparison to the control group, the group receiving combined IL-7 and sip-T treatment showed decreased levels of the prostate cancer marker PSA at week 6. Additionally, there was an increase in the number of patients with PSADTs longer than 6 months.
The combination of IL-7 with antibodies has also shown promising anti-tumour effects in recent studies. Gou et al. [99] found that IL-7 in combination with oxaliplatin inhibited the growth of metastatic tumours and enhanced the anti-tumour activity of oxaliplatin in a mouse model of lung and peritoneal metastases. Combinations of IL-7 with temozolomide for the treatment of glioblastoma [64] and with atelizumab for the treatment of skin cancer [100], melanoma, and squamous cell carcinoma of the skin [101] are under ongoing investigation. A clinical trial of IL-7 and atezolizumab for the treatment of urothelial carcinoma (NCT03513952) is also ongoing, and IL-7-Fc is being used in combination with pembrolizumab in a clinical trial of triple-negative breast cancer [102].
Recent studies have identified a novel IL-7 agonist with the pharmacologically active peptide MDK1472 forming MDK-703, a complex that induces an immune cell profile in an NSG mouse model very similar to that produced by IL-7, with properties more suitable for therapeutic applications than natural IL-7. MDK-703 was administered to cynomolgus monkeys through a single dose of 1 mg/kg via intravenous, subcutaneous, or intramuscular injection. MDK-703 was well tolerated with no adverse effects, suggesting that MDK-703 may be a clinical candidate for oncological treatment [103]. A trial of MDK-703 with patients with advanced solid tumours is currently underway (NCT05716295).
4.2.3. Combination of Oncolytic Viruses
Oncolytic virus therapy is an emerging approach to tumour immunotherapy. Oncolytic viruses (OVs) are specifically designed to target and destroy tumour cells, leaving healthy cells unharmed [104]. Not only can oncolytic viruses lyse cells directly, but the lysed tumour cells can also release tumour-associated antigens [4], as well as induce tumour immunity by releasing cytokines and exhibiting damage-associated molecular patterns to enhance antigen presentation and immune activation [105].
IL-7 has shown promising results in clinical trials with the combination therapy of IL-7 with lysosomal viruses used to enhance anti-tumour effects. The construction of a lysosomal adenovirus Ad5/3-E2F-d24-hIL-7 expressing IL-7 targets the tumour microenvironment for high expressions, overcoming systemic delivery problems and improving therapeutic efficacy. The effects of this recombinant virus on tumour growth, immune cell activation, and cytokine profiles in the tumour microenvironment were evaluated in three clinically relevant animal models. The results showed that Ad5/3-E2F-d24-hIL-7 promoted pro-inflammatory cytokine expression and CD4+ and CD8+ T cell activation and migration. Local treatment in hormone-treated mice significantly reduced pancreatic cancer growth [106].
Nakao et al. developed a lysogenic poxvirus co-expressing the cytokines IL-7 and IL-12 to enhance the anti-tumour effect. When this recombinant virus was treated via intratumoral administration, anti-tumour activity was shown in all three tumour models, with a 92.9% inhibition of tumour growth in the B16-F10 melanoma model and 53.3% inhibition of tumour growth in CT26. There were mice in the B16-F10 melanoma model that showed tumour regression, and mice with complete tumour regression were resistant to rechallenge with the same tumour cells; it has been suggested that the injection of the virus results in the establishment of long-term memory specific to the tumour [107].
In a recent study, several immunotherapeutic genes were inserted into the viral genome to enhance its oncolytic activity. Herpes simplex virus-2 (HSV-2) vectors are a promising type of lysogenic virus. oHSV2 co-expressing IL-7 and the chemokine CCL19 was constructed by Hu et al. [108], and its anti-tumour efficacy was tested in 4T1- and CT26-loaded mouse models. The results showed that oHSV2-IL7-CCL19 significantly inhibited tumour growth in mice compared to controls.
The above results indicate that IL-7 combined with lysosomal viruses has good tumour-killing and anti-tumour immune effects in the treatment of malignant tumours and has good prospects for clinical application.
4.2.4. Combined CAR-T Cells
Chimeric antigen receptor (CAR-T) cells are engineered receptors that are added to normal T cells, allowing them to effectively target and kill tumour cells expressing specific antigens. In recent years, IL-7, in combination with CAR-T cells, has been widely used in preclinical and clinical trials for a variety of solid tumours and haematological cancers, with promising results [109,110,111,112,113,114,115,116,117].
Studies have demonstrated that IL-7 promotes the in vitro expansion of CAR-T cells [118], and IL-7 has also been shown to enhance the memory properties of CAR-T cells [119,120]. When IL-7 was combined with CAR-T cells in relapsed and refractory haematological malignancies, tumour growth was significantly inhibited [121]. A clinical trial involving IL-7 × CCL19 CAR-T cells combined with tirilizumab was conducted on 11 patients with relapsed or refractory large B cell lymphoma. The results demonstrated that the autologous 7 × 19 CAR-T cells, in combination with tirilizumab, displayed good efficacy in treating relapsed and refractory large B cell lymphoma while also causing manageable side effects [122]. After 30 days of receiving 7 × 19 CAR-T cell injections at the tumour site, a patient with advanced hepatocellular carcinoma experienced the complete disappearance of the tumour. In a separate case, a patient with advanced pancreatic cancer experienced a near-total disappearance of the tumour after 240 days of intravenous injections [109]. Wang SY et al. [123] showed that IL-7 in combination with (NICE) CD19 CAR-T cells exhibited good therapeutic efficacy in a mouse model of B cell lymphoma compared to the control group. The tumour growth rate was significantly reduced, and survival was improved in the treatment group receiving intravenous IL-7.
Due to the substantial anti-tumour activity displayed by IL-7 and CAR-T cells in haematological malignancies, their combination has been incorporated into clinical trials for diverse cancer immunotherapies. However, previous studies have shown that CAR-T calls have poor efficacy in solid tumours and T cell recruitment; survival and proliferation are important limiting factors for CAR-T cell therapy in solid tumours [124]. CAR-T cells armed with IL-7 prolong the persistence of CD4+ T cells and enhance the anti-tumour response. Therefore, Luo et al. [115] developed a CAR-T cell co-expressing the cytokines IL-7 and CCL21 (7 × 21 CAR-T). The results of the study showed that 7 × 21 CAR-T cells significantly inhibited tumour growth and even achieved tumour elimination after treatment compared with conventional CAR-T cells in the PANC02-A2 pancreatic cancer model. The co-expression of IL-7 and IL-7 Flt3L CAR-T cells improves the therapeutic efficacy of heterogeneous glioblastoma in mice [119]. A novel CAR-T cell targeting GPC3 induced the co-expression of IL-7 and high levels of CCL19. In a humanised NSG mouse xenograft model, GPC3-CAR-T killed tumour cells at twice the rate of conventional CAR-T cells, and this led to tumour regression. Therefore, a phase I clinical trial was initiated in which GPC3-7-19 CAR T cells showed good safety and anti-tumour efficacy in patients with hepatocellular carcinoma (HCC) [125].
Triple-negative breast cancer (TNBC) is a very aggressive malignancy for which there are no effective targeted therapies. To use CAR-T cells for the treatment of TNBC, the anti-tumour efficacy of CAR-T cells was enhanced with the IL-7 receptor (C7R). It was shown that C7R-enhanced CAR-T cells exhibited significant anti-tumour activity in the NOD/SCID mouse model of TNBC, with a significant reduction in tumour volume compared to the PBS group, and a prolonged survival time of CAR-T cells in mice [114]. Recombinant protein technology could be a potential approach due to its advantages of reduced toxicity and lower cost. The most recent study was a recombinant protein targeting HER-2, which is a fusion of the anti-HER-2 single-chain fragment variable structural domain CCL19 with IL-7. The results of the study showed that in a humanised NSG mouse model of gastric cancer, control tumours consistently increased in volume and 2/5 mice developed skin ulcerations localised to the burdened tumour. On the other hand, following three treatments with HER2 scFv-CCL19-IL-7, there was a marked reduction in tumour volume and consistent inhibition of tumour growth, indicating potent and sustained tumour suppression [126].
Furthermore, IL-7 has been shown to have anti-tumour effects in T cell receptor (TCR-T) cells, in addition to its known impact on CAR-T calls. TCR-modified T cells for open T cell therapy have shown promise in the treatment of metastatic melanoma and other malignancies. A common protocol for TCR-modified T cells involves activating the T cells through CD3 stimulation, allowing for the efficient transfer of tumour-responsive receptors using viral vectors [127]. It has been demonstrated that using IL-7 to generate TCR-modified T cells in the absence of CD3 activation is a feasible strategy for enhancing over-the-counter T cell therapy for melanoma and other types of cancer [127]. A study was conducted to genetically engineer TCR-T cells (7 × 19 P1A T) to express both IL-7 and CCL19, which resulted in complete tumour regression and prolonged survival in half of the mice after 7 × 19 P1A T treatment in a murine mast cell tumour P815 model [109].
Adoptive T cell therapy (ACT) has demonstrated remarkable effectiveness in combating tumours. This treatment depends on a reservoir of functional T cells capable of continually targeting and destroying tumour cells [128]. However, a persistent shortage of functional T cells has been recognised as a significant obstacle to achieving a lasting response. Equipping infused T cells with stem-like qualities to produce memory T cells with the ability to self-renew and differentiate in multiple ways, akin to pluripotent stem cells, is crucial and holds great promise for improving T cell function and sustaining anti-tumour immunity [128].
The findings above suggest that combining CAR T cell therapy with IL-7 expression could have a positive anti-tumour immune effect, making it a promising option for clinical use.
4.2.5. Other
IL-7 can also be used as an adjuvant in combination with tumour vaccine therapy to increase T lymphocyte infiltration into the tumour microenvironment, thereby enhancing the anti-tumour effect of cancer vaccines. IL-7 adjuvants also help to prolong the survival of activated T cells, enhance effector responses, and increase cytokine production, thereby improving the immune effect of tumour vaccines. Current examples of IL-7 in combination with different tumour vaccines in clinical trials are summarised in Table 3. Clinical trials of rhIL-7-hyFc in combination with various tumour vaccines are also underway [98]. IL-7 promotes antibody and T cell responses to HCV DNA vaccines [129]. In addition, the same adjuvant effect of IL-7 has been shown to promote the efficacy of DC vaccines. Thus, the combination of IL-7 and tumour vaccines has great potential for use in tumour therapy.

Table 3.
Presents clinical trials investigating cancer vaccines used in combination with IL-7 as an adjuvant for the treatment of the malignancy.
5. Conclusions and Outlook
The results of a large number of preclinical and clinical studies have shown that IL-7 can enhance anti-tumour efficacy by promoting lymphocyte infiltration and improving the tumour microenvironment. Whether used as monotherapy or in combination with tumour vaccines, oncolytic viruses, or CAR T cell therapy, IL-7 enhances T cell infiltration, thereby improving anti-tumour efficacy. In addition, some studies have shown that not only does IL-7 not induce Treg proliferation, but it also reduces Treg levels. This modulation to improve or reverse the state of tumour immunosuppression and enhance the body’s immune response to tumours should be the focus and hotspot of current tumour research. IL-7 has a promising application in tumour immunotherapy because of its antagonistic effect on the state of immunosuppression.
CAR-T cells have achieved remarkable results in the treatment of various cancers, but some challenges remain. Although patients treated with CAR-T cells may achieve initial responses, many patients still experience frequent relapses and do not achieve complete eradication. Therefore, improving the proliferation and cytotoxicity of CAR-T cells, reducing depletion, and increasing infiltration remain pressing issues that need to be addressed urgently. IL-7 promotes the proliferation as well as increases the toxicity of CAR-T, and therefore, IL-7 with CAR-T cells may improve their efficacy against solid tumours and hold the promise of achieving the complete eradication of tumour cells.
The role of IL-7 in cancer cachexia continues to be a topic of great interest in the field of oncology. Further study is needed to fully elucidate the role of IL-7 in cancer cachexia and to determine whether targeting the IL-7 signalling pathway is a viable therapeutic strategy for mitigating the devastating effects of cachexia in cancer patients. In the meantime, a combination of immunotherapy and other therapeutic agents may be able to reverse the malignant process to some extent while improving the quality of life and survival of cancer patients. Some studies have also suggested that IL-7-based therapy may improve the prognosis of patients, but this idea still needs to be evaluated in more in-depth studies in the future [132]. The tumour microenvironment is a crucial factor in the development, progression, and prognosis of lung adenocarcinoma. It is worth noting that IL-7 inhibits tumour growth by regulating the proportion of immune iso-cell infiltration in the tumour immune microenvironment. Therefore, IL-7 can be used as a beneficial prognostic marker with the potential to be applied in the treatment of lung adenocarcinoma patients.
Author Contributions
C.F.: investigation, visualisation, and writing—original draft; X.Z. (Xinqiang Zhang): writing—original draft; X.Z. (Xinyu Zhang): writing—original draft; D.W.: writing—original draft; S.H.: funding acquisition, and writing—review and editin; Z.M.: conceptualisation, funding acquisition, project administration, supervision, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 31140018) and Tianchi Talent Introduction Plan Innovative Leader.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hjerrild, K.J.; Jerzy, R.; Clevenger, W.; Gillis, S.; Cosman, D.; Namen, A.E. Human Interleukin 7: Molecular Cloning and Growth Factor Activity on Human and Murine B-Lineage Cells. Proc. Natl. Acad. Sci. USA 1989, 86, 302–306. [Google Scholar] [CrossRef]
- Barata, J.T.; Durum, S.K.; Seddon, B. Flip the Coin: IL-7 and IL-7R in Health and Disease. Nat. Immunol. 2019, 20, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Li, W.Q.; Aiello, F.B.; Mazzucchelli, R.; Asefa, B.; Khaled, A.R.; Durum, S.K. Cell Biology of IL-7, a Key Lymphotrophin. Cytokine Growth Factor Rev. 2005, 16, 513–533. [Google Scholar] [CrossRef] [PubMed]
- Leilei, Z.; Kewen, Z.; Biao, H.; Fang, H.; Yigang, W. The Role of Chemokine IL-7 in Tumor and Its Potential Antitumor Immunity. J. Interf. Cytokine Res. 2022, 42, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Winer, H.; Rodrigues, G.O.L.; Hixon, J.A.; Aiello, F.B.; Hsu, T.C.; Wachter, B.T.; Li, W.; Durum, S.K. IL-7: Comprehensive Review. Cytokine 2022, 160, 156049. [Google Scholar] [CrossRef] [PubMed]
- Mackall, C.L.; Fry, T.J.; Gress, R.E. Harnessing the Biology of IL-7 for Therapeutic Application. Nat. Rev. Immunol. 2011, 11, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Baizan-Edge, A.; Stubbs, B.A.; Stubbington, M.J.T.; Bolland, D.J.; Tabbada, K.; Andrews, S.; Corcoran, A.E. IL-7R Signaling Activates Widespread VH and DH Gene Usage to Drive Antibody Diversity in Bone Marrow B Cells. Cell Rep. 2021, 36, 109349. [Google Scholar] [CrossRef]
- Fernandez-Botran, R. Soluble Cytokine Receptors: Basic Immunology and Clinical Applications. Crit. Rev. Clin. Lab. Sci. 1999, 36, 165–224. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R Package and Shiny App for Producing PRISMA 2020-compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Chou, W.-C.; Levy, D.E.; Lee, C.-K. STAT3 Positively Regulates an Early Step in B-Cell Development. Blood 2006, 108, 3005–3011. [Google Scholar] [CrossRef]
- Kikuchi, K.; Lai, A.Y.; Hsu, C.-L.; Kondo, M. IL-7 Receptor Signaling Is Necessary for Stage Transition in Adult B Cell Development through up-Regulation of EBF. J. Exp. Med. 2005, 201, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Dittel, B.N.; LeBien, T.W. The Growth Response to IL-7 during Normal Human B Cell Ontogeny Is Restricted to B-Lineage Cells Expressing CD34. J. Immunol. 1995, 154, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Parrish, Y.K.; Baez, I.; Milford, T.-A.; Benitez, A.; Galloway, N.; Rogerio, J.W.; Sahakian, E.; Kagoda, M.; Huang, G.; Hao, Q.-L.; et al. IL-7 Dependence in Human B Lymphopoiesis Increases during Progression of Ontogeny from Cord Blood to Bone Marrow1. J. Immunol. 2009, 182, 4255–4266. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Chen, Y.; Zeng, H.; Zheng, Y.; Fu, G.; Zhu, W.; Broeckel, U.; Aggarwal, P.; Turner, A.; Neale, G.; et al. PLCγ-Dependent mTOR Signalling Controls IL-7-Mediated Early B Cell Development. Nat. Commun. 2017, 8, 1457. [Google Scholar] [CrossRef] [PubMed]
- Schlissel, M.S.; Durum, S.D.; Muegge, K. The Interleukin 7 Receptor Is Required for T Cell Receptor Gamma Locus Accessibility to the V(D)J Recombinase. J. Exp. Med. 2000, 191, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Durum, S.K.; Candèias, S.; Nakajima, H.; Leonard, W.J.; Baird, A.M.; Berg, L.J.; Muegge, K. Interleukin 7 Receptor Control of T Cell Receptor Gamma Gene Rearrangement: Role of Receptor-Associated Chains and Locus Accessibility. J. Exp. Med. 1998, 188, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Takada, I.; Hidano, S.; Takahashi, S.; Yanaka, K.; Ogawa, H.; Tsuchiya, M.; Yokoyama, A.; Sato, S.; Ochi, H.; Nakagawa, T.; et al. Transcriptional Coregulator Ess2 Controls Survival of Post-Thymic CD4+ T Cells through the Myc and IL-7 Signaling Pathways. J. Biol. Chem. 2022, 298, 102342. [Google Scholar] [CrossRef] [PubMed]
- Duy, C.; Yu, J.J.; Nahar, R.; Swaminathan, S.; Kweon, S.-M.; Polo, J.M.; Valls, E.; Klemm, L.; Shojaee, S.; Cerchietti, L.; et al. BCL6 Is Critical for the Development of a Diverse Primary B Cell Repertoire. J. Exp. Med. 2010, 207, 1209–1221. [Google Scholar] [CrossRef]
- Shenoy, A.R.; Kirschnek, S.; Häcker, G. IL-15 Regulates Bcl-2 Family Members Bim and Mcl-1 through JAK/STAT and PI3K/AKT Pathways in T Cells. Eur. J. Immunol. 2014, 44, 2500–2507. [Google Scholar] [CrossRef]
- Ta, V.B.T.; de Bruijn, M.J.W.; Matheson, L.; Zoller, M.; Bach, M.P.; Wardemann, H.; Jumaa, H.; Corcoran, A.; Hendriks, R.W. Highly Restricted Usage of Ig H Chain VH14 Family Gene Segments in Slp65-Deficient Pre-B Cell Leukemia in Mice. J. Immunol. 2012, 189, 4842–4851. [Google Scholar] [CrossRef]
- Li, W.Q.; Guszczynski, T.; Hixon, J.A.; Durum, S.K. Interleukin-7 Regulates Bim Proapoptotic Activity in Peripheral T-Cell Survival. Mol. Cell. Biol. 2010, 30, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Shi, S.X.; Zhu, X.; Borazanci, A.; Shi, F.-D.; Gan, Y. Interleukin-7 Expression and Its Effect on Natural Killer Cells in Patients with Multiple Sclerosis. J. Neuroimmunol. 2014, 276, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ezeh, P.C.; Xu, H.; Lauer, F.T.; Liu, K.J.; Hudson, L.G.; Burchiel, S.W. Monomethylarsonous Acid (MMA+3) Inhibits IL-7 Signaling in Mouse Pre-B Cells. Toxicol. Sci. 2016, 149, 289–299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Peydró, M.; de Yébenes, V.G.; Toribio, M.L. Notch1 and IL-7 Receptor Interplay Maintains Proliferation of Human Thymic Progenitors While Suppressing Non-T Cell Fates. J. Immunol. 2006, 177, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, S.; Li, X.; Wang, L.; Zhang, J.; Xu, J.; Huo, S.; Cao, X.; Zhong, Z.; Zhong, F. Mutual Enhancement of IL-2 and IL-7 on DNA Vaccine Immunogenicity Mainly Involves Regulations on Their Receptor Expression and Receptor-Expressing Lymphocyte Generation. Vaccine 2015, 33, 3480–3487. [Google Scholar] [CrossRef]
- Tang, J.-C.; Shen, G.-B.; Wang, S.-M.; Wan, Y.-S.; Wei, Y.-Q. IL-7 Inhibits Tumor Growth by Promoting T Cell-Mediated Antitumor Immunity in Meth A Model. Immunol. Lett. 2014, 158, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Vladyka, O.; Vrabcova, P.; Reiterova, M.; Parackova, Z.; Haesler, R.; Sediva, A.; Kalina, T.; Klocperk, A. Th1/Interferon-γ Bias in 22q11.2 Deletion Syndrome Is Driven by Memory T Cells and Exacerbated by IL-7. Clin. Immunol. 2023, 256, 109793. [Google Scholar] [CrossRef] [PubMed]
- Brenchley, J.M.; Douek, D.C.; Ambrozak, D.R.; Chatterji, M.; Betts, M.R.; Davis, L.S.; Koup, R.A. Expansion of Activated Human Naïve T-Cells Precedes Effector Function. Clin. Exp. Immunol. 2002, 130, 432–440. [Google Scholar] [CrossRef]
- Min, H.; Valente, L.A.; Xu, L.; O’Neil, S.M.; Begg, L.R.; Kurtzberg, J.; Filiano, A.J. Improving Thymus Implantation for Congenital Athymia with Interleukin-7. Clin. Transl. Immunol. 2023, 12, e1475. [Google Scholar] [CrossRef]
- Drake, A.; Kaur, M.; Iliopoulou, B.P.; Phennicie, R.; Hanson, A.; Chen, J. Interleukins 7 and 15 Maintain Human T Cell Proliferative Capacity through STAT5 Signaling. PLoS ONE 2016, 11, e0166280. [Google Scholar] [CrossRef]
- Kim, H.K.; Waickman, A.T.; Castro, E.; Flomerfelt, F.A.; Hawk, N.V.; Kapoor, V.; Telford, W.G.; Gress, R.E. Distinct IL-7 Signaling in Recent Thymic Emigrants versus Mature Naïve T Cells Controls T-Cell Homeostasis. Eur. J. Immunol. 2016, 46, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Luckey, M.A.; Park, J.-H. Intrathymic IL-7: The Where, When, and Why of IL-7 Signaling during T Cell Development. Semin. Immunol. 2012, 24, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, H.; Ito, M.; Sudo, T.; Hattori, M.; Kano, S.; Katsura, Y.; Minato, N. IL-7 Promotes Thymocyte Proliferation and Maintains Immunocompetent Thymocytes Bearing Alpha Beta or Gamma Delta T-Cell Receptors in Vitro: Synergism with IL-2. J. Immunol. 1989, 143, 2917–2922. [Google Scholar] [CrossRef] [PubMed]
- Conlon, P.; Morrissey, P.; Nordan, R.; Grabstein, K.; Prickett, K.; Reed, S.; Goodwin, R.; Cosman, D.; Namen, A. Murine Thymocytes Proliferate in Direct Response to Interleukin-7. Blood 1989, 74, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Huang, J.; Li, W.Q.; Cavinato, T.; Keller, J.R.; Durum, S.K. Role of the Intracellular Domain of IL-7 Receptor in T Cell Development1. J. Immunol. 2007, 178, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, C.; Hozumi, K.; Silva-Santos, B.; Bruno, L.; Hayday, A.C.; Owen, M.J.; Pennington, D.J. Pre-TCR Signaling Regulates IL-7 Receptor α Expression Promoting Thymocyte Survival at the Transition from the Double-Negative to Double-Positive Stage. Eur. J. Immunol. 2003, 33, 1968–1977. [Google Scholar] [CrossRef] [PubMed]
- Tani-ichi, S.; Shimba, A.; Wagatsuma, K.; Miyachi, H.; Kitano, S.; Imai, K.; Hara, T.; Ikuta, K. Interleukin-7 Receptor Controls Development and Maturation of Late Stages of Thymocyte Subpopulations. Proc. Natl. Acad. Sci. USA 2013, 110, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Gast, C.E.; Silk, A.D.; Zarour, L.; Riegler, L.; Burkhart, J.G.; Gustafson, K.T.; Parappilly, M.S.; Roh-Johnson, M.; Goodman, J.R.; Olson, B.; et al. Cell Fusion Potentiates Tumor Heterogeneity and Reveals Circulating Hybrid Cells That Correlate with Stage and Survival. Sci. Adv. 2018, 4, eaat7828. [Google Scholar] [CrossRef]
- Lu, L.; Chaudhury, P.; Osmond, D.G. Regulation of Cell Survival during B Lymphopoiesis: Apoptosis and Bcl-2/Bax Content of Precursor B Cells in Bone Marrow of Mice with Altered Expression of IL-7 and Recombinase-Activating Gene-21. J. Immunol. 1999, 162, 1931–1940. [Google Scholar] [CrossRef]
- Lali, F.V.; Crawley, J.; McCulloch, D.A.; Foxwell, B.M.J. A Late, Prolonged Activation of the Phosphatidylinositol 3-Kinase Pathway Is Required for T Cell Proliferation1. J. Immunol. 2004, 172, 3527–3534. [Google Scholar] [CrossRef]
- Geiselhart, L.A.; Humphries, C.A.; Gregorio, T.A.; Mou, S.; Subleski, J.; Komschlies, K.L. IL-7 Administration Alters the CD4:CD8 Ratio, Increases T Cell Numbers, and Increases T Cell Function in the Absence of Activation1. J. Immunol. 2001, 166, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- ElKassar, N.; Gress, R.E. An Overview of IL-7 Biology and Its Use in Immunotherapy. J. Immunotoxicol. 2010, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grabstein, K.H.; Namen, A.E.; Shanebeck, K.; Voice, R.F.; Reed, S.G.; Widmer, M.B. Regulation of T Cell Proliferation by IL-7. J. Immunol. 1990, 144, 3015–3020. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Tong, J.; Zhu, S.; Batista, P.J.; Duffy, E.E.; Zhao, J.; Bailis, W.; Cao, G.; Kroehling, L.; Chen, Y.; et al. m6A mRNA Methylation Controls T Cell Homeostasis by Targeting the IL-7/STAT5/SOCS Pathways. Nature 2017, 548, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Melão, A.; van Boxtel, R.; Santos, C.I.; Silva, A.; Silva, M.C.; Cardoso, B.A.; Coffer, P.J.; Barata, J.T. STAT5 Is Essential for IL-7–Mediated Viability, Growth, and Proliferation of T-Cell Acute Lymphoblastic Leukemia Cells. Blood Adv. 2018, 2, 2199–2213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, B.; Bai, Q.; Wang, P.; Wei, G.; Li, Z.; Hu, L.; Tian, Q.; Zhou, J.; Huang, Q.; et al. The lncRNA Snhg1-Vps13D Vesicle Trafficking System Promotes Memory CD8 T Cell Establishment via Regulating the Dual Effects of IL-7 Signaling. Signal Transduct. Target. Ther. 2021, 6, 126. [Google Scholar] [CrossRef] [PubMed]
- Kaech, S.M.; Tan, J.T.; Wherry, E.J.; Konieczny, B.T.; Surh, C.D.; Ahmed, R. Selective Expression of the Interleukin 7 Receptor Identifies Effector CD8 T Cells That Give Rise to Long-Lived Memory Cells. Nat. Immunol. 2003, 4, 1191–1198. [Google Scholar] [CrossRef]
- Park, J.-H.; Adoro, S.; Guinter, T.; Erman, B.; Alag, A.S.; Catalfamo, M.; Kimura, M.Y.; Cui, Y.; Lucas, P.J.; Gress, R.E.; et al. Signaling by Intrathymic Cytokines, Not T Cell Antigen Receptors, Specifies CD8 Lineage Choice and Promotes the Differentiation of Cytotoxic-Lineage T Cells. Nat. Immunol. 2010, 11, 257–264. [Google Scholar] [CrossRef]
- Armitage, R.J.; Namen, A.E.; Sassenfeld, H.M.; Grabstein, K.H. Regulation of Human T Cell Proliferation by IL-7. J. Immunol. 1990, 144, 938–941. [Google Scholar] [CrossRef]
- Buentke, E.; Mathiot, A.; Tolaini, M.; Di Santo, J.; Zamoyska, R.; Seddon, B. Do CD8 Effector Cells Need IL-7R Expression to Become Resting Memory Cells? Blood 2006, 108, 1949–1956. [Google Scholar] [CrossRef]
- Chazen, G.D.; Pereira, G.M.; LeGros, G.; Gillis, S.; Shevach, E.M. Interleukin 7 Is a T-Cell Growth Factor. Proc. Natl. Acad. Sci. USA 1989, 86, 5923–5927. [Google Scholar] [CrossRef] [PubMed]
- Schluns, K.S.; Kieper, W.C.; Jameson, S.C.; Lefrançois, L. Interleukin-7 Mediates the Homeostasis of Naïve and Memory CD8 T Cells in Vivo. Nat. Immunol. 2000, 1, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Bevington, S.L.; Keane, P.; Soley, J.K.; Tauch, S.; Gajdasik, D.W.; Fiancette, R.; Matei-Rascu, V.; Willis, C.M.; Withers, D.R.; Cockerill, P.N. IL-2/IL-7-inducible Factors Pioneer the Path to T Cell Differentiation in Advance of Lineage-defining Factors. EMBO J. 2020, 39, e105220. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.; Halim, L.; Chandele, A.; Perry, C.J.; Kim, S.H.; Kim, S.-U.; Byun, Y.; Yuk, S.H.; Kaech, S.M.; Jung, Y.W. IL-7 Plays a Critical Role for the Homeostasis of Allergen-Specific Memory CD4 T Cells in the Lung and Airways. Sci. Rep. 2017, 7, 11155. [Google Scholar] [CrossRef] [PubMed]
- Surh, C.D.; Sprent, J. Regulation of Mature T Cell Homeostasis. Semin. Immunol. 2005, 17, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tu, H.; Yang, Y.; Jiang, X.; Hu, X.; Luo, Q.; Li, J. Bone Marrow–Derived Mesenchymal Stromal Cells Promote Resistance to Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia via the IL-7/JAK1/STAT5 Pathway. J. Biol. Chem. 2019, 294, 12167–12179. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.L.; Albuquerque, A.S.; Serra-Caetano, A.; Foxall, R.B.; Pires, A.R.; Matoso, P.; Fernandes, S.M.; Ferreira, J.; Cheynier, R.; Victorino, R.M.M.; et al. Human Naïve Regulatory T-Cells Feature High Steady-State Turnover and Are Maintained by IL-7. Oncotarget 2016, 7, 12163–12175. [Google Scholar] [CrossRef] [PubMed]
- Le Campion, A.; Pommier, A.; Delpoux, A.; Stouvenel, L.; Auffray, C.; Martin, B.; Lucas, B. IL-2 and IL-7 Determine the Homeostatic Balance between the Regulatory and Conventional CD4+ T Cell Compartments during Peripheral T Cell Reconstitution. J. Immunol. 2012, 189, 3339–3346. [Google Scholar] [CrossRef]
- Fan, M.Y.; Low, J.S.; Tanimine, N.; Finn, K.K.; Priyadharshini, B.; Germana, S.K.; Kaech, S.M.; Turka, L.A. Differential Roles of IL-2 Signaling in Developing versus Mature Tregs. Cell Rep. 2018, 25, 1204–1213.e4. [Google Scholar] [CrossRef]
- Simonetta, F.; Gestermann, N.; Martinet, K.Z.; Boniotto, M.; Tissières, P.; Seddon, B.; Bourgeois, C. Interleukin-7 Influences FOXP3+CD4+ Regulatory T Cells Peripheral Homeostasis. PLoS ONE 2012, 7, e36596. [Google Scholar] [CrossRef]
- Liu, W.; Putnam, A.L.; Xu-yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; de St. Groth, B.F.; et al. CD127 Expression Inversely Correlates with FoxP3 and Suppressive Function of Human CD4+ T Reg Cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.-G.; Xiong, Y.; Chen, F. NFAT Gene Family in Inflammation and Cancer. Curr. Mol. Med. 2013, 13, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Iolyeva, M.; Aebischer, D.; Proulx, S.T.; Willrodt, A.-H.; Ecoiffier, T.; Häner, S.; Bouchaud, G.; Krieg, C.; Onder, L.; Ludewig, B.; et al. Interleukin-7 Is Produced by Afferent Lymphatic Vessels and Supports Lymphatic Drainage. Blood 2013, 122, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Nayar, S.; Campos, J.; Chung, M.M.; Navarro-Núñez, L.; Chachlani, M.; Steinthal, N.; Gardner, D.H.; Rankin, P.; Cloake, T.; Caamaño, J.H.; et al. Bimodal Expansion of the Lymphatic Vessels Is Regulated by the Sequential Expression of IL-7 and Lymphotoxin A1β2 in Newly Formed Tertiary Lymphoid Structures. J. Immunol. 2016, 197, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cornelissen, F.; Papazian, N.; Reijmers, R.M.; Llorian, M.; Cupedo, T.; Coles, M.; Seddon, B. IL-7–Dependent Maintenance of ILC3s Is Required for Normal Entry of Lymphocytes into Lymph Nodes. J. Exp. Med. 2018, 215, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Chappaz, S.; Finke, D. The IL-7 Signaling Pathway Regulates Lymph Node Development Independent of Peripheral Lymphocytes. J. Immunol. 2010, 184, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Hong, C.; Park, J.-H. Seeing Is Believing: Illuminating the Source of In Vivo Interleukin-7. Immune Netw. 2011, 11, 1. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kang, M.C.; Seo, Y.B.; Namkoong, H.; Park, Y.; Choi, D.-H.; Suh, Y.S.; Lee, S.-W.; Sung, Y.C.; Jin, H.-T. Intravaginal Administration of Fc-Fused IL7 Suppresses the Cervicovaginal Tumor by Recruiting HPV DNA Vaccine-Induced CD8 T Cells. Clin. Cancer Res. 2016, 22, 5898–5908. [Google Scholar] [CrossRef]
- Neitzke, D.J.; Bowers, J.S.; Andrijauskaite, K.; O’Connell, N.S.; Garrett-Mayer, E.; Wrangle, J.; Li, Z.; Paulos, C.M.; Cole, D.J.; Rubinstein, M.P. Murine Th17 Cells Utilize IL-2 Receptor Gamma Chain Cytokines but Are Resistant to Cytokine Withdrawal-Induced Apoptosis. Cancer Immunol. Immunother. 2017, 66, 737–751. [Google Scholar] [CrossRef]
- Amezcua Vesely, M.C.; Pallis, P.; Bielecki, P.; Low, J.S.; Zhao, J.; Harman, C.C.D.; Kroehling, L.; Jackson, R.; Bailis, W.; Licona-Limón, P.; et al. Effector TH17 Cells Give Rise to Long-Lived TRM Cells That Are Essential for an Immediate Response against Bacterial Infection. Cell 2019, 178, 1176–1188.e15. [Google Scholar] [CrossRef]
- Chen, Y.; Chauhan, S.K.; Tan, X.; Dana, R. Interleukin-7 and -15 Maintain Pathogenic Memory Th17 Cells in Autoimmunity. J. Autoimmun. 2017, 77, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Shireman, J.M.; Gonugunta, N.; Zhao, L.; Pattnaik, A.; Distler, E.; Her, S.; Wang, X.; Das, R.; Galipeau, J.; Dey, M. GM-CSF and IL-7 Fusion Cytokine Engineered Tumor Vaccine Generates Long-term Th-17 Memory Cells and Increases Overall Survival in Aged Syngeneic Mouse Models of Glioblastoma. Aging Cell 2023, 22, e13864. [Google Scholar] [CrossRef] [PubMed]
- Saout, C.L.; Hasley, R.B.; Imamichi, H.; Tcheung, L.; Hu, Z.; Luckey, M.A.; Park, J.-H.; Durum, S.K.; Smith, M.; Rupert, A.W.; et al. Chronic Exposure to Type-I IFN under Lymphopenic Conditions Alters CD4 T Cell Homeostasis. PLoS Pathog. 2014, 10, e1003976. [Google Scholar] [CrossRef] [PubMed]
- Maimela, N.R.; Liu, S.; Zhang, Y. Fates of CD8+ T Cells in Tumor Microenvironment. Comput. Struct. Biotechnol. J. 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, X.; Wu, H.; Su, X.; Wang, G.; Zhu, L.; Ji, Z. Prognostic Impact of Aberrantly Expressed Protein-Coding Gene Associated with Gastric Cancer’s Regulatory T Cells, Based on Online Databases. Altern. Ther. Health Med. 2023, 29, 160. [Google Scholar] [PubMed]
- Dwyer, C.J.; Knochelmann, H.M.; Smith, A.S.; Wyatt, M.M.; Rangel Rivera, G.O.; Arhontoulis, D.C.; Bartee, E.; Li, Z.; Rubinstein, M.P.; Paulos, C.M. Fueling Cancer Immunotherapy with Common Gamma Chain Cytokines. Front. Immunol. 2019, 10, 440788. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Kim, N.A.; Lim, D.G.; Eun, C.; Choi, D.; Jeong, S.H. Biophysical Stability of hyFc Fusion Protein with Regards to Buffers and Various Excipients. Int. J. Biol. Macromol. 2016, 86, 622–629. [Google Scholar] [CrossRef]
- Heo, M.; Sohn, J.; Lee, M.A.; Shin, E.-C.; Park, S.-H.; Kim, S.J.; Oh, Y.-K.; Jeong, S.; Woo, J.; Sung, Y.C.; et al. Phase 1b Study of GX-I7, a Long-Acting Interleukin-7, Evaluating the Safety, Pharmacokinetics and Pharmacodynamics Profiles in Patients with Advanced Solid Cancers. J. Immunother. Cancer 2019, 7, 1. [Google Scholar]
- Yu, E.M.; Cho, E.; Singh, R.; Kim, S.-H.; Han, C.; Han, S.; Lee, D.G.; Kim, Y.H.; Kwon, B.S.; Choi, B.K. IL7-Fc Enhances the Efficacy of Adoptive T Cell Therapy under Lymphopenic Conditions in a Murine Melanoma Model. Cells 2021, 10, 2018. [Google Scholar] [CrossRef]
- Ahn, S.; Park, J.; Kim, H.; Heo, M.; Sung, Y.C.; Jeun, S. Compassionate Use of Recombinant Human IL-7-hyFc as a Salvage Treatment for Restoring Lymphopenia in Patients with Recurrent Glioblastoma. Cancer Med. 2022, 12, 6778–6787. [Google Scholar] [CrossRef]
- Campian, J.L.; Ghosh, S.; Kapoor, V.; Yan, R.; Thotala, S.; Jash, A.; Hu, T.; Mahadevan, A.; Rifai, K.; Page, L.; et al. Long-Acting Recombinant Human Interleukin-7, NT-I7, Increases Cytotoxic CD8 T Cells and Enhances Survival in Mouse Glioma Models. Clin. Cancer Res. 2022, 28, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Neuendorff, N.R.; Khan, A.; Ullrich, F.; Yates, S.; Devarakonda, S.; Lin, R.J.; von Tresckow, B.; Cordoba, R.; Artz, A.; Rosko, A.E. Cellular Therapies in Older Adults with Hematological Malignancies: A Case-Based, State-of-the-Art Review. J. Geriatr. Oncol. 2024, 15, 101734. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Long, Z.; Jia, R.; Wang, M.; Zhu, D.; Liu, M.; Chen, S.; Zhao, X.; Yang, Q.; Wu, Y.; et al. The Broad Immunomodulatory Effects of IL-7 and Its Application in Vaccines. Front. Immunol. 2021, 12, 680442. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, I.H.; Rahman, S.; Afroze, Y.J.; Shovah, S.T. IUPHAR ECR Review: Cancer-Related Anorexia-Cachexia in Cancer Patients: Pathophysiology and Treatment. Pharmacol. Res. 2024, 203, 107129. [Google Scholar] [CrossRef]
- Costa, R.G.F.; Caro, P.L.; de Matos-Neto, E.M.; Lima, J.D.C.C.; Radloff, K.; Alves, M.J.; Camargo, R.G.; Pessoa, A.F.M.; Simoes, E.; Gama, P.; et al. Cancer Cachexia Induces Morphological and Inflammatory Changes in the Intestinal Mucosa. J. Cachexia Sarcopenia Muscle 2019, 10, 1116–1127. [Google Scholar] [CrossRef]
- Zidi, B.; Vincent-Fabert, C.; Pouyet, L.; Seillier, M.; Vandevelde, A.; N’guessan, P.; Poplineau, M.; Guittard, G.; Mancini, S.J.C.; Duprez, E.; et al. TP53INP1 Deficiency Maintains Murine B Lymphopoiesis in Aged Bone Marrow through Redox-Controlled IL-7R/STAT5 Signaling. Proc. Natl. Acad. Sci. USA 2019, 116, 211–216. [Google Scholar] [CrossRef]
- Castro, C.C.M.; Silva, S.P.; Rabelo, L.N.; Queiroz, J.P.G.; Campos, L.D.; Silva, L.C.; Fiuza, F.P. Age, Education Years, and Biochemical Factors Are Associated with Selective Neuronal Changes in the Elderly Hippocampus. Cells 2022, 11, 4033. [Google Scholar] [CrossRef]
- Cao, L.; Coventry, B.; Goreshnik, I.; Huang, B.; Sheffler, W.; Park, J.S.; Jude, K.M.; Marković, I.; Kadam, R.U.; Verschueren, K.H.G.; et al. Design of Protein-Binding Proteins from the Target Structure Alone. Nature 2022, 605, 551–560. [Google Scholar] [CrossRef]
- Williams, J.H.; Udata, C.; Ganguly, B.J.; Bucktrout, S.L.; Joh, T.; Shannon, M.; Wong, G.Y.; Levisetti, M.; Garzone, P.D.; Meng, X. Model-Based Characterization of the Pharmacokinetics, Target Engagement Biomarkers, and Immunomodulatory Activity of PF-06342674, a Humanized mAb Against IL-7 Receptor-α, in Adults with Type 1 Diabetes. AAPS J. 2020, 22, 23. [Google Scholar] [CrossRef]
- Caushi, J.X.; Zhang, J.; Ji, Z.; Vaghasia, A.; Zhang, B.; Hsiue, E.H.-C.; Mog, B.J.; Hou, W.; Justesen, S.; Blosser, R.; et al. Transcriptional Programs of Neoantigen-Specific TIL in Anti-PD-1-Treated Lung Cancers. Nature 2021, 596, 126–132. [Google Scholar] [CrossRef]
- Courtois, L.; Cabannes-Hamy, A.; Kim, R.; Delecourt, M.; Pinton, A.; Charbonnier, G.; Feroul, M.; Smith, C.; Tueur, G.; Pivert, C.; et al. IL-7 Receptor Expression Is Frequent in T-Cell Acute Lymphoblastic Leukemia and Predicts Sensitivity to JAK Inhibition. Blood 2023, 142, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Sereti, I.; Estes, J.D.; Thompson, W.L.; Morcock, D.R.; Fischl, M.A.; Croughs, T.; Beq, S.; Lafaye de Micheaux, S.; Yao, M.D.; Ober, A.; et al. Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration. PLoS Pathog. 2014, 10, e1003890. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Watson, R.A.; Tong, O.; Ye, W.; Nassiri, I.; Gilchrist, J.J.; de los Aires, A.V.; Sharma, P.K.; Koturan, S.; Cooper, R.A.; et al. IL7 Genetic Variation and Toxicity to Immune Checkpoint Blockade in Patients with Melanoma. Nat. Med. 2022, 28, 2592–2600. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, M.; Yamashita, M.; Arai, Y.; Nakamura, T.; Nakao, S. IL-7 Coupled with IL-12 Increases Intratumoral T Cell Clonality, Leading to Complete Regression of Non-Immunogenic Tumors. Cancer Immunol. Immunother. 2021, 70, 3557–3571. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, Y.; Hu, R.; Su, M.; Rood, D.; Lai, L. In Vivo Antitumor Activity of a Recombinant IL7/IL15 Hybrid Cytokine in Mice. Mol. Cancer Ther. 2016, 15, 2413–2421. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Niu, J.; Wang, L.; Zhang, W.; He, X.; Zhang, X.; Hu, W.; Tang, Y.; Yang, H.; Sun, J.; et al. An Injectable Hydrogel Microsphere-Integrated Training Court to Inspire Tumor-Infiltrating T Lymphocyte Potential. Biomaterials 2024, 306, 122475. [Google Scholar] [CrossRef]
- Ke, B.; Wei, T.; Huang, Y.; Gong, Y.; Wu, G.; Liu, J.; Chen, X.; Shi, L. Interleukin-7 Resensitizes Non-Small-Cell Lung Cancer to Cisplatin via Inhibition of ABCG2. Mediat. Inflamm. 2019, 2019, 7241418. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Morishima, C.; Szmulewitz, R.; Harshman, L.; Appleman, L.; Monk, P.; Bitting, R.L.; Kucuk, O.; Millard, F.; Seigne, J.D.; et al. IL-7 Expands Lymphocyte Populations and Enhances Immune Responses to Sipuleucel-T in Patients with Metastatic Castration-Resistant Prostate Cancer (mCRPC). J. Immunother. Cancer 2021, 9, e002903. [Google Scholar] [CrossRef]
- Gou, H.-F.; Huang, J.; Shi, H.-S.; Chen, X.; Wang, Y.-S. Chemo-Immunotherapy with Oxaliplatin and Interleukin-7 Inhibits Colon Cancer Metastasis in Mice. PLoS ONE 2014, 9, e85789. [Google Scholar] [CrossRef]
- Gastman, B.; Fling, S.; Ansstas, G.; Funchain, P.; Silk, A.W.; Friedlander, P.A.; Curti, B.D.; Xing, Y.; Nguyen, O.; Christensen, A.; et al. A Phase 1b/2a Study of Safety and Efficacy of NT-I7 in Combination with Anti-PD-L1 (Atezolizumab) in Patients with Anti-PD-1/PD-L1 Naïve or Relapsed/Refractory (R/R) High-Risk Skin Cancers: The Phase 1b Report. J. Clin. Oncol. 2022, 40, 9561. [Google Scholar] [CrossRef]
- Burns, C.; Kubicki, S.; Nguyen, Q.-B.; Aboul-Fettouh, N.; Wilmas, K.M.; Chen, O.M.; Doan, H.Q.; Silapunt, S.; Migden, M.R. Advances in Cutaneous Squamous Cell Carcinoma Management. Cancers 2022, 14, 3653. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kong, L.; Kim, S.; Lee, S.; Oh, S.; Jo, S.; Jang, I.; Kim, T.-D. The Role of IL-7 and IL-7R in Cancer Pathophysiology and Immunotherapy. Int. J. Mol. Sci. 2022, 23, 10412. [Google Scholar] [CrossRef] [PubMed]
- Dower, W.J.; Park, A.I.; Bakker, A.V.; Cwirla, S.E.; Pongtornpipat, P.; Williams, B.M.; Joshi, P.; Baxter, B.A.; Needels, M.C.; Barrett, R.W. A Mechanistically Novel Peptide Agonist of the IL-7 Receptor That Addresses Limitations of IL-7 Cytokine Therapy. PLoS ONE 2023, 18, e0286834. [Google Scholar] [CrossRef] [PubMed]
- Ltd, B.P.G. Correction: Oncolytic Virus Expressing PD-1 Inhibitors Activates a Collaborative Intratumoral Immune Response to Control Tumor and Synergizes with CTLA-4 or TIM-3 Blockade. J. Immunother. Cancer 2024, 12, e004762corr1. [Google Scholar] [CrossRef]
- Shalhout, S.Z.; Miller, D.M.; Emerick, K.S.; Kaufman, H.L. Therapy with Oncolytic Viruses: Progress and Challenges. Nat. Rev. Clin. Oncol. 2023, 20, 160–177. [Google Scholar] [CrossRef]
- Kudling, T.V.; Clubb, J.H.A.; Quixabeira, D.C.A.; Santos, J.M.; Havunen, R.; Kononov, A.; Heiniö, C.; Cervera-Carrascon, V.; Pakola, S.; Basnet, S.; et al. Local Delivery of Interleukin 7 with an Oncolytic Adenovirus Activates Tumor-Infiltrating Lymphocytes and Causes Tumor Regression. Oncoimmunology 2022, 11, 2096572. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Arai, Y.; Tasaki, M.; Yamashita, M.; Murakami, R.; Kawase, T.; Amino, N.; Nakatake, M.; Kurosaki, H.; Mori, M.; et al. Intratumoral Expression of IL-7 and IL-12 Using an Oncolytic Virus Increases Systemic Sensitivity to Immune Checkpoint Blockade. Sci. Transl. Med. 2020, 12, eaax7992. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, S.; Cai, L.; Duan, H.; Li, Y.; Yang, J.; Wang, Y.; Liu, B.; Dong, S.; Fang, Z.; et al. A Novel Cocktail Therapy Based on Quintuplet Combination of Oncolytic Herpes Simplex Virus-2 Vectors Armed with Interleukin-12, Interleukin-15, GM-CSF, PD1v, and IL-7 × CCL19 Results in Enhanced Antitumor Efficacy. Virol. J. 2022, 19, 74. [Google Scholar] [CrossRef]
- Tokunaga, Y.; Sasaki, T.; Goto, S.; Adachi, K.; Sakoda, Y.; Tamada, K. Enhanced Antitumor Responses of Tumor Antigen-Specific TCR T Cells Genetically Engineered to Produce IL7 and CCL19. Mol. Cancer Ther. 2022, 21, 138–148. [Google Scholar] [CrossRef]
- He, C.; Zhou, Y.; Li, Z.; Farooq, M.A.; Ajmal, I.; Zhang, H.; Zhang, L.; Tao, L.; Yao, J.; Du, B.; et al. Co-Expression of IL-7 Improves NKG2D-Based CAR T Cell Therapy on Prostate Cancer by Enhancing the Expansion and Inhibiting the Apoptosis and Exhaustion. Cancers 2020, 12, 1969. [Google Scholar] [CrossRef]
- Goto, S.; Sakoda, Y.; Adachi, K.; Sekido, Y.; Yano, S.; Eto, M.; Tamada, K. Enhanced Anti-Tumor Efficacy of IL-7/CCL19-Producing Human CAR-T Cells in Orthotopic and Patient-Derived Xenograft Tumor Models. Cancer Immunol. Immunother. 2021, 70, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-Loaded Oncolytic Adenovirus Improves CAR-T Cell Therapy for Glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Wang, K.; Wei, C.; Feng, D.; Liu, Y.; He, Q.; Xu, X.; Wang, C.; Zhao, S.; Lv, L.; et al. The BCMA-Targeted Fourth-Generation CAR-T Cells Secreting IL-7 and CCL19 for Therapy of Refractory/Recurrent Multiple Myeloma. Front. Immunol. 2021, 12, 609421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Liu, W.; Li, X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Triple-Negative Breast Cancer. BioMed Res. Int. 2020, 2020, e4795171. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Su, J.; Sun, R.; Sun, Y.; Wang, Y.; Dong, Y.; Shi, B.; Jiang, H.; Li, Z. Coexpression of IL7 and CCL21 Increases Efficacy of CAR-T Cells in Solid Tumors without Requiring Preconditioned Lymphodepletion. Clin. Cancer Res. 2020, 26, 5494–5505. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Xi, J.; Liu, Q.; Wang, C.; Jiang, Z.; Yue, S.-Y.; Shi, L.; Rong, Y. Co-Expression of IL-7 and PH20 Promote Anti-GPC3 CAR-T Tumour Suppressor Activity in Vivo and in Vitro. Liver Int. 2021, 41, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.; Shi, J.; Qin, L.; Chen, A.; Tang, Y.; Yang, H.; Huang, Y.; Wu, Q.; Li, X.; He, B.; et al. IL-7 and CCL19-Secreting CAR-T Cell Therapy for Tumors with Positive Glypican-3 or Mesothelin. J. Hematol. Oncol. 2021, 14, 118. [Google Scholar] [CrossRef]
- Joedicke, J.J.; Großkinsky, U.; Gerlach, K.; Künkele, A.; Höpken, U.E.; Rehm, A. Accelerating Clinical-Scale Production of BCMA CAR T Cells with Defined Maturation Stages. Mol. Ther.—Methods Clin. Dev. 2022, 24, 181–198. [Google Scholar] [CrossRef]
- Swan, S.L.; Mehta, N.; Ilich, E.; Shen, S.H.; Wilkinson, D.S.; Anderson, A.R.; Segura, T.; Sanchez-Perez, L.; Sampson, J.H.; Bellamkonda, R.V. IL7 and IL7 Flt3L Co-Expressing CAR T Cells Improve Therapeutic Efficacy in Mouse EGFRvIII Heterogeneous Glioblastoma. Front. Immunol. 2023, 14, 1085547. [Google Scholar] [CrossRef]
- Belarif, L.; Mary, C.; Jacquemont, L.; Mai, H.L.; Danger, R.; Hervouet, J.; Minault, D.; Thepenier, V.; Nerrière-Daguin, V.; Nguyen, E.; et al. IL-7 Receptor Blockade Blunts Antigen-Specific Memory T Cell Responses and Chronic Inflammation in Primates. Nat. Commun. 2018, 9, 4483. [Google Scholar] [CrossRef]
- Kann, M.C.; Schneider, E.M.; Almazan, A.J.; Lane, I.C.; Bouffard, A.A.; Supper, V.M.; Takei, H.N.; Tepper, A.; Leick, M.B.; Larson, R.C.; et al. Chemical Genetic Control of Cytokine Signaling in CAR-T Cells Using Lenalidomide-Controlled Membrane-Bound Degradable IL-7. Leukemia 2023, 38, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, H.; Lei, W.; Chen, P.; Zhao, A.; Yuan, X.; Gao, J.; Qian, W. Efficacy and safety of fourth-generation CD19 CAR-T expressing IL7 and CCL19 along with PD-1 monoclonal antibody for relapsed or refractory large B-cell lymphoma. Zhonghua Xue Ye Xue Za Zhi 2023, 44, 820–824. [Google Scholar] [PubMed]
- Wang, S.-Y.; Scurti, G.M.; Dalheim, A.V.; Quinn, S.; Stiff, P.J.; Nishimura, M.I. Nonactivated and IL-7 Cultured CD19-Specific CAR T Cells Are Enriched in Stem Cell Phenotypes and Functionally Superior. Blood Adv. 2023, 8, 324–335. [Google Scholar] [CrossRef]
- Adachi, K.; Kano, Y.; Nagai, T.; Okuyama, N.; Sakoda, Y.; Tamada, K. IL-7 and CCL19 Expression in CAR-T Cells Improves Immune Cell Infiltration and CAR-T Cell Survival in the Tumor. Nat. Biotechnol. 2018, 36, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-L.; Xiao, S.; Lin, Z.; Bai, J.; Li, W.; Song, Z.; Zhou, Y.; Lu, B.; Wu, W.-Z. GPC3-IL7-CCL19-CAR-T Primes Immune Microenvironment Reconstitution for Hepatocellular Carcinoma Therapy. Cell Biol. Toxicol. 2023, 39, 3101–3119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ye, X.; Wen, J.; Cai, Z.; Li, Y.; Zhang, M.; Shen, L.; Cai, J. Anti-HER2 scFv-CCL19-IL7 Recombinant Protein Inhibited Gastric Tumor Growth in Vivo. Sci. Rep. 2022, 12, 10461. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Moore, T.V.; Dalheim, A.V.; Scurti, G.M.; Nishimura, M.I. Melanoma Reactive TCR-Modified T Cells Generated without Activation Retain a Less Differentiated Phenotype and Mediate a Superior in Vivo Response. Sci. Rep. 2021, 11, 13327. [Google Scholar] [CrossRef]
- Luo, M.; Gong, W.; Zhang, Y.; Li, H.; Ma, D.; Wu, K.; Gao, Q.; Fang, Y. New Insights into the Stemness of Adoptively Transferred T Cells by Γc Family Cytokines. Cell Commun. Signal 2023, 21, 347. [Google Scholar] [CrossRef]
- Greenberg, Z.J.; Monlish, D.A.; Bartnett, R.L.; Yang, Y.; Shen, G.; Li, W.; Bednarski, J.J.; Schuettpelz, L.G. The Tetraspanin CD53 Regulates Early B Cell Development by Promoting IL-7R Signaling. J. Immunol. 2020, 204, 58–67. [Google Scholar] [CrossRef]
- Merchant, M.S.; Bernstein, D.; Amoako, M.; Baird, K.; Fleisher, T.A.; Morre, M.; Steinberg, S.M.; Sabatino, M.; Stroncek, D.F.; Venkatasan, A.M.; et al. Adjuvant Immunotherapy to Improve Outcome in High-Risk Pediatric Sarcomas. Clin. Cancer Res. 2016, 22, 3182–3191. [Google Scholar] [CrossRef]
- Volz, B.; Schmidt, M.; Heinrich, K.; Kapp, K.; Schroff, M.; Wittig, B. Design and Characterization of the Tumor Vaccine MGN1601, Allogeneic Fourfold Gene-Modified Vaccine Cells Combined with a TLR-9 Agonist. Mol. Ther. Oncolytics 2016, 3, 15023. [Google Scholar] [CrossRef][Green Version]
- Van der Sijde, F.; Dik, W.A.; Mustafa, D.A.M.; Vietsch, E.E.; Besselink, M.G.; Debets, R.; Koerkamp, B.G.; Haberkorn, B.C.M.; Homs, M.Y.V.; Janssen, Q.P.; et al. Serum Cytokine Levels Are Associated with Tumor Progression during FOLFIRINOX Chemotherapy and Overall Survival in Pancreatic Cancer Patients. Front. Immunol. 2022, 13, 898498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).