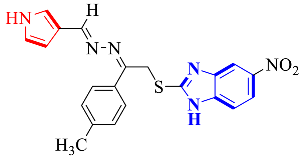
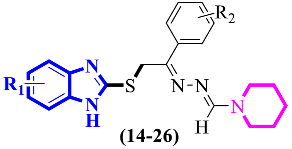
Abstract
Benzimidazole-based pyrrole/piperidine analogs (1–26) were synthesized and then screened for their acetylcholinesterase and butyrylcholinesterase activities. All the analogs showed good to moderate cholinesterase activities. Synthesized compounds (1–13) were screened in cholinesterase enzyme inhibition assays and showed AChE activities in the range of IC50 = 19.44 ± 0.60 µM to 36.05 ± 0.4 µM against allanzanthane (IC50 = 16.11 ± 0.33 µM) and galantamine (IC50 = 19.34 ± 0.62 µM) and varied BuChE inhibitory activities, with IC50 values in the range of 21.57 ± 0.61 µM to 39.55 ± 0.03 µM as compared with standard allanzanthane (IC50 = 18.14 ± 0.05 µM) and galantamine (IC50 = 21.45 ± 0.21 µM). Similarly, synthesized compounds (14–26) were also subjected to tests to determine their in vitro AChE inhibitory activities, and the results obtained corroborated that all the compounds showed varied activities in the range of IC50 = 22.07 ± 0.13 to 42.01 ± 0.02 µM as compared to allanzanthane (IC50 = 20.01 ± 0.12 µM) and galantamine (IC50 = 18.05 ± 0.31 µM) and varied BuChE inhibitory activities, with IC50 values in the range of 26.32 ± 0.13 to 47.03 ± 0.15 µM as compared to standard allanzanthane (IC50 = 18.14 ± 0.05 µM) and galantamine (IC50 = 21.45 ± 0.21 µM). Binding interactions of the most potent analogs were confirmed through molecular docking studies. The active analogs 2, 4, 10 and 13 established numerous interactions with the active sites of targeted enzymes, with docking scores of −10.50, −9.3, −7.73 and −7.8 for AChE and −8.97, −8.2, −8.20 and −7.6 for BuChE, respectively.
1. Introduction
Alzheimer’s disease (AD) is a chronic neurological disorder characterized by memory impairment, cognitive dysfunction, behavioral disturbances and deficits in activities of daily living [1,2,3]. AD has been found to be associated with a cholinergic deficit in the postmortem brain characterized by a significant decrease in the amount of acetylcholine [4,5]. AD has become a major problem, particularly in developed countries, due to increasing old-age populations with a high quality of life. Acetylcholine is a neurotransmitter inhibited, primarily, by acetylcholinesterase (AChE) and, secondarily, by butyrylcholinesterase (BuChE), which are considered to play a role in the pathology of AD [6]. Both enzymes are present in the brain and are detected in neurofibrillary tangles and neurotic plaques [7]. Despite the unknown etiology of AD, elevation of acetylcholine amounts through AChE enzyme inhibition has been accepted as the most effective treatment strategy against AD [8]. Therefore, AChE and BuChE inhibitors have become remarkable alternatives in the treatment of AD. However, the present drugs (e.g., tacrine, galantamine, donepezil and rivastigmine) (Figure 1) with AChE inhibitory activity possess some side effects and are effective only against the mild type of AD, and currently there is no drug with BuChE inhibitory activity available [9,10].
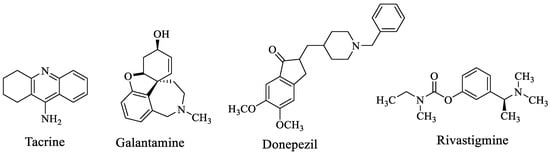
Figure 1.
Available drugs for AD with AChE activity.
Benzimidazole possesses a diverse range of biological activities, such as anti-hypertensive [11,12], anti-Alzheimer’s [13], anti-microbial, anti-viral [14], anti-diabetic [15,16] and anti-cancer activities [17]. There are some drug molecules, such as benoxaprofen, albendazole, enviradine, bendamastine, omeprazole and astemizole, which contain a benzimidazole moiety in their skeleton (Figure 2) [18].
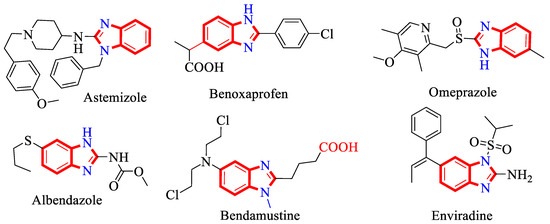
Figure 2.
Benzimidazole analogs bearing bioactive drugs.
Pyrrole derivatives have been found to exhibit a wide range of biological activities, including anti-bacterial, anti-fungal, anti-viral, anti-inflammatory, analgesic, anti-tumor and anti-convulsant activities. Pyrrole-containing drugs, such as atorvastatin, are used to treat hypercholesterolemia [19], and tolmetin is used to treat osteoarthritis and rheumatoid arthritis [20]. Nargenicin is an antibacterial drug that contains a pyrrole ring [21]. Prodigiosin is an anti-cancer compound that contains three pyrrole scaffolds [22]. Piperidine and its derivatives have great biological significance in medicinal chemistry and are widely used in the development of drugs for the treatment of various diseases. Their unique chemical properties and ability to interact with a variety of biological targets make them important scaffolds for drug design [23], including anti-cancer drugs [24,25,26,27,28], antibiotics [29], analgesics [30], antidepressants [31] and antioxidants [32]. Keeping in view the biological significance of benzimidazole, pyrrole and piperidine moieties, in this study, we designed and synthesized hybrid scaffolds of benzimidazole-bearing pyrroles and piperidines as potential inhibitors of AChE and BuChE for the treatment of Alzheimer’s disease in the search for lead molecules, and the results obtained corroborated that these compounds could be considered as potential lead molecules for the development of improved AChE and BuChE inhibitors.
2. Results and Discussion
2.1. Chemistry
The synthesis of targeted benzimidazole-based pyrrole/piperidine derivatives (1–26) was achieved in a series of steps. Initially, 2-marcaptobenzimidazole (I) was reacted with differently substituted phenacyl bromides in ethanol and triethylamine, and then a reaction mixture was refluxed for 4 h to give S-substituted benzimidazole (II), which was further redissolved in a hydrazine hydrate solution while being stirred in acetic acid. After being refluxed for 3 h, the solvent was evaporated, and the resulting solid residue (III) was reacted in two different ways:
- (1)
- Substrate (III) was put in ethanol and triethylamine, followed by the addition of 1H-pyrrole-3-carbaldehyde. The reaction mixture was refluxed and stirred for 7 h to obtain targeted benzimidazole-based pyrrole derivatives (1–13).
- (2)
- Further, the intermediate (III) was reacted with piperidine-1-carbaldehyde in ethanol along with a catalytic amount of triethylamine, and the resulting solid residue was heated over a sand bath for 8 h to afford targeted benzimidazole-based piperidine derivatives (14–26) (Scheme 1).
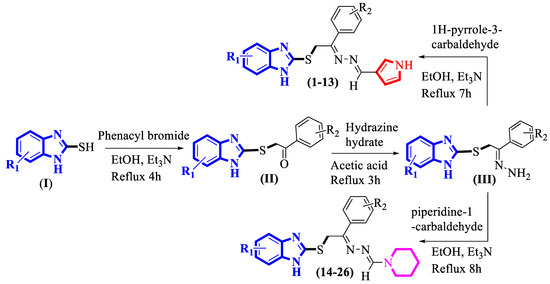 Scheme 1. Synthesis of targeted benzimidazole-based hybrid compounds (1–26).
Scheme 1. Synthesis of targeted benzimidazole-based hybrid compounds (1–26).
2.2. In Vitro Acetylcholinesterase and Butyrylcholinesterase Activities
The synthesized scaffolds based on benzimidazole-bearing pyrroles/piperidines (1–26) were subjected to in vitro acetylcholinesterase and butyrylcholinesterase inhibitory activity assays. All the newly synthesized compounds showed good to moderate acetylcholinesterase and butyrylcholinesterase inhibitory activities. Target compounds (1–13) were screened in assays for cholinesterase enzyme inhibition and showed AChE activities in the range of IC50 = 19.44 ± 0.60 µM–36.05 ± 0.4 µM against allanzanthane (IC50 = 16.11 ± 0.33 µM) and galantamine (IC50 = 19.34 ± 0.62 µM) and varied BuChE inhibitory activities, with IC50 values in the range of 21.57 ± 0.61 µM–39.55 ± 0.03 µM. The results were compared with those of standard allanzanthane (IC50 = 18.14 ± 0.05 µM) and galantamine (IC50 = 21.45 ± 0.21 µM). Similarly, target compounds (14–26) were also subjected to in vitro AChE inhibitory activity assays, and the results obtained corroborated that all the compounds showed varied activities in the range of IC50 = 22.07 ± 0.13–42.01 ± 0.02 µM against allanzanthane (IC50 = 20.01 ± 0.12 µM) and galantamine (IC50 = 18.05 ± 0.31 µM) and varied BuChE inhibitory activities, with IC50 values in the range of 26.32 ± 0.13–47.03 ± 0.15 µM as compared to standard allanzanthane (IC50 = 18.14 ± 0.05 µM) and galantamine (IC50 = 21.45 ± 0.21 µM).
Structure–Activity Relationship (SAR) Studies for Acetylcholinesterase and Butyrylcholinesterase Activities
By inspecting the influence of various moieties (R1 and R2) on AChE and BuChE activities, SAR studies were established. Among the series (1–13), compound 10 (bearing a NO2 at the 5-position of the benzimidazole ring and a m-NO2 substitution on the aryl ring) emerged as the most potent inhibitor of AChE and BuChE enzymes. Furthermore, compound 2 (that has a –OCH3 group at the 5-position of the benzimidazole ring and a p-OCH3 substitution on the aryl ring) also showed great inhibitory potential against the targeted enzymes. However, an alteration in inhibitory potential was observed by changing the position of the –OCH3 group around the aryl ring. Therefore, compound 4 (that has a –OCH3 group at the 5-position of the benzimidazole ring and a o-OCH3 substitution on the aryl ring), which holds substituents of a similar nature but has the –OCH3 group in a different position on the aryl ring, demonstrated a lesser inhibition profile. Moreover, compound 8 (with an unsubstituted benzimidazole ring and a o-OCH3 substitutedaryl ring) showed great inhibitory potential when compared to compound 9 (with an unsubstituted benzimidazole ring and p-OCH3 substituted in the aryl ring), indicating that a change in the position of the substituent around the aryl ring may cause an alteration to the inhibitory potential (Table 1).

Table 1.
Different substituents (R1 and R2) and in vitro acetylcholinesterase and butyrylcholinesterase inhibitory activities of synthesized benzimidazole-based pyrrole/piperidine derivatives (1–26).
It was noteworthy that analogs (10–13) bearing a –NO2 substituent at the 5-position of the benzimidazole ring and various groups, like –NO2, –Ph, –OCH3 and –CH3, at the variable position of the other aryl ring were identified as encouraging the inhibition of AChE and BuChE enzymes. Among these analogs, analog 10 was shown as a potent inhibitor of the targeted enzymes. Further, the comparison of analog 12 (which has a 5-NO2 substitution at the benzimidazole ring and a p-OCH3 substituted at the aryl ring) with compounds 11 (which has a 5-NO2 substitution at the benzimidazole ring and a p-Ph moiety at the aryl ring) and 13 (which has a 5-NO2 substitution at the benzimidazole ring and a p-CH3 group on the aryl ring) demonstrated that analog 12 showed a better inhibitory potential than its counterparts. This difference in inhibitory potential may perhaps be owing to the different nature of the attached substituents around the aryl ring, which cause an alteration in the inhibitory potential (Table 1).
Among the series (14–26), compound 26 (which has a NO2 at the 5-position of the benzimidazole ring and a m-NO2 substitution on the aryl ring) emerged as the most active inhibitor of AChE and BuChE enzymes, followed by compound 17, bearing a OCH3 at the 5-position of the benzimidazole ring and a m-NO2 substitution on the aryl ring). Compounds 15 and 16, which have the same 5-OCH3 group on the benzimidazole ring and o-OCH3 and p-OCH3 substitutions on the aryl ring, also exhibited good inhibition against both AChE and BuChE. When the substituents on the aryl ring were replaced by p-CH3 and p-phenyl in compounds 18 and 14, respectively, a slight change in their inhibitory activities was observed. Compound 20, bearing a benzimidazole ring and a m-NO2 group on the phenyl ring showed good inhibition. When the m-NO2 group was replaced by p-CH3, o-OCH3 and p-OCH3 in compounds 19, 21 and 22, respectively, a decline in inhibitory activities was observed. Among compounds 23–26 (which have the same 5-NO2 substitution at the benzimidazole ring but different substituents, like p-Ph, p-OCH3, p-CH3 and m-NO2, at the phenyl ring), compound 26 exhibited significant inhibitory potential, while the remaining three compounds exhibited inhibition levels in the following order: 24 > 25 > 23 (Table 1). The variation in inhibitory potential could be attributed to the distinct characteristics of the substituents attached around the aryl ring, which potentially led to alterations in inhibitory effectiveness.
2.3. In Silico Molecular Docking Approach
2.3.1. Docking Study for Benzimidazole-Based Pyrrole Analogs
Molecular docking studies were performed for the compounds (bioactive moieties) in the synthesized series. Different software packages were used in order to sketch out the molecules in the protein complexes, namely, the auto dock tool, Pymol (2.5.2) and the Discovery studio visualizer (DSV). Our research groups have already worked on a similar package while using varied heterocyclic moieties [33,34,35]. Ligands were docked in the same places on the amino acids, where co-crystallized ligands were present in order to check the binding interactions of the synthesized moieties. For the purpose of docking, proteins were downloaded from the RCSB protein data bank (PDB) using the code 1ACL for AChE and 1P0P for BuChE, and both the proteins and ligand molecules were prepared in the DSV and the files were transferred to the auto dock tool, where polar hydrogens and charges were added, and the configuration files were saved in PDBQT format. Upon completion of these steps, the docking procedures were run and all the poses were visualized. After the successful completion of the procedures, nine different poses were obtained for each molecule along with binding affinities. Among these, the poses with very negative values (binding affinity) were selected. All the biologically potent molecules were docked into the binding pocket of the corresponding protein (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9 and Figure 10). Docking scores and binding distances were also calculated (Table 2). Two- and three-dimensional binding visualizations are shown in the figures below.
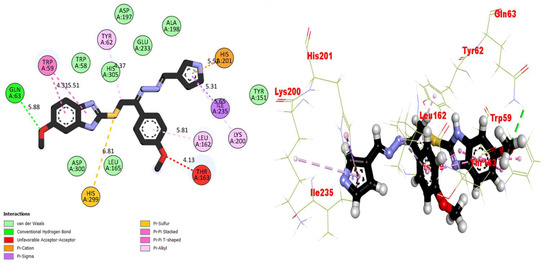
Figure 3.
Representations of the protein–ligand interaction (PLI) profile of compound 2 in the AChE complex.
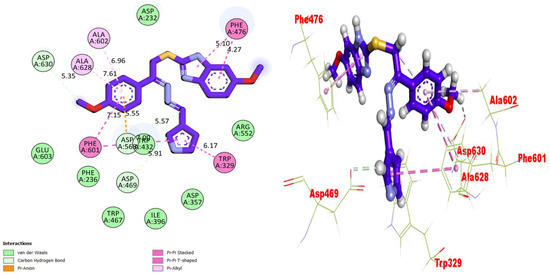
Figure 4.
Representations of the protein–ligand interaction (PLI) profile of compound 2 in the BuChE complex.
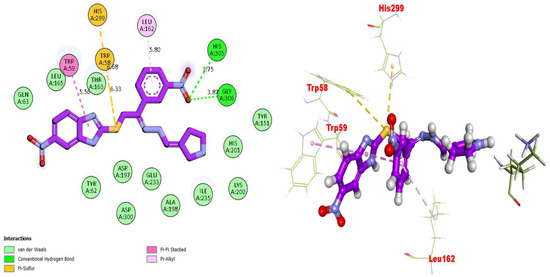
Figure 5.
Representations of the protein–ligand interaction (PLI) profile of compound 10 in the AChE complex.
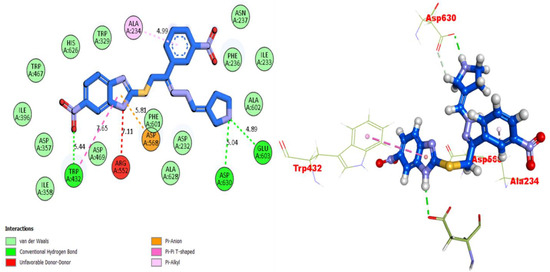
Figure 6.
Representations of the protein–ligand interaction (PLI) profile of compound 10 in the BuChE complex.
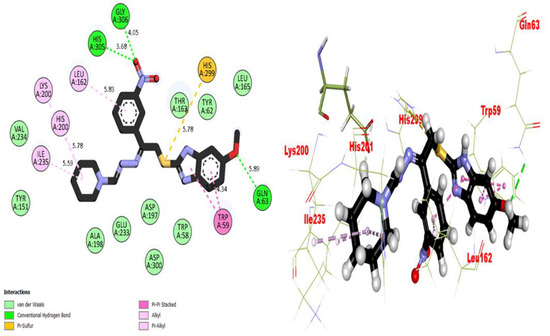
Figure 7.
Representations of the protein–ligand interaction (PLI) profile of compound 4 in the AChE complex.
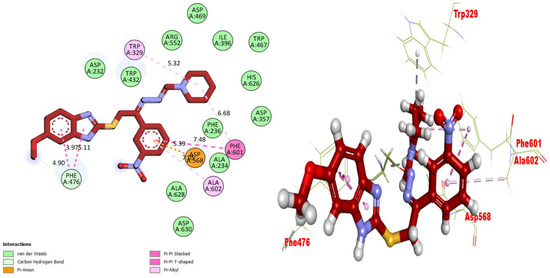
Figure 8.
Representations of the protein–ligand interaction (PLI) profile of compound 4 in the BuChE complex.
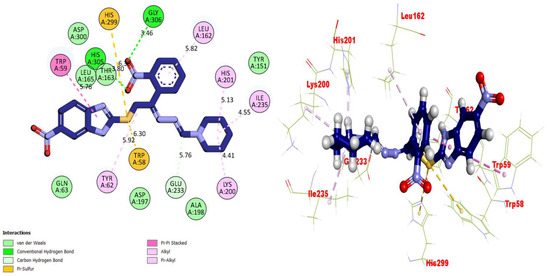
Figure 9.
Representations of the protein–ligand interaction (PLI) profile of compound 13 in the AChE complex.
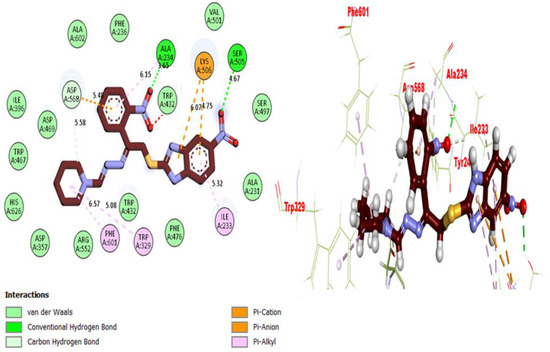
Figure 10.
Representations of the protein–ligand interaction (PLI) profile of compound 13 in the BuChE complex.

Table 2.
The interaction profiles of the subjected analogs (2 and 10) in AChE and BuChE complexes along with docking scores.
2.3.2. Docking Study for Benzimidazole-Based Piperidine Analogs
The selected compounds (bioactive moieties) in the synthesized series were subjected to molecular docking experiments. To draw out the molecules in the protein complexes, various software packages were employed, including the auto dock tool, PyMol and Discovery studio visualizer (DSV). Our research groups have previously worked on a comparable package with other heterocyclic moieties [33,34,35]. This type of study is used to investigate protein–ligand interactions (PLIs) and is one of the simplest ways to detect the fundamental interaction of molecules with a certain amino acid in a protein complex. Ligands were docked in the same amino acid position as the co-crystallized ligand to test the binding interactions of the synthesized moieties. Proteins were retrieved using the code 1ACL for AChE and 1P0P for BuChE from the RCSB protein data bank (PDB) for docking purposes, and both protein and ligand molecules were created in the DSV before being transferred to the auto dock tool, where polar hydrogen and charges were added and configuration files were produced in PDBQT format. After completing these steps, the docking operations were conducted, and all the positions were visualized. After successfully completing the task, nine different poses for each molecule were obtained along with binding affinities (Table 3). Among them, the pose with the most negative value (binding affinity) was chosen for binding visualization. All the potent molecules were docked into the binding pocket of the corresponding protein. Docking scores and binding distances were also calculated. Two- and three-dimensional representations are shown in the figures below.

Table 3.
The interaction profiles of the subjected analogs (4 and 13) in AChE and BuChE complexes along with docking scores.
2.4. ADME Analysis
Absorption, Distribution, Metabolism and Excretion (ADME) analysis was conducted to evaluate the drug likeness properties of the synthesized potent benzimidazole-bearing pyrrole and piperidine hybrid derivatives using an online tool, Swiss ADME (Web online server). Significant outcomes of the ADME analysis further enhanced the molecular docking and biological activity studies. The ADME analysis also showed that marketed drugs violate drug likeness, whereas the potent compounds of the current novel series were reported with violations. These rules include PAINS, log Kp, lead likeness, Brenk alert, Veber, Ghose, Lipinski, Egan, bioavailability score and Muegge violations. The active compounds 2, 4, 10 and 13 of the novel series exhibited excellent inhibitory potency and showed remarkable drug likeness properties, which are illustrated in Figure 11, Figure 12, Figure 13 and Figure 14.
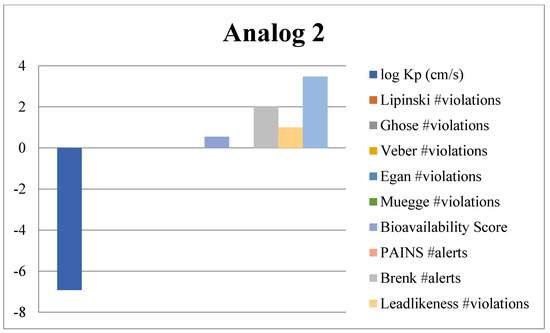
Figure 11.
ADME predictions of analog 2.
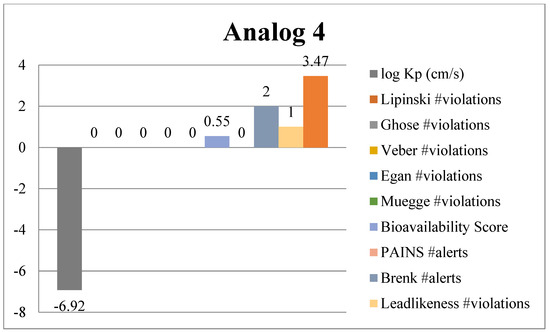
Figure 12.
ADME predictions of analog 4.
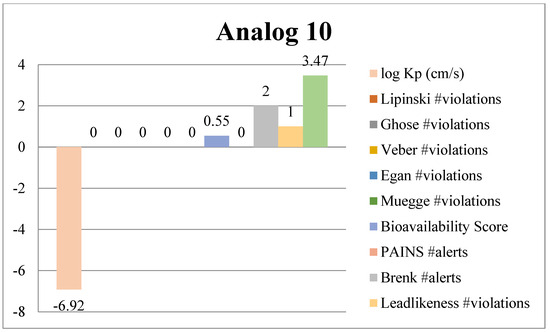
Figure 13.
ADME predictions of analog 10.
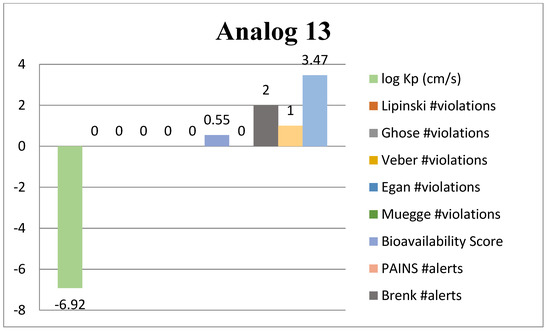
Figure 14.
ADME predictions of analog 13.
3. Material and Methods
3.1. General Information
All the chemicals were purchased from Sigma Aldrich®, St. Louis, MO, USA. For NMR experiments, an Advance Bruker AM-500 MHz instrument was used. To obtain high-resolution electron impact mass spectra (HR-EIMS), a Finnigan MAT-311A (Bremen, Germany) mass spectrometer was used. Thin-layer chromatography (TLC) was performed on pre-coated silica gel aluminum plates (Kieselgel 60, 254, E. Merck, Darmstadt, Germany). To visualize the chromatograms, UV at 254 and 365 nm was used. The solvent that was used for the reaction was of analytical grade (Merck, Germany), while commercial solvents (for washing purposes) were purchased from a local supplier and were distilled before use.
3.2. General Procedure for the Synthesis of Benzimidazole-Based Pyrrole/Piperidine Derivatives
Targeted pyrrole/piperidine derivatives (1–26) based on benzimidazole were afforded via several steps. 2-Marcaptobenzimidazole (I, 1 equivalent) was treated with variously substituted phenacyl bromides (1 equivalent) in ethanol (10 mL) and triethylamine (catalyst), and the resulting reaction mixture was then refluxed for 4 h to offer S-substituted benzimidazole (II, 1 equivalent), which was then further redissolved in hydrazine hydrate (5 mL) solution while being agitated in acetic acid (10 mL). The solvent was evaporated after 3 h of refluxing, and the resultant solid residue (III) underwent two distinct reactions. Substrate (III, 1 equivalent) was first placed in ethanol (10 mL) and triethylamine (catalyst), and then 1H-pyrrole-3-carbaldehyde (1 equivalent) was added. For seven hours, the reaction mixture was refluxed and stirred to form the desired pyrrole derivatives (1–13) based on benzimidazole. Additionally, for the benzimidazole-based piperidine derivatives (14–26), the intermediate (III, 1 equivalent) was again reacted with piperidine-1-carbaldehyde (1 equivalent) in ethanol (10 mL) along with catalytic amounts of triethylamine.
3.3. Spectral Analysis (1–26)
3.3.1. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(p-tolyl)ethyl)thio)-5-methoxy-1H-benzo[d]imidazole (1)
Yield: 62%, 1H-NMR (600 MHz, DMSO-d6): δ 7.71 (d, J = 7.8 Hz, 1H, Ar-H), 7.51 (m, J = 7.6, 1.7 Hz 1H, NH), 7.49 (m, J = 7.6 Hz, J = 1.3 Hz, 1H, Ar-H), 7.30 (d, J = 7.9 Hz, 2Hs, Ar-H), 6.99 (d, J = 7.4 Hz, 1H, Ar-H), 6.8 (m, J = 7.8 Hz, J = 3.1, 2Hs, NH), 6.1 (t, J = 7.3 Hz, 1H, Ar-H), 5.1 (s, 2H, NH), 3.79 (s, 3H), 3.20 (s, 2H), 2.30 (s, 3H). 13C-NMR (150 MHz, DMSO-d6): 167.9, 155.7, 145.7, 139.2, 138.9, 133.0, 130.4, 129.4, 128.4, 128.4, 127, 127, 122.9, 120.4, 119.8, 116.2, 115, 108.2, 55.8, 55.8, 37.7, 37.7, 21.17, 21.17. HREI-MS: m/z calcd for C22H21N5OS [M]+ 403.1698, found 403.1707.
3.3.2. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(4-methoxyphenyl)ethyl)thio)-5-methoxy-1H-benzo[d]imidazole (2)
Yield: 60%, 1H-NMR (600 MHz, DMSO-d6): δ 7.9 (d, J = 7.4 Hz, 2Hs, Ar-H), 7.52 (s, 1H, Ar-H), 7.49 (dd, J = 7.8, 1.8 Hz, 1H, Ar-H), 7.14 (s, 1H, Ar-H), 7.08 (d, J = 7.3 Hz, 1H, Ar-H), 6.91 (d = 7.2 Hz, 1H, Ar-H), 5.9 (d, J = 7.3 Hz, 1H, Ar-H), 5.1 (s, 2H, NH), 3.70 (s, 3H), 3.16 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.7, 156.4, 149.3, 147.4, 140.4, 139.5, 131.3, 131.3, 129.2, 129.2, 127.3, 127.3, 122.3, 120.4, 119.4, 116.2, 111.2, 108.1, 100.4, 55.2, 37.3, 21.5. HREI-MS: m/z calcd for C22H21N5OS [M]+ 419.1698, found 419.1707.
3.3.3. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-([1,1′-biphenyl]-4-yl)ethyl)thio)-5-methoxy-1H-benzo[d]imidazole (3)
Yield: 64%, 1H-NMR (600 MHz, DMSO-d6): δ 12.11 (s, 1H, NH), 8.78 (d, J = 7.4 Hz, 2H, Ar-H), 8.05 (d, J = 7.4 Hz, 2H, Ar-H), 7.93 (d, J = 7.5 Hz, 2H, Ar-H), 7.87 (d, J = 7.3 Hz, 2H, Ar-H), 7.72 (s, 1H), 7.49 (d, J = 7.6 Hz, 1H, Ar-H), 7.40 (m, J = 7.6, 1.3 Hz, 1H, Ar-H), 7.19 (s, 1H, Ar-H), 7.08 (d, J = 7.4 Hz, 1H, Ar-H), 7.03 (m, J = 7.4, 1.3 Hz, 2H, Ar-H), 6.92 (d, J = 7.4 Hz), 6.84 (s, 2H, NH), 4.80 (s, 3H), 3.78 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.5, 156.5, 149.5, 147.2, 143.2, 140.7, 139.8, 132.7, 131.5, 129.5, 129.4, 129.4, 129.4, 128.4, 128.4, 127.8, 127.8, 127.5, 122.5, 120.6, 119.6, 116.6, 111.6, 108.6, 100.6, 55.5, 37.4; HREI-MS: m/z calcd for C27H23N5OS [M]+ 465.1698, found 467.1707.
3.3.4. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(2-methoxyphenyl)ethyl)thio)-5-methoxy-1H-benzo[d]imidazole (4)
Yield: 61%, 1H-NMR (600 MHz, DMSO-d6): δ 7.61 (m, J = 7.6, 1.3 Hz, 1H, Ar-H), 7.49 (s, 1H, Ar-H), 7.46 (d, J = 7.4, 1H, Ar-H), 7.42 (m, J = 7.8, 1.4 Hz, 1H, Ar-H), 7.29 (m, J = 7.4, 1.3 Hz, 1H, Ar-H), 7.16 (s, 1H, Ar-H), 7.01 (m, J = 7.8, 1.7 Hz, 1H, Ar-H), 6.9 (d, J = 7.4, 1.3, 2H, Ar-H), 6.0 (d, J = 7.4, 1H, Ar-H), 5.0 (s, 2H, NH), 3.90 (s, 3H), 3.12 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 147.2, 122.7, 119.3, 139.3, 131.3, 120.5, 108.4, 156.4, 160.4, 100.4, 116.4, 117.3, 111.4, 111.4, 131.6, 132.1, 121.1, 38.1, 55.9, 55.9, 149.3, 164.0 HREI-MS: m/z calcd for C22H21N5OS [M]+ 419.1698, found 419.1707.
3.3.5. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-([1,1′-biphenyl]-4-yl)ethyl)thio)-1H-benzo[d]imidazole (5)
Yield: 65%, 1H-NMR (600 MHz, DMSO-d6): δ 7.90 (d, J = 7.4, 2H, Ar-H), 7.88 (d, J = 7.6, 2H, Ar-H), 7.61 (m, J = (7.7, 1.3 Hz, 2H, Ar-H), 7.5 (m, J = 7.6, 1.3 Hz, 2H, Ar-H), 7.49 (m, J = 7.5, 1.3, 2H, Ar-H), 7.43 (m, J = 7.5, 1.7 Hz, 2H, Ar-H), 7.24 (m, J = 7.5, 1.3 Hz, 2H, Ar-H), 6.8 (m, 2H, Ar-H), 6.2 (d, J = 7.3 Hz, 1H, ArH), 5.2 (s, 2H, NH), 3.14 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 147.2, 143.2, 140.7, 138.8, 138.8, 132.8, 129.8, 128.2, 128.2, 127.7, 127.7, 123.8, 123.8, 122.5, 120.5, 119.7, 115.6, 115.6, 108.4. HREI-MS: m/z calcd for C26H21N5OS [M]+ 45,355.198, found 455.5507.
3.3.6. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(3-nitrophenyl)ethyl)thio)-1H-benzo[d]imidazole (6)
Yield: 69%, 1H-NMR (600 MHz, DMSO-d6): δ 8.54 (m, J = 7.8, 1.4 Hz, 1H, Ar-H), 8.36, (m, J = 7.8, 1.3 Hz, 1H, Ar-H), 8.16 (m, J = 7.8, 1.2 Hz, 1H, Ar-H, 7.80 (m, J = 7.4, 1.2 Hz, 1H, Ar-H), 7.61 (m, J = 7.4, 1.2 Hz, 2H, Ar-H), 7.51 (s, 1H), 7.23 (m, J = 7.1, 1.7 Hz, 2H, Ar-H), 6.8 (d, J = 7.3 Hz, 1H, Ar-H), 6.7 (t, J = 7.3 Hz, 1H, Ar-H), 5.9 (d, J = 7.5 Hz, 1H, Ar-H), 3.14 (s, 1H). 13C-NMR (150 MHz, DMSO-d6): 168.0, 156.3, 148.0, 139.0, 138.7, 134.8, 134.6, 132.9, 132.9, 129.6, 124.4, 123.7, 122.2, 120.8, 119.7, 115.6, 115.6, 108.9, 39.1. HREI-MS: m/z calcd for C20H16N6O2S [M]+ 404.198, found 405.5507.
3.3.7. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(p-tolyl)ethyl)thio)-1H-benzo[d]imidazole (7)
Yield: 72%, 1H-NMR (600 MHz, DMSO-d6): δ 7.89 (d, J = 7.8 Hz, 2H, Ar-H), 7.86 (d, J = 7.9 Hz, 2H, Ar-H), 7.60 (d, J = 7.7 Hz, 2H, Ar-H), 7.56 (m, J = 7.4 Hz, 1.9 Hz, 2H, Ar-H), 7.54 (m, J = 7.8 Hz, 1.9 Hz, 2H, Ar-H), 7.52 (s, 1H, Ar-H), 7.44 (m, J = 7.6 Hz, 1.8 Hz, 1H, Ar-H), 7.24 (d, J = 7.9 Hz, 2H, Ar-H). 13C-NMR (150 MHz, DMSO-d6): 164.7, 149.5, 147.8, 143.8, 140.9, 138.7, 138.7, 132.7, 129.8, 129.8, 129.3, 129.3, 128.1, 128.1, 127.8, 127.8, 127.7, 123.1, 123.1, 122.9, 120.7, 119.7, 115.2, 115.2, 108.2 HREI-MS: m/z calcd for C21H19N S [M]+ 405.198, found 405.5507.
3.3.8. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(2-methoxyphenyl)ethyl)thio)-1H-benzo[d]imidazole (8)
Yield: 70%, 1H-NMR (600 MHz, DMSO-d6): δ 7.60 (m, J = 7.7, 1.5 Hz, 2H, Ar-H), 7.57 (m, J = 7.6, 1.3 Hz, 1H, Ar-H), 7.52 (s, 1H, Ar-H), 7.43 (m, J = 7.4, 1.3 Hz, 1H, Ar-H), 7.26 (m, J = 7.6, 1.7 Hz, 2H, Ar-H), 6.99 (m, J = 7.6, 1.9 Hz, 1H, Ar-H), 6.7 (m, J = 7.8, 1.6 Hz, 1H, Ar-H), 6.2 (d, J = 7.6 Hz, 1H, Ar-H), 5.0 (s, 2H, NH), 3.86 (s, 2H), 3.20 (s, 3H). 13C-NMR (150 MHz, DMSO-d6): 164.5, 160.8, 149.9, 147.2, 138.7, 138.7, 132.1, 131.8, 123.7, 123.7, 122.7, 121.8, 120.7, 119.6, 117.5, 115.2, 115.6, 111.7, 108.6. HREI-MS: m/z calcd for C21H19N S [M]+ 389.138, found 389.1507.
3.3.9. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(4-methoxyphenyl)ethyl)thio)-1H-benzo[d]imidazole (9)
Yield: 62%, 1H-NMR (600 MHz, DMSO-d6): δ 7.89 (d, J = 7.8 Hz, 2H, Ar-H), 7.61 (m, J = 7.6, 1.5 Hz, 2H, Ar-H), 7.23 (m, J = 7.9, 1.9 Hz, 2H, Ar-H), 7.10 (d, J = 7.5 Hz, 1H, Ar-H), 6.8 (m, J = 7.6, 2.1 Hz, 2H, Ar-H), 6.0 (d, J = 7.6 Hz, 1H, Ar-H), 5.0 (s, 2H, NH, Ar-H), 3.90 (s, 3H), 3.20 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.8, 162.7, 149.5, 147.7, 138.8, 138.8, 128.8, 128.8, 126.2, 123.1, 123.1, 122.6, 120.5, 119.7, 115.4, 115.4, 114.6, 114.6, 108.4, 55.6, 37.8 HREI-MS: m/z calcd for C20H15N7O4 S [M]+ 449.038, found 449.94.
3.3.10. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(3-nitrophenyl)ethyl)thio)-5-nitro-1H-benzo[d]imidazole (10)
Yield: 68%, 1H-NMR (600 MHz, DMSO-d6): δ 8.54 (s, 1H, Ar-H) 8.40 (s, 1H, Ar-H), 8.36 (d, J = 7.5, 2.8 Hz, 1H, Ar-H), 8.17 (m, J = 7.9, 3.1 Hz, NH), 8.12 (m, J = 7.3, 1.8 Hz, 1H, Ar-H), 7.80 (t, J = 7.5 Hz, 1H, Ar-H), 7.66 (d, J = 7.9 Hz, 1H, NH), 7.55 (s, 1H, Ar-H), 6.7 (m, J = 7.5, 2.1 Hz, 2H, Ar-H), 6.1 (d, J = 7.6, 1H, Ar-H), 5.1 (s, 2H, NH, Ar-H), 3.18 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 171.7, 164.5, 149.5, 147.4, 145.4, 142.5, 137.3, 132.2, 132.2, 131.2, 131.2, 126.1, 125.3, 122.8, 120.3, 119.7, 118.9, 116.2, 112.8, 109.2, 45.7. HREI-MS: m/z calcd for C20H15N7O4 S [M]+ 449.038, found 449.94.
3.3.11. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-([1,1′-biphenyl]-4-yl)ethyl)thio)-5-nitro-1H-benzo[d]imidazole (11)
Yield: 67%, 1H-NMR (600 MHz, DMSO-d6): δ 8.40 (s, 1H, Ar-H), 8.1 (d, J = 7.6 Hz, 1H, Ar-H), 7.88 (d, J = 7.6 Hz, 2H, Ar-H), 7.86 (d, J = 7.8 Hz, 2H, Ar-H), 7.70 (d, J = 7.6 Hz, 1H, Ar-H), 7.54 (m, J = 7.5, 2.8 Hz, 2H, Ar-H), 7.53 (m, J = 7.7, 1.8 Hz, 2H, Ar-H), 7.54 (s, 1H, Ar-H), 7.41 (m, 7.8, 1.9 Hz, 2H, Ar-H), 6.5 (m, J = 7.4, 1.8 Hz 2H, Ar-H), 6.2 (d, J = 7.5 Hz, 1H, Ar-H), 5.1 (s, 2H, NH Ar-H), 3.18 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.5, 149.4, 147.7, 145.6, 144.6, 143.4, 140.5, 139.7, 132.8, 129.8, 129.7, 129.8, 129.7, 128.8, 128.6, 127.7, 127.7, 127.7, 122.9, 120.7, 119.7, 118.7, 116.7, 112.6, 108.7, 37.5. HREI-MS: m/z calcd for C26H20N6O2 S [M]+ 480.138, found 480.59.
3.3.12. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(p-tolyl)ethyl)thio)-5-methoxy-1H-benzo[d]imidazole (12)
Yield: 65%, 1H-NMR (600 MHz, DMSO-d6): δ 8.38 (s, 1H, Ar-H), 8.12 (d, J = 7.5 Hz, 1H, Ar-H), 7.93 (d, J = 7.4 Hz, 2H, Ar-H), 7.68 (d, 7.3 Hz, 1H, Ar-H), 7.53 (s, 1H, Ar-H), 7.11 (d, J = 7.5 Hz, 1H, Ar-H), 6.7 (m, J = 7.6, 1.8 Hz, 2H, Ar-H), 5.9 (d, J = 7.5 Hz, 1H, Ar-H), 5.1 (s, 2H, NH, Ar-H), 3.84 (s, 3H), 3.18 (s, 2H). 13C-NMR (150 MHz, DMSO-d6): 171.7, 162.9, 149.4, 147.1, 145.0, 142.5, 137.3, 135.3, 132.2, 126.3, 122.9, 120.4, 118.9, 118.6, 116.1, 114.4, 114.4, 112.9, 109.2, 55.8, 45.6. HREI-MS: m/z calcd for C21H18N6O34S [M]+ 434.1, found 434.4.
3.3.13. 2-(((E)-2-(((E)-(1H-pyrrol-3-yl)methylene)hydrazono)-2-(p-tolyl)ethyl)thio)-5-nitro-1H-benzo[d]imidazole (13)
Yield: 70%, 1H-NMR (600 MHz, DMSO-d6): δ 8.40 (s, 1H, Ar-H), 8.13 (d, J = 7.4 Hz, 1H, Ar-H), 7.74 (d, J = 7.5 Hz, 2H, Ar-H), 7.52 (s, 1H, Ar-H), 7.30 (d, J = 7.6 Hz, 1H, Ar-H), 6.5 (m, 2H, Ar-H), 5.9 (d, J = 7.5 Hz, 1H, Ar-H), 5.1 (s, 2H, NH, Ar-H), 3.18 (s, 2H), 2.84 (s, 3H). 13C-NMR (150 MHz, DMSO-d6): 147.8, 122.8, 119.7, 164.8, 149.7, 145.5, 144.8, 140.8, 139.7, 131.7, 129.6, 129.6, 127.1, 127.1, 120.1, 118.7, 116.2, 112.2, 108.2, 37.6, 21.5. HREI-MS: m/z calcd for C21H18N6O2S [M]+ 418.1, found 418.4.
3.3.14. 2-(((E)-2-([1,1′-biphenyl]-4-yl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl)thio)-5-methoxy-1H-benzo[d]imidazole (14)
Yield: 63%, 1H NMR (600 MHz, DMSO-d6):δ 7.59 (m, 1H, Ar-H), 7.52 (s, 1H, Ar-H), 7.47 (d, J = 7.6 Hz, 1H, Ar-H), 7.42 (m, J = 7.7, 1.9 Hz 1H, Ar-H), 7.30 (m, J = 7.4, 1.7 Hz, 1H, Ar-H), 7.17 (d, J = 7.4 Hz 1H, Ar-H), 7.0 (d, J = 7.6 Hz, 1H, Ar-H), 7.4 (m, J = 7.6, 1.9 Hz, 1H, Ar-H), 5.1 (s, 1H, NH, Ar-H), 3.86 (s, 1H), 3.52 (dd, J = 7.6, 1.9 Hz, 3H), 1.60 (dd, 7.8, 1.7 Hz 1H), 1.56 (dd, 7.8, 1.8 Hz 2H). 13C-NMR (150 MHz, DMSO-d6): 164.5, 156.2, 155.1, 147.4, 143.7, 140.8, 139.8, 132.8, 131.3, 129.7, 129.8, 129.6, 129.7, 128.6, 128.6, 127.6, 127.6, 127.6, 116.3, 111.7, 100.9, 48.8, 48.6, 25.6, 25.6, 24.4, 37.6, 55.7. HREI-MS: m/z calcd for C28H29N5OS [M]+ 483.2, found 483.4.
3.3.15. 5-methoxy-2-(((E)-2-(2-methoxyphenyl)-2-(((E)-piperidin-1-ylmethylene)hydrazono) ethyl)thio)-1H-benzo[d]imidazole (15)
Yield: 66%, 1H-NMR (600 MHz, DMSO-d6): δ 7.60 (m, J = 7.6, 1.9 Hz, 1H, Ar-H), 7.51 (s, 1H, Ar-H), 7.45 (d, J = 7.6 Hz, 1H, Ar-H), 7.43 (m, J = 7.4, 1.6 Hz, 1H, Ar-H), 7.30 (m, J = 7.7, 1.9 Hz, 1H, Ar-H), 7.17 (d, J = 7.4 Hz, 1H, Ar-H), 7.0 (d, 7.6 Hz, 1H, Ar-H), 7.4 (m, J = 7.8, 1.9 Hz, 1H, Ar-H), 5.1 (s, 1H, NH, Ar-H), 3.86 (s, 1H), 3.51 (m, 3H), 1.60 (m, 1H), 1.56 (m, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.7, 160.8, 156.4, 155.6, 147.5, 139. 1, 132.8, 131.7, 131.8, 121.6, 117.4, 116.7, 111.8, 111.9, 100.7, 55.7, 55.7, 48.8, 48.8, 38.7, 25.7, 24.6, 25.8; HREI-MS: m/z calcd for C23H27N5OS [M]+ 437.2, found 437.4.
3.3.16. 5-methoxy-2-(((E)-2-(4-methoxyphenyl)-2-(((E)-piperidin-1ylmethylene) hydrazono)ethyl)thio)-1H-benzo[d]imidazole (16)
Yield: 68%, 1H-NMR (600 MHz, DMSO-d6): δ 7.60 (m, 1H, Ar-H), 7.5 1 (s, 1H, Ar-H), 7.45 (d, J = 7.6 Hz, 1H, Ar-H), 7.43 (m, J = 7.8, 1.7 Hz 1H, Ar-H), 7.30 (m J = 7.7, 1.8 Hz, 1H, Ar-H), 7.17 (d, J = 7.4 Hz 1H, Ar-H), 7.0 (d, J = 7.6 Hz, 1.4 Hz, 1H, Ar-H), 7.4 (m, J = 7.8, 1.9 Hz, 1H, Ar-H), 5.1 (s, 1H, NH, Ar-H), 3.86 (s, 3H), 3.51 (dd, J = 7.8, 2.9 Hz, 1H), 1.60 (dd, J = 7.7, 1.9 Hz, 1H), 1.56 (m J = 7.6, 1.8 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.5, 162.8, 156.2, 155.1, 147.2, 139.8, 131.3, 128.8, 128.8, 126.4, 116.5, 114.6, 114.6, 111.7, 100.6, 55.6, 55.6, 48.8, 48.9, 37.7, 25.7, 24.2, 25.7. HREI-MS: m/z calcd for C23H27N5OS [M]+ 437.2, found 437.4.
3.3.17. 5-methoxy-2-(((E)-2-(3-nitrophenyl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl) thio)-1H-benzo[d]imidazole (17)
Yield: 60%, 1H NMR (600 MHz, DMSO-d6):δ 7.93 (d, J = 7.6 Hz Ar-H), 7.51 (s, 1H, Ar-H), 7.46 (d, J = 7.6 Hz, 1H, Ar-H), 7.17 (d, J = 7.4 Hz 1H, Ar-H), 7.08 (d, J = 7.5 Hz, 1H, Ar-H), 7.0 (d, J = 7.6 Hz, 1H, Ar-H), 5.1 (s, 1H, NH, Ar-H), 3.86 (s, 3H), 3.51 (m, J = 7.8, 1.9 Hz, 1H, Ar-H), 3.16 (s, 2H), 1.61 (m, J = 7.9, 1.7 Hz, 1H, Ar-H), 1.56 (m, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.8, 156.5, 155.6, 147.8, 139.7, 134.8, 134.8, 131.6, 131.4, 126.6, 125.7, 116.0, 111.7, 100.6, 55.7, 48.8, 48.8, 37.8, 25.6, 25.6, 24.3. HREI-MS: m/z calcd for C22H24N6O3S [M]+ 452.2, found 452.4.
3.3.18. 5-methoxy-2-(((E)-2-(((E)-piperidin-1-ylmethylene)hydrazono)-2-(p-tolyl)ethyl)thio)-1H-benzo[d]imidazole (18)
Yield: 63%, 1H-NMR (600 MHz, DMSO-d6): δ 8.54 (m, 1H, Ar-H), 8.34 (m, 1H, Ar-H), 7.80 (t, 1H, Ar-H), 7.50 (s, 1H, Ar-H), 7.46 (d, J = 7.5 Hz, 1H, Ar-H), 7.0 (d, J = 7.5 Hz 1H, Ar-H), 5.2 (s, 1H, NH, Ar-H), 3.53 (m J = 7.8, 1.9 Hz, 1H, Ar-H), 3.16 (s, 2Hs), 1.61 (m, J = 7.7, 1.6 Hz, 1H 1H), 1.56 (m J = 7.7, 1.9 Hz, 1H, Ar-H). 13C-NMR (150 MHz, DMSO-d6): 164.5, 156.3, 155.4, 147.6, 140.6, 139.7, 131.5, 131.8, 129.7, 129.7, 127.8, 127.8, 116.6, 111.7, 100.7, 55.7, 48.8, 48.0, 37.8, 25.9, 25.9, 24.6, 21.4. HREI-MS: m/z calcd for C23H27N5OS [M]+ 421.2, found 421.4
3.3.19. 2-(((E)-2-(((E)-piperidin-1-ylmethylene)hydrazono)-2-(p-tolyl)ethyl)thio)-1H-benzo[d]imidazole (19)
Yield: 66%, 1H-NMR (600 MHz, DMSO-d6): δ 7.74 (d, J = 7.5 Hz, Ar-H), 7.51 (d, J = 7.5, 1H), 7.36 (d, J = 7.7 Hz, 1H, Ar-H), 7.16 (d, J = 7.4 Hz, 1H, Ar-H), 7.02 (d, J = 7.5 Hz, 1H, Ar-H), 5.1 (s, 1H, NH, Ar-H), 3.86 (s, 3H), 3.51 (m, J = 7.6, 1.7 Hz, 1H, 2H), 3.16 (s, 2H), 1.60 (m, J = 7.6, 1.3 Hz, 1H), 1.54 (m, J = 7.2, 1.6 Hz, 1H, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.0, 155.0, 147.1, 140.7, 139.9, 138.9, 138.9, 131.2, 131.0, 129.1, 129.1, 127.0, 127.0, 123.0, 123.0, 116.2, 115.5, 48.7, 48.7, 37.7, 25.7, 25.7, 24.2, 21.3.HREI-MS: m/z calcd for C22H25N5S [M]+ 391.2, found 391.4
3.3.20. 2-(((E)-2-(3-nitrophenyl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl)thio)-1H-benzo[d]imidazole (20)
Yield: 65%, 1H-NMR (600 MHz, DMSO-d6): δ 8.54 (d, J = 7.5 Hz, 1H, Ar-H), 8.34 (m, 1H, Ar-H), 8.17 (m, 1H, Ar-H), 7.80 (m, 2H, Ar-H), 7.61 (d, J = 7.5 Hz, 1H, Ar-H), 7.28 (m, J = 7.2, 1.6 Hz 1H, Ar-H), 5.1 (s, 1H, NH Ar-H), 3.51 (m, J = 7.2, 1.9 Hz, 2H), 3.15 (s, 2H), 1.60 (m, J = 7.2, 1.3 Hz, 1H), 1.56 (m, J = 7.3, 1.3 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.8, 155.0, 147.1, 140.7, 138.9, 138.9, 138.9, 134.3, 134.9, 131.2, 131.0, 126.1, 125.1, 123.0, 123.0, 116.2, 115.5, 48.7, 48.7, 37.7, 25.7, 25.7, 24.2, 21.3. HREI-MS: m/z calcd for C21H22N6O2S [M]+ 422.2, found 422.4.
3.3.21. 2-(((E)-2-(2-methoxyphenyl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl)thio)-1H-benzo[d]imidazole (21)
Yield: 62%, 1H-NMR (600 MHz, DMSO-d6): δ 7.60 (m, 2H, Ar-H), 7.57 (m, J = 7.5, 1.6 Hz, 1H), 7.46 (m, J = 7.5, 1.3 Hz, 1H, Ar-H), 7.29 (m, J = 7.5 Hz, 1.7 Hz, 1H, Ar-H), 7.24 (m, J = 7.2 Hz, 1.7 Hz, 2H, Ar-H), 7.0 (d, J = 7.5 Hz, 1H, Ar-H), 5.1 (s, 1H, NH), 3.86 (s, 3H), 3.51 (m J = 7.2, 1.6 Hz, 2H), 3.16 (s, 2H), 1.60 (m, J = 7.2, 1.6 Hz, 1H), 1.54 (m, J = 7.2, 1.6 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.3, 160.3, 155.3, 147.3, 140.7, 138.8, 138.7, 138.7, 132.1, 131.1, 131.1, 123.2, 123.2, 123.2, 121.2, 117.3, 116.3, 115.3, 111.4, 55.4, 48.4, 48.4, 37.4, 25.4, 25.6, 24.1, HREI-MS: m/z calcd for C22H25N5OS [M]+ 407.2, found 407.18.
3.3.22. 2-(((E)-2-(4-methoxyphenyl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl)thio)-1H-benzo[d]imidazole (22)
Yield: 61%, 1H-NMR (600 MHz, DMSO-d6): δ 7.94 (d, J = 7.5 Hz, 1H, Ar-H), 7.60 (m, 2H, Ar-H), 7.7 (m, J = 7.3 Hz, 1.2 Hz, 1H, Ar-H), 7.10 (d, J = 7.5 Hz, 2H, Ar-H), 5.1 (s, 1H, NH), 3.85 (s, 3Hs), 3.51 (m, J = 7.3 Hz, 1.2 Hz, 2H), 3.15 (s, 2H), 1.60 (m, J = 7.3 Hz, 1.4 Hz, 1H), 1.56 (m, J = 7.9 Hz, 1.2 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.5, 162.8, 155.1, 147.2, 138.4, 128.5, 128.5, 126.4, 123.4, 123.1, 115.3, 115.3, 114.3, 114.3, 55.8, 48.7, 48.7, 37.7, 25.6, 25.6, 24.1. HREI-MS: m/z calcd for C22H25N5OS [M]+ 407.2, found 407.18
3.3.23. 2-(((E)-2-([1,1′-biphenyl]-3-yl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl)thio)-5-nitro-1H-benzo[d]imidazole (23)
Yield: 63%, 1H-NMR (600 MHz, DMSO-d6): δ 8.44 (d, J = 7.5 Hz, 1H, Ar-H), 8.14 (d, J = 7.5, 1H, Ar-H), 8.09 (s, 1H, Ar-H), 7.96 (m, J = 7.4 Hz, 1.8 Hz, 2H, Ar-H), 7.86 (m, J = 7.9 Hz, 1.3 Hz 2H, Ar-H), 7.61 (d, J = 7.5 Hz, 1H, Ar-H), 7.53 (m, J = 7.4 Hz, 1.5 Hz, 2H, Ar-H), 7.5 (m, J = 7.4 Hz, 1.3 Hz, 1H, Ar-H), 7.51 (d, J = 7.5 Hz, 1H), 7.42 (m, J = 7.3 Hz, 1.6 Hz, 1H, Ar-H), 5.1 (s, 1H, NH, Ar-H), 3.51 (m, J = 7.1 Hz, 1.2 Hz, 2H), 3.15 (s, 2H), 1.60 (m, J = 7.4 Hz, 1.3 Hz 1H), 1.56 (m, J = 7.6 Hz, 1.9 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.4, 155.1, 147.4, 145.3, 144.4, 141.8, 140.9, 139.8, 134.8, 130.1, 127.8, 127.8, 127.8, 118.7, 37.8, 25.8, 25.9, 24.8. HREI-MS: m/z calcd for C27H26N6O2S [M]+ 498.2, found 489.68
3.3.24. 2-(((E)-2-(4-methoxyphenyl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl)thio)-5-nitro-1H-benzo[d]imidazole (24)
Yield: 58%, 1H-NMR (600 MHz, DMSO-d6): δ 8.44 (d, J = 7.5 Hz, 1H, Ar-H), 8.10 (d, J = 7.5 Hz, 1H, Ar-H), 7.93 (d, J = 7.5, 1H), 7.67 (d, J = 7.5 Hz, 1H, Ar-H), 7.10 (d, J = 7.5 Hz, 2H, Ar-H), 7.51 (s, 2H, Ar-H), 5.1 (s, 1H, NH, Ar-H), 3.85 (s, 3Hs), 3.51 (m, J = 7.2 Hz, 1.9 Hz, 2H), 3.15 (s, 2Hs), 1.60 (m, J = 7.4 Hz, 1.3 Hz, 1H), 1.56 (m, J = 7.2 Hz, 1.6 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.3, 162.3, 155.1, 147.2, 138.2, 128.7, 128.7, 126.8, 123.8, 123.8, 115.5, 115.5, 114.3, 114.3, 55.9, 48.8, 48.8, 37.6, 25.5, 25.5, 24.6. HREI-MS: m/z calcd for C22H24N6O3S [M]+ 452.2, found 452.68
3.3.25. 5-nitro-2-(((E)-2-(((E)-piperidin-1-ylmethylene)hydrazono)-2-(p-tolyl)ethyl)thio)-1H-benzo[d]imidazole (25)
Yield: 71%, 1H-NMR (600 MHz, DMSO-d6): δ 8.40 (s, 1H, Ar-H), 8.12 (d, J = 7.5, 1H, Ar-H), 7.73 (d, J = 7.5, 2H, Ar-H), 7.51 (s, 2H, Ar-H), 7.69 (d, J = 7.5, 1H, Ar-H), 7.27 (d, J = 7.5 Hz, 2H, Ar-H), 7.10 (d, J = 7.5 Hz, 2H, Ar-H), 5.1 (s, 1H, NH), 3.85 (s, 3H), 3.51 (m, J = 7.4 Hz, 1.3 Hz, 2H), 3.15 (s, 2Hs), 2.38 (s, 3H), 1.60 (m, J = 7.5 Hz, 1.4 Hz, 1H), 1.56 (m, J = 7.3 Hz, 1.4 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6):164.3, 155.3, 147.3, 145.1, 144.1, 140.1, 139.1, 131.1, 129.4, 129.4, 127.4, 127.4, 118.7, 116.2, 112.2, 48.2, 48.2, 37.2, 25.6, 25.6, 21.5, 24.3 HREI-MS: m/z calcd for C22H24N6O2S [M]+ 436.2, found 436.68.
3.3.26. 5-nitro-2-(((E)-2-(2-nitrophenyl)-2-(((E)-piperidin-1-ylmethylene)hydrazono)ethyl)thio)-1H-benzo[d]imidazole (26)
Yield: 69%, 1H-NMR (600 MHz, DMSO-d6): δ 8.41 (s, 1H, Ar-H), 8.11 (d, J = 7.5, 1H, Ar-H), 8.09 (m, 1H, Ar-H), 7.99 (m, 1H, Ar-H), 7.93 (m, 1H, Ar-H), 7.69 (d, J = 7.5, 1H), 7.57 (m, J = 7.5 Hz, 1.6 Hz 1H, Ar-H), 7.51 (s, 2H, Ar-H), 5.1 (s, 1H, NH), 3.85 (s, 3H), 3.51 (m, J = 7.6 Hz, 1.2 Hz, 2H), 3.15 (s, 2H), 2.38 (s, 3H), 1.60 (m, J = 7.5 Hz, 1.4 Hz, 1H), 1.56 (m, J = 7.4 Hz, 1.3 Hz, 2H). 13C-NMR (150 MHz, DMSO-d6): 164.6, 155.0, 147.1, 145.0, 144.3, 141.7, 140.8, 139.8, 134.5, 130.2, 130.0, 129.2, 129.2, 127.9, 127.9, 127.6, 127.1, 126.3, 118.6, 116.0, 112.0, 48.7, 48.7, 37.7, 25.7, 24.2, 25.7. HREI-MS: m/z calcd for C21H21N7O4S [M]+ 436.2, found 467.68.
3.4. Acetylcholinesterase and Butyrylcholinesterase Inhibition Assays
The enzyme inhibition activities for acetylcholinesterase and butyrylcholinesterase were determined using the method of Ellman et al. [36] with slight modifications. The 62.0 mM sodium phosphate buffer (pH 8.0, 880 μL) used contained 0.002 M of DTNB (5,5′-dithiobis-2-nitrobenzoic acid). AChE and BuChE solutions (40 μL) were added with the test compounds (40 μL) and kept at 25 °C for 10–15 min. The acetylthiocholine or butyrylthiocholine (40 μL) were added just before the initiation of the catalytic reaction. The enzymatic hydrolysis of the substrates encompassing the formation of yellow 5-thio-2-nitrobenzoate anion products was monitored at 412 nm (15 min). The inhibition reactions were repeated in triplicate with a Shimadzu spectrophotometer (Kyoto, Japan).
3.5. Assay Protocol for Molecular Docking Study
This assay was carried out according to our previously reported work [37,38,39].
4. Conclusions
In the present study, a series of benzimidazole-based pyrrole and piperidine derivatives (1–26) were synthesized and characterized through 1H NMR, 13C NMR and HRMS (EI) analysis and evaluated for in vitro AChE and BuChE inhibitory potential. The evaluation results for these synthesized derivatives showed that compound 10 revealed good inhibitory potential against AChE and BuChE, with an IC50 even lower than the standard. Moreover, it binds with the active regions of target proteins with good binding affinity. So, based on the in vitro inhibitory and molecular docking results, it can be concluded that compound 10 might be a potential lead candidate for further studies in the development of AChE and BuChE inhibitors.
Author Contributions
Conceptualization, H.U.; methodology, S.T.; software, R.H.; validation, S.K., M.S. and N.I.; formal analysis, M.U.K. and M.K.F.; writing—original draft preparation, H.U. and F.R.; writing—review and editing, W.R., A.H. and M.A.B.; visualization, S.A.A.S.; supervision, F.R.; project administration, F.R.; funding acquisition, M.A.B. All authors have read and agreed to the published version of the manuscript.
Funding
Researchers supporting project number (RSPD2024R740), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We are thankful to the researchers supporting project number (RSPD2024R740), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jann, M.W. Preclinical pharmacology of metrifonate. Pharmacotherapy 1998, 18, 55–67. [Google Scholar] [CrossRef]
- Adams, R.L.; Craig, P.L.; Parsons, O.A. Neuropsychology of dementia. Neurol. Clin. 1986, 4, 387–404. [Google Scholar] [CrossRef]
- Aisen, P.S.; Davis, K.L. The search for disease-modifying treatment for Alzheimer’s disease. Neurology 1997, 48 (Suppl. 6), 35S–41S. [Google Scholar] [CrossRef]
- Bachman, D.L.; Wolf, P.A.; Linn, R.; Knoefel, J.E.; Cobbs, J.; Belanger, A.; D’agostino, R.B.; White, L.R. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology 1992, 42, 115. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D.; Katzman, R.K. Senile dementia of the Alzheimer type. Ann. Neurol. 1983, 14, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Scherr, P.A.; Beckett, L.A.; Albert, M.S.; Pilgrim, D.M.; Chown, M.J.; Funkenstein, H.H.; Evans, D.A. Age-specific incidence of Alzheimer’s disease in a community population. JAMA 1995, 273, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.M.; Kokmen, E.; O’brien, P.C.; Kurland, L.T. The prevalence of dementia is changing over time in Rochester, Minnesota. Neurology 1995, 45, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Kumar, A. Reversible dementias. Med. Clin. North Am. 1993, 77, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S. Treatment of Alzheimer’s disease with cholinesterase inhibitors. Clin. Geriatr. Med. 2001, 17, 337–358. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Nordberg, A.; Arnold, S.E. A preclinical view of cholinesterase inhibitors in neuroprotection: Do they provide more than symptomatic benefits in Alzheimer’s disease? Trends Pharmacol. Sci. 2005, 26, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Sethy, S.; Mandal, S.K.; Ewies, E.F.; Dhiman, N.; Garg, A. Synthesis, Characterization and Biological Evaluation of Benzimidazole and Benzindazole Derivatives as Anti-hypertensive Agents. Egypt J. Chem. 2021, 64, 3659–3664. [Google Scholar] [CrossRef]
- Wu, Z.; Xia, M.B.; Bertsetseg, D.; Wang, Y.H.; Bao, X.L.; Zhu, W.B.; Chen, P.R.; Tang, H.S.; Yan, Y.J.; Chen, Z.L. Design, synthesis and biological evaluation of novel fluoro-substituted benzimidazole derivatives with anti-hypertension activities. Bioorg. Chem. 2020, 101, 104042. [Google Scholar] [CrossRef]
- Hussain, R.; Rahim, F.; Ullah, H.; Khan, S.; Sarfraz, M.; Iqbal, R.; Suleman, F.; Al-Sadoon, M.K. Design, Synthesis, In Vitro Biological Evaluation and In Silico Molecular Docking Study of Benzimidazole-Based Oxazole Analogues: A Promising Acetylcholinesterase and Butyrylcholinesterase Inhibitors. Molecules 2023, 28, 7015. [Google Scholar] [CrossRef] [PubMed]
- Marinescu, M. Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review. Antibiotics 2023, 12, 1220. [Google Scholar] [CrossRef]
- Patagar, D.N.; Batakurki, S.R.; Kusanur, R.; Patra, S.M.; Saravanakumar, S.; Ghate, M. Synthesis, antioxidant and anti-diabetic potential of novel benzimidazole substituted coumarin-3-carboxamides. J. Mol. Struct. 2023, 1274, 134589. [Google Scholar] [CrossRef]
- Hayat, S.; Ullah, H.; Rahim, F.; Ullah, I.; Taha, M.; Iqbal, N.; Khan, F.; Khan, M.S.; Shah, S.A.A.; Wadood, A.; et al. Synthesis, biological evaluation and molecular docking study of benzimidazole derivatives as α-glucosidase inhibitors and anti-diabetes candidates. J. Mol. Struct. 2023, 1276, 134774. [Google Scholar] [CrossRef]
- Rashid, M. Design, synthesis and ADMET prediction of bis-benzimidazole as anticancer agent. Bioorg. Chem. 2020, 96, 103576. [Google Scholar] [CrossRef] [PubMed]
- Bansal, Y.; Silakari, O.; Lapinsky, D.J.; Kusayanagi, T.; Tsukuda, S.; Shimura, S.; Manita, D.; Iwakiri, K.; Kamisuki, S.; Takakusagi, Y.; et al. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6199–6236. [Google Scholar] [CrossRef]
- Carvalho, R.C.; Martins, W.A.; Silva, T.P.; Kaiser, C.R.; Bastos, M.M.; Pinheiro, L.C.; Krettli, A.U.; Boechat, N. New pentasubstituted pyrrole hybrid atorvastatin–quinoline derivatives with antiplasmodial activity. Bioorg Med. Chem. Lett. 2016, 26, 1881–1884. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Elbakry, A.M.; Esmaeil, A.H.; Khaleel, S.A. Pharmaceutical and pharmacokinetic evaluation of novel rectal mucoadhesive hydrogels containing tolmetin sodium. J. Pharm. Investig. 2018, 48, 673–683. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Zhou, L.; Yu, S.; Su, Z.; Song, J.; Sun, Q.; Sha, O.; Wang, X.; Jiang, W.; et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13150–13155. [Google Scholar] [CrossRef]
- Vardanyan, R. Classes of Piperidine-Based Drugs. In Piperidine-Based Drug Discovery; Elsevier: Amsterdam, The Netherlands, 2017; pp. 299–332. [Google Scholar]
- Goel, P.; Alam, O.; Naim, M.J.; Nawaz, F.; Iqbal, M.; Alam, M.I. Recent advancement of piperidine moiety in treatment of cancer- A review. Eur. J. Med. Chem. 2018, 157, 480–502. [Google Scholar] [CrossRef]
- Martens, U.M. (Ed.) Small Molecules in Hematology; Springer: Berlin/Heidelberg, Germany, 2018; p. 1. [Google Scholar]
- Milling, R.V.; Grimm, D.; Krüger, M.; Grosse, J.; Kopp, S.; Bauer, J.; Infanger, M.; Wehland, M. Pazopanib, Cabozantinib, and Vandetanib in the Treatment of Progressive Medullary Thyroid Cancer with a Special Focus on the Adverse Effects on Hypertension. Int. J. Mol. Sci. 2018, 19, 3258. [Google Scholar] [CrossRef]
- Coricello, A.; Mesiti, F.; Lupia, A.; Maruca, A.; Alcaro, S. Inside Perspective of the Synthetic and Computational Toolbox of JAK Inhibitors: Recent Updates. Molecules 2020, 25, 3321. [Google Scholar] [CrossRef]
- Shaw, V.; Srivastava, S.; Srivastava, S.K. Repurposing antipsychotics of the diphenylbutylpiperidine class for cancer therapy. Semin. Cancer Biol. 2021, 68, 75–83. [Google Scholar] [CrossRef]
- Ezelarab, H.A.A.; Abbas, S.H.; Hassan, H.A.; Abuo-Rahma, G.E.-D.A. Recent updates of fluoroquinolones as antibacterial agents. Arch. Pharm. 2018, 351, 1800141. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Qin, W.; Tian, S.; Xu, Q.; Wold, E.A.; Zhou, J.; Zhen, X.-C. Small Molecules Selectively Targeting Sigma-1 Receptor for the Treatment of Neurological Diseases. J. Med. Chem. 2020, 63, 15187–15217. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.H. Pharmacological interventions for psychosis in Parkinson’s disease patients. Expert Opin. Pharmacother. 2018, 19, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Kantrowitz, J.T. Targeting Serotonin 5-HT2A Receptors to Better Treat Schizophrenia: Rationale and Current Approaches. CNS Drugs 2020, 34, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Rk, M.; Begum, S.; Begum, A.; Koganti, B. Antioxidant potential of piperidine containing compounds—A short review. Asian J. Pharm. Clin. Res. 2018, 11, 66. [Google Scholar]
- Khan, S.; Ullah, H.; Taha, M.; Rahim, F.; Sarfraz, M.; Iqbal, R.; Iqbal, N.; Hussain, R.; Ali Shah, S.A.; Ayub, K.; et al. Synthesis, DFT Studies, molecular docking and biological activity evaluation of thiazole-sulfonamide derivatives as potent Alzheimer’s inhibitors. Molecules 2023, 28, 559. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, H.; Rahim, F.; Nawaz, M.; Hussain, R.; Rasheed, L. Synthesis, in vitro α-amylase, α-glucosidase activities and molecular docking study of new benzimidazole bearing thiazolidinone derivatives. J. Mol. Struct. 2022, 1269, 133812. [Google Scholar] [CrossRef]
- Khan, S.; Ullah, H.; Hussain, R.; Khan, Y.; Khan, M.U.; Khan, M.; Sattar, A.; Khan, M.S. Synthesis, in vitro bio-evaluation, and molecular docking study of thiosemicarbazone-based isatin/bis-Schiff base hybrid analogues as effective cholinesterase inhibitors. J. Mol. Struct. 2023, 1284, 135351. [Google Scholar] [CrossRef]
- Rahim, F.; Ullah, H.; Taha, M.; Hussain, R.; Sarfraz, M.; Iqbal, R.; Iqbal, N.; Khan, S.; Ali Shah, S.A.; Albalawi, M.A.; et al. Synthesis of new triazole-based thiosemicarbazone derivatives as anti-Alzheimer’s disease candidates: Evidence-based in vitro study. Molecules 2022, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Kshatriya, R.; Kambale, D.; Mali, S.; Jejurkar, V.P.; Lokhande, P.; Chaudhari, H.K.; Saha, S. Brønsted acid catalyzed domino synthesis of functionalized 4H-chromens and their ADMET, molecular docking and antibacterial studies. Chem. Select. 2019, 4, 7943–7948. [Google Scholar] [CrossRef]
- Jejurkar, V.P.; Mali, S.N.; Kshatriya, R.; Chaudhari, H.K.; Saha, S. Synthesis, antimicrobial screening and in silico appraisal of iminocarbazole derivatives. Chem. Select. 2019, 4, 9470–9475. [Google Scholar] [CrossRef]
- Mishra, V.R.; Ghanavatkar, C.W.; Mali, S.N.; Qureshi, S.I.; Chaudhari, H.K.; Sekar, N. Design, synthesis, antimicrobial activity and computational studies of novel azo linked substituted benzimidazole, benzoxazole and benzothiazole derivatives. Comput. Biol. Chem. 2019, 78, 330–337. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).