The Association between Molecular Initiating Events and Drug-Induced Hiccups
Abstract
1. Introduction
2. Results
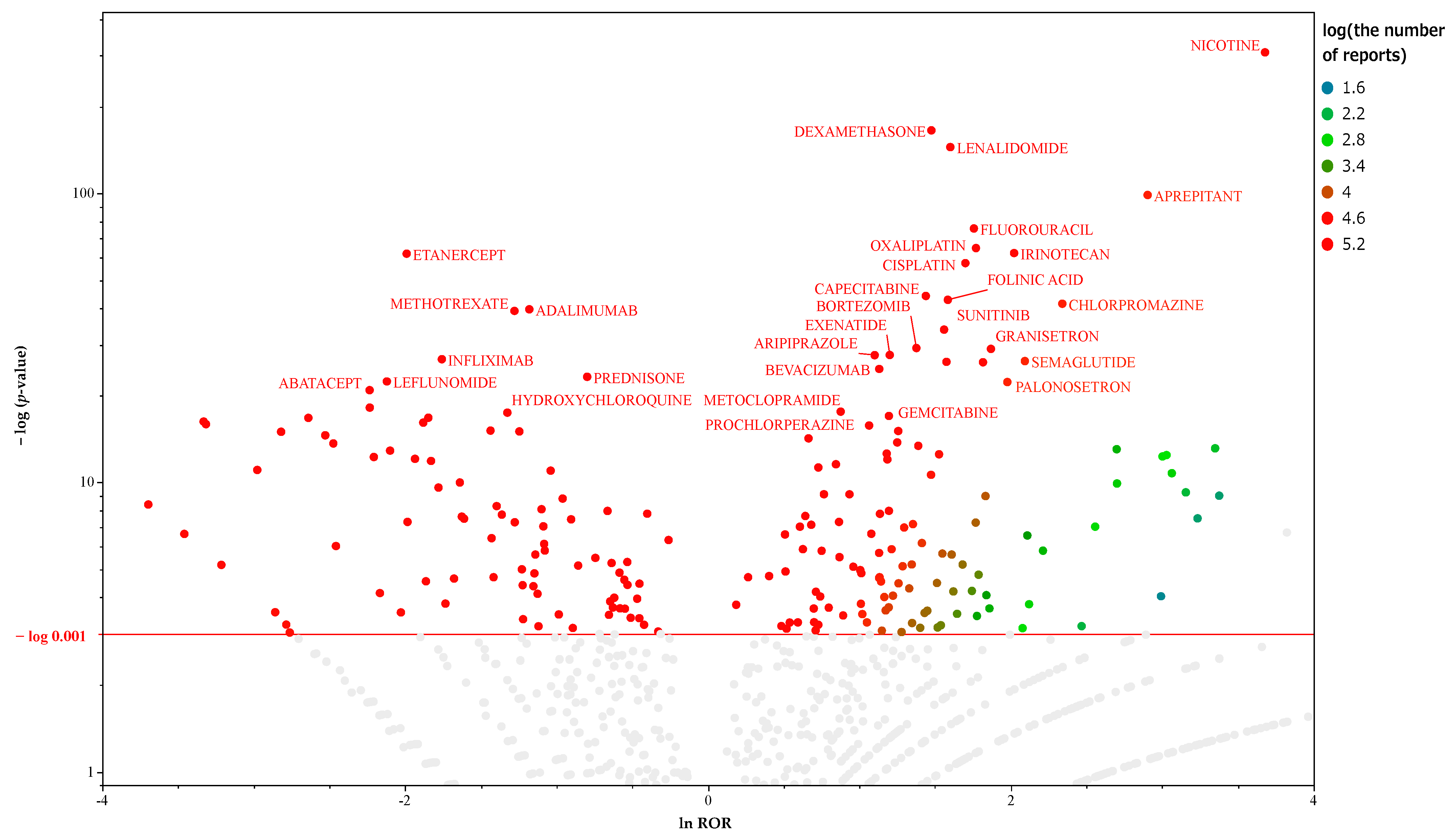
2.1. Prediction of MIEs Associated with Hiccups
2.2. Prediction of MIEs Associated with Hiccups in Male Patients
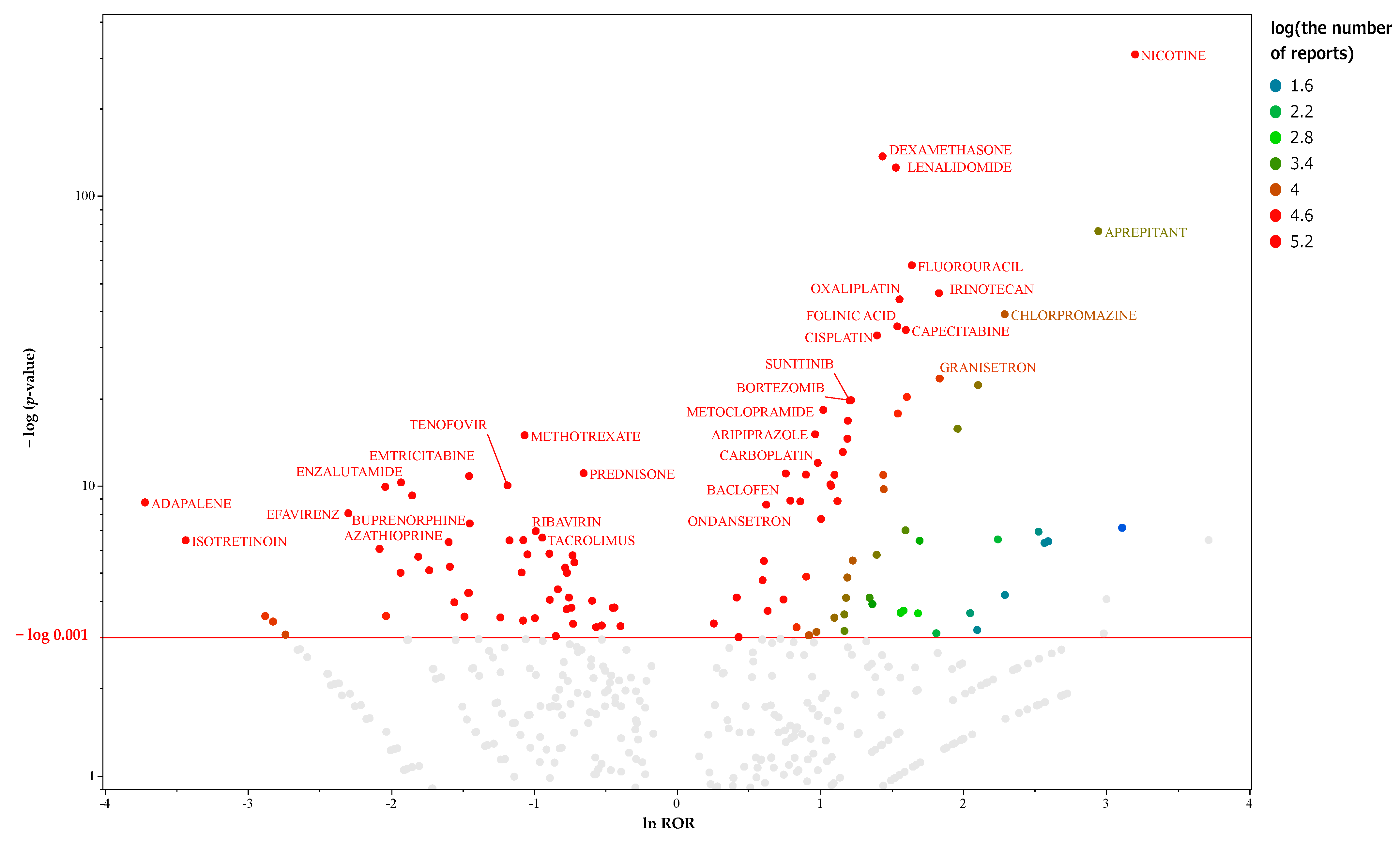
2.3. Prediction of MIEs Associated with Hiccups in Female Patients
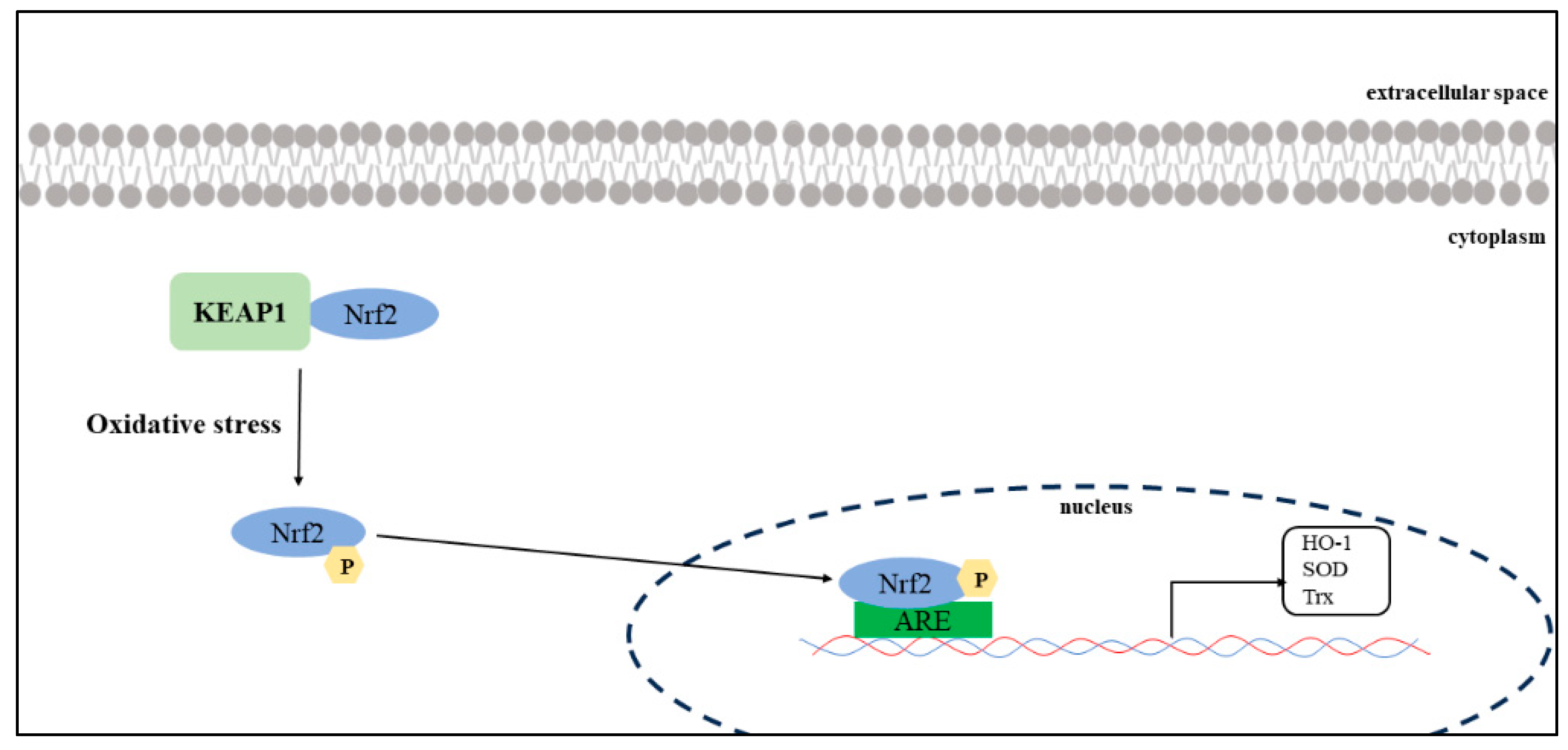
3. Discussion
4. Materials and Methods
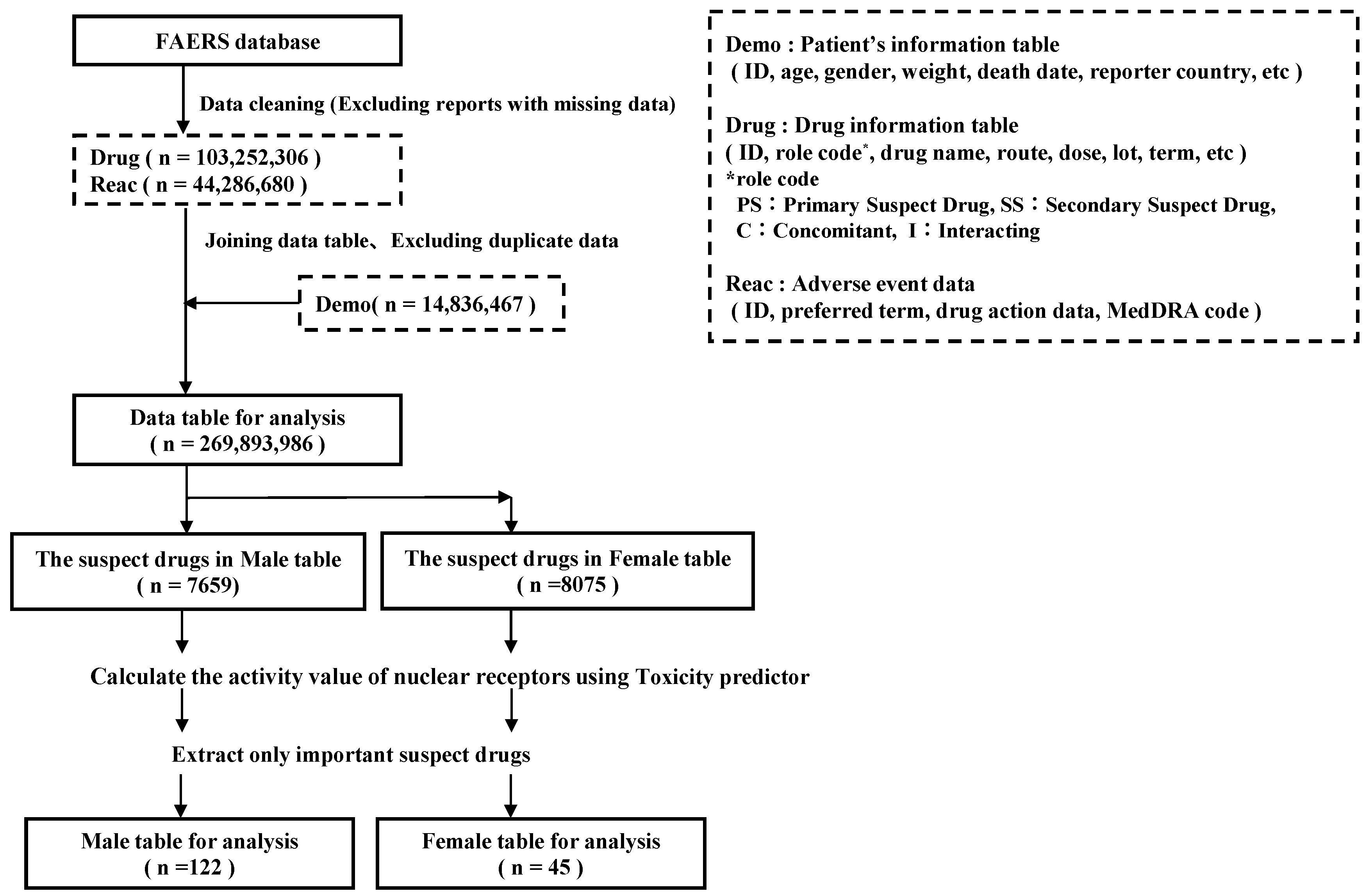
4.1. Database Information
4.2. Definitions of Adverse Events and Suspected Drugs
4.3. Extraction of Drugs Suspected of Causing Hiccups
4.4. MIE Activity Prediction Using Toxicity Predictor
4.5. MIEs Associated with Hiccups
4.6. Statistical Analysis
4.7. Limitation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, D.E. Nausea, Vomiting, and Hiccups: A Review of Mechanisms and Treatment. Anesth. Prog. 2010, 57, 150–157. [Google Scholar] [CrossRef]
- Kolodzik, P.W.; Filers, M.A. Hiccups (Singultus): Review and Approach to Management. Ann. Emerg. Med. 1991, 20, 565–573. [Google Scholar] [CrossRef]
- Kondo, T.; Toyooka, H.; Arita, H. Hiccup Reflex Is Mediated by Pharyngeal Branch of Glossopharyngeal Nerve in Cats. Neurosci. Res. 2003, 47, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Nausheen, F.; Mohsin, H.; Lakhan, S.E. Neurotransmitters in Hiccups. Springerplus 2016, 5, 1357. [Google Scholar] [CrossRef]
- Oshima, T.; Sakamoto, M.; Tatsuta, H.; Arita, H. GABAergic Inhibition of Hiccup-like Reflex Induced by Electrical Stimulation in Medulla of Cats. Neurosci. Res. 1998, 30, 287–293. [Google Scholar] [CrossRef]
- Lee, G.W.; Kim, R.B.; Go, S.I.; Cho, H.S.; Lee, S.J.; Hui, D.; Bruera, E.; Kang, J.H. Gender Differences in Hiccup Patients: Analysis of Published Case Reports and Case-Control Studies. J. Pain Symptom Manag. 2016, 51, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, R.; Tanaka, I.; Ishii-Nozawa, R.; Amino, T.; Kamata, T.; Hino, S.; Kagaya, H.; Uesawa, Y. Risk Factors for Cancer Chemotherapy-Induced Hiccups (CIH). Pharmacol. Pharm. 2018, 9, 331–343. [Google Scholar] [CrossRef]
- Liaw, C.C.; Wang, C.H.; Chang, H.K.; Wang, H.M.; Huang, J.S.; Lin, Y.C.; Chen, J.S. Cisplatin-Related Hiccups: Male Predominance, Induction by Dexamethasone, and Protection against Nausea and Vomiting. J. Pain Symptom Manag. 2005, 30, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Sugimura, H.; Suga, Y.; Kawahara, M.; Aimiya, K.; Miyamoto, K. Study on Risk Factors for Hiccups Induced by Cisplatin-Based Chemotherapy. Iryo Yakugaku (Jpn. J. Pharm. Health Care Sci.) 2009, 35, 89–95. [Google Scholar] [CrossRef][Green Version]
- Kang, J.H.; Hui, D.; Kim, M.J.; Kim, H.G.; Kang, M.H.; Lee, G.W.; Bruera, E. Corticosteroid Rotation to Alleviate Dexamethasone-Induced Hiccup: A Case Series at a Single Institution. J. Pain Symptom Manag. 2012, 43, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, R.; Uesawa, Y.; Ishii-Nozawa, R.; Kagaya, H. Analysis of Factors Associated with Hiccups Based on the Japanese Adverse Drug Event Report Database. PLoS ONE 2017, 12, e0172057. [Google Scholar] [CrossRef]
- Hosoya, R.; Ishii-nozawa, R.; Kurosaki, K.; Uesawa, Y. Analysis of Factors Associated with Hiccups Using the FAERS Database. Pharmaceuticals 2022, 15, 27. [Google Scholar] [CrossRef]
- Okunaka, M.; Kano, D.; Uesawa, Y. Nuclear Receptor and Stress Response Pathways Associated with Antineoplastic Agent-Induced Diarrhea. Int. J. Mol. Sci. 2022, 23, 12407. [Google Scholar] [CrossRef]
- Olefsky, J.M. Nuclear Receptor Minireview Series. J. Biol. Chem. 2001, 276, 36863–36864. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Kurosaki, K.; Wu, R.; Uesawa, Y. A Toxicity Prediction Tool for Potential Agonist/Antagonist Activities in Molecular Initiating Events Based on Chemical Structures. Int. J. Mol. Sci. 2020, 21, 7853. [Google Scholar] [CrossRef]
- Oh, J.M.; Kim, I. Drug Repositioning Prediction for Psoriasis Using the Adverse Event Reporting Database. Front. Med. 2023, 10, 1159453. [Google Scholar] [CrossRef]
- Kinoshita, S.; Hosomi, K.; Yokoyama, S.; Takada, M. Inverse Association between Metformin and Amiodarone-Associated Extracardiac Adverse Events. Int. J. Med. Sci. 2020, 17, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Fujimoto, M.; Motomura, H.; Hosomi, K. Inverse Association between Sodium Channel-Blocking Antiepileptic Drug Use and Cancer: Data Mining of Spontaneous Reporting and Claims Databases. Int. J. Med. Sci. 2016, 13, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Yamazaki, H.; Manabe, Y.; Fukuda, C.; Hanai, K.; Fushiki, T. Transforming Growth Factor-Beta Activated during Exercise in Brain Depresses Spontaneous Motor Activity of Animals. Relevance to Central Fatigue. Brain Res. 1999, 846, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, K.M.; Wade, F.M.; Mahesh, V.B.; Brann, D.W. Astrocyte-Derived Transforming Growth Factor-β Mediates the Neuroprotective Effects of 17β-Estradiol: Involvement of Nonclassical Genomic Signaling Pathways. Endocrinology 2005, 146, 2749–2759. [Google Scholar] [CrossRef]
- Wahl, S.M.; Allen, J.B.; McCartney-Francis, N.; Morganti-Kossmann, M.C.; Kossmann, T.; Ellingsworth, L.; Mai, U.E.H.; Mergenhagen, S.E.; Orenstein, J.M. Macrophage- and Astrocyte-Derived Transforming Growth Factor β as a Mediator of Central Nervous System Dysfunction in Acquired Immune Deficiency Syndrome. J. Exp. Med. 1991, 173, 981–991. [Google Scholar] [CrossRef]
- Kondo, W.; Okuno, M.; Kojima, S. Molecular Mechanism and Regulation of Latent TGF-ΒActivation. Jpn. J. Thromb. Hemost. 2003, 14, 210–219. [Google Scholar] [CrossRef]
- Katsuno, M.; Adachi, H.; Banno, H.; Suzuki, K.; Tanaka, F.; Sobue, G. Transforming Growth Factor-β Signaling in Motor Neuron Diseases. Curr. Mol. Med. 2011, 11, 48–56. [Google Scholar] [CrossRef]
- Chang, F.Y.; Lu, C.L. Hiccup: Mystery, Nature and Treatment. J. Neurogastroenterol. Motil. 2012, 18, 123–130. [Google Scholar] [CrossRef]
- Muñoz, M.D.; de la Fuente, N.; Sánchez-Capelo, A. TGF-β/Smad3 Signalling Modulates GABA Neurotransmission: Implications in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 590. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, R.; Luo, H.; Peng, Y.; Chen, P.; Ren, S.; Liu, G. Dysfunction of Nrf2-ARE Signaling Pathway: Potential Pathogenesis in the Development of Neurocognitive Impairment in Patients with Moderate to Severe Obstructive Sleep Apnea-Hypopnea Syndrome. Oxid. Med. Cell. Longev. 2018, 2018, 3529709. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging Roles of Nrf2 and Phase II Antioxidant Enzymes in Neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef]
- Mishra, A.; Chaturvedi, P.; Datta, S.; Sinukumar, S.; Joshi, P.; Garg, A. Harmful Effects of Nicotine. Indian J. Med. Paediatr. Oncol. 2015, 36, 24–31. [Google Scholar] [CrossRef]
- Fathi, M.; Saeedyan, S.; Kaoosi, M. Gamma-Amino Butyric Acid (GABA) Supplementation Alleviates Dexamethasone Treatment-Induced Oxidative Stress and Inflammation Response in Broiler Chickens. Stress 2023, 26, 2185861. [Google Scholar] [CrossRef]
- Sun, J.K.; Iwata, T.; Zigler, J.S.; Carper, D.A. Differential Gene Expression in Male and Female Rat Lenses Undergoing Cataract Induction by Transforming Growth Factor-β (TGF-β). Exp. Eye Res. 2000, 70, 169–181. [Google Scholar] [CrossRef] [PubMed]
- FDA FAERS. Available online: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/fda-adverse-event-reporting-system-faers-public-dashboard (accessed on 15 March 2024).
- MedDRA. Available online: https://www.meddra.org/26 (accessed on 15 March 2024).
- Department of Medical Molecular Informatics, M.P.U. Toxicity Predictor. Available online: http://mmi-03.my-pharm.ac.jp/tox1/prediction_groups/new (accessed on 8 May 2023).
- Maeda, R. JADER from Pharmacovigilance Point of View. Jpn. J. Pharmacoepidemiol. 2014, 19, 51–56. [Google Scholar] [CrossRef]
- Avillach, P.; Salvo, F.; Thiessard, F.; Miremont-Salamé, G.; Fourrier-Reglat, A.; Haramburu, F.; Bégaud, B.; Moore, N.; Pariente, A. Pilot Evaluation of an Automated Method to Decrease False-Positive Signals Induced by Co-Prescriptions in Spontaneous Reporting Databases. Pharmacoepidemiol. Drug Saf. 2014, 23, 186–194. [Google Scholar] [CrossRef] [PubMed]



| Univariate Analysis | Multivariate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIEs | Activity Type | 95% CI | 95% CI | |||||||
| Odds Ratio | Lower | Upper | p-Value (Fisher’s Exact Test) | Odds Ratio | Lower | Upper | p-Value (Likelihood Ratio Test) | p-Value (Wald Test) | ||
| ERaant | agonist | 0.21 * | 0.06 | 0.69 | 0.010 | 0.28 | 0.06 | 1.26 | 0.088 | 0.097 |
| PR | antagonist | 0.43 * | 0.23 | 0.82 | 0.011 | 0.61 | 0.30 | 1.26 | 0.181 | 0.181 |
| ARaant | agonist | 0.33 * | 0.14 | 0.76 | 0.011 | 0.65 | 0.21 | 1.97 | 0.445 | 0.443 |
| TGFb | agonist | 3.37 * | 1.30 | 8.75 | 0.014 | 4.59 * | 1.54 | 13.67 | 0.002 | 0.006 |
| PR | agonist | 0.20 * | 0.05 | 0.76 | 0.014 | 0.66 | 0.12 | 3.55 | 0.625 | 0.628 |
| Shh | agonist | 0.40 * | 0.18 | 0.89 | 0.026 | 0.63 | 0.25 | 1.60 | 0.330 | 0.329 |
| Univariate Analysis | Multivariate Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MIEs | Activity Type | 95% CI | 95% CI | |||||||
| Odds Ratio | Lower | Upper | p-Value (Fisher’s Exact Test) | Odds Ratio | Lower | Upper | p-Value (Likelihood Ratio Test) | p-Value (Wald Test) | ||
| PR | agonist | 0.326 * | 0.152 | 0.698 | 0.005 | 0.60 | 0.22 | 1.62 | 0.318 | 0.318 |
| CAR | antagonist | 0.347 * | 0.160 | 0.753 | 0.009 | 0.56 | 0.23 | 1.41 | 0.222 | 0.220 |
| Shh | agonist | 0.389 * | 0.176 | 0.861 | 0.021 | 0.70 | 0.25 | 1.97 | 0.504 | 0.502 |
| ARlbd | antagonist | 0.401 * | 0.189 | 0.851 | 0.025 | 0.62 | 0.22 | 1.70 | 0.351 | 0.348 |
| TGFb | agonist | 2.857 * | 1.100 | 7.423 | 0.030 | 3.67 * | 1.29 | 10.45 | 0.011 | 0.015 |
| MIEs | Activity Type | Univariate Analysis | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||||
| Odds Ratio | Lower | Upper | p-Value (Fisher’s Exact Test) | Odds Ratio | Lower | Upper | p-Value (Likelihood Ratio Test) | p-Value (Wald Test) | ||
| ARE | agonist | 0.08 * | 0.01 | 0.70 | 0.01 | 0.12 * | 0.01 | 1.32 | 0.048 | 0.083 |
| CAR | antagonist | 0.18 * | 0.03 | 0.94 | 0.04 | 0.50 | 0.07 | 3.62 | 0.483 | 0.489 |
| ARant | agonist | 0.20 * | 0.05 | 0.88 | 0.05 | 0.24 | 0.04 | 1.20 | 0.073 | 0.080 |
| Hiccups | Other Adverse Events | |
|---|---|---|
| Suspected drugs | n11 | n12 |
| Other drugs | n21 | n22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosoya, R.; Ishii-Nozawa, R.; Terajima, T.; Kagaya, H.; Uesawa, Y. The Association between Molecular Initiating Events and Drug-Induced Hiccups. Pharmaceuticals 2024, 17, 379. https://doi.org/10.3390/ph17030379
Hosoya R, Ishii-Nozawa R, Terajima T, Kagaya H, Uesawa Y. The Association between Molecular Initiating Events and Drug-Induced Hiccups. Pharmaceuticals. 2024; 17(3):379. https://doi.org/10.3390/ph17030379
Chicago/Turabian StyleHosoya, Ryuichiro, Reiko Ishii-Nozawa, Tomoko Terajima, Hajime Kagaya, and Yoshihiro Uesawa. 2024. "The Association between Molecular Initiating Events and Drug-Induced Hiccups" Pharmaceuticals 17, no. 3: 379. https://doi.org/10.3390/ph17030379
APA StyleHosoya, R., Ishii-Nozawa, R., Terajima, T., Kagaya, H., & Uesawa, Y. (2024). The Association between Molecular Initiating Events and Drug-Induced Hiccups. Pharmaceuticals, 17(3), 379. https://doi.org/10.3390/ph17030379







