The Effect of Heparin and Other Exogenous Glycosaminoglycans (GAGs) in Reducing IL-1β-Induced Pro-Inflammatory Cytokine IL-8 and IL-6 mRNA Expression and the Potential Role for Reducing Inflammation
Abstract
1. Introduction
2. Results
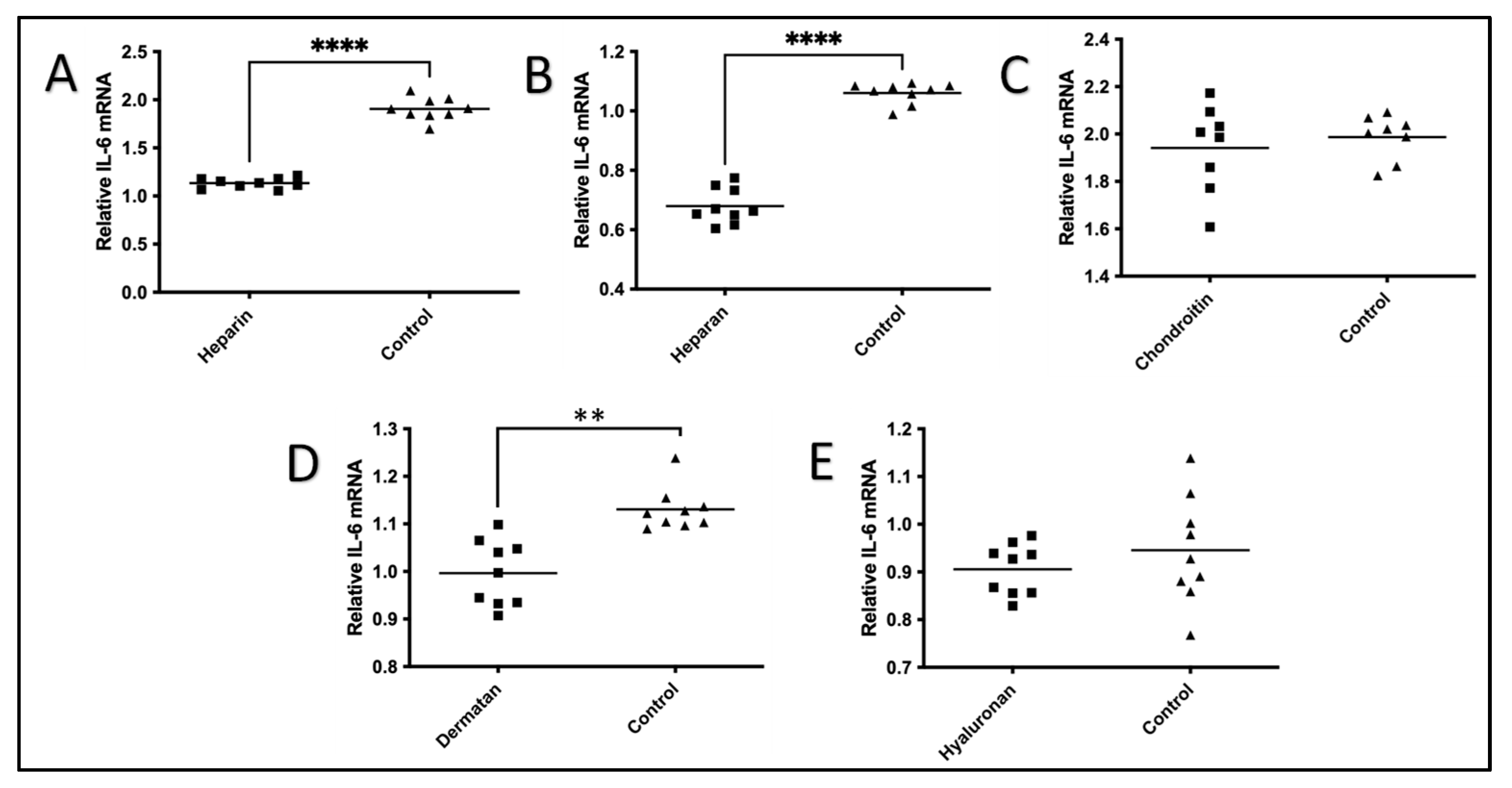
2.1. The Effect of GAGs on IL-1β-Stimulated Expression of IL-6 mRNA
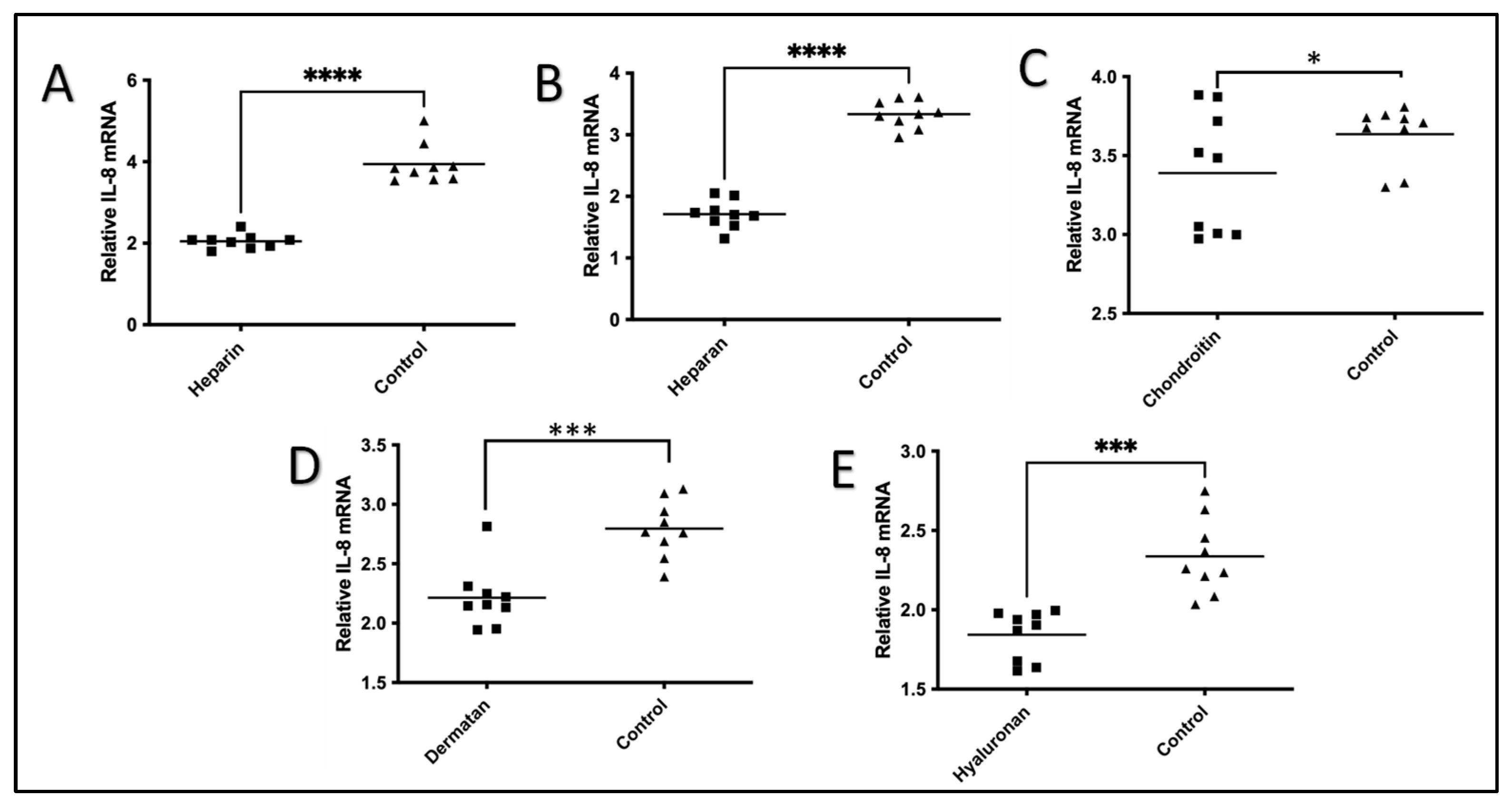
2.2. The Effect of GAGs on IL-1β-Stimulated Expression of IL-8 mRNA
3. Discussion
4. Materials and Methods
4.1. HeLa Cells and Culture Preparation
4.2. Fibronectin
4.3. IL-1β
4.4. The Preparation of GAGs
4.5. Experimental Procedures
4.6. Real-Time Quantitative Reverse-Transcription PCR (qRT-PCR)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Debarre, F.; Bonhoeffer, S.; Regoes, R.R. The effect of population structure on the emergence of drug resistance during influenza pandemics. J. R. Soc. Interface 2007, 4, 893–906. [Google Scholar] [CrossRef]
- Tumova, S.; Woods, A.; Couchman, J.R. Heparan sulfate proteoglycans on the cell surface: Versatile coordinators of cellular functions. Int. J. Biochem. Cell Biol. 2000, 32, 269–288. [Google Scholar] [CrossRef]
- Raman, R.; Sasisekharan, V.; Sasisekharan, R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem. Biol. 2005, 12, 267–277. [Google Scholar] [CrossRef]
- Prydz, K. Determinants of Glycosaminoglycan (GAG) Structure. Biomolecules 2015, 5, 2003–2022. [Google Scholar] [CrossRef]
- Sasisekharan, R.; Venkataraman, G. Heparin and heparan sulfate: Biosynthesis, structure and function. Curr. Opin. Chem. Biol. 2000, 4, 626–631. [Google Scholar] [CrossRef]
- Sugahara, K.; Mikami, T.; Uyama, T.; Mizuguchi, S.; Nomura, K.; Kitagawa, H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr. Opin. Struct. Biol. 2003, 13, 612–620. [Google Scholar] [CrossRef]
- Kobayashi, T.; Chanmee, T.; Itano, N. Hyaluronan: Metabolism and Function. Biomolecules 2020, 10, 1525. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Albadawi, H.; Watkins, M.T.; Edelman, E.R.; Baker, A.B. Syndecan-4 proteoliposomes enhance fibroblast growth factor-2 (FGF-2)-induced proliferation, migration, and neovascularization of ischemic muscle. Proc. Natl. Acad. Sci. USA 2012, 109, 1679–1684. [Google Scholar] [CrossRef]
- ten Dijke, P.; Arthur, H.M. Extracellular control of TGFbeta signalling in vascular development and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 857–869. [Google Scholar] [CrossRef]
- Jiang, Z.; Georgel, P.; Du, X.; Shamel, L.; Sovath, S.; Mudd, S.; Huber, M.; Kalis, C.; Keck, S.; Galanos, C.; et al. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 2005, 6, 565–570. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A. How Toll-like receptors signal: What we know and what we don’t know. Curr. Opin. Immunol. 2006, 18, 3–9. [Google Scholar] [CrossRef]
- Gay, N.J.; Gangloff, M. Structure and function of Toll receptors and their ligands. Annu. Rev. Biochem. 2007, 76, 141–165. [Google Scholar] [CrossRef]
- Beutler, B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature 2004, 430, 257–263. [Google Scholar] [CrossRef]
- Vallés, S.; Tsoi, C.; Huang, W.Y.; Wyllie, D.; Carlotti, F.; Askari, J.A.; Humphries, M.J.; Dower, S.K.; Qwarnström, E.E. Recruitment of a heparan sulfate subunit to the interleukin-1 receptor complex. Regulation by fibronectin attachment. J. Biol. Chem. 1999, 274, 20103–20109. [Google Scholar] [CrossRef]
- Zhang, X.; Shephard, F.; Kim, H.B.; Palmer, I.R.; McHarg, S.; Fowler, G.J.; O’Neill, L.A.; Kiss-Toth, E.; Qwarnstrom, E.E. TILRR, a novel IL-1RI co-receptor, potentiates MyD88 recruitment to control Ras-dependent amplification of NF-kappaB. J. Biol. Chem. 2010, 285, 7222–7232. [Google Scholar] [CrossRef]
- Hudson, R.C.; Gray, C.; Kiss-Toth, E.; Chico, T.J.; Qwarnstrom, E.E. Bioinformatics Analysis of the FREM1 Gene-Evolutionary Development of the IL-1R1 Co-Receptor, TILRR. Biology 2012, 1, 484–494. [Google Scholar] [CrossRef]
- Smith, S.A.; Samokhin, A.O.; Alfaidi, M.; Murphy, E.C.; Rhodes, D.; Holcombe, W.M.; Kiss-Toth, E.; Storey, R.F.; Yee, S.P.; Francis, S.E.; et al. The IL-1RI Co-Receptor TILRR (FREM1 Isoform 2) Controls Aberrant Inflammatory Responses and Development of Vascular Disease. JACC Basic Transl. Sci. 2017, 2, 398–414. [Google Scholar] [CrossRef]
- Kashem, M.A.; Li, H.; Toledo, N.P.; Omange, R.W.; Li, L.; Kindrachuk, J.; Plummer, F.A.; Luo, M. Toll-like Interleukin 1 Receptor Regulator Is an Important Modulator of Inflammation Responsive Genes. Front. Immunol. 2019, 10, 272. [Google Scholar] [CrossRef]
- Xu, X.Y.; Guo, W.J.; Pan, S.H.; Zhang, Y.; Gao, F.L.; Wang, J.T.; Zhang, S.; Li, H.Y.; Wang, R.; Zhang, X. TILRR (FREM1 isoform 2) is a prognostic biomarker correlated with immune infiltration in breast cancer. Aging 2020, 12, 19335–19351. [Google Scholar] [CrossRef]
- Baggiolini, M.; Clark-Lewis, I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992, 307, 97–101. [Google Scholar] [CrossRef]
- Harada, A.; Sekido, N.; Akahoshi, T.; Wada, T.; Mukaida, N.; Matsushima, K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Kawamura, T.; Wakusawa, R.; Okada, K.; Inada, S. Elevation of cytokines during open heart surgery with cardiopulmonary bypass: Participation of interleukin 8 and 6 in reperfusion injury. Can. J. Anaesth. J. Can. D’anesthesie 1993, 40, 1016–1021. [Google Scholar] [CrossRef]
- Yu, H.H.; Li, M.; Li, Y.B.; Lei, B.B.; Yuan, X.; Xing, X.K.; Xie, Y.F.; Wang, M.; Wang, L.; Yang, H.J.; et al. Benzoylaconitine Inhibits Production of IL-6 and IL-8 via MAPK, Akt, NF-kappaB Signaling in IL-1beta-Induced Human Synovial Cells. Biol. Pharm. Bull. 2020, 43, 334–339. [Google Scholar] [CrossRef]
- Mizuno, M.; Nakano, R.; Nose, S.; Matsumura, M.; Nii, Y.; Kurogochi, K.; Sugiya, H.; Uechi, M. Canonical NF-kappaB p65, but Not p105, Contributes to IL-1beta-Induced IL-8 Expression in Cardiac Fibroblasts. Front. Immunol. 2022, 13, 863309. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Masuda, K.; Kishimoto, T. Regulation of IL-6 in Immunity and Diseases. Adv. Exp. Med. Biol. 2016, 941, 79–88. [Google Scholar]
- Catanzaro, J.M.; Sheshadri, N.; Pan, J.A.; Sun, Y.; Shi, C.; Li, J.; Powers, R.S.; Crawford, H.C.; Zong, W.X. Oncogenic Ras induces inflammatory cytokine production by upregulating the squamous cell carcinoma antigens SerpinB3/B4. Nat. Commun. 2014, 5, 3729. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; Freeman, G.L. Induction of nuclear factor kappaB and activation protein 1 in postischemic myocardium. FEBS Lett. 1997, 401, 30–34. [Google Scholar] [CrossRef]
- Steinberg, J.B.; Kapelanski, D.P.; Olson, J.D.; Weiler, J.M. Cytokine and complement levels in patients undergoing cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1993, 106, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Chong, G.L.; Baigrie, R.J.; Pillai, R.; Westaby, S.; Rocker, G.M. Cytokine responses to cardiopulmonary bypass with membrane and bubble oxygenation. Ann. Thorac. Surg. 1992, 53, 833–838. [Google Scholar] [CrossRef]
- Finn, A.; Naik, S.; Klein, N.; Levinsky, R.J.; Strobel, S.; Elliott, M. Interleukin-8 release and neutrophil degranulation after pediatric cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1993, 105, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kalfin, R.E.; Engelman, R.M.; Rousou, J.A.; Flack, J.E., 3rd; Deaton, D.W.; Kreutzer, D.L.; Das, D.K. Induction of interleukin-8 expression during cardiopulmonary bypass. Circulation 1993, 88, II401–II406. [Google Scholar]
- Casey, L.C. Role of cytokines in the pathogenesis of cardiopulmonary-induced multisystem organ failure. Ann. Thorac. Surg. 1993, 56 (Suppl. S5), S92–S96. [Google Scholar] [CrossRef]
- Mustapha, S.; Kirshner, A.; De Moissac, D.; Kirshenbaum, L.A. A direct requirement of nuclear factor-kappa B for suppression of apoptosis in ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H939–H945. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Haudek, S.B.; Knuefermann, P.; Vallejo, J.G.; Chen, Z.J.; Michael, L.H.; Sivasubramanian, N.; Olson, E.N.; Entman, M.L.; Mann, D.L. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation 2003, 108, 3075–3078. [Google Scholar] [CrossRef]
- Maekawa, N.; Wada, H.; Kanda, T.; Niwa, T.; Yamada, Y.; Saito, K.; Fujiwara, H.; Sekikawa, K.; Seishima, M. Improved myocardial ischemia/reperfusion injury in mice lacking tumor necrosis factor-alpha. J. Am. Coll. Cardiol. 2002, 39, 1229–1235. [Google Scholar] [CrossRef]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol. Cell Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-kappaB in the heart: To be or not to NF-kappaB. Circ. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef]
- Hamid, T.; Guo, S.Z.; Kingery, J.R.; Xiang, X.; Dawn, B.; Prabhu, S.D. Cardiomyocyte NF-kappaB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc. Res. 2011, 89, 129–138. [Google Scholar] [CrossRef]
- Nian, M.; Lee, P.; Khaper, N.; Liu, P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004, 94, 1543–1553. [Google Scholar] [CrossRef]
- Wan, S.; DeSmet, J.M.; Barvais, L.; Goldstein, M.; Vincent, J.L.; LeClerc, J.L. Myocardium is a major source of proinflammatory cytokines in patients undergoing cardiopulmonary bypass. J. Thorac. Cardiovasc. Surg. 1996, 112, 806–811. [Google Scholar] [CrossRef]
- Neumann, F.J.; Ott, I.; Gawaz, M.; Richardt, G.; Holzapfel, H.; Jochum, M.; Schömig, A. Cardiac release of cytokines and inflammatory responses in acute myocardial infarction. Circulation 1995, 92, 748–755. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Smith, C.W.; Entman, M.L. The inflammatory response in myocardial infarction. Cardiovasc. Res. 2002, 53, 31–47. [Google Scholar] [CrossRef]
- Akasaka, Y.; Morimoto, N.; Ishikawa, Y.; Fujita, K.; Ito, K.; Kimura-Matsumoto, M.; Ishiguro, S.; Morita, H.; Kobayashi, Y.; Ishii, T. Myocardial apoptosis associated with the expression of proinflammatory cytokines during the course of myocardial infarction. Mod. Pathol. 2006, 19, 588–598. [Google Scholar] [CrossRef]
- Hammond, M.E.; Lapointe, G.R.; Feucht, P.H.; Hilt, S.; Gallegos, C.A.; Gordon, C.A.; Giedlin, M.A.; Mullenbach, G.; Tekamp-Olson, P. IL-8 induces neutrophil chemotaxis predominantly via type I IL-8 receptors. J. Immunol. 1995, 155, 1428–1433. [Google Scholar] [CrossRef]
- Taub, D.D.; Anver, M.; Oppenheim, J.J.; Longo, D.L.; Murphy, W.J. T lymphocyte recruitment by interleukin-8 (IL-8). IL-8-induced degranulation of neutrophils releases potent chemoattractants for human T lymphocytes both in vitro and in vivo. J. Clin. Investig. 1996, 97, 1931–1941. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Dinarello, C.A.; van der Meer, J.W. Treating inflammation by blocking interleukin-1 in humans. Semin. Immunol. 2013, 25, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Kontos, M.C.; Grizzard, J.D.; Biondi-Zoccai, G.G.; Van Tassell, B.W.; Robati, R.; Roach, L.M.; Arena, R.A.; Roberts, C.S.; Varma, A.; et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am. J. Cardiol. 2010, 105, 1371–1377.e1. [Google Scholar] [CrossRef] [PubMed]
- Dower, S.K.; Kronheim, S.R.; Hopp, T.P.; Cantrell, M.; Deeley, M.; Gillis, S.; Henney, C.S.; Urdal, D.L. The cell surface receptors for interleukin-1 alpha and interleukin-1 beta are identical. Nature 1986, 324, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Tahtinen, S.; Tong, A.J.; Himmels, P.; Oh, J.; Paler-Martinez, A.; Kim, L.; Wichner, S.; Oei, Y.; McCarron, M.J.; Freund, E.C.; et al. IL-1 and IL-1ra are key regulators of the inflammatory response to RNA vaccines. Nat. Immunol. 2022, 23, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Gresnigt, M.S.; Bozza, S.; Becker, K.L.; Joosten, L.A.; Abdollahi-Roodsaz, S.; van Der Berg, W.B.; Dinarello, C.A.; Netea, M.G.; Fontaine, T.; De Luca, A.; et al. A polysaccharide virulence factor from Aspergillus fumigatus elicits anti-inflammatory effects through induction of Interleukin-1 receptor antagonist. PLoS Pathog. 2014, 10, e1003936. [Google Scholar] [CrossRef]
- Ramsden, L.; Rider, C.C. Selective and differential binding of interleukin (IL)-1 alpha, IL-1 beta, IL-2 and IL-6 to glycosaminoglycans. Eur. J. Immunol. 1992, 22, 3027–3031. [Google Scholar] [CrossRef] [PubMed]
- Kuschert, G.S.; Coulin, F.; Power, C.A.; Proudfoot, A.E.; Hubbard, R.E.; Hoogewerf, A.J.; Wells, T.N. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 1999, 38, 12959–12968. [Google Scholar] [CrossRef] [PubMed]
- Kashem, M.A.; Lischynski, J.; Stojak, B.; Li, L.; Yuan, X.-Y.; Liang, B.; Kimani, J.; Plummer, F.A.; Luo, M. High level of plasma TILRR protein is associated with faster HIV seroconversion. EBioMedicine 2022, 78, 103955. [Google Scholar] [CrossRef] [PubMed]
- Kashem, M.A.; Yuan, X.Y.; Li, L.; Kimani, J.; Plummer, F.; Luo, M. TILRR (Toll-like Interleukin-1 Receptor Regulator), an Important Modulator of Inflammatory Responsive Genes, is Circulating in the Blood. J. Inflamm. Res. 2021, 14, 4927–4943. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, L.D.; Ferneini, E.M. Heparin and Lovenox: What the Oral and Maxillofacial Surgeon Needs to Know. Oral Maxillofac. Surg. Clin. N. Am. 2016, 28, 507–513. [Google Scholar] [CrossRef]
- Bounameaux, H.; Goldhaber, S.Z. Uses of low-molecular-weight heparin. Blood Rev. 1995, 9, 213–219. [Google Scholar] [CrossRef]

| Glycosaminoglycan (GAG) | Disaccharide Repeat |
|---|---|
| Heparin | α4GlcNAc-β4GlcUA |
| Heparan sulfate | α4GlcNAc-β4GlcUA(SO4) |
| Dermatan sulfate | β3GalNAc-β4GlcUA(SO4) |
| Chondroitin sulfate | β3GalNAc-β4GlcUA(SO4) |
| Hyaluronan | β3GlcNAc-β4GlcUA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafri, M.; Li, L.; Liang, B.; Luo, M. The Effect of Heparin and Other Exogenous Glycosaminoglycans (GAGs) in Reducing IL-1β-Induced Pro-Inflammatory Cytokine IL-8 and IL-6 mRNA Expression and the Potential Role for Reducing Inflammation. Pharmaceuticals 2024, 17, 371. https://doi.org/10.3390/ph17030371
Jafri M, Li L, Liang B, Luo M. The Effect of Heparin and Other Exogenous Glycosaminoglycans (GAGs) in Reducing IL-1β-Induced Pro-Inflammatory Cytokine IL-8 and IL-6 mRNA Expression and the Potential Role for Reducing Inflammation. Pharmaceuticals. 2024; 17(3):371. https://doi.org/10.3390/ph17030371
Chicago/Turabian StyleJafri, Murtaza, Lin Li, Binhua Liang, and Ma Luo. 2024. "The Effect of Heparin and Other Exogenous Glycosaminoglycans (GAGs) in Reducing IL-1β-Induced Pro-Inflammatory Cytokine IL-8 and IL-6 mRNA Expression and the Potential Role for Reducing Inflammation" Pharmaceuticals 17, no. 3: 371. https://doi.org/10.3390/ph17030371
APA StyleJafri, M., Li, L., Liang, B., & Luo, M. (2024). The Effect of Heparin and Other Exogenous Glycosaminoglycans (GAGs) in Reducing IL-1β-Induced Pro-Inflammatory Cytokine IL-8 and IL-6 mRNA Expression and the Potential Role for Reducing Inflammation. Pharmaceuticals, 17(3), 371. https://doi.org/10.3390/ph17030371







