Efficacy Analysis and Prognostic Impact of Sivelestat Sodium in Coronavirus Disease 2019-Related Acute Respiratory Distress Syndrome
Abstract
1. Introduction
2. Results
2.1. Baseline Data Analysis
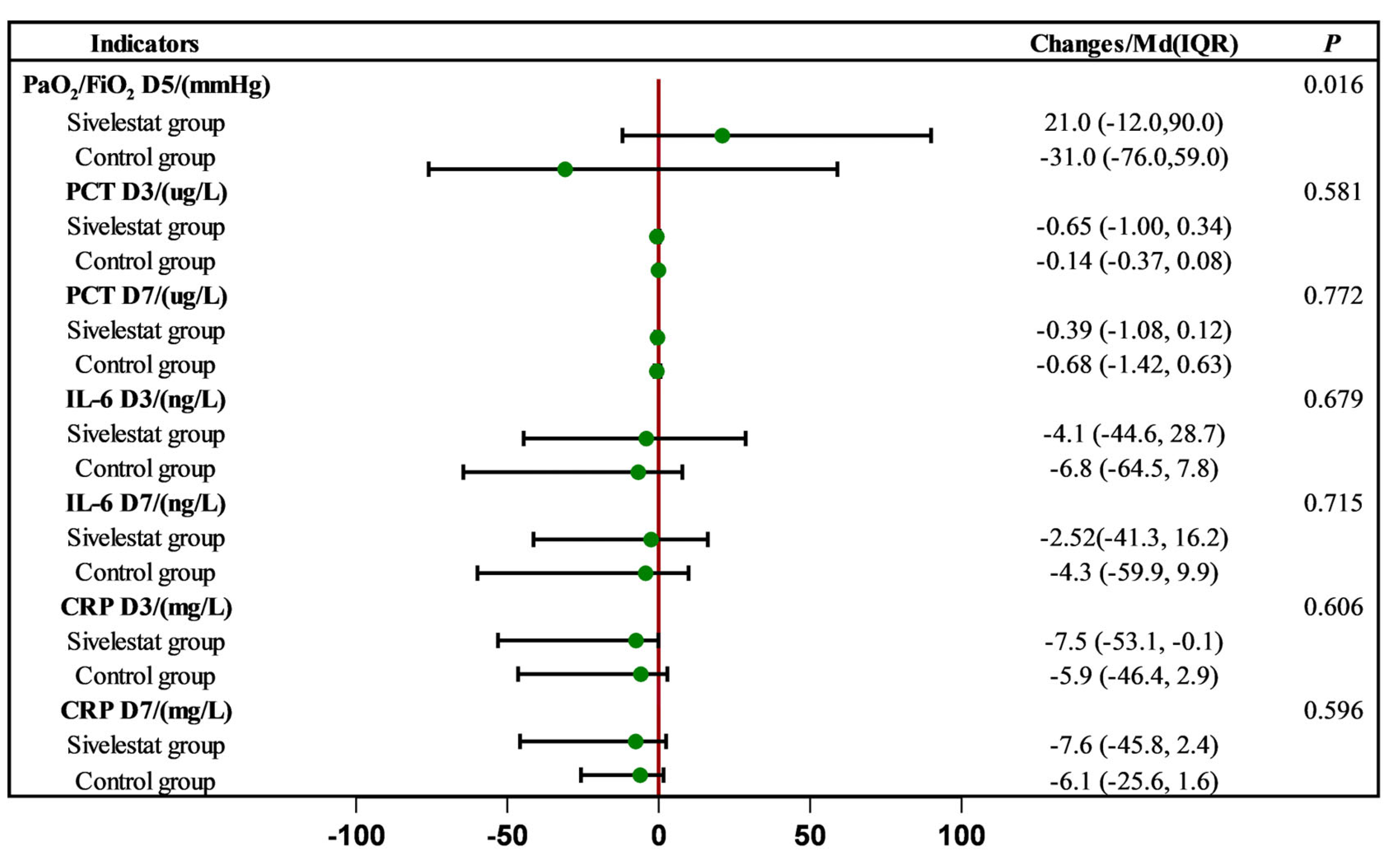
2.2. Effect of Sivelestat Sodium on Inflammatory Indices and Oxygenation Indices
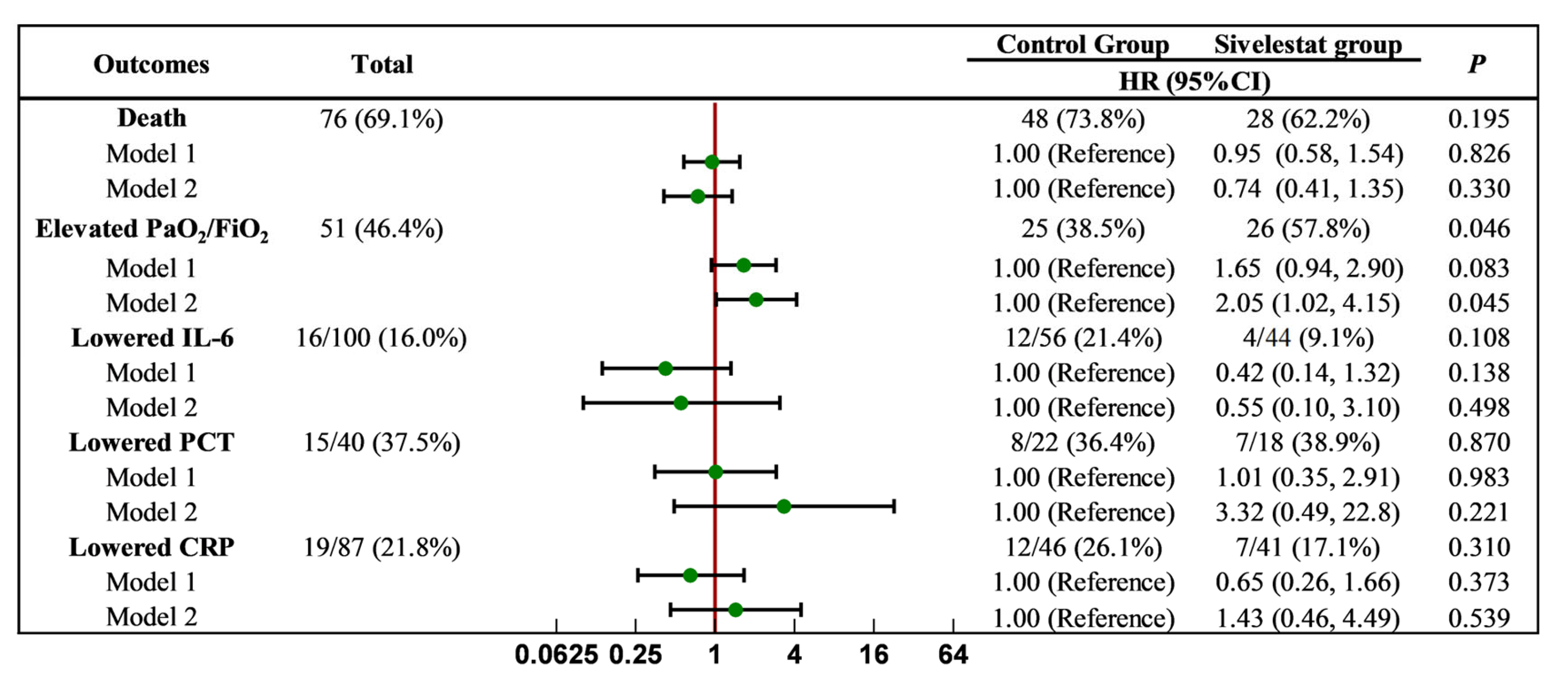
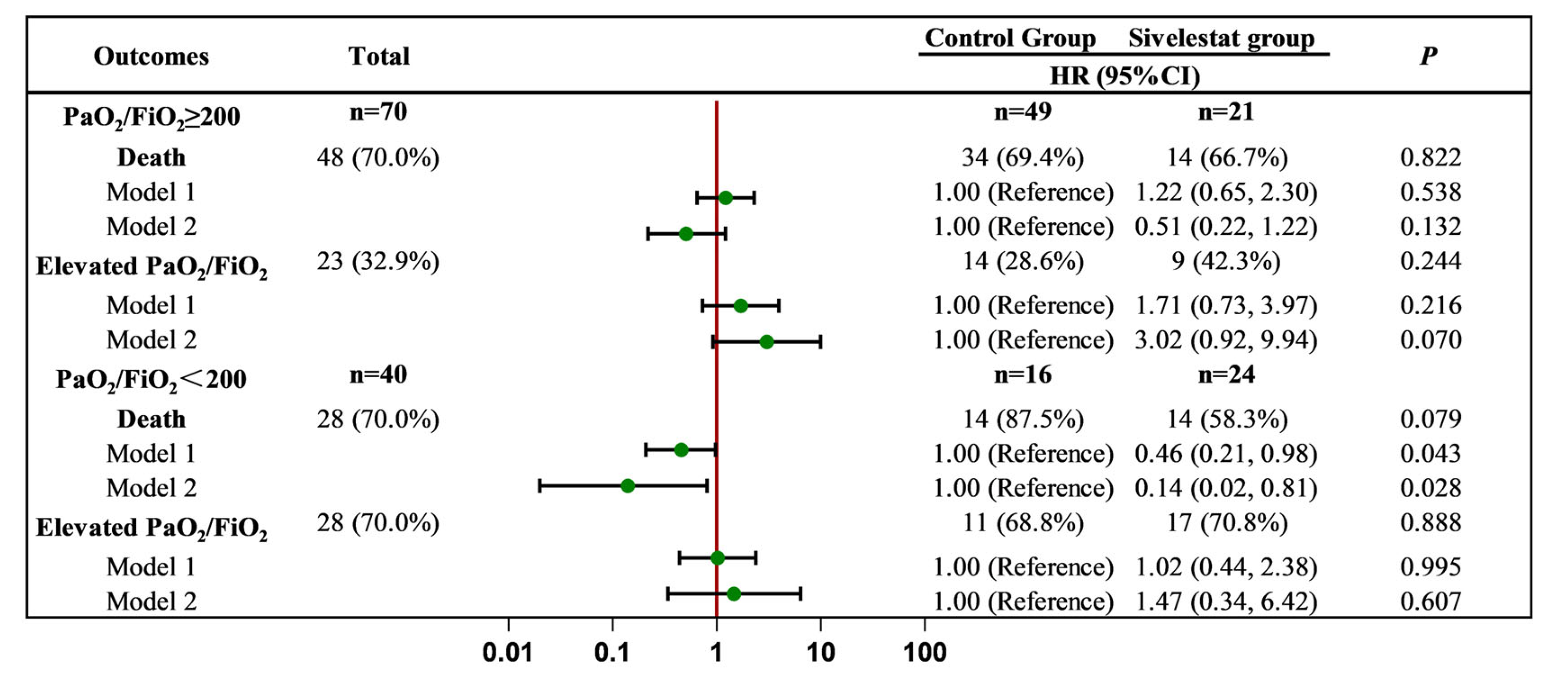
2.3. Effect of Sivelestat Sodium on Clinical Endpoint Events
3. Discussion
4. Methods
4.1. Patients
- All patients were diagnosed with severe or critical types of COVID-19 based on the Diagnostic and Treatment Protocol for COVID-19 (Trial 10th Edition).
- All patients met the diagnostic criteria for ARDS as defined in Berlin definition.
- All patients were 18 years of age or older.
- Minor patients (below 18 years old).
- Breastfeeding patients or pregnant women.
- Patients who did not complete a full course of treatment with sivelestat sodium but refused to continue its use.
- Patients who did not receive sivelestat sodium within 24 h of admission but used it in the middle and late stages of the disease.
- Individuals who experienced adverse drug reactions to sivelestat sodium.
4.2. Diagnosis of COVID-19 and ARDS
4.3. Grouping and Method
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. China Medical Treatment Expert Group for COVID-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Michalski, J.E.; Kurche, J.S.; Schwartz, D.A. From ards to pulmonary fibrosis: The next phase of the COVID-19 pandemic? Transl. Res. J. Lab. Clin. Med. 2022, 241, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hu, H.B.; Sun, Y.; Zhao, X.; Zeng, Z.; Liu, Y.; Wu, G. Sivelestat sodium for treatment of patients with COVID-19-associated acute respiratory distress syndrome in intensive care unit: A single-center retrospective cohort study. Nan Fang Yi Ke Da Xue Xue Bao 2023, 43, 1259–1267. [Google Scholar] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Jia’an, X.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet. Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Cao, W.; Fang, Z.; Hou, G.; Han, M.; Xu, X.; Dong, J.; Zheng, J. The psychological impact of the COVID-19 epidemic on college students in China. Psychiatry Res. 2020, 287, 112934. [Google Scholar] [CrossRef]
- Zambon, M.; Vincent, J.-L. Mortality Rates for Patients with Acute Lung Injury/ARDS Have Decreased Over Time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef]
- Phua, J.; Badia, J.R.; Adhikari, N.K.; Friedrich, J.O.; Fowler, R.A.; Singh, J.M.; Scales, D.C.; Stather, D.R.; Li, A.; Jones, A.; et al. Has mortality from acute respiratory distress syndrome decreased over time? A systematic review. Am. J. Respir. Crit. Care Med. 2009, 179, 220–227. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, R.; Lei, Z.; Guo, X.; Yang, Y.; Tian, J.; Huang, L. Efficacy, safety, and pharmacoeconomics of sivelestat sodium in the treatment of septic acute respiratory distress syndrome: A retrospective cohort study. Ann. Palliat. Med. 2021, 10, 11910–11917. [Google Scholar] [CrossRef]
- Kido, T.; Muramatsu, K.; Yatera, K.; Asakawa, T.; Otsubo, H.; Kubo, T.; Fujino, Y.; Matsuda, S.; Mayumi, T.; Mukae, H. Efficacy of early sivelestat administration on acute lung injury and acute respiratory distress syndrome. Respirology 2017, 22, 708–713. [Google Scholar] [CrossRef] [PubMed]
- Duggal, A.; Ganapathy, A.; Ratnapalan, M.; Adhikari, N.K. Pharmacological treatments for acute respiratory distress syndrome: Systematic review. Minerva Anestesiol. 2014, 81, 567–588. [Google Scholar] [PubMed]
- Briel, M.; Meade, M.; Mercat, A.; Brower, R.G.; Talmor, D.; Walter, S.D.; Slutsky, A.S.; Pullenayegum, E.; Zhou, Q.; Cook, D.; et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: Systematic review and meta-analysis. JAMA 2010, 303, 865–873. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone Positioning in Severe Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, Y.; Yang, C.; Li, X.; Yu, X. Clinical utility of the sivelestat for the treatment of ali/ards: Moving on in the controversy? Intens. Care Res. 2023, 3, 12–17. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Zeiher, B.G.; Artigas, A.; Vincent, J.-L.; Dmitrienko, A.; Jackson, K.; Thompson, B.T.; Bernard, G. Neutrophil elastase inhibition in acute lung injury: Results of the STRIVE study. Crit. Care Med. 2004, 32, 1695–1702. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Miyoshi, S.; Hamada, H.; Ito, R.; Katayama, H.; Irifune, K.; Suwaki, T.; Nakanishi, N.; Kanematsu, T.; Dote, K.; Aibiki, M.; et al. Usefulness of a selective neutrophil elastase inhibitor, sivelestat, in acute lung injury patients with sepsis. Drug Des. Dev. Ther. 2013, ume 7, 305–316. [Google Scholar] [CrossRef]
- Hoshi, K.; Kurosawa, S.; Kato, M.; Andoh, K.; Satoh, D.; Kaise, A. Sivelestat, a Neutrophil Elastase Inhibitor, Reduces Mortality Rate of Critically Ill Patients. Tohoku J. Exp. Med. 2005, 207, 143–148. [Google Scholar] [CrossRef]
- Aikawa, N.; Ishizaka, A.; Hirasawa, H.; Shimazaki, S.; Yamamoto, Y.; Sugimoto, H.; Shinozaki, M.; Taenaka, N.; Endo, S.; Ikeda, T.; et al. Reevaluation of the efficacy and safety of the neutrophil elastase inhibitor, Sivelestat, for the treatment of acute lung injury associated with systemic inflammatory response syndrome; a phase IV study. Pulm. Pharmacol. Ther. 2011, 24, 549–554. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ards): Clinical features and differences from typical pre-COVID-19 ards. Med. J. Aust. 2020, 213, 54–56. [Google Scholar] [CrossRef]
- Tzotzos, S.J.; Fischer, B.; Fischer, H.; Zeitlinger, M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: A global literature survey. Crit. Care 2020, 24, 1–4. [Google Scholar] [CrossRef]
- Zeng, W.; Song, Y.; Wang, R.; He, R.; Wang, T. Neutrophil elastase: From mechanisms to therapeutic potential. J. Pharm. Anal. 2023, 13, 355–366. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.; Ni, L.; Di, X.; Ma, B.; Niu, S.; Liu, C.; Reiter, R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020, 250, 117583. [Google Scholar] [CrossRef]
- Petrosillo, N.; Viceconte, G.; Ergonul, O.; Ippolito, G.; Petersen, E. COVID-19, sars and mers: Are they closely related? Clin. Microbiol. Infect. 2020, 26, 729–734. [Google Scholar] [CrossRef]
- Lee, J.M.; Yeo, C.D.; Lee, H.Y.; Rhee, C.K.; Kim, I.K.; Lee, D.G.; Lee, S.H.; Kim, J.W. Inhibition of neutrophil elastase contributes to attenuation of lipopolysaccharide-induced acute lung injury during neutropenia recovery in mice. J. Anesth. 2017, 31, 397–404. [Google Scholar] [CrossRef]
- Ding, Q.; Wang, Y.; Yang, C.; Tuerxun, D.; Yu, X. Effect of Sivelestat in the Treatment of Acute Lung Injury and Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Intensiv. Care Res. 2023, 3, 140–149. [Google Scholar] [CrossRef]
- Okayama, N.; Kakihana, Y.; Setoguchi, D.; Imabayashi, T.; Omae, T.; Matsunaga, A. Clinical effects of a neutrophil elastase inhibitor, sivelestat, in patients with acute respiratory distress syndrome. J. Anesth. 2006, 20, 6–10. [Google Scholar] [CrossRef]
- Hayashida, K.; Fujishima, S.; Sasao, K.; Orita, T.; Toyoda, Y.; Kitano, M.; Hori, S. Early Administration of Sivelestat, the Neutrophil Elastase Inhibitor, in Adults for Acute Lung Injury Following Gastric Aspiration. Shock 2011, 36, 223–227. [Google Scholar] [CrossRef]
- Kadoi, Y.; Hinohara, H.; Kunimoto, F.; Saito, S.; Goto, F.; Kosaka, T.; Ieta, K. Pilot Study of the Effects of ONO-5046 in Patients with Acute Respiratory Distress Syndrome. Obstet. Anesth. Dig. 2004, 99, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The berlin definition. JAMA 2012, 307, 2526–2533. [Google Scholar]

| Total (n = 110) | Control Group (n = 65) | Sivelestat Group (n = 45) | p Value | |
|---|---|---|---|---|
| Age/Md(IQR), (years) | 84.0 (69.0, 91.0) | 88.0 (71.5, 91.5) | 77.0 (68.5, 88.0) | 0.055 |
| Male/(n,%) | 87 (79.1%) | 55 (84.6%) | 32 (71.1%) | 0.087 |
| Length of hospitalization/Md(IQR), (days) | 17.0 (10.0, 33.25) | 19.0 (8.5, 35.5) | 16.0 (13.0, 29.0) | 0.910 |
| Sivelestat-using days/Md(IQR), (days) | 6.0 (5.0, 8.5) | |||
| Smoking (n,%) | 33 (30.0%) | 20 (30.8%) | 13 (28.9%) | 0.832 |
| Alcohol (n,%) | 21 (19.1%) | 12 (18.5%) | 9 (20.0%) | 0.840 |
| Treatments (n,%) | ||||
| Mechanical ventilation | 110 (100%) | 65 (100%) | 45 (100%) | |
| CRRT | 36 (32.7%) | 20 (30.8%) | 16 (35.6%) | 0.599 |
| Antibiotics | 109 (99.1%) | 64 (98.5%) | 45 (100.0%) | 0.403 |
| Glucocorticoid | 104 (94.5%) | 63 (96.6%) | 41 (91.1%) | 0.187 |
| Immunoglobulin | 19 (17.3%) | 11 (16.9%) | 8 (17.8%) | 0.907 |
| Vasopressor | 78 (70.9%) | 46 (70.8%) | 32 (71.1%) | 0.969 |
| Paxlovid | 57 (51.8%) | 37 (56.9%) | 20 (44.4%) | 0.198 |
| Comorbidities (n,%) | ||||
| Hypertension | 68 (61.8%) | 43 (66.2%) | 25 (55.6%) | 0.261 |
| Diabetes | 44 (40.0%) | 30 (46.2%) | 14 (31.1%) | 0.113 |
| Congestive heart failure | 45 (40.9%) | 30 (46.2%) | 15 (33.3%) | 0.179 |
| Chronic pulmonary disease | 17 (15.5%) | 12 (18.5%) | 5 (11.1%) | 0.294 |
| Chronic renal disease | 17 (15.5%) | 9 (13.8%) | 8 (17.8%) | 0.575 |
| Liver disease | 18 (16.4%) | 9 (13.8%) | 9 (20.0%) | 0.391 |
| Tumor | 17 (15.5%) | 13 (20.0%) | 4 (8.9%) | 0.113 |
| Cerebrovascular disease | 36 (32.7%) | 22 (33.8%) | 14 (31.1%) | 0.764 |
| Others | 57 (51.8%) | 33 (50.8%) | 24 (53.3%) | 0.791 |
| Death (n,%) | 76 (69.1%) | 48 (73.8%) | 28 (62.2%) | 0.195 |
| Vital sign ( ± s) | ||||
| Body temperature (°C) | 36.9 ± 0.7 | 36.9 ± 0.7 | 37.0 ± 0.8 | 0.695 |
| Heart rate (/min) | 86.6 ± 16.0 | 88.0 ± 16.4 | 84.6 ± 17.8 | 0.311 |
| Systolic blood pressure (mmHg) | 133.4 ± 23.5 | 134.6 ± 21.6 | 131.8 ± 26.3 | 0.552 |
| PaO2/FiO2 (mmHg) D0 | 178.0 (109.0, 261.0) | 202.0 (112.0, 295.0) | 157.0 (104.0, 213.0) | 0.044 |
| PaO2/FiO2 (mmHg) D5 | 206.0 (135.0, 280.0) | 192.0 (129.0, 256.0) | 211.0 (160.0, 272.0) | 0.430 |
| Laboratory indicators/Md(IQR), ( ± s) | ||||
| WBC(×109/L) | 9.79 ± 5.60 | 8.39 ± 4.06 | 11.80 ± 6.83 | 0.004 |
| Hb(g/L) | 121.4 ± 22.3 | 125.0 ± 21.7 | 118.9 ± 23.2 | 0.336 |
| PLT/(×109/L) | 157.3 ± 39.4 | 161.7 ± 42.7 | 150.9 ± 36.0 | 0.484 |
| Alb/(g/L) | 30.2 ± 4.3 | 30.7 ± 4.0 | 29.3 ± 4.6 | 0.076 |
| ALT/(U/L) | 25.0 (17.0, 45.9) | 23.1 (17.4, 44.2) | 28.4 (16.1, 47.5) | 0.308 |
| AST/(U/L) | 36.5 (24.8, 61.3) | 35.2 (23.4, 49.1) | 38.9 (26.6, 75.5) | 0.315 |
| Cr/(umol/L) | 80.5 (59.8, 120.3) | 82.8 (66.2, 121.5) | 67.5 (58.9, 126.7) | 0.335 |
| BUN/(mmol/L) | 8.87 (6.71, 15.30) | 8.37 (6.79, 14.54) | 9.22 (6.35, 16.13) | 0.615 |
| PT/(s) | 14.6 ± 5.3 | 14.3 ± 2.8 | 15.2 ± 7.6 | 0.399 |
| CRP/(mg/L) | ||||
| D0 | 34.7 (10.4, 91.5) | 33.5 (10.3, 96.1) | 35.1 (10.0, 90.4) | 0.870 |
| D3 | 19.3 (6.0, 50.2) | 25.7 (7.7, 50.8) | 15.1 (5.7, 50.3) | 0.373 |
| D7 | 18.5 (4.0, 56.3) | 23.4 (5.1, 77.0) | 16.3 (3.7, 51.5) | 0.352 |
| IL-6/(ng/L) | ||||
| D0 | 36.3 (21.1, 121.5) | 33.7 (16.1, 135.3) | 39.2 (20.3, 91.6) | 0.51 |
| D3 | 21.8 (13.2, 69.0) | 20.5 (9.4, 72.7) | 24.2 (12.7, 68.9) | 0.332 |
| D7 | 24.0 (16.9, 92.6) | 22.2 (7.9, 76.4) | 26.8 (17.2, 115.0) | 0.236 |
| PCT/(ug/L) | ||||
| D0 | 0.28 (0.07, 1.28) | 0.27 (0.08, 1.00) | 0.31 (0.04, 1.99) | 0.447 |
| D3 | 0.18 (0.05, 0.82) | 0.18 (0.05, 0.69) | 0.24 (0.03, 0.91) | 0.329 |
| D7 | 0.21 (0.06, 0.93) | 0.12 (0.08, 0.84) | 0.34 (0.03, 1.25) | 0.336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Che, X.; Hu, W.; Zhang, Z.; Wang, L.; Xu, Z.; Wang, F. Efficacy Analysis and Prognostic Impact of Sivelestat Sodium in Coronavirus Disease 2019-Related Acute Respiratory Distress Syndrome. Pharmaceuticals 2024, 17, 368. https://doi.org/10.3390/ph17030368
Che X, Hu W, Zhang Z, Wang L, Xu Z, Wang F. Efficacy Analysis and Prognostic Impact of Sivelestat Sodium in Coronavirus Disease 2019-Related Acute Respiratory Distress Syndrome. Pharmaceuticals. 2024; 17(3):368. https://doi.org/10.3390/ph17030368
Chicago/Turabian StyleChe, Xiao, Wei Hu, Ziying Zhang, Lexiao Wang, Zhe Xu, and Fusheng Wang. 2024. "Efficacy Analysis and Prognostic Impact of Sivelestat Sodium in Coronavirus Disease 2019-Related Acute Respiratory Distress Syndrome" Pharmaceuticals 17, no. 3: 368. https://doi.org/10.3390/ph17030368
APA StyleChe, X., Hu, W., Zhang, Z., Wang, L., Xu, Z., & Wang, F. (2024). Efficacy Analysis and Prognostic Impact of Sivelestat Sodium in Coronavirus Disease 2019-Related Acute Respiratory Distress Syndrome. Pharmaceuticals, 17(3), 368. https://doi.org/10.3390/ph17030368






