The Current Therapeutic Landscape for Metastatic Prostate Cancer
Abstract
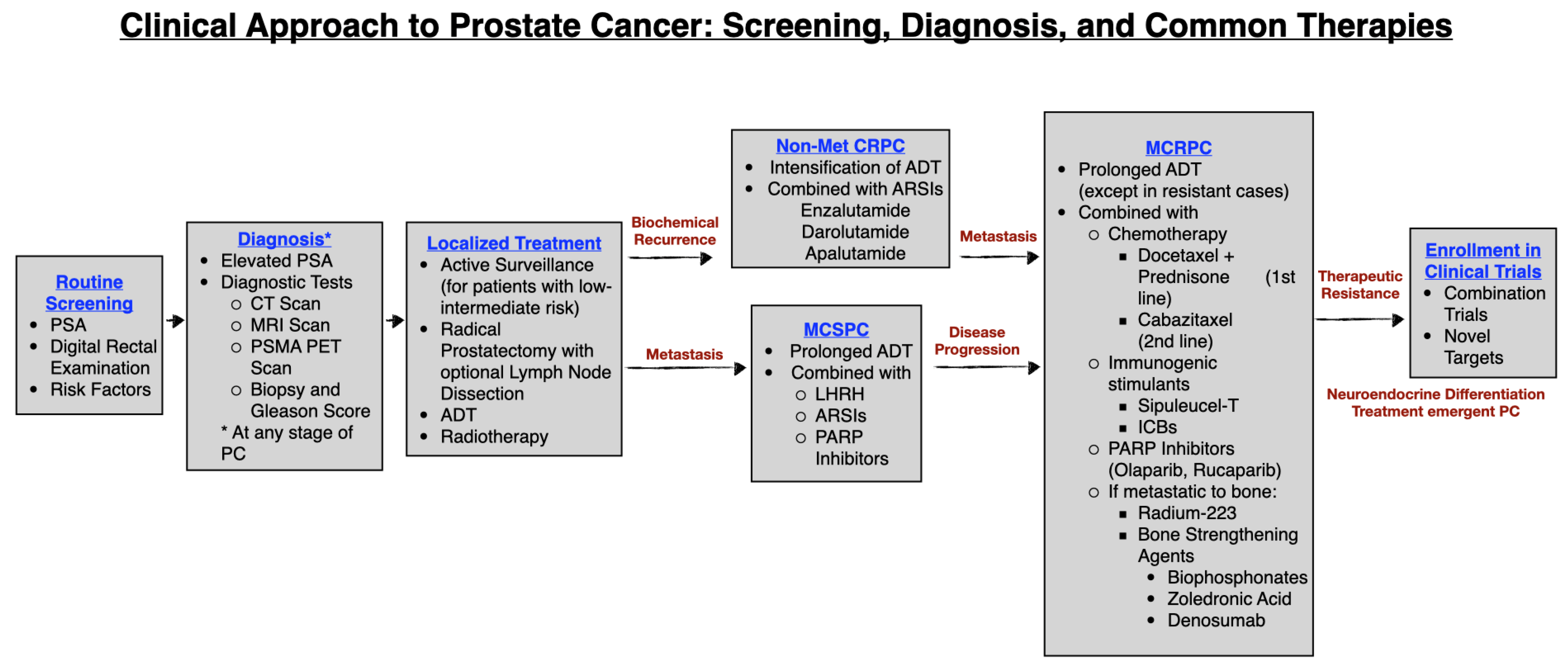
1. Introduction
1.1. Prostate Cancer Statistics
1.2. Diagnosis of Prostate Cancer
1.2.1. Diagnostic Tests
1.2.2. Biopsy and Gleason Score
1.2.3. Histological Classification
1.2.4. Imaging
1.3. Common Lines of Treatment: Localized Prostate Cancer
1.4. Non-Metastatic Castration Resistant Prostate Cancer (NMCRPC)
1.5. Advanced or Metastatic PC
1.6. Metastatic Castration Sensitive Prostate Cancer (MCSPC)
1.7. Metastatic Castration Resistant Prostate Cancer (MCRPC)
1.8. Metastatic NEPC (MNEPC)
2. Clinical Trials for Late-Stage Neuroendocrine Prostate Cancer
2.1. Combination Clinical Trials
2.2. Novel Targets for Neuroendocrine Prostate Cancer
2.2.1. Target: DLL3
2.2.2. Target: EZH2
2.2.3. Target: B7-H3
2.2.4. Target: Aurora Kinase
2.2.5. Target: PD-1 and PD-L1
2.2.6. Target: Receptor Tyrosine Kinases
3. Discussion
3.1. Combination Trials
3.2. Novel Target Trials
3.3. Necessity of Biomarkers for Prevention and Understanding PC
3.4. Landscape of NEPC: Knowledge and Therapy
3.5. Limitations and Concerns
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ferlay, J.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. International Agency for Research on Cancer, Lyon, France. 2024. Available online: https://gco.iarc.fr/today/en/dataviz/pie?mode=population&group_populations=0&cancers=27 (accessed on 12 January 2024).
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA A Cancer J Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Mukherjee, S.; Papadopoulos, D.; Norris, J.M.; Wani, M.; Madaan, S. Comparison of Outcomes of Active Surveillance in Intermediate-Risk Versus Low-Risk Localised Prostate Cancer Patients: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 2732. [Google Scholar] [CrossRef]
- Kramer, B.S. Prostate Cancer Screening: What We Know and What We Need To Know. Ann. Intern. Med. 1993, 119, 914. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B.; Albertsen, P.C.; Barry, M.J.; Etzioni, R.; Freedland, S.J.; Greene, K.L.; Holmberg, L.; Kantoff, P.; Konety, B.R.; Murad, M.H.; et al. Early Detection of Prostate Cancer: AUA Guideline. J. Urol. 2013, 190, 419–426. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901. [Google Scholar] [CrossRef] [PubMed]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and Overtreatment of Prostate Cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef]
- Catalona, W.J.; Richie, J.P.; Ahmann, F.R.; Hudson, M.A.; Scardino, P.T.; Flanigan, R.C.; Dekernion, J.B.; Ratliff, T.L.; Kavoussi, L.R.; Dalkin, B.L.; et al. Comparison of Digital Rectal Examination and Serum Prostate Specific Antigen in the Early Detection of Prostate Cancer: Results of a Multicenter Clinical Trial of 6630 Men. J. Urol. 1994, 151, 1283–1290. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 161, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.T.; D’Elia, G.; Caliendo, G.; Resse, M.; Casamassimi, A.; Passariello, L.; Albanese, L.; Cioffi, M.; Molinari, A.M. Hereditary Prostate Cancer: Genes Related, Target Therapy and Prevention. Int. J. Mol. Sci. 2021, 22, 3753. [Google Scholar] [CrossRef]
- Kweldam, C.F.; van Leenders, G.J.; van der Kwast, T. Grading of Prostate Cancer: A Work in Progress. Histopathology 2019, 74, 146–160. [Google Scholar] [CrossRef]
- Gleason, D.F. Histologic Grading of Prostate Cancer: A Perspective. Hum. Pathol. 1992, 23, 273–279. [Google Scholar] [CrossRef]
- Lester, S.C. Manual of Surgical Pathology, 3rd ed.; Elsevier Saunders: Philadelphia, PA, USA, 2010. [Google Scholar]
- Stark, J.R.; Perner, S.; Stampfer, M.J.; Sinnott, J.A.; Finn, S.; Eisenstein, A.S.; Ma, J.; Fiorentino, M.; Kurth, T.; Loda, M.; et al. Gleason Score and Lethal Prostate Cancer: Does 3 + 4 = 4 + 3? J. Clin. Oncol. 2009, 27, 3459–3464. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Amin, M.B.; Beltran, H.; Lotan, T.L.; Mosquera, J.-M.; Reuter, V.E.; Robinson, B.D.; Troncoso, P.; Rubin, M.A. Proposed Morphologic Classification of Prostate Cancer With Neuroendocrine Differentiation. Am. J. Surg. Pathol. 2014, 38, 756–767. [Google Scholar] [CrossRef]
- Beltran, H.; Demichelis, F. Therapy Considerations in Neuroendocrine Prostate Cancer: What Next? Endocr.-Relat. Cancer 2021, 28, T67–T78. [Google Scholar] [CrossRef] [PubMed]
- Fanti, S.; Minozzi, S.; Antoch, G.; Banks, I.; Briganti, A.; Carrio, I.; Chiti, A.; Clarke, N.; Eiber, M.; De Bono, J.; et al. Consensus on Molecular Imaging and Theranostics in Prostate Cancer. Lancet Oncol. 2018, 19, e696–e708. [Google Scholar] [CrossRef]
- Salembier, C.; Villeirs, G.; De Bari, B.; Hoskin, P.; Pieters, B.R.; Van Vulpen, M.; Khoo, V.; Henry, A.; Bossi, A.; De Meerleer, G.; et al. ESTRO ACROP Consensus Guideline on CT- and MRI-Based Target Volume Delineation for Primary Radiation Therapy of Localized Prostate Cancer. Radiother. Oncol. 2018, 127, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Ferrante di Ruffano, L.; Takwoingi, Y.; Cheung, S.T.; Nathan, P.; Matin, R.N.; Chuchu, N.; Chan, S.A.; Durack, A.; Bayliss, S.E.; et al. Ultrasound, CT, MRI, or PET-CT for Staging and Re-Staging of Adults with Cutaneous Melanoma. Cochrane Database Syst. Rev. 2019, 7, CD012806. [Google Scholar] [CrossRef]
- Ceci, F.; Fiorentino, M.; Castellucci, P.; Fanti, S. Molecular Imaging and Precision Medicine in Prostate Cancer. PET Clin. 2017, 12, 83–92. [Google Scholar] [CrossRef]
- Pianou, N.K.; Stavrou, P.Z.; Vlontzou, E.; Rondogianni, P.; Exarhos, D.N.; Datseris, I.E. More Advantages in Detecting Bone and Soft Tissue Metastases from Prostate Cancer Using 18F-PSMA PET/CT. Hell. J. Nucl. Med. 2019, 22, 6–9. [Google Scholar]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van Der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef]
- Bangma, C.H.; Valdagni, R.; Carroll, P.R.; van Poppel, H.; Klotz, L.; Hugosson, J. Active Surveillance for Low-Risk Prostate Cancer: Developments to Date. Eur. Urol. 2015, 67, 646–648. [Google Scholar] [CrossRef]
- Roehl, K.A.; Han, M.; Ramos, C.G.; Antenor, J.A.V.; Catalona, W.J. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3478 consecutive patients: Long-term results. J. Urol. 2004, 172, 910–914. [Google Scholar] [CrossRef]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of Prostate Cancer–Specific Mortality Following Biochemical Recurrence After Radical Prostatectomy. JAMA 2005, 294, 433. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.C.; James, N.D.; Brawley, C.D.; Clarke, N.W.; Hoyle, A.P.; Ali, A.; Ritchie, A.W.S.; Attard, G.; Chowdhury, S.; Cross, W.; et al. Radiotherapy to the Primary Tumour for Newly Diagnosed, Metastatic Prostate Cancer (STAMPEDE): A Randomised Controlled Phase 3 Trial. Lancet 2018, 392, 2353–2366. [Google Scholar] [CrossRef] [PubMed]
- Gay, H.A.; Michalski, J.M. Radiation Therapy for Prostate Cancer. Mo. Med. 2018, 115, 146–150. [Google Scholar] [PubMed]
- Stish, B.J.; Pisansky, T.M.; Harmsen, W.S.; Davis, B.J.; Tzou, K.S.; Choo, R.; Buskirk, S.J. Improved Metastasis-Free and Survival Outcomes with Early Salvage Radiotherapy in Men With Detectable Prostate-Specific Antigen After Prostatectomy for Prostate Cancer. J. Clin. Oncol. 2016, 34, 3864–3871. [Google Scholar] [CrossRef] [PubMed]
- Widmark, A.; Klepp, O.; Solberg, A.; Damber, J.-E.; Angelsen, A.; Fransson, P.; Lund, J.-Å.; Tasdemir, I.; Hoyer, M.; Wiklund, F.; et al. Endocrine Treatment, with or without Radiotherapy, in Locally Advanced Prostate Cancer (SPCG-7/SFUO-3): An Open Randomised Phase III Trial. Lancet 2009, 373, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Golabek, T.; Belsey, J.; Drewa, T.; Kołodziej, A.; Skoneczna, I.; Milecki, P.; Dobruch, J.; Słojewski, M.; Chłosta, P.L. Evidence-Based Recommendations on Androgen Deprivation Therapy for Localized and Advanced Prostate Cancer. Cent. Eur. J. Urol. 2016, 69, 131–138. [Google Scholar] [CrossRef]
- Gunner, C.; Gulamhusein, A.; Rosario, D.J. The Modern Role of Androgen Deprivation Therapy in the Management of Localised and Locally Advanced Prostate Cancer. J. Clin. Urol. 2016, 9, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.P.; Weber, M.; Iravani, A.; Hofman, M.S.; Calais, J.; Czernin, J.; Ilhan, H.; Saad, F.; Small, E.J.; Smith, M.R.; et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 7448–7454. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, C.N.; Fizazi, K.; Saad, F.; Shore, N.D.; De Giorgi, U.; Penson, D.F.; Ferreira, U.; Efstathiou, E.; Madziarska, K.; Kolinsky, M.P.; et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide Treatment and Metastasis-Free Survival in Prostate Cancer. N. Engl. J. Med. 2018, 378, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Fizazi, K.; Saad, F.; Rathenborg, P.; Shore, N.; Ferreira, U.; Ivashchenko, P.; Demirhan, E.; Modelska, K.; Phung, D.; et al. Enzalutamide in Men with Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2018, 378, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the Castration-Resistant Prostate Cancer Population: A Systematic Review: The Epidemiology of CRPC. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N. Engl. J. Med. 2020, 383, 1040–1049. [Google Scholar] [CrossRef]
- Smith, M.R.; Saad, F.; Chowdhury, S.; Oudard, S.; Hadaschik, B.A.; Graff, J.N.; Olmos, D.; Mainwaring, P.N.; Lee, J.Y.; Uemura, H.; et al. Apalutamide and Overall Survival in Prostate Cancer. Eur. Urol. 2021, 79, 150–158. [Google Scholar] [CrossRef]
- Schultz, N.M.; O’Day, K.; Sugarman, R.; Ramaswamy, K. Budget Impact of Enzalutamide for Nonmetastatic Castration-Resistant Prostate Cancer. J. Manag. Care Spec. Pharm. 2020, 26, 538–549. [Google Scholar] [CrossRef]
- Gravis, G.; Fizazi, K.; Joly, F.; Oudard, S.; Priou, F.; Esterni, B.; Latorzeff, I.; Delva, R.; Krakowski, I.; Laguerre, B.; et al. Androgen-Deprivation Therapy Alone or with Docetaxel in Non-Castrate Metastatic Prostate Cancer (GETUG-AFU 15): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2013, 14, 149–158. [Google Scholar] [CrossRef]
- Ong, S.; O’Brien, J.; Medhurst, E.; Lawrentschuk, N.; Murphy, D.; Azad, A. Current Treatment Options for Newly Diagnosed Metastatic Hormone-Sensitive Prostate Cancer—A Narrative Review. Transl. Androl. Urol. 2021, 10, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Virgo, K.S.; Rumble, R.B.; De Wit, R.; Mendelson, D.S.; Smith, T.J.; Taplin, M.-E.; Wade, J.L.; Bennett, C.L.; Scher, H.I.; Nguyen, P.L.; et al. Initial Management of Noncastrate Advanced, Recurrent, or Metastatic Prostate Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 1274–1305. [Google Scholar] [CrossRef] [PubMed]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic Genomic Alterations, Clinical Phenotypes, and Outcomes in Metastatic Castration-Sensitive Prostate Cancer. Clin. Cancer Res. 2020, 26, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Bossi, A.; Davis, I.D.; De Bono, J.; Fizazi, K.; James, N.D.; Mottet, N.; Shore, N.; Small, E.; Smith, M.; et al. Management of Patients with Advanced Prostate Cancer. Part I: Intermediate-/High-Risk and Locally Advanced Disease, Biochemical Relapse, and Side Effects of Hormonal Treatment: Report of the Advanced Prostate Cancer Consensus Conference 2022. Eur. Urol. 2023, 83, 267–293. [Google Scholar] [CrossRef] [PubMed]
- Formaggio, N.; Rubin, M.A.; Theurillat, J.-P. Loss and Revival of Androgen Receptor Signaling in Advanced Prostate Cancer. Oncogene 2021, 40, 1205–1216. [Google Scholar] [CrossRef]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging Mechanisms of Resistance to Androgen Receptor Inhibitors in Prostate Cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef]
- Beltran, H.; Prandi, D.; Mosquera, J.M.; Benelli, M.; Puca, L.; Cyrta, J.; Marotz, C.; Giannopoulou, E.; Chakravarthi, B.V.S.K.; Varambally, S.; et al. Divergent Clonal Evolution of Castration-Resistant Neuroendocrine Prostate Cancer. Nat. Med. 2016, 22, 298–305. [Google Scholar] [CrossRef]
- Wade, C.A.; Kyprianou, N. Profiling Prostate Cancer Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 904. [Google Scholar] [CrossRef]
- Smith, M.R.; Kabbinavar, F.; Saad, F.; Hussain, A.; Gittelman, M.C.; Bilhartz, D.L.; Wynne, C.; Murray, R.; Zinner, N.R.; Schulman, C.; et al. Natural History of Rising Serum Prostate-Specific Antigen in Men with Castrate Nonmetastatic Prostate Cancer. J. Clin. Oncol. 2005, 23, 2918–2925. [Google Scholar] [CrossRef]
- Kishan, A.U.; Wang, X.; Seiferheld, W.; Collette, L.; Sandler, K.A.; Sandler, H.M.; Bolla, M.; Maingon, P.; De Reijke, T.; Hanks, G.E.; et al. Association of Gleason Grade With Androgen Deprivation Therapy Duration and Survival Outcomes: A Systematic Review and Patient-Level Meta-Analysis. JAMA Oncol. 2019, 5, 91. [Google Scholar] [CrossRef]
- Bolla, M.; De Reijke, T.M.; Van Tienhoven, G.; Van Den Bergh, A.C.M.; Oddens, J.; Poortmans, P.M.P.; Gez, E.; Kil, P.; Akdas, A.; Soete, G.; et al. Duration of Androgen Suppression in the Treatment of Prostate Cancer. N. Engl. J. Med. 2009, 360, 2516–2527. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef]
- Narayan, V.; Ross, A.E.; Parikh, R.B.; Nohria, A.; Morgans, A.K. How to Treat Prostate Cancer with Androgen Deprivation and Minimize Cardiovascular Risk. JACC CardioOncology 2021, 3, 737–741. [Google Scholar] [CrossRef]
- Shahinian, V.B.; Kuo, Y.-F.; Freeman, J.L.; Goodwin, J.S. Risk of Fracture after Androgen Deprivation for Prostate Cancer. N. Engl. J. Med. 2005, 352, 154–164. [Google Scholar] [CrossRef]
- Beltran, H.; Hruszkewycz, A.; Scher, H.I.; Hildesheim, J.; Isaacs, J.; Yu, E.Y.; Kelly, K.; Lin, D.; Dicker, A.; Arnold, J.; et al. The Role of Lineage Plasticity in Prostate Cancer Therapy Resistance. Clin. Cancer Res. 2019, 25, 6916–6924. [Google Scholar] [CrossRef]
- Aggarwal, R.; Huang, J.; Alumkal, J.J.; Zhang, L.; Feng, F.Y.; Thomas, G.V.; Weinstein, A.S.; Friedl, V.; Zhang, C.; Witte, O.N.; et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-Institutional Prospective Study. J. Clin. Oncol. 2018, 36, 2492–2503. [Google Scholar] [CrossRef]
- de Bono, J.S.; Logothetis, C.J.; Molina, A.; Fizazi, K.; North, S.; Chu, L.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Saad, F.; et al. Abiraterone and Increased Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2011, 364, 1995–2005. [Google Scholar] [CrossRef]
- Oudard, S.; Fizazi, K.; Sengeløv, L.; Daugaard, G.; Saad, F.; Hansen, S.; Hjälm-Eriksson, M.; Jassem, J.; Thiery-Vuillemin, A.; Caffo, O.; et al. Cabazitaxel Versus Docetaxel As First-Line Therapy for Patients with Metastatic Castration-Resistant Prostate Cancer: A Randomized Phase III Trial-FIRSTANA. J. Clin. Oncol. 2017, 35, 3189–3197. [Google Scholar] [CrossRef]
- Agarwal, N.; Padmanabh, S.; Vogelzang, N.J. Development of Novel Immune Interventions for Prostate Cancer. Clin. Genitourin. Cancer 2012, 10, 84–92. [Google Scholar] [CrossRef]
- Bieńkowski, M.; Tomasik, B.; Braun, M.; Jassem, J. PARP Inhibitors for Metastatic Castration-Resistant Prostate Cancer: Biological Rationale and Current Evidence. Cancer Treat. Rev. 2022, 104, 102359. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Wang, R.; Han, Y.; Zhao, Z.; Yang, F.; Chen, T.; Zhou, W.; Wang, X.; Qi, L.; Zhao, W.; Guo, Z.; et al. Link Synthetic Lethality to Drug Sensitivity of Cancer Cells. Brief. Bioinform. 2019, 20, 1295–1307. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- de Bono, J.; Mateo, J.; Fizazi, K.; Saad, F.; Shore, N.; Sandhu, S.; Chi, K.N.; Sartor, O.; Agarwal, N.; Olmos, D.; et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2020, 382, 2091–2102. [Google Scholar] [CrossRef]
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Zheng, M.; et al. Long-Term Efficacy of Zoledronic Acid for the Prevention of Skeletal Complications in Patients with Metastatic Hormone-Refractory Prostate Cancer. J. Natl. Cancer Inst. 2004, 96, 879–882. [Google Scholar] [CrossRef]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus Zoledronic Acid for Treatment of Bone Metastases in Men with Castration-Resistant Prostate Cancer: A Randomised, Double-Blind Study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A Randomized, Placebo-Controlled Trial of Zoledronic Acid in Patients with Hormone-Refractory Metastatic Prostate Carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gorin, M.A.; Pomper, M.G. Imaging of Prostate-Specific Membrane Antigen with Small-Molecule PET Radiotracers: From the Bench to Advanced Clinical Applications. Annu. Rev. Med. 2019, 70, 461–477. [Google Scholar] [CrossRef]
- Sartor, O.; De Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef]
- James, N.D.; De Bono, J.S.; Spears, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Ritchie, A.W.S.; Amos, C.L.; Gilson, C.; Jones, R.J.; et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N. Engl. J. Med. 2017, 377, 338–351. [Google Scholar] [CrossRef]
- James, N.D.; Sydes, M.R.; Clarke, N.W.; Mason, M.D.; Dearnaley, D.P.; Spears, M.R.; Ritchie, A.W.S.; Parker, C.C.; Russell, J.M.; Attard, G.; et al. Addition of Docetaxel, Zoledronic Acid, or Both to First-Line Long-Term Hormone Therapy in Prostate Cancer (STAMPEDE): Survival Results from an Adaptive, Multiarm, Multistage, Platform Randomised Controlled Trial. Lancet 2016, 387, 1163–1177. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.; Matsubara, N.; Rodriguez-Antolin, A.; Alekseev, B.Y.; Özgüroğlu, M.; Ye, D.; Feyerabend, S.; Protheroe, A.; et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N. Engl. J. Med. 2017, 377, 352–360. [Google Scholar] [CrossRef]
- Puca, L.; Gavyert, K.; Sailer, V.; Conteduca, V.; Dardenne, E.; Sigouros, M.; Isse, K.; Kearney, M.; Vosoughi, A.; Fernandez, L.; et al. Delta-like Protein 3 Expression and Therapeutic Targeting in Neuroendocrine Prostate Cancer. Sci. Transl. Med. 2019, 11, eaav0891. [Google Scholar] [CrossRef]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A.; et al. A DLL3-Targeted Antibody-Drug Conjugate Eradicates High-Grade Pulmonary Neuroendocrine Tumor-Initiating Cells In Vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef]
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M.; et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results From the Phase II TRINITY Study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef]
- Ku, S.-Y.; Gleave, M.E.; Beltran, H. Towards Precision Oncology in Advanced Prostate Cancer. Nat. Rev. Urol. 2019, 16, 645–654. [Google Scholar] [CrossRef]
- Li, Y.; Donmez, N.; Sahinalp, C.; Xie, N.; Wang, Y.; Xue, H.; Mo, F.; Beltran, H.; Gleave, M.; Wang, Y.; et al. SRRM4 Drives Neuroendocrine Transdifferentiation of Prostate Adenocarcinoma Under Androgen Receptor Pathway Inhibition. Eur. Urol. 2017, 71, 68–78. [Google Scholar] [CrossRef]
- Flores-Morales, A.; Bergmann, T.B.; Lavallee, C.; Batth, T.S.; Lin, D.; Lerdrup, M.; Friis, S.; Bartels, A.; Kristensen, G.; Krzyzanowska, A.; et al. Proteogenomic Characterization of Patient-Derived Xenografts Highlights the Role of REST in Neuroendocrine Differentiation of Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 595–608. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Vourda, A.; Syggelos, S.; Gyftopoulos, K. Cell Plasticity and Prostate Cancer: The Role of Epithelial–Mesenchymal Transition in Tumor Progression, Invasion, Metastasis and Cancer Therapy Resistance. Cancers 2021, 13, 2795. [Google Scholar] [CrossRef]
- Davies, A.; Zoubeidi, A.; Selth, L.A. The Epigenetic and Transcriptional Landscape of Neuroendocrine Prostate Cancer. Endocr. Relat. Cancer 2020, 27, R35–R50. [Google Scholar] [CrossRef]
- Dardenne, E.; Beltran, H.; Benelli, M.; Gayvert, K.; Berger, A.; Puca, L.; Cyrta, J.; Sboner, A.; Noorzad, Z.; MacDonald, T.; et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016, 30, 563–577. [Google Scholar] [CrossRef]
- Li, G.; Quan, Y.; Che, F.; Wang, L. B7-H3 in Tumors: Friend or Foe for Tumor Immunity? Cancer Chemother. Pharmacol. 2018, 81, 245–253. [Google Scholar] [CrossRef]
- Ross, A.; Benzon, B.; Zhao, S.; Takhar, M.; Haffner, M.; Erho, N.; Hurley, P.; Tosoian, J.J.; Alshalalfa, M.; Glavaris, S.; et al. The Relationship of B7H3 Expression to Androgen and Prostate Cancer Outcomes in a Large Natural History Cohort of Men Undergoing Prostatectomy. J. Clin. Oncol. 2016, 34, 256. [Google Scholar] [CrossRef]
- Guo, C.; Sharp, A.; Gurel, B.; Crespo, M.; Figueiredo, I.; Jain, S.; Vogl, U.; Rekowski, J.; Rouhifard, M.; Gallagher, L.; et al. Targeting Myeloid Chemotaxis to Reverse Prostate Cancer Therapy Resistance. Nature 2023, 623, 1053–1061. [Google Scholar] [CrossRef]
- Shi, X.; Day, A.; Bergom, H.E.; Tape, S.; Baca, S.C.; Sychev, Z.E.; Larson, G.; Bozicevich, A.; Drake, J.M.; Zorko, N.; et al. Integrative Molecular Analyses Define Correlates of High B7-H3 Expression in Metastatic Castrate-Resistant Prostate Cancer. npj Precis. Oncol. 2022, 6, 80. [Google Scholar] [CrossRef]
- Mou, P.K.; Yang, E.J.; Shi, C.; Ren, G.; Tao, S.; Shim, J.S. Aurora Kinase A, a Synthetic Lethal Target for Precision Cancer Medicine. Exp. Mol. Med. 2021, 53, 835–847. [Google Scholar] [CrossRef]
- Beltran, H.; Oromendia, C.; Danila, D.C.; Montgomery, B.; Hoimes, C.; Szmulewitz, R.Z.; Vaishampayan, U.; Armstrong, A.J.; Stein, M.; Pinski, J.; et al. A Phase II Trial of the Aurora Kinase A Inhibitor Alisertib for Patients with Castration-Resistant and Neuroendocrine Prostate Cancer: Efficacy and Biomarkers. Clin. Cancer Res. 2019, 25, 43–51. [Google Scholar] [CrossRef]
- Fourcade, J.; Sun, Z.; Benallaoua, M.; Guillaume, P.; Luescher, I.F.; Sander, C.; Kirkwood, J.M.; Kuchroo, V.; Zarour, H.M. Upregulation of Tim-3 and PD-1 Expression Is Associated with Tumor Antigen-Specific CD8+ T Cell Dysfunction in Melanoma Patients. J. Exp. Med. 2010, 207, 2175–2186. [Google Scholar] [CrossRef]
- Karwacz, K.; Bricogne, C.; MacDonald, D.; Arce, F.; Bennett, C.L.; Collins, M.; Escors, D. PD-L1 Co-Stimulation Contributes to Ligand-Induced T Cell Receptor down-Modulation on CD8+ T Cells. EMBO Mol. Med. 2011, 3, 581–592. [Google Scholar] [CrossRef]
- DeAngelis, N.; Ferrante, C.; Powers, G.; Sendecki, J.; Mattson, B.; Pizutti, D.; Packman, K.; Wang, W.; Trouba, K.; Nanjunda, R.; et al. Discovery and Pharmacological Characterization of Cetrelimab (JNJ-63723283), an Anti-Programmed Cell Death Protein-1 (PD-1) Antibody, in Human Cancer Models. Cancer Chemother. Pharmacol. 2022, 89, 515–527. [Google Scholar] [CrossRef]
- Su, R.; Chen, L.; Jiang, Z.; Yu, M.; Zhang, W.; Ma, Z.; Ji, Y.; Shen, K.; Xin, Z.; Qi, J.; et al. Comprehensive Analysis of Androgen Receptor Status in Prostate Cancer with Neuroendocrine Differentiation. Front. Oncol. 2022, 12, 955166. [Google Scholar] [CrossRef]
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J.M. Antibodies to Watch in 2020. mAbs 2020, 12, 1703531. [Google Scholar] [CrossRef]
- Collins, J.M.; Gulley, J.L. Product Review: Avelumab, an Anti-PD-L1 Antibody. Hum. Vaccin. Immunother. 2018, 15, 891–908. [Google Scholar] [CrossRef]
- Labrecque, M.P.; Brown, L.G.; Coleman, I.M.; Nguyen, H.M.; Lin, D.W.; Corey, E.; Nelson, P.S.; Morrissey, C. Cabozantinib Can Block Growth of Neuroendocrine Prostate Cancer Patient-Derived Xenografts by Disrupting Tumor Vasculature. PLoS ONE 2021, 16, e0245602. [Google Scholar] [CrossRef]
- Smith, M.; De Bono, J.; Sternberg, C.; Le Moulec, S.; Oudard, S.; De Giorgi, U.; Krainer, M.; Bergman, A.; Hoelzer, W.; De Wit, R.; et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J. Clin. Oncol. 2016, 34, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.M.; Scholz, M.C.; De Bono, J.S.; Vogelzang, N.J.; De Souza, P.L.; Marx, G.M.; Vaishampayan, U.N.; George, S.; Schwarz, J.K.; Antonarakis, E.S.; et al. Final Analysis of COMET-2: Cabozantinib (Cabo) versus Mitoxantrone/Prednisone (MP) in Metastatic Castration-Resistant Prostate Cancer (MCRPC) Patients (Pts) with Moderate to Severe Pain Who Were Previously Treated with Docetaxel (D) and Abiraterone (A) and/or Enzalutamide (E). J. Clin. Oncol. 2015, 33, 141. [Google Scholar] [CrossRef]
- Sonpavde, G.P.; Pond, G.R.; Fizazi, K.; de Bono, J.S.; Basch, E.M.; Scher, H.I.; Smith, M.R. Cabozantinib for Progressive Metastatic Castration-Resistant Prostate Cancer Following Docetaxel: Combined Analysis of Two Phase 3 Trials. Eur. Urol. Oncol. 2020, 3, 540–543. [Google Scholar] [CrossRef]
- Stultz, J.; Fong, L. How to Turn up the Heat on the Cold Immune Microenvironment of Metastatic Prostate Cancer. Prostate Cancer Prostatic Dis. 2021, 24, 697–717. [Google Scholar] [CrossRef] [PubMed]
- Klempner, S.J.; Borghei, A.; Hakimian, B.; Ali, S.M.; Ou, S.-H.I. Intracranial Activity of Cabozantinib in MET Exon 14–Positive NSCLC with Brain Metastases. J. Thorac. Oncol. 2017, 12, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A Phase 2 and Biomarker Study of Cabozantinib in Patients with Advanced Cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef]
- Deluce, J.E.; Cardenas, L.; Lalani, A.-K.; Maleki Vareki, S.; Fernandes, R. Emerging Biomarker-Guided Therapies in Prostate Cancer. Curr. Oncol. 2022, 29, 5054–5076. [Google Scholar] [CrossRef]
- Sanhueza, C.; Kohli, M. Clinical and Novel Biomarkers in the Management of Prostate Cancer. Curr. Treat. Options Oncol. 2018, 19, 8. [Google Scholar] [CrossRef]
- Cresta Morgado, P.; Mateo, J. Clinical Implications of Homologous Recombination Repair Mutations in Prostate Cancer. Prostate 2022, 82 (Suppl. S1), S45–S59. [Google Scholar] [CrossRef]
- Kohaar, I.; Petrovics, G.; Srivastava, S. A Rich Array of Prostate Cancer Molecular Biomarkers: Opportunities and Challenges. Int. J. Mol. Sci. 2019, 20, 1813. [Google Scholar] [CrossRef]
- Park, J.W.; Lee, J.K.; Sheu, K.M.; Wang, L.; Balanis, N.G.; Nguyen, K.; Smith, B.A.; Cheng, C.; Tsai, B.L.; Cheng, D.; et al. Reprogramming Normal Human Epithelial Tissues to a Common, Lethal Neuroendocrine Cancer Lineage. Science 2018, 362, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Alabi, B.R.; Liu, S.; Stoyanova, T. Current and Emerging Therapies for Neuroendocrine Prostate Cancer. Pharmacol. Ther. 2022, 238, 108255. [Google Scholar] [CrossRef] [PubMed]
- Beltran, H.; Rickman, D.S.; Park, K.; Chae, S.S.; Sboner, A.; MacDonald, T.Y.; Wang, Y.; Sheikh, K.L.; Terry, S.; Tagawa, S.T.; et al. Molecular Characterization of Neuroendocrine Prostate Cancer and Identification of New Drug Targets. Cancer Discov. 2011, 1, 487–495. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Pilié, P.G.; Geng, C.; Park, S.; Manyam, G.C.; Lu, Y.; Yang, G.; Tang, Z.; Kondraganti, S.; et al. PARP and CDK4/6 Inhibitor Combination Therapy Induces Apoptosis and Suppresses Neuroendocrine Differentiation in Prostate Cancer. Mol. Cancer Ther. 2021, 20, 1680–1691. [Google Scholar] [CrossRef]
- Quintanal-Villalonga, A.; Durani, V.; Sabet, A.; Redin, E.; Kawasaki, K.; Shafer, M.; Karthaus, W.R.; Zaidi, S.; Zhan, Y.A.; Manoj, P.; et al. Exportin 1 Inhibition Prevents Neuroendocrine Transformation through SOX2 Down-Regulation in Lung and Prostate Cancers. Sci. Transl. Med. 2023, 15, eadf7006. [Google Scholar] [CrossRef]
- Hu, C.-Y.; Wu, K.-Y.; Lin, T.-Y.; Chen, C.-C. The Crosstalk of Long Non-Coding RNA and MicroRNA in Castration-Resistant and Neuroendocrine Prostate Cancer: Their Interaction and Clinical Importance. Int. J. Mol. Sci. 2021, 23, 392. [Google Scholar] [CrossRef]
- Vogl, U.M.; Beer, T.M.; Davis, I.D.; Shore, N.D.; Sweeney, C.J.; Ost, P.; Attard, G.; Bossi, A.; De Bono, J.; Drake, C.G.; et al. Lack of Consensus Identifies Important Areas for Future Clinical Research: Advanced Prostate Cancer Consensus Conference (APCCC) 2019 Findings. Eur. J. Cancer 2022, 160, 24–60. [Google Scholar] [CrossRef]


| Trial Identifier | Status | Phase | Drug 1 | Drug 2 | Drug 3 | Drug 4 | Drug 5 |
|---|---|---|---|---|---|---|---|
| NCT02485691 | Completed | 4 | Enzalutamide | Abiraterone | Cabazitaxel | Docetaxel | |
| NCT00973882 | Completed, no results posted | 2 | Carboplatin | Etoposide | |||
| NCT00014456 | Completed, no results posted | 1 | Filgrastim | Docetaxel | Gemcitabine Hydrochloride | ||
| NCT04848337 | Recruiting | 2 | Pembrolizumab | Lanvatinib | |||
| NCT03910660 | Active, not recruiting | 1/2 | BXCL701 | Pembrolizumab | |||
| NCT04926181 | Active, not recruiting | 2 | Apalutamide | Cetrelimab | |||
| NCT03582475 | Completed, no results posted | 1 | Pembrolizumab | Carboplatin | Cisplatin | Docetaxel | Etoposide |
| NCT02893917 | Active, not recruiting | 2 | Cediranib | Olaparib | |||
| NCT04592237 | Recruiting | 2 | Cabazitaxel | Carboplatin | Cetrelimab | Niraparib | Cetrelimab (again) |
| NCT03902951 | Active, not recruiting | 2 | Abiraterone | Apalutamide | Leuprorelin | Stereotactic Body Radiation Therapy | |
| NCT05582031 | Withdrawn | 2 | Regorafenib | Tislelizumab | |||
| NCT03649841 | Terminated due to low accrual. | 2 | Antiandrogen therapy (unclear which drug/method was used) | Abiraterone | Prednisone | Radiation Therapy | |
| NCT05000294 | Recruiting | 1/2 | Atezolizumab | Tivozanib | |||
| NCT03333616 | Active, not recruiting | 2 | Nivolumab | Ipilimumab | |||
| NCT03896503 | Active, not recruiting | 2 | Topotecan | Berzosertib | |||
| NCT03365791 | Completed | 2 | Spartalizumab (PDR001) | Leramilimab | |||
| NCT03866382 | Recruiting | 2 | Nivolumab | Ipilimumab | Cabozantinib | ||
| DRKS00004797 | Completed, no results posted | Unknown | Docetaxel | Cabazitaxel | |||
| NCT03480646 | Unknown status | 1/2 | CPI-1205 | Enzalutamide | Abiraterone | Prednisone | |
| NCT02381314 | Completed | 1 | Enoblituzumab (MGA271) | Ipilimumab | |||
| NCT05293496 | Recruiting | 1/2 | Vobramitamab Duocarmazine (MGC018) | Lorigerlimab | |||
| NCT01848067 | Completed | 1 | Alisertib (MLN8237) | Docetaxel |
| Trial Identifier | Status | Phase | Drug/Treatment | Target | Additional Drugs | NEPC Exclusive Cohorts |
|---|---|---|---|---|---|---|
| NCT02709889 | Terminated | 1 | Rovalpituzumab tesirine (SC16LD6.5) | DLL3 | Yes | |
| NCT05652686 | Recruiting | 1 | PT217 | DLL3 | Yes | |
| NCT04702737 | Active, not recruiting | 1 | AMG 757 | DLL3 | Yes | |
| NCT03480646 | Unknown status | 1/2 | CPI-1205 | EZH2 | Enzalutamide, Abiraterone/ Prednisone | Yes |
| NCT02875548 | Active, not recruiting | 1/2 | Tazemetostat | EZH2 | No | |
| NCT02381314 | Completed | 1 | Enoblituzumab (MGA271) | B7-H3 | Ipilimumab | No |
| NCT05293496 | Recruiting | 1/2 | Vobramitamab Duocarmazine (MGC018) | B7-H3 | Lorigerlimab | No |
| NCT01094288 | Completed | 1 | Alisertib (MLN8237) | Aurora-A | Docetaxel | No |
| NCT01799278 | Completed | 2 | Alisertib (MLN8237) | Aurora-A | Yes | |
| NCT03179410 | Completed | 2 | Avelumab | PD-L1 | Yes | |
| NCT03365791 | Completed | 2 | PDR001 | PD-1 | Lag525 | No |
| NCT04926181 | Active, not recruiting | 2 | Cetrelimab (JNJ-63723283) | PD-1 | Apalutamide | Yes |
| NCT04592237 | Recruiting | 2 | Cetrelimab (JNJ-63723283) | PD-1 | Cabazitaxel, carboplatin, followed by nirparib | Yes |
| NCT03866382 | Recruiting | 2 | Cabozantinib | Receptor Tyrosine Kinases | Yes | |
| COMET-1, -2 | Completed | 3 | Cabozantinib | Receptor Tyrosine Kinases | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernal, A.; Bechler, A.J.; Mohan, K.; Rizzino, A.; Mathew, G. The Current Therapeutic Landscape for Metastatic Prostate Cancer. Pharmaceuticals 2024, 17, 351. https://doi.org/10.3390/ph17030351
Bernal A, Bechler AJ, Mohan K, Rizzino A, Mathew G. The Current Therapeutic Landscape for Metastatic Prostate Cancer. Pharmaceuticals. 2024; 17(3):351. https://doi.org/10.3390/ph17030351
Chicago/Turabian StyleBernal, Anastasia, Alivia Jane Bechler, Kabhilan Mohan, Angie Rizzino, and Grinu Mathew. 2024. "The Current Therapeutic Landscape for Metastatic Prostate Cancer" Pharmaceuticals 17, no. 3: 351. https://doi.org/10.3390/ph17030351
APA StyleBernal, A., Bechler, A. J., Mohan, K., Rizzino, A., & Mathew, G. (2024). The Current Therapeutic Landscape for Metastatic Prostate Cancer. Pharmaceuticals, 17(3), 351. https://doi.org/10.3390/ph17030351






