Marine-Derived Bioactive Metabolites as a Potential Therapeutic Intervention in Managing Viral Diseases: Insights from the SARS-CoV-2 In Silico and Pre-Clinical Studies
Abstract
1. Introduction
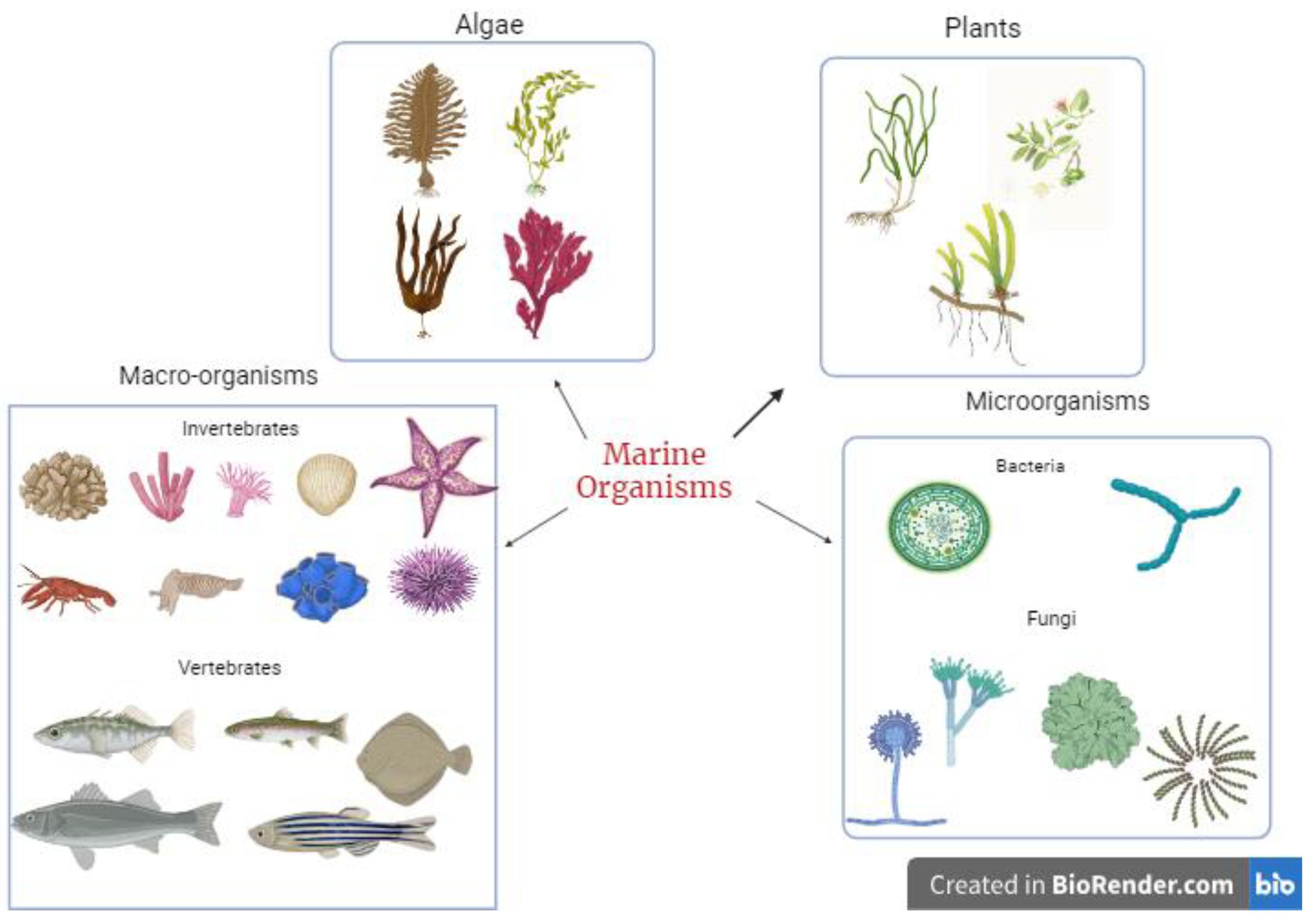
2. Metabolites from Marine Organisms
3. General Antiviral Activities of Metabolites from Different Marine Sources
3.1. Marine Microorganisms
3.1.1. Bacteria
3.1.2. Marine Fungi
3.1.3. Marine Algae
3.2. Marine Plants
3.3. Marine Macro-Organisms
3.3.1. Marine Invertebrates
3.3.2. Marine Vertebrates
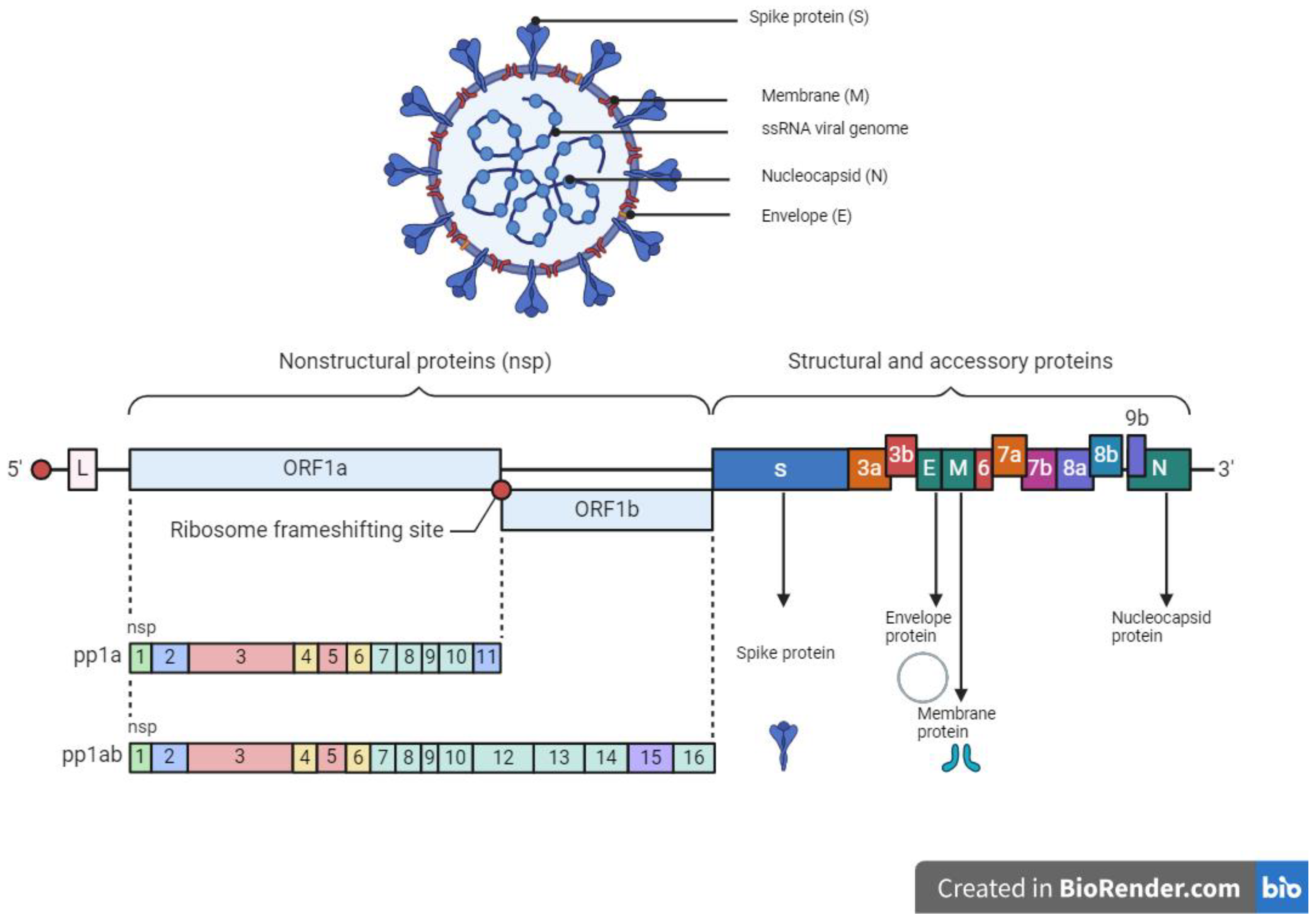
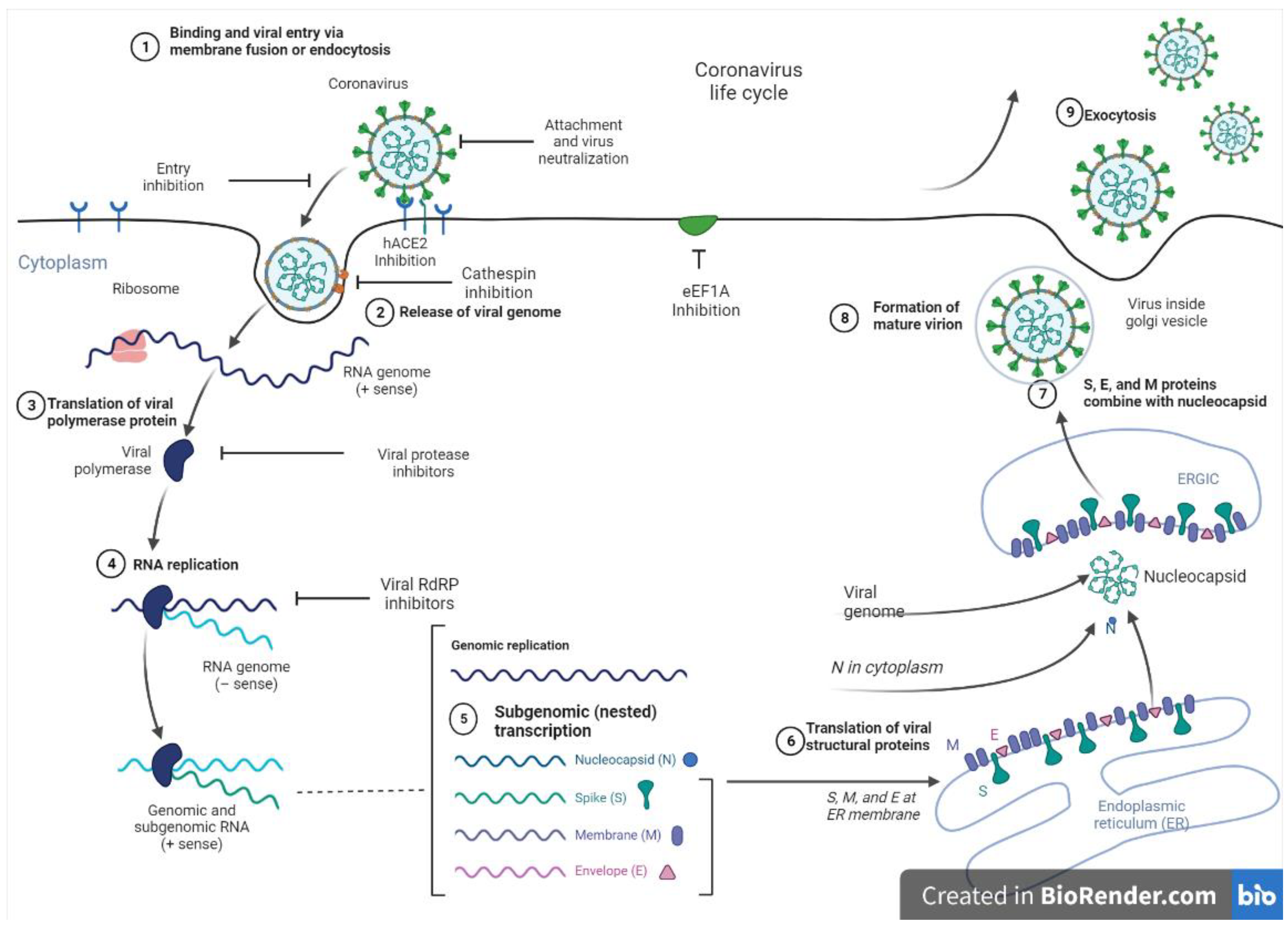
4. SARS-CoV-2 Virology and Mode of Entry
Therapeutic Target Site to Inhibit SARS-CoV-2 Entry and Replication
5. Potential In Silico and Pre-Clinical Studies of Marine-Derived Metabolites against Target Sites of SARS-CoV-2 as Therapeutics
5.1. In Silico, In Vitro, and In Vivo Studies of Major Classes of Metabolites against Entry and Replication of SARS-CoV-2
5.1.1. Polysaccharides
- a.
- Sulfated Polysaccharide (SP)
- b.
- Non-sulfated polysaccharides
5.1.2. Proteins
- a.
- Peptides
- b.
- Lectins
- c.
- Protein-bound pigments
5.1.3. Lipids
5.2. In Silico Studies of Other Secondary Metabolites (Phytochemicals) with Potential Antiviral and Therapeutic Properties against SARS-CoV-2
5.2.1. Polyphenols
5.2.2. Alkaloids
5.2.3. Terpenes
5.2.4. Other Metabolites
6. A Promising Future for Marine Bioactive Metabolites to Tackle SARS-CoV-2
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahajan, M.; Bhardwaj, K. Anthropogenic Ecological Changes and Spill Over of Viruses—A Review. Curr. World Environ. 2021, 16, 594–599. [Google Scholar] [CrossRef]
- Lawler, O.K.; Allan, H.L.; Baxter, P.W.J.; Castagnino, R.; Tor, M.C.; Dann, L.E.; Hungerford, J.; Karmacharya, D.; Lloyd, T.J.; López-Jara, M.J.; et al. The COVID-19 Pandemic Is Intricately Linked to Biodiversity Loss and Ecosystem Health. Lancet Planet. Health 2021, 5, e840–e850. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Rovira, J. Effects of Air Pollutants on the Transmission and Severity of Respiratory Viral Infections. Environ. Res. 2020, 187, 109650. [Google Scholar] [CrossRef] [PubMed]
- Neiderud, C.-J. How Urbanization Affects the Epidemiology of Emerging Infectious Diseases. Infect. Ecol. Epidemiol. 2015, 5, 27060. [Google Scholar] [CrossRef]
- Vijayaraj, R.; Altaff, K.; Rosita, A.S.; Ramadevi, S.; Revathy, J. Bioactive Compounds from Marine Resources against Novel Corona Virus (2019-NCoV): In Silico Study for Corona Viral Drug. Nat. Prod. Res. 2020, 35, 5525–5529. [Google Scholar] [CrossRef] [PubMed]
- Irving, W.L. Ebola Virus Transmission. Int. J. Exp. Pathol. 1995, 76, 225–226. [Google Scholar] [PubMed]
- Salmón-Mulanovich, G.; Vásquez, A.; Albújar, C.; Guevara, C.; Laguna-Torres, A.; Salazar, M.; Zamalloa, H.; Cáceres, M.; Gómez-Benavides, J.; Pacheco, V.; et al. Human Rabies and Rabies in Vampire and Nonvampire Bat Species, Southeastern Peru, 2007. Emerg. Infect. Dis. 2009, 15, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G.; Heymann, D.L.; Lloyd, G.; McCormick, J.B.; Miatudila, M.; Murphy, F.A.; Muyembé-Tamfun, J.-J.; Piot, P.; Ruppol, J.-F.; Sureau, P.; et al. Discovery and Description of Ebola Zaire Virus in 1976 and Relevance to the West African Epidemic During 2013–2016. J. Infect. Dis. 2016, 214, S93–S101. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vézinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-Lymphotropic Retrovirus from a Patient at Risk for Acquired Immune Deficiency Syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, F.; Wang, R.; Guan, K.; Jiang, T.; Xu, G.; Sun, J.; Chang, C. The Deadly Coronaviruses: The 2003 SARS Pandemic and the 2020 Novel Coronavirus Epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-T.; Hsu, W.-C.; Lin, C.-C. Antiviral Natural Products and Herbal Medicines. J. Tradit. Complement. Med. 2014, 4, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Ma, A.; Wang, X.; Yang, C.; Chen, X.; Li, G.; Qiu, F. Research Progress on the Antiviral Activities of Natural Products and Their Derivatives: Structure–Activity Relationships. Front. Chem. 2022, 10, 1005360. [Google Scholar] [CrossRef]
- Kamzeeva, P.N.; Aralov, A.V.; Alferova, V.A.; Korshun, V.A. Recent Advances in Molecular Mechanisms of Nucleoside Antivirals. Curr. Issues Mol. Biol. 2023, 45, 6851–6879. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.J.; Cheng, S.M.S.; Leung, K.; Lee, C.K.; Hachim, A.; Tsang, L.C.H.; Yam, K.W.H.; Chaothai, S.; Kwan, K.K.H.; Chai, Z.Y.H.; et al. Real-World COVID-19 Vaccine Effectiveness against the Omicron BA.2 Variant in a SARS-CoV-2 Infection-Naive Population. Nat. Med. 2023, 29, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, I.J.d.S.; Silva, L.R.; da Silva-Júnior, E.F. Challenges in Designing Antiviral Agents. In Viral Infections and Antiviral Therapies; Dhara, A.K., Nayak, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 169–209. [Google Scholar]
- Zhao, J.-H.; Wang, Y.-W.; Yang, J.; Tong, Z.-J.; Wu, J.-Z.; Wang, Y.-B.; Wang, Q.-X.; Li, Q.-Q.; Yu, Y.-C.; Leng, X.-J.; et al. Natural Products as Potential Lead Compounds to Develop New Antiviral Drugs over the Past Decade. Eur. J. Med. Chem. 2023, 260, 115726. [Google Scholar] [CrossRef] [PubMed]
- Demicheli, V.; Jefferson, T.; Di Pietrantonj, C.; Ferroni, E.; Thorning, S.; Thomas, R.E.; Rivetti, A. Vaccines for Preventing Influenza in the Elderly. Cochrane Database Syst. Rev. 2018, 2, CD004876. [Google Scholar] [CrossRef]
- Riccio, G.; Ruocco, N.; Mutalipassi, M.; Costantini, M.; Zupo, V.; Coppola, D.; de Pascale, D.; Lauritano, C. Ten-Year Research Update Review: Antiviral Activities from Marine Organisms. Biomolecules 2020, 10, 1007. [Google Scholar] [CrossRef]
- Teng, Y.-F.; Xu, L.; Wei, M.-Y.; Wang, C.-Y.; Gu, Y.-C.; Shao, C.-L. Recent Progresses in Marine Microbial-Derived Antiviral Natural Products. Arch. Pharm. Res. 2020, 43, 1215–1229. [Google Scholar] [CrossRef]
- Donia, M.; Hamann, M.T. Marine Natural Products and Their Potential Applications as Anti-Infective Agents. Lancet Infect. Dis. 2003, 3, 338–348. [Google Scholar] [CrossRef]
- Almeida, M.C.; Resende, D.I.S.P.; da Costa, P.M.; Pinto, M.M.M.; Sousa, E. Tryptophan Derived Natural Marine Alkaloids and Synthetic Derivatives as Promising Antimicrobial Agents. Eur. J. Med. Chem. 2021, 209, 112945. [Google Scholar] [CrossRef] [PubMed]
- Festa, M.; Sansone, C.; Brunet, C.; Crocetta, F.; Di Paola, L.; Lombardo, M.; Bruno, A.; Noonan, D.M.; Albini, A. Cardiovascular Active Peptides of Marine Origin with ACE Inhibitory Activities: Potential Role as Anti-Hypertensive Drugs and in Prevention of SARSCoV-2 Infection. Int. J. Mol. Sci. 2020, 21, 8364. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.; Nikzad, S.; Kadir, H.; Abubakar, S.; Zandi, K. Potential Antiviral Agents from Marine Fungi: An Overview. Mar. Drugs 2015, 13, 4520–4538. [Google Scholar] [CrossRef] [PubMed]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers. Viruses 2023, 15, 647. [Google Scholar] [CrossRef]
- Nepali, K.; Sharma, R.; Sharma, S.; Thakur, A.; Liou, J.-P. Beyond the Vaccines: A Glance at the Small Molecule and Peptide-Based Anti-COVID19 Arsenal. J. Biomed. Sci. 2022, 29, 65. [Google Scholar] [CrossRef] [PubMed]
- El-Demerdash, A.; Metwaly, A.M.; Hassan, A.; El-Aziz, T.M.A.; Elkaeed, E.B.; Eissa, I.H.; Arafa, R.K.; Stockand, J.D. Comprehensive Virtual Screening of the Antiviral Potentialities of Marine Polycyclic Guanidine Alkaloids against SARS-CoV-2 (COVID-19). Biomolecules 2021, 11, 460. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef]
- Nollet, L.M.L. (Ed.) Marine Microorganisms Extraction and Analysis of Bioactive Compounds; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498702560. [Google Scholar]
- Ameen, F.; AlNadhari, S.; Al-Homaidan, A.A. Marine Microorganisms as an Untapped Source of Bioactive Compounds. Saudi J. Biol. Sci. 2021, 28, 224–231. [Google Scholar] [CrossRef]
- Raveh, A.; Delekta, P.C.; Dobry, C.J.; Peng, W.; Schultz, P.J.; Blakely, P.K.; Tai, A.W.; Matainaho, T.; Irani, D.N.; Sherman, D.H.; et al. Discovery of Potent Broad Spectrum Antivirals Derived from Marine Actinobacteria. PLoS ONE 2013, 8, e82318. [Google Scholar] [CrossRef]
- Suthindhiran, K.; Sarath Babu, V.; Kannabiran, K.; Ishaq Ahmed, V.P.; Sahul Hameed, A.S. Anti-Fish Nodaviral Activity of Furan-2-Yl Acetate Extracted from Marine Streptomyces spp. Nat. Prod. Res. 2011, 25, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, S.; Huang, H.; Li, H.; Wang, W.; Li, W. Generation of Methylated Violapyrones with Improved Anti-Influenza A Virus Activity by Heterologous Expression of a Type III PKS Gene in a Marine Streptomyces Strain. Bioorg. Med. Chem. Lett. 2018, 28, 2865–2868. [Google Scholar] [CrossRef] [PubMed]
- Strand, M.; Carlsson, M.; Uvell, H.; Islam, K.; Edlund, K.; Cullman, I.; Altermark, B.; Mei, Y.F.; Elofsson, M.; Willassen, N.P.; et al. Isolation and Characterization of Anti-Adenoviral Secondary Metabolites from Marine Actinobacteria. Mar. Drugs 2014, 12, 799. [Google Scholar] [CrossRef] [PubMed]
- Al-Nahas, M.O.; Darwish, M.M.; Ali, A.E.; Amin, M.A. Characterization of an Exopolysaccharide-Producing Marine Bacterium, Isolate Pseudoalteromonas sp. AM. Afr. J. Microbiol. Res. 2011, 5, 3823–3831. [Google Scholar] [CrossRef]
- Manimaran, M.; Rajkumar, T.; Vimal, S.; Taju, G.; Abdul Majeed, S.; Sahul Hameed, A.S.; Kannabiran, K. Antiviral Activity of 9(10H)-Acridanone Extracted from Marine Streptomyces fradiae Strain VITMK2 in Litopenaeus vannamei Infected with White Spot Syndrome Virus. Aquaculture 2018, 488, 66–73. [Google Scholar] [CrossRef]
- Huang, H.; Song, Y.; Zang, R.; Wang, X.; Ju, J. Octyl Substituted Butenolides from Marine-Derived Streptomyces koyangensis. Nat. Prod. Res. 2019, 35, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xi, L.; Liu, P.; Wang, Y.; Wang, W.; Huang, Y.; Zhu, W. Diketopiperazine Derivatives from the Marine-Derived Actinomycete Streptomyces sp. FXJ7.328. Mar. Drugs 2013, 11, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, Y.; Li, X.; Wang, X.; Ling, C.; Qin, X.; Zhou, Z.; Li, Q.; Wei, X.; Ju, J. Abyssomicin Monomers and Dimers from the Marine-Derived Streptomyces koyangensis SCSIO 5802. J. Nat. Prod. 2018, 81, 1892–1898. [Google Scholar] [CrossRef]
- Imhoff, J.F. Natural Products from Marine Fungi—Still an Underrepresented Resource. Mar. Drugs 2016, 14, 19. [Google Scholar] [CrossRef]
- Liu, S.; Su, M.; Song, S.-J.; Jung, J. Marine-Derived Penicillium Species as Producers of Cytotoxic Metabolites. Mar. Drugs 2017, 15, 329. [Google Scholar] [CrossRef]
- Nicoletti, R.; Trincone, A. Bioactive Compounds Produced by Strains of Penicillium and Talaromyces of Marine Origin. Mar. Drugs 2016, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. New Rubrolides from the Marine-Derived Fungus Aspergillus Terreus OUCMDZ-1925. J. Antibiot. 2013, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zhang, X.; Du, L.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Sorbicatechols A and B, Antiviral Sorbicillinoids from the Marine-Derived Fungus Penicillium Chrysogenum PJX-17. J. Nat. Prod. 2014, 77, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guo, W.; Wang, Q.; Zhang, L.; Zhu, M.; Zhu, T.; Gu, Q.; Wang, W.; Li, D. Aspulvinones from a Mangrove Rhizosphere Soil-Derived Fungus Aspergillus Terreus Gwq-48 with Anti-Influenza A Viral (H1N1) Activity. Bioorg. Med. Chem. Lett. 2013, 23, 1776–1778. [Google Scholar] [CrossRef]
- Kong, F.D.; Ma, Q.Y.; Huang, S.Z.; Wang, P.; Wang, J.F.; Zhou, L.M.; Yuan, J.Z.; Dai, H.F.; Zhao, Y.X. Chrodrimanins K-N and Related Meroterpenoids from the Fungus Penicillium sp. SCS-KFD09 Isolated from a Marine Worm, Sipunculus Nudus. J. Nat. Prod. 2017, 80, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Q.; Lin, X.P.; Wang, Z.; Zhou, X.F.; Qin, X.C.; Kaliyaperumal, K.; Zhang, T.Y.; Tu, Z.C.; Liu, Y. Asteltoxins with Antiviral Activities from the Marine Sponge-Derived Fungus Aspergillus sp. SCSIO XWS02F40. Molecules 2015, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, Y.; Liu, P.; Fu, P.; Zhu, T.; Wang, W.; Zhu, W. Indole-Diterpenoids with Anti-H1N1 Activity from the Aciduric Fungus Penicillium Camemberti OUCMDZ-1492. J. Nat. Prod. 2013, 76, 1328–1336. [Google Scholar] [CrossRef]
- He, F.; Bao, J.; Zhang, X.Y.; Tu, Z.C.; Shi, Y.M.; Qi, S.H. Asperterrestide A, a Cytotoxic Cyclic Tetrapeptide from the Marine-Derived Fungus Aspergillus Terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef]
- Sun, Y.L.; Wang, J.; Wang, Y.F.; Zhang, X.Y.; Nong, X.H.; Chen, M.Y.; Xu, X.Y.; Qi, S.H. Cytotoxic and Antiviral Tetramic Acid Derivatives from the Deep-Sea-Derived Fungus Trichobotrys Effuse DFFSCS021. Tetrahedron 2015, 71, 9328–9332. [Google Scholar] [CrossRef]
- Yu, G.; Zhou, G.; Zhu, M.; Wang, W.; Zhu, T.; Gu, Q.; Li, D. Neosartoryadins A and B, Fumiquinazoline Alkaloids from a Mangrove-Derived Fungus Neosartorya Udagawae HDN13-313. Org. Lett. 2016, 18, 244–247. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Hao, X.; Tan, J.; Li, F.; Qiao, X.; Chen, S.; Xiao, C.; Chen, M.; Peng, Z.; et al. Raistrickindole A, an Anti-HCV Oxazinoindole Alkaloid from Penicillium Raistrickii IMB17-034. J. Nat. Prod. 2019, 82, 1391–1395. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Hao, X.; Li, S.; Jia, J.; Guan, Y.; Peng, Z.; Bi, H.; Xiao, C.; Cen, S.; et al. Broad-Spectrum Antiviral Natural Products from the Marine-Derived Penicillium sp. IMB17-046. Molecules 2019, 24, 2821. [Google Scholar] [CrossRef]
- Huang, Z.; Nong, X.; Ren, Z.; Wang, J.; Zhang, X.; Qi, S. Anti-HSV-1, Antioxidant and Antifouling Phenolic Compounds from the Deep-Sea-Derived Fungus Aspergillus Versicolor SCSIO 41502. Bioorg. Med. Chem. Lett. 2017, 27, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Q.; Li, X.M.; Li, X.; Li, H.L.; Meng, L.H.; Wang, B.G. New Citrinin Analogues Produced by Coculture of the Marine Algal-Derived Endophytic Fungal Strains Aspergillus Sydowii EN-534 and Penicillium Citrinum EN-535. Phytochem. Lett. 2018, 25, 191–195. [Google Scholar] [CrossRef]
- Jin, Y.; Qin, S.; Gao, H.; Zhu, G.; Wang, W.; Zhu, W.; Wang, Y. An Anti-HBV Anthraquinone from Aciduric Fungus Penicillium sp. OUCMDZ-4736 under Low PH Stress. Extremophiles 2018, 22, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, A.; Kumar, A.P.; Viswanath, B.; Saigopal, D.V.R.; Narasimha, G. Production of Bioactive Compounds by Actinomycetes and Their Antioxidant Properties. Biotechnol. Res. Int. 2014, 2014, 217030. [Google Scholar] [CrossRef]
- Janardhan, A.; Kumar, A.P.; Viswanath, B.; Gopal, D.S.; Narasimha, G. Antiviral and Larvicidal Properties of Novel Bioactive Compounds Produced from Marine Actinomycetes. Russ. J. Mar. Biol. 2018, 44, 424–428. [Google Scholar] [CrossRef]
- Niu, S.; Liu, D.; Shao, Z.; Proksch, P.; Lin, W. Eutypellazines A–M, Thiodiketopiperazine-Type Alkaloids from Deep Sea Derived Fungus Eutypella sp. MCCC 3A00281. RSC Adv. 2017, 7, 33580–33590. [Google Scholar] [CrossRef]
- Luo, M.; Zang, R.; Wang, X.; Chen, Z.; Song, X.; Ju, J.; Huang, H. Natural Hydroxamate-Containing Siderophore Acremonpeptides A-D and an Aluminum Complex of Acremonpeptide D from the Marine-Derived Acremonium Persicinum SCSIO 115. J. Nat. Prod. 2019, 82, 2594–2600. [Google Scholar] [CrossRef]
- Liang, X.; Nong, X.H.; Huang, Z.H.; Qi, S.H. Antifungal and Antiviral Cyclic Peptides from the Deep-Sea-Derived Fungus Simplicillium Obclavatum EIODSF 020. J. Agric. Food Chem. 2017, 65, 5114–5121. [Google Scholar] [CrossRef]
- Chen, X.; Si, L.; Liu, D.; Proksch, P.; Zhang, L.; Zhou, D.; Lin, W. Neoechinulin B and Its Analogues as Potential Entry Inhibitors of Influenza Viruses, Targeting Viral Hemagglutinin. Eur. J. Med. Chem. 2015, 93, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.L.; Wei, M.Y.; Chen, H.Y.; Guan, F.F.; Wang, C.Y.; Shao, C.L. (+)- and (−)-Pestaloxazine A, a Pair of Antiviral Enantiomeric Alkaloid Dimers with a Symmetric Spiro[Oxazinane-Piperazinedione] Skeleton from Pestalotiopsis sp. Org. Lett. 2015, 17, 4216–4219. [Google Scholar] [CrossRef]
- Ma, X.; Nong, X.H.; Ren, Z.; Wang, J.; Liang, X.; Wang, L.; Qi, S.H. Antiviral Peptides from Marine Gorgonian-Derived Fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Jia, Y.L.; Guan, F.F.; Ma, J.; Wang, C.Y.; Shao, C.L. Pestalotiolide A, a New Antiviral Phthalide Derivative from a Soft Coral-Derived Fungus Pestalotiopsis sp. Nat. Prod. Sci. 2015, 21, 227–230. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, L.; Liu, D.; Proksch, P.; Zhou, D.; Lin, W. Truncateols A–N, New Isoprenylated Cyclohexanols from the Sponge-Associated Fungus Truncatella Angustata with Anti-H1N1 Virus Activities. Tetrahedron 2015, 71, 2708–2718. [Google Scholar] [CrossRef]
- Liu, F.A.; Lin, X.; Zhou, X.; Chen, M.; Huang, X.; Yang, B.; Tao, H. Xanthones and Quinolones Derivatives Produced by the Deep-Sea-Derived Fungus Penicillium sp. SCSIO Ind16F01. Molecules 2017, 22, 1999. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Si, L.; Liu, D.; Zhou, A.; Zhang, Z.; Shao, Z.; Wang, S.; Zhang, L.; Zhou, D.; Lin, W. Spiromastilactones: A New Class of Influenza Virus Inhibitors from Deep-Sea Fungus. Eur. J. Med. Chem. 2016, 108, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.H.; Zhang, H.B.; Zhong, M.J.; Ma, L.Y.; Liu, D.S.; Liu, W.Z.; Ren, H. Potential Antiviral Xanthones from a Coastal Saline Soil Fungus Aspergillus Iizukae. Mar. Drugs 2018, 16, 449. [Google Scholar] [CrossRef]
- Yu, M.L.; Guan, F.F.; Cao, F.; Jia, Y.L.; Wang, C.Y. A New Antiviral Pregnane from a Gorgonian-Derived Cladosporium sp. Fungus. Nat. Prod. Res. 2018, 32, 1260–1266. [Google Scholar] [CrossRef]
- Pang, X.; Lin, X.; Wang, J.; Liang, R.; Tian, Y.; Salendra, L.; Luo, X.; Zhou, X.; Yang, B.; Tu, Z.; et al. Three New Highly Oxygenated Sterols and One New Dihydroisocoumarin from the Marine Sponge-Derived Fungus Cladosporium sp. SCSIO41007. Steroids 2018, 129, 41–46. [Google Scholar] [CrossRef]
- Cao, X.; Shi, Y.; Wu, X.; Wang, K.; Huang, S.; Sun, H.; Dickschat, J.S.; Wu, B. Talaromyolides A-D and Talaromytin: Polycyclic Meroterpenoids from the Fungus Talaromyces sp. CX11. Org. Lett. 2019, 21, 6539–6542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Jia, C.; Lang, J.; Niaz, S.I.; Li, J.; Yuan, J.; Yu, J.; Chen, S.; Liu, L. Antiviral and Anti-Inflammatory Meroterpenoids: Stachybonoids A–F from the Crinoid-Derived Fungus Stachybotrys Chartarum 952. RSC Adv. 2017, 7, 49910–49916. [Google Scholar] [CrossRef]
- Qin, C.; Lin, X.; Lu, X.; Wan, J.; Zhou, X.; Liao, S.; Tu, Z.; Xu, S.; Liu, Y. Sesquiterpenoids and Xanthones Derivatives Produced by Sponge-Derived Fungus Stachybotry sp. HH1 ZSDS1F1-2. J. Antibiot. 2014, 68, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, F.; Liu, Y.; Liu, Y.; Li, K.; Yang, X.; Liu, S.; Zhou, X.; Yang, J. Spirostaphylotrichin X from a Marine-Derived Fungus as an Anti-Influenza Agent Targeting RNA Polymerase PB2. J. Nat. Prod. 2018, 81, 2722–2730. [Google Scholar] [CrossRef] [PubMed]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine Fucoidans: Structural, Extraction, Biological Activities and Their Applications in the Food Industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.-B.; Atsumi, K.; Kanazashi, M.; Shibayama, T.; Okamoto, K.; Kawahara, T.; Hayashi, T. In Vitro and in Vivo Anti-Herpes Simplex Virus Activity of Monogalactosyl Diacylglyceride from Coccomyxa sp. KJ (IPOD FERM BP-22254), a Green Microalga. PLoS ONE 2019, 14, e0219305. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Influence of Sulphate on the Composition and Antibacterial and Antiviral Properties of the Exopolysaccharide from Porphyridium Cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Kim, M.; Yim, J.H.; Kim, S.Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.K. In Vitro Inhibition of Influenza A Virus Infection by Marine Microalga-Derived Sulfated Polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef]
- Gastineau, R.; Pouvreau, J.B.; Hellio, C.; Morançais, M.; Fleurence, J.; Gaudin, P.; Bourgougnon, N.; Mouget, J.L. Biological Activities of Purified Marennine, the Blue Pigment Responsible for the Greening of Oysters. J. Agric. Food Chem. 2012, 60, 3599–3605. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and Comparison of the Structural Features, Immune-Modulatory and Anti-Avian Influenza Virus Activities Conferred by Three Algal Sulfated Polysaccharides. Mar. Drugs 2015, 14, 4. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Hou, L.; Qin, L.; He, M.; Li, W.; Mao, W. A Sulfated Glucuronorhamnan from the Green Seaweed Monostroma Nitidum: Characteristics of Its Structure and Antiviral Activity. Carbohydr. Polym. 2020, 227, 115280. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Briseño, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodríguez-Padilla, C.; Trejo-Avila, L.M. Sulphated Polysaccharides from Ulva Clathrata and Cladosiphon Okamuranus Seaweeds Both Inhibit Viral Attachment/Entry and Cell-Cell Fusion, in NDV Infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CL(pro) Inhibitor, Isolated from the Edible Brown Algae Ecklonia Cava. Bioorg. Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.R.; Robaina, M.C.S.; Mendes, G.S.; Silva, T.S.L.; Gestinari, L.M.S.; Pamplona, O.S.; Yoneshigue-Valentin, Y.; Kaiser, C.R.; Romanos, M.T.V. Antiviral Activity of Extracts from Brazilian Seaweeds against Herpes Simplex Virus. Rev. Bras. Farmacogn. 2012, 22, 714–723. [Google Scholar] [CrossRef]
- Hamdy, A.H.A.; Mettwallya, W.S.A.; El Fotouh, M.A.; Rodriguez, B.; El-Dewany, A.I.; El-Toumy, S.A.A.; Hussein, A.A. Bioactive Phenolic Compounds from the Egyptian Red Sea Seagrass Thalassodendron Ciliatum. Z. Naturforschung Sect. C J. Biosci. 2012, 67C, 291–296. [Google Scholar] [CrossRef]
- Mohammed, M.M.D.; Hamdy, A.H.A.; El-Fiky, N.M.; Mettwally, W.S.A.; El-Beih, A.A.; Kobayashi, N. Anti-Influenza A Virus Activity of a New Dihydrochalcone Diglycoside Isolated from the Egyptian Seagrass Thalassodendron Ciliatum (Forsk.) Den Hartog. Nat. Prod. Res. 2014, 28, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Abou El-Kassem, L.T. Thalassiolin D: A New Flavone O-Glucoside Sulphate from the Seagrass Thalassia Hemprichii. Nat. Prod. Res. 2017, 31, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.L.; Huang, X.Y.; Li, J.; Xin, G.R.; Guo, Y.W. Absolute Configurations of Integracins A, B, and 15′-Dehydroxy-Integracin B. Chirality 2012, 24, 459–462. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Z.; Shen, L.; Pedpradab, P.; Bruhn, T.; Wu, J.; Bringmann, G. Antiviral Limonoids Including Khayanolides from the Trang Mangrove Plant Xylocarpus Moluccensis. J. Nat. Prod. 2015, 78, 1570–1578. [Google Scholar] [CrossRef]
- Tietjen, I.; Williams, D.E.; Read, S.; Kuang, X.T.; Mwimanzi, P.; Wilhelm, E.; Markle, T.; Kinloch, N.N.; Naphen, C.N.; Tenney, K.; et al. Inhibition of NF-ΚB-Dependent HIV-1 Replication by the Marine Natural Product Bengamide A. Antivir. Res. 2018, 152, 94–103. [Google Scholar] [CrossRef]
- Shin, H.J.; Rashid, M.A.; Cartner, L.K.; Bokesch, H.R.; Wilson, J.A.; McMahon, J.B.; Gustafson, K.R. Stellettapeptins A and B, HIV-Inhibitory Cyclic Depsipeptides from the Marine Sponge Stelletta sp. Tetrahedron Lett. 2015, 56, 4215–4219. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Niu, S.; Gao, J.; Zuo, H.; Yuan, J.; Weng, S.; He, J.; Xu, X. A Single WAP Domain (SWD)-Containing Protein with Antiviral Activity from Pacific White Shrimp Litopenaeus Vannamei. Fish. Shellfish. Immunol. 2018, 73, 167–174. [Google Scholar] [CrossRef]
- Tripoteau, L.; Bedoux, G.; Gagnon, J.; Bourgougnon, N. In Vitro Antiviral Activities of Enzymatic Hydrolysates Extracted from Byproducts of the Atlantic Holothurian Cucumaria Frondosa. Process Biochem. 2015, 50, 867–875. [Google Scholar] [CrossRef]
- González-Almela, E.; Sanz, M.A.; García-Moreno, M.; Northcote, P.; Pelletier, J.; Carrasco, L. Differential Action of Pateamine A on Translation of Genomic and Subgenomic MRNAs from Sindbis Virus. Virology 2015, 484, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, J.; Liu, Y.; Lu, A.; Wang, Z.; Li, Y.; Yang, S.; Wang, Q. Marine-Natural-Product Development: First Discovery of Nortopsentin Alkaloids as Novel Antiviral, Anti-Phytopathogenic-Fungus, and Insecticidal Agents. J. Agric. Food Chem. 2018, 66, 4062–4072. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, Z.; Li, G.; Liu, Y.; Xie, Y.; Wang, Q. First Discovery of Polycarpine, Polycarpaurines A and C, and Their Derivatives as Novel Antiviral and Antiphytopathogenic Fungus Agents. J. Agric. Food Chem. 2016, 64, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Hsieh, M.K.; Duh, C.Y. New Diterpenoids from Soft Coral Sarcophyton Ehrenbergi. Mar. Drugs 2013, 11, 4318. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.K.; Duh, C.Y. Secocrassumol, a Seco-Cembranoid from the Dongsha Atoll Soft Coral Lobophytum Crassum. Mar. Drugs 2014, 12, 6028–6037. [Google Scholar] [CrossRef]
- Cao, F.; Shao, C.L.; Chen, M.; Zhang, M.Q.; Xu, K.X.; Meng, H.; Wang, C.Y. Antiviral C-25 Epimers of 26-Acetoxy Steroids from the South China Sea Gorgonian Echinogorgia Rebekka. J. Nat. Prod. 2014, 77, 1488–1493. [Google Scholar] [CrossRef]
- Gong, K.K.; Tang, X.L.; Zhang, G.; Cheng, C.L.; Zhang, X.W.; Li, P.N.; Li, G.Q. Polyhydroxylated Steroids from the South China Sea Soft Coral Sarcophyton sp. and Their Cytotoxic and Antiviral Activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef]
- Pujol, C.A.; Sepúlveda, C.S.; Richmond, V.; Maier, M.S.; Damonte, E.B. Polyhydroxylated Sulfated Steroids Derived from 5α-Cholestanes as Antiviral Agents against Herpes Simplex Virus. Arch. Virol. 2016, 161, 1993–1999. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Jiang, L.; Wu, J.; Liu, Z.; Wu, Y. Anti-Tumor and Anti-Virus Activity of Polysaccharides Extracted from Sipunculus Nudus(SNP) on Hepg2.2.15. Int. J. Biol. Macromol. 2016, 87, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Wijanarko, A.; Lischer, K.; Hermansyah, H.; Pratami, D.K.; Sahlan, M. Antiviral Activity of Acanthaster Planci Phospholipase A2 against Human Immunodeficiency Virus. Vet. World 2018, 11, 824. [Google Scholar] [CrossRef]
- Lum, K.Y.; Carroll, A.R.; Ekins, M.G.; Read, S.; Haq, Z.; Tietjen, I.; St John, J.; Davis, R.A. Capillasterin A, a Novel Pyrano[2,3-f]Chromene from the Australian Crinoid Capillaster Multiradiatus. Mar. Drugs 2019, 17, 26. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Fujimoto, Y.; Tamaki, M.; Setiawan, A.; Tanaka, T.; Okuyama-Dobashi, K.; Kasai, H.; Watashi, K.; Wakita, T.; Toyama, M.; et al. Identification of Antiviral Agents Targeting Hepatitis B Virus Promoter from Extracts of Indonesian Marine Organisms by a Novel Cell-Based Screening Assay. Mar. Drugs 2015, 13, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Tamaki, M.; Kasai, H.; Tanaka, T.; Otoguro, T.; Ryo, A.; Maekawa, S.; Enomoto, N.; de Voogd, N.J.; Tanaka, J.; et al. Inhibitory Effects of Metachromin A on Hepatitis B Virus Production via Impairment of the Viral Promoter Activity. Antivir. Res. 2017, 145, 136–145. [Google Scholar] [CrossRef]
- Salam, K.A.; Furuta, A.; Noda, N.; Tsuneda, S.; Sekiguchi, Y.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; Tani, H.; et al. Inhibition of Hepatitis C Virus NS3 Helicase by Manoalide. J. Nat. Prod. 2012, 75, 650–654. [Google Scholar] [CrossRef]
- Salam, K.A.; Furuta, A.; Noda, N.; Tsuneda, S.; Sekiguchi, Y.; Yamashita, A.; Moriishi, K.; Nakakoshi, M.; Tsubuki, M.; Tani, H.; et al. Psammaplin A Inhibits Hepatitis C Virus NS3 Helicase. J. Nat. Med. 2013, 67, 765–772. [Google Scholar] [CrossRef]
- Yu, H.B.; Yang, F.; Sun, F.; Li, J.; Jiao, W.H.; Gan, J.H.; Hu, W.Z.; Lin, H.W. Aaptamine Derivatives with Antifungal and Anti-HIV-1 Activities from the South China Sea Sponge Aaptos Aaptos. Mar. Drugs 2014, 12, 6003. [Google Scholar] [CrossRef]
- Li, G.; Guo, J.; Wang, Z.; Liu, Y.; Song, H.; Wang, Q. Marine Natural Products for Drug Discovery: First Discovery of Kealiinines A-C and Their Derivatives as Novel Antiviral and Antiphytopathogenic Fungus Agents. J. Agric. Food Chem. 2018, 66, 7310–7318. [Google Scholar] [CrossRef]
- Dolashka, P.; Dolashka, P.; Nesterova, N.; Zagorodnya, S.; Dolashki, A.; Baranova, G.; Golovan, A.; Voelter, W. Antiviral Activity of Hemocyanin Rapana Venosa and Its Isoforms Against Epstein-Barr Virus. Glob. J. Pharmacol. 2014, 8, 206–212. [Google Scholar]
- Talaei Zanjani, N.; Miranda-Saksena, M.; Valtchev, P.; Diefenbach, R.J.; Hueston, L.; Diefenbach, E.; Sairi, F.; Gomes, V.G.; Cunningham, A.L.; Dehghani, F. Abalone Hemocyanin Blocks the Entry of Herpes Simplex Virus 1 into Cells: A Potential New Antiviral Strategy. Antimicrob. Agents Chemother. 2015, 60, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Robinson, N.; Chataway, T.; Benkendorff, K.; O’Connor, W.; Speck, P. Evidence That the Major Hemolymph Protein of the Pacific Oyster, Crassostrea Gigas, Has Antiviral Activity against Herpesviruses. Antivir. Res. 2014, 110, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Novoa, B.; Romero, A.; Álvarez, Á.L.; Moreira, R.; Pereiro, P.; Costa, M.M.; Dios, S.; Estepa, A.; Parra, F.; Figueras, A. Antiviral Activity of Myticin C Peptide from Mussel: An Ancient Defense against Herpesviruses. J. Virol. 2016, 90, 7692–7702. [Google Scholar] [CrossRef] [PubMed]
- Lillsunde, K.E.; Festa, C.; Adel, H.; De Marino, S.; Lombardi, V.; Tilvi, S.; Nawrot, D.A.; Zampella, A.; D’Souza, L.; D’Auria, M.V.; et al. Bioactive Cembrane Derivatives from the Indian Ocean Soft Coral, Sinularia Kavarattiensis. Mar. Drugs 2014, 12, 4045–4068. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.T.; Wang, S.K.; Dai, C.F.; Duh, C.Y. Briacavatolides A-C, New Briaranes from the Taiwanese Octocoral Briareum Excavatum. Mar. Drugs 2012, 10, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.K.; Yeh, T.T.; Duh, C.Y. Briacavatolides D-F, New Briaranes from the Taiwanese Octocoral Briareum Excavatum. Mar. Drugs 2012, 10, 2103–2110. [Google Scholar] [CrossRef]
- Ahmed, S.; Ibrahim, A.; Arafa, A.S. Anti-H5N1 Virus Metabolites from the Red Sea Soft Coral, Sinularia Candidula. Tetrahedron Lett. 2013, 54, 2377–2381. [Google Scholar] [CrossRef]
- Du, Z.-Q.; Ren, Q.; Huang, A.M.; Fang, W.H.; Zhou, J.F.; Gao, L.J.; Li, X.C. A Novel Peroxinectin Involved in Antiviral and Antibacterial Immunity of Mud Crab, Scylla Paramamosain. Mol. Biol. Rep. 2013, 40, 6873–6881. [Google Scholar] [CrossRef]
- Peng, H.; Liu, H.P.; Chen, B.; Hao, H.; Wang, K.J. Optimized Production of Scygonadin in Pichia Pastoris and Analysis of Its Antimicrobial and Antiviral Activities. Protein Expr. Purif. 2012, 82, 37–44. [Google Scholar] [CrossRef]
- Liu, H.P.; Chen, R.Y.; Zhang, Q.X.; Wang, Q.Y.; Li, C.R.; Peng, H.; Cai, L.; Zheng, C.Q.; Wang, K.J. Characterization of Two Isoforms of Antiliopolysacchride Factors (Sp-ALFs) from the Mud Crab Scylla Paramamosain. Fish. Shellfish. Immunol. 2012, 33, 1–10. [Google Scholar] [CrossRef]
- Zhan, S.; Aweya, J.J.; Wang, F.; Yao, D.; Zhong, M.; Chen, J.; Li, S.; Zhang, Y. Litopenaeus Vannamei Attenuates White Spot Syndrome Virus Replication by Specific Antiviral Peptides Generated from Hemocyanin. Dev. Comp. Immunol. 2019, 91, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Q.; Wang, Y.; Ma, H.Y.; Shen, X.L.; Wang, K.; Du, J.; Yu, X.D.; Fang, W.H.; Li, X.C. A New Crustin Homologue (SpCrus6) Involved in the Antimicrobial and Antiviral Innate Immunity in Mud Crab, Scylla Paramamosain. Fish. Shellfish. Immunol. 2019, 84, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Khong, H.Y.; Mao, W.; Chen, X.; Bao, L.; Wen, X.; Xu, Y. Tunicates as Sources of High-Quality Nutrients and Bioactive Compounds for Food/Feed and Pharmaceutical Applications: A Review. Foods 2023, 12, 3684. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Harper, M.K.; Pond, C.D.; Barrows, L.R.; Ireland, C.M.; Van Wagoner, R.M. Thiazoline Peptides and a Tris-Phenethyl Urea from Didemnum Molle with Anti-HIV Activity. J. Nat. Prod. 2012, 75, 1436–1440. [Google Scholar] [CrossRef] [PubMed]
- Smitha, D.; Kumar, M.M.K.; Ramana, H.; Rao, D.V. Rubrolide R: A New Furanone Metabolite from the Ascidian Synoicum of the Indian Ocean. Nat. Prod. Res. 2014, 28, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.E.; Pond, C.D.; Pierce, E.; Harmer, Z.P.; Kwan, J.; Zachariah, M.M.; Harper, M.K.; Wyche, T.P.; Matainaho, T.K.; Bugni, T.S.; et al. Accessing Chemical Diversity from the Uncultivated Symbionts of Small Marine Animals. Nat. Chem. Biol. 2018, 14, 179–185. [Google Scholar] [CrossRef]
- Migliolo, L.; Silva, O.N.; Silva, P.A.; Costa, M.P.; Costa, C.R.; Nolasco, D.O.; Barbosa, J.A.R.G.; Silva, M.R.R.; Bemquerer, M.P.; Lima, L.M.P.; et al. Structural and Functional Characterization of a Multifunctional Alanine-Rich Peptide Analogue from Pleuronectes Americanus. PLoS ONE 2012, 7, e47047. [Google Scholar] [CrossRef]
- Vilas Boas, L.C.P.; de Lima, L.M.P.; Migliolo, L.; Mendes, G.d.S.; de Jesus, M.G.; Franco, O.L.; Silva, P.A. Linear Antimicrobial Peptides with Activity against Herpes Simplex Virus 1 and Aichi Virus. Biopolymers 2017, 108, e22871. [Google Scholar] [CrossRef]
- Sommeng, A.N.; Muhammad Yusuf Arya, R.; Ginting, M.J.; Pratami, D.K.; Hermansyah, H.; Sahlan, M.; Wijanarko, A. Antiretroviral Activity of Pterois Volitans (Red Lionfish) Venom in the Early Development of Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome Antiretroviral Alternative Source. Vet. World 2019, 12, 309–315. [Google Scholar] [CrossRef]
- Xin, Y.; Li, W.; Lu, L.; Zhou, L.; Victor, D.W.; Xuan, S. Antiviral Effects of Stichopus Japonicus Acid Mucopolysaccharide on Hepatitis B Virus Transgenic Mice. J. Ocean. Univ. China 2016, 15, 719–725. [Google Scholar] [CrossRef]
- Gentile, D.; Patamia, V.; Scala, A.; Sciortino, M.T.; Piperno, A.; Rescifina, A. Putative Inhibitors of SARS-CoV-2 Main Protease from a Library of Marine Natural Products: A Virtual Screening and Molecular Modeling Study. Mar. Drugs 2020, 18, 225. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A.D.S.; Wiedemann, L.S.M.; Veiga-Junior, V.F. Natural Products’ Role against COVID-19. RSC Adv. 2020, 10, 23379–23393. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Skrzypczak, D.; Mikula, K.; Młynarz, P. Phytochemicals Containing Biologically Active Polyphenols as an Effective Agent against COVID-19-Inducing Coronavirus. J. Funct. Foods 2020, 73, 104146. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Ghosh, S.; Garg, S.; Ghosh, S.; Jana, A.; Samat, R.; Mukherjee, N.; Roy, R.; Ghosh, S. An Overview of Key Potential Therapeutic Strategies for Combat in the COVID-19 Battle. RSC Adv. 2020, 10, 28243–28266. [Google Scholar] [CrossRef]
- Twomey, J.D.; Luo, S.; Dean, A.Q.; Bozza, W.P.; Nalli, A.; Zhang, B. COVID-19 Update: The Race to Therapeutic Development. Drug Resist. Updates 2020, 53, 100733. [Google Scholar] [CrossRef] [PubMed]
- Thoms, M.; Buschauer, R.; Ameismeier, M.; Koepke, L.; Denk, T.; Hirschenberger, M.; Kratzat, H.; Hayn, M.; MacKens-Kiani, T.; Cheng, J.; et al. Structural Basis for Translational Shutdown and Immune Evasion by the Nsp1 Protein of SARS-CoV-2. Science 2020, 369, 1249–1256. [Google Scholar] [CrossRef]
- Emrani, J.; Ahmed, M.; Jeffers-Francis, L.; Teleha, J.C.; Mowa, N.; Newman, R.H.; Thomas, M.D. SARS-CoV-2, Infection, Transmission, Transcription, Translation, Proteins, and Treatment: A Review. Int. J. Biol. Macromol. 2021, 193, 1249–1273. [Google Scholar] [CrossRef]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the Novel Coronavirus from the Ongoing Wuhan Outbreak and Modeling of Its Spike Protein for Risk of Human Transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef]
- Millet, J.K.; Whittaker, G.R. Physiological and Molecular Triggers for SARS-CoV Membrane Fusion and Entry into Host Cells. Virology 2018, 517, 3–8. [Google Scholar] [CrossRef]
- Mengist, H.M.; Dilnessa, T.; Jin, T. Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease. Front. Chem. 2021, 9, 622898. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, D.; Pandav, K.; Patel, M.; Riva-Moscoso, A.; Singh, B.M.; Patel, A.; Min, Z.C.; Singh-Makkar, S.; Sana, M.K.; Sanchez-Dopazo, R.; et al. Omega 3 Fatty Acids and COVID-19: A Comprehensive Review. Infect. Chemother. 2020, 52, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef] [PubMed]
- De Lira, S.P.; Seleghim, M.H.R.; Williams, D.E.; Marion, F.; Hamill, P.; Jean, F.; Andersen, R.J.; Hajdu, E.; Berlinck, R.G.S. A SARS-Coronovirus 3CL Protease Inhibitor Isolated from the Marine Sponge Axinella Cf. Corrugata: Structure Elucidation and Synthesis. J. Braz. Chem. Soc. 2007, 18, 440–443. [Google Scholar] [CrossRef][Green Version]
- Ahmed, S.A.; Abdelrheem, D.A.; El-Mageed, H.R.A.; Mohamed, H.S.; Rahman, A.A.; Elsayed, K.N.M.; Ahmed, S.A. Destabilizing the Structural Integrity of COVID-19 by Caulerpin and Its Derivatives along with Some Antiviral Drugs: An in Silico Approaches for a Combination Therapy. Struct. Chem. 2020, 31, 2391–2412. [Google Scholar] [CrossRef]
- Al Adem, K.; Shanti, A.; Stefanini, C.; Lee, S. Inhibition of SARS-CoV-2 Entry into Host Cells Using Small Molecules. Pharmaceuticals 2020, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Arora, P.; Prajapati, S.K. Can Algal Derived Bioactive Metabolites Serve as Potential Therapeutics for the Treatment of SARS-CoV-2 Like Viral Infection? Front. Microbiol. 2020, 11, 596374. [Google Scholar] [CrossRef]
- Pendyala, B.; Patras, A.; Dash, C. Phycobilins as Potent Food Bioactive Broad-Spectrum Inhibitors Against Proteases of SARS-CoV-2 and Other Coronaviruses: A Preliminary Study. Front. Microbiol. 2021, 12, 645713. [Google Scholar] [CrossRef]
- Gyebi, G.A.; Ogunro, O.B.; Adegunloye, A.P.; Ogunyemi, O.M.; Afolabi, S.O. Potential Inhibitors of Coronavirus 3-Chymotrypsin-like Protease (3CL pro): An in Silico Screening of Alkaloids and Terpenoids from African Medicinal Plants. J. Biomol. Struct. Dyn. 2020, 39, 3396–3408. [Google Scholar] [CrossRef]
- Erlina, L.; Paramita, R.I.; Kusuma, W.A.; Fadilah, F.; Tedjo, A.; Pratomo, I.P.; Ramadhanti, N.S.; Nasution, A.K.; Surado, F.K.; Fitriawan, A.; et al. Virtual Screening of Indonesian Herbal Compounds as COVID-19 Supportive Therapy: Machine Learning and Pharmacophore Modeling Approaches. BMC Complement. Med. Ther. 2022, 22, 207. [Google Scholar] [CrossRef]
- Jiao, X.; Jin, X.; Ma, Y.; Yang, Y.; Li, J.; Liang, L.; Liu, R.; Li, Z. A Comprehensive Application: Molecular Docking and Network Pharmacology for the Prediction of Bioactive Constituents and Elucidation of Mechanisms of Action in Component-Based Chinese Medicine. Comput. Biol. Chem. 2021, 90, 107402. [Google Scholar] [CrossRef] [PubMed]
- Surti, M.; Patel, M.; Adnan, M.; Moin, A.; Ashraf, S.A.; Siddiqui, A.J.; Snoussi, M.; Deshpande, S.; Reddy, M.N. Ilimaquinone (Marine Sponge Metabolite) as a Novel Inhibitor of SARS-CoV-2 Key Target Proteins in Comparison with Suggested COVID-19 Drugs: Designing, Docking and Molecular Dynamics Simulation Study. RSC Adv. 2020, 10, 37707–37720. [Google Scholar] [CrossRef]
- Vivar-Sierra, A.; Araiza-Macías, M.J.; Hernández-Contreras, J.P.; Vergara-Castañeda, A.; Ramírez-Vélez, G.; Pinto-Almazán, R.; Salazar, J.R.; Loza-Mejía, M.A. In Silico Study of Polyunsaturated Fatty Acids as Potential SARS-CoV-2 Spike Protein Closed Conformation Stabilizers: Epidemiological and Computational Approaches. Molecules 2021, 26, 711. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, L.; Wen, H.; Li, Y. Herbal Combinations against COVID-19: A Network Pharmacology, Molecular Docking and Dynamics Study. J. Integr. Med. 2023, 21, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Li, H.; Li, W.; Wu, X.; Li, Z. Application of Network Pharmacology in the Prevention and Treatment of COVID-19 by Traditional Chinese Medicine. Tradit. Med. Res. 2022, 7, 21. [Google Scholar] [CrossRef]
- Zaporozhets, T.S.; Besednova, N.N. Biologically Active Compounds from Marine Organisms in the Strategies for Combating Coronaviruses. AIMS Microbiol. 2020, 6, 470–494. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chauhan, N.; Kuddus, M. Exploring the Therapeutic Potential of Marine-Derived Bioactive Compounds against COVID-19. Environ. Sci. Pollut. Res. 2021, 28, 52798–52809. [Google Scholar] [CrossRef]
- Arunkumar, M.; Gunaseelan, S.; Kubendran Aravind, M.; Mohankumar, V.; Anupam, P.; Harikrishnan, M.; Siva, A.; Ashokkumar, B.; Varalakshmi, P. Marine Algal Antagonists Targeting 3CL Protease and Spike Glycoprotein of SARS-CoV-2: A Computational Approach for Anti-COVID-19 Drug Discovery. J. Biomol. Struct. Dyn. 2021, 40, 8961–8988. [Google Scholar] [CrossRef]
- Kwon, P.S.; Oh, H.; Kwon, S.-J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated Polysaccharides Effectively Inhibit SARS-CoV-2 in Vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef]
- Morokutti-Kurz, M.; Fröba, M.; Graf, P.; Große, M.; Grassauer, A.; Auth, J.; Schubert, U.; Prieschl-Grassauer, E. Iota-Carrageenan Neutralizes SARS-CoV-2 and Inhibits Viral Replication in Vitro. PLoS ONE 2021, 16, e0237480. [Google Scholar] [CrossRef]
- Reynolds, D.; Huesemann, M.; Edmundson, S.; Sims, A.; Hurst, B.; Cady, S.; Beirne, N.; Freeman, J.; Berger, A.; Gao, S. Viral Inhibitors Derived from Macroalgae, Microalgae, and Cyanobacteria: A Review of Antiviral Potential throughout Pathogenesis. Algal Res. 2021, 57, 102331. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, H.; Wang, Q.; Liu, Z.; Dong, X.; Wen, C.; Ai, C.; Zhang, Y.; Wang, Z.; Zhu, B. Inhibitory Activities of Marine Sulfated Polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Panggabean, J.A.; Adiguna, S.P.; Rahmawati, S.I.; Ahmadi, P.; Zainuddin, E.N.; Bayu, A.; Putra, M.Y. Antiviral Activities of Algal-Based Sulfated Polysaccharides. Molecules 2022, 27, 1178. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Shin, H.; Lee, M.K.; Kwon, O.S.; Shin, J.S.; Kim, Y.I.; Kim, C.W.; Lee, H.R.; Kim, M. Antiviral Activity of Lambda-Carrageenan against Influenza Viruses and Severe Acute Respiratory Syndrome Coronavirus 2. Sci. Rep. 2021, 11, 821. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.K.; Kim, K.; Kim, I.; Chun, S.H.; Oh, T.H.; Kim, J.U.; Kim, J.; Jung, W.; Moon, H.; Ku, B.; et al. Inhibition of SARS-CoV-2 Virus Entry by the Crude Polysaccharides of Seaweeds and Abalone Viscera in Vitro. Mar. Drugs 2021, 19, 219. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Ferrandis-Montesinos, M.; Wang, R. Antiviral Properties of Alginate-Based Biomaterials: Promising Antiviral Agents against SARS-CoV-2. ACS Appl. Bio. Mater. 2021, 4, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Cano-Vicent, A.; Hashimoto, R.; Takayama, K.; Serrano-Aroca, Á. Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2. Polymers 2022, 14, 1483. [Google Scholar] [CrossRef]
- You, Y.; Song, H.; Wang, L.; Peng, H.; Sun, Y.; Ai, C.; Wen, C.; Zhu, B.; Song, S. Structural Characterization and SARS-CoV-2 Inhibitory Activity of a Sulfated Polysaccharide from Caulerpa Lentillifera. Carbohydr. Polym. 2022, 280, 119006. [Google Scholar] [CrossRef]
- Yao, Y.; Luo, Z.; Zhang, X. In Silico Evaluation of Marine Fish Proteins as Nutritional Supplements for COVID-19 Patients. Food Funct. 2020, 11, 5565–5572. [Google Scholar] [CrossRef]
- Riyadi, P.H.; Tanod, W.A.; Wahyudi, D.; Susanto, E.; Fahmi, A.S.; Aisiah, S. Potential of Tilapia (Oreochromis Niloticus) Viscera Bioactive Peptides as Antiviral for SARS-CoV-2 (COVID 19). IOP Conf. Ser. Earth Environ. Sci. 2020, 584, 012004. [Google Scholar] [CrossRef]
- White, K.M.; Rosales, R.; Yildiz, S.; Kehrer, T.; Miorin, L.; Moreno, E.; Jangra, S.; Uccellini, M.B.; Rathnasinghe, R.; Coughlan, L.; et al. Plitidepsin Has Potent Preclinical Efficacy against SARS-CoV-2 by Targeting the Host Protein EEF1A. Science 2021, 371, 926–931. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, X.; Hao, L.; Zhao, M.; Hua, Q.; An, F. Bioactive Indolyl Diketopiperazines from the Marine Derived Endophytic Aspergillus Versicolor DY180635. Mar. Drugs 2020, 18, 338. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Calapoğlu, F.; Ozmen, I. Didemnins Inhibit COVID-19 Main Protease (MPRO). Biointerface Res. Appl. Chem. 2021, 11, 8204–8209. [Google Scholar] [CrossRef]
- Ashhurst, A.S.; Tang, A.H.; Fajtová, P.; Yoon, M.C.; Aggarwal, A.; Bedding, M.J.; Stoye, A.; Beretta, L.; Pwee, D.; Drelich, A.; et al. Potent Anti-SARS-CoV-2 Activity by the Natural Product Gallinamide A and Analogues via Inhibition of Cathepsin L. J. Med. Chem. 2022, 65, 2956–2970. [Google Scholar] [CrossRef] [PubMed]
- Ahmmed, M.K.; Bhowmik, S.; Giteru, S.G.; Zilani, M.N.H.; Adadi, P.; Islam, S.S.; Kanwugu, O.N.; Haq, M.; Ahmmed, F.; Ng, C.C.W.; et al. An Update of Lectins from Marine Organisms: Characterization, Extraction Methodology, and Potential Biofunctional Applications. Mar. Drugs 2022, 20, 430. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, R.P.; Kumar, I.; Yadav, P.; Singh, S.K.; Kaushalendra; Singh, P.K.; Gupta, R.K.; Singh, S.M.; Kesawat, M.S.; et al. Algal Metabolites Can Be an Immune Booster against COVID-19 Pandemic. Antioxidants 2022, 11, 452. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, W.; Gu, C.; Cai, X.; Qu, D.; Lu, L.; Xie, Y.; Jiang, S. Griffithsin with A Broad-Spectrum Antiviral Activity by Binding Glycans in Viral Glycoprotein Exhibits Strong Synergistic Effect in Combination with A Pan-Coronavirus Fusion Inhibitor Targeting SARS-CoV-2 Spike S2 Subunit. Virol. Sin. 2020, 35, 857–860. [Google Scholar] [CrossRef]
- Pendyala, B.; Patras, A. In Silico Screening of Food Bioactive Compounds to Predict Potential Inhibitors of COVID-19 Main Protease (Mpro) and RNA-Dependent RNA Polymerase (RdRp). ChemRxiv 2020. [Google Scholar] [CrossRef]
- Petit, L.; Vernès, L.; Cadoret, J.P. Docking and in Silico Toxicity Assessment of Arthrospira Compounds as Potential Antiviral Agents against SARS-CoV-2. J. Appl. Phycol. 2021, 33, 1579–1602. [Google Scholar] [CrossRef]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant Activities of Phycocyanobilin Prepared from Spirulina Platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Raj, K.T.; Ranjithkumar, R.; Kanthesh, B.M.; Gopenath, T.S. Algal Metabolites Can Be an Immune Booster against COVID-19 Pandemic. Int. J. Pharm. Sci. Res. 2020, 11, 4271–4278. [Google Scholar] [CrossRef]
- Toelzer, C.; Gupta, K.; Yadav, S.K.N.; Borucu, U.; Davidson, A.D.; Williamson, M.K.; Shoemark, D.K.; Garzoni, F.; Staufer, O.; Milligan, R.; et al. Free Fatty Acid Binding Pocket in the Locked Structure of SARS-CoV-2 Spike Protein. Science 2020, 370, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Zahran, E.M.; Albohy, A.; Khalil, A.; Ibrahim, A.H.; Ahmed, H.A.; El-Hossary, E.M.; Bringmann, G.; Abdelmohsen, U.R. Bioactivity Potential of Marine Natural Products from Scleractinia-Associated Microbes and In Silico Anti-SARS-CoV-2 Evaluation. Mar. Drugs 2020, 18, 645. [Google Scholar] [CrossRef] [PubMed]
- Tassakka, A.C.M.A.R.; Sumule, O.; Massi, M.N.; Sulfahri; Manggau, M.; Iskandar, I.W.; Alam, J.F.; Permana, A.D.; Liao, L.M. Potential Bioactive Compounds as SARS-CoV-2 Inhibitors from Extracts of the Marine Red Alga Halymenia Durvillei (Rhodophyta)—A Computational Study. Arab. J. Chem. 2021, 14, 103393. [Google Scholar] [CrossRef] [PubMed]
- Aatif, M.; Muteeb, G.; Alsultan, A.; Alshoaibi, A.; Khelif, B.Y. Dieckol and Its Derivatives as Potential Inhibitors of SARS-CoV-2 Spike Protein (Uk Strain: VUI 202012/01): A Computational Study. Mar. Drugs 2021, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Ali, A.; Wang, Q.; Irfan, M.; Khan, A.; Zeb, M.T.; Zhang, Y.-J.; Chinnasamy, S.; Wei, D.-Q. Marine Natural Compounds as Potents Inhibitors against the Main Protease of SARS-CoV-2—A Molecular Dynamic Study. J. Biomol. Struct. Dyn. 2020, 39, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Youssef, F.S.; Alshammari, E.; Ashour, M.L. Bioactive Alkaloids from Genus Aspergillus: Mechanistic Interpretation of Their Antimicrobial and Potential SARS-CoV-2 Inhibitory Activity Using Molecular Modelling. Int. J. Mol. Sci. 2021, 22, 1866. [Google Scholar] [CrossRef]
- Muteeb, G.; Alshoaibi, A.; Aatif, M.; Rehman, M.T.; Qayyum, M.Z. Screening Marine Algae Metabolites as High-Affinity Inhibitors of SARS-CoV-2 Main Protease (3CLpro): An in Silico Analysis to Identify Novel Drug Candidates to Combat COVID-19 Pandemic. Appl. Biol. Chem. 2020, 63, 79. [Google Scholar] [CrossRef]
- Abdelrheem, D.A.; Ahmed, S.A.; Abd El-Mageed, H.R.; Mohamed, H.S.; Rahman, A.A.; Elsayed, K.N.M.; Ahmed, S.A. The Inhibitory Effect of Some Natural Bioactive Compounds against SARS-CoV-2 Main Protease: Insights from Molecular Docking Analysis and Molecular Dynamic Simulation. J. Environ. Sci. Health Part. A 2020, 55, 1373–1386. [Google Scholar] [CrossRef]
- Gaudêncio, S.P.; Pereira, F. A Computer-Aided Drug Design Approach to Predict Marine Drug-Like Leads for SARS-CoV-2 Main Protease Inhibition. Mar. Drugs 2020, 18, 633. [Google Scholar] [CrossRef]
- Kumar, V.; Parate, S.; Yoon, S.; Lee, G.; Lee, K.W. Computational Simulations Identified Marine-Derived Natural Bioactive Compounds as Replication Inhibitors of SARS-CoV-2. Front. Microbiol. 2021, 12, 647295. [Google Scholar] [CrossRef] [PubMed]
- Sepay, N.; Sekar, A.; Halder, U.C.; Alarifi, A.; Afzal, M. Anti-COVID-19 Terpenoid from Marine Sources: A Docking, Admet and Molecular Dynamics Study. J. Mol. Struct. 2021, 1228, 129433. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual Screening of Natural Products against Type II Transmembrane Serine Protease (TMPRSS2), the Priming Agent of Coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef]
- Bharathi, M.; Sivamaruthi, B.S.; Kesika, P.; Thangaleela, S.; Chaiyasut, C. In Silico Screening of Bioactive Compounds of Representative Seaweeds to Inhibit SARS-CoV-2 ACE2-Bound Omicron B.1.1.529 Spike Protein Trimer. Mar. Drugs 2022, 20, 148. [Google Scholar] [CrossRef] [PubMed]
- Ambarwati, R.; Rahayu, D.A.; Khaleyla, F.; Wisanti; Putri, E.K. Angiotensin-Converting Enzyme 2 (ACE2) of Marine Biota: A Preliminary Study of Potential Therapy for SARS-CoV-2 Infectiontle. J. Phys. Conf. Ser. 2020; in press. [Google Scholar] [CrossRef]
- Fayed, M.A.A.; El-Behairy, M.F.; Abdallah, I.A.; Abdel-Bar, H.M.; Elimam, H.; Mostafa, A.; Moatasim, Y.; Abouzid, K.A.M.; Elshaier, Y.A.M.M. Structure- and Ligand-Based in Silico Studies towards the Repurposing of Marine Bioactive Compounds to Target SARS-CoV-2. Arab. J. Chem. 2021, 14, 103092. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.K.; Jaiswal, J.; Kumar, U. In Silico Bioprospecting of Antiviral Compounds from Marine Fungi and Mushroom for Rapid Development of Nutraceuticals against SARS-CoV-2. J. Biomol. Struct. Dyn. 2021, 41, 1574–1585. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Tolba, E.; Lieberwirth, I.; Wang, S.; Schröder, H.C.; Müller, W.E.G. The Inorganic Polymer, Polyphosphate, Blocks Binding of SARS-CoV-2 Spike Protein to ACE2 Receptor at Physiological Concentrations. Biochem. Pharmacol. 2020, 182, 114215. [Google Scholar] [CrossRef]
- Geahchan, S.; Ehrlich, H.; Rahman, M.A. The Anti-Viral Applications of Marine Resources for COVID-19 Treatment: An Overview. Mar. Drugs 2021, 19, 409. [Google Scholar] [CrossRef]
- Müller, W.E.G.; Neufurth, M.; Wang, S.; Tan, R.; Schröder, H.C.; Wang, X. Morphogenetic (Mucin Expression) as Well as Potential Anti-Corona Viral Activity of the Marine Secondary Metabolite Polyphosphate on A549 Cells. Mar. Drugs 2020, 18, 639. [Google Scholar] [CrossRef]
- Neufurth, M.; Wang, X.; Wang, S.; Schröder, H.C.; Müller, W.E.G. Caged Dexamethasone/Quercetin Nanoparticles, Formed of the Morphogenetic Active Inorganic Polyphosphate, Are Strong Inducers of MUC5AC. Mar. Drugs 2021, 19, 64. [Google Scholar] [CrossRef]

| Group of Compounds | Compound Name and Structural Formula (PubChem CID) | Marine Microorganism(s) | Source of Microorganism(s) | Antiviral Activity | Refs. |
|---|---|---|---|---|---|
| Alkaloid | 9(10H) acridanone (Acridone) (9) (2015) 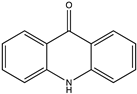 | Streptomyces fradiae VITMK2 | Marine soil sediment collected from mangrove forest region of Pichavaram Tamil Nadu, India | Antiviral activity against WSSV in shrimps with a survival rate of 88.9%, 83.3%, and 55.6% at doses of 500 μg, 250 μg, and 125 μg/animal | [37] |
| Butenolide | (4S)-10-hydroxy-10- methyl-11-oxo-dodec-2-en-1,4-olide (Avenolide) (10) (129320493) 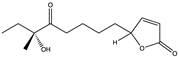 | Streptomyces koyangensis SCSIO 5802 | Isolated from the south China sea | Antiviral activity against HSV (EC50 25.4 μM) | [38] |
| Amide (diketopiperazines) | (3Z,6Z)-3-(4-hydroxybenzylidene)-6-isobutylidenepiperazine-2,5-dione (11) (135034789) 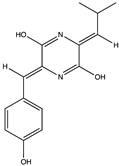 | Streptomyces sp. FXJ7.328 | Coastal sediment collected at Huanghai Beach, Dalian, China | Anti-H1N1 activity at IC50 of 41.5 ± 4.5 μM | [39] |
| Polyketide abyssomicin | Neoabyssomicin D (12) (139590101) 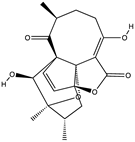 | Streptomyces koyangensis SCSIO5802 | Sediment sample collected from the South China Sea | Antiviral activity against HSV at a concentration of 10 μM | [40] |
| Group of Compounds | Compound Name and Structural Formula (PubChem CID) | Marine Microorganism(s) | Source of Microorganism(s) | Antiviral Activity | Refs. |
|---|---|---|---|---|---|
| Alkaloid | Trichobotrysin A (27) (132594639) 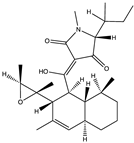 Trichobotrysin B (28) (132594640) 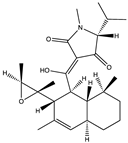 Trichobotrysin D (29) (132594642) 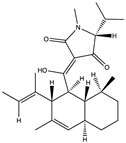 | Trichobotrys effuse DFFSCS021 | Deep sea sediment collected from the South China Sea | Anti-HSV-1 activity at TC0 with IC50 values of 3.1, 9.4, and 3.1 μM | [51] |
| Fumiquinazoline Alkaloid | Neosartoryadin A (30) (132552299) 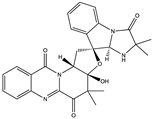 Neosartoryadin B (31) (132552300) 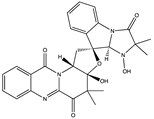 | Neosartorya udagawae HDN13-313 | Isolated from the root of the mangrove plant Avicennia marina | Anti-H1N1 activity with IC50 values of 66 and 58 μM | [52] |
| Indole diketopiperazine alkaloid | Raistrickindole A (32) (145720909) 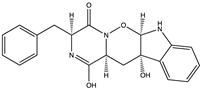 Raistrickin (33) (135666745) 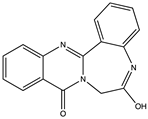 | Penicilliumraistrickii IMB17-034 | Marine sediment collected from mangrove swamp | Anti-HCV activity at EC50 values of 5.7 and 7.0 μM (against VX-950 positive 0.05 μM control) and CC50 values of >200 μM | [53] |
| Trypilepyrazinol (34) (146682634) 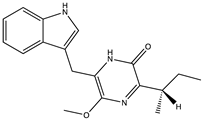 | Penicillium sp. IMB17-046 | Marine sediments collected from a mangrove swamp in Sanya, Hainan province, China | Anti-HIV and anti-HCV activities with IC50 values of 4.6 and 7.7 μM and CC50 values of 44.3 and 116.1 μM, respectively | [54] | |
| Quinone | Aspergilol H (35) (137655878) 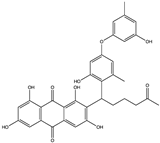 Aspergilol I (36) (137640339) 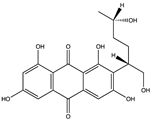 Coccoquinone A (37) (132512004) 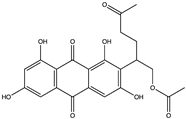 | Aspergillus versicolor SCSIO41502 | Deep sea sediment collected from the South China Sea | Anti-HSV-1 activity at EC50 values of 4.7, 6.3, and 3.1 µM and CC50 values of 108.6, and 50.7 µM, respectively | [55] |
| Seco-penicitrinol A (38) (146683966) 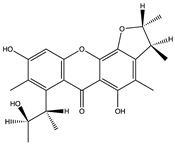 | Aspergillus sydowii EN-534 and Penicillium citrinum EN-535 | Isolated from the same fresh tissue of the marine red alga Laurencia okamurai. | Inhibitory activity against influenza neuraminidase with an IC50 value of 24.7 nM | [56] | |
| (–)-2′R-1-hydroxyisorhodoptilometrin (39) (132279362) 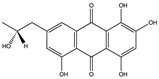 Methyl 6,8-dihydroxy-3-methyl-9-oxo-9H-xanthene-1-carboxylate (40) (132278119) 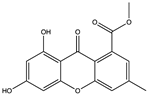 | Penicillium sp. OUCMDZ-4736 | Soil sediment around roots of mangrove plant, Acanthus ilicifolius | Anti-hepatitis B virus activity by inhibiting HBsAg and HBeAg secretion in HepG2.2.15 cells (IC50 values of 4.6 and 11. 4 µM and no cytotoxicity at 20 µM) | [57] | |
| (Z)-1-((1-hydroxypenta-2,4-dien-1-yl) oxy) anthracene-9,10-dione (41) (134822307) 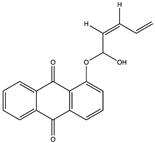 | Nocardia alba KC710971 | Mangrove soil collected from Nellore region of Andhra Pradesh, India | In ovo antiviral activity against two poultry viruses NDV and IBDV, at doses of 25–100 mg | [58,59] | |
| Peptides thiodiketopiper-azine-type alkaloid | Eutypellazines A-L (42–53) (129909516) (see Figure S1 in Supplementary File) | Eutypella sp. MCCC 3A00281 | Deep sea sediment collected from the South Atlantic Ocean | Anti-HIV activity with IC50 ranging between 1 and 19 µM and CC50 > 100 µM | [60] |
| Acremonpeptide A (54) (145721238) 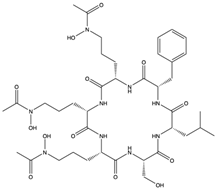 Acremonpeptide B (55) (145721239) 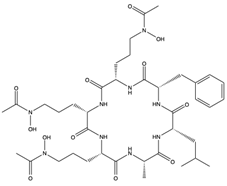 Al(III)-acremonpeptide D (56) (145721242) 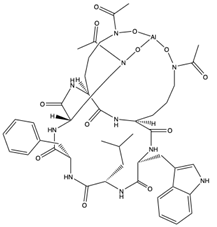 | Acremonium persicinum SCSIO 115 | Marine sediment South China Sea | Anti-HSV-1 activity (EC50 values of 16, 8.7, and 14 µM) | [61] | |
Simplicilliumtide J (57)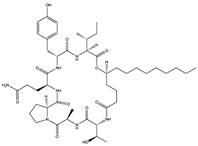 Verlamelin A (58) (139588823) 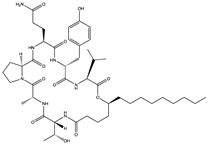 Verlamelin B (59) (139588455) 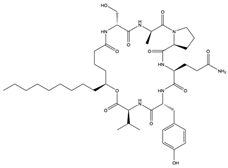 | Simplicillium obclavatum EIODSF 020 | Marine sediment samples collected in the East Indian Ocean | Anti-HSV-1 activity against Vero cells (IC50 values of 14.0, 16.7, and 15.6 µM), TC0 and TC50 values of 25.1 and 204 µM for simplicilliumtide J, 57.2 and 137.0 µM for verlamelin A, and 49.4 and 101.1 µM for verlamelin B | [62] | |
| Prenylated indole diketopiperazine | Neoechinulin B (60) (23425626) 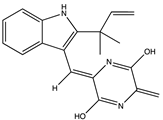 | Eurotium rubrum F33 (MCCC3A00287) | Sediment collected from 2067 m depth under the South Atlantic Ocean | Anti-influenza A/WSN/33 virus activity (EC50 27.4 μM, CC50 > 200 μM) | [63] |
| Enantiomeric alkaloid dimer | (+)-pestaloxazine A (61) (145256980) 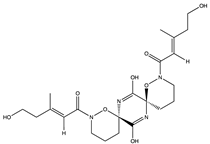 | Pestalotiopsis sp. (ZJ-2009-7-6) | Derived from a soft coral from the South China Sea | Antiviral activity against EV71 (IC50 value of 14.2 ± 1.3 μM compared to ribavirin at IC50 256.1 ± 15.1 μM) and a TC50 value of 130.2 ± 10.1 μM | [64] |
| Aspergillipeptide D (Aspergillide D) (62) (132496356) 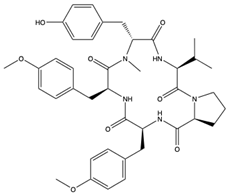 Aspergillipeptide E (Aspergillide E) (63) (139591200) 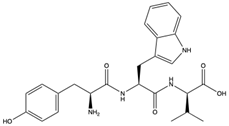 | Aspergillus sp. | China South Sea Gorgonian Melitodes squamata. | Anti-HSV-1 activity with IC50 values of 9.5 and 19.8 μM and TC0 and TC50 values of 81.9 and 204.4 mM for aspergillide D and 153.2 and 346.0 mM for spergillide E | [65] | |
| Polyketone | Pestalotiolide A (64) (156581239) 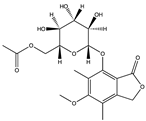 | Pestalotiopsis sp. (ZJ-2009-7-6) | Soft coral Sarcophyton sp. collected from Yongxing Island in the South China Sea | Anti-EV71 activity at IC50 of 27.7 μM and TC50 value of 254.9 μM (Ribavirin IC50 value 418.0 μM) | [66] |
| Truncateol M (65) (156580490) 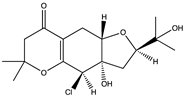 | Truncatella angustata | Finger sponge Amphimedon sp. collected from the reef in Yongxing Island in the South China Sea | Anti-H1N1 activity with an IC50 value of 8.8 µM and CC50 > 100 µM | [67] | |
| Epiremisporine B (66) (139584953) 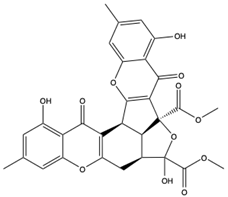 | Penicillium sp. SCSIO | Deep sea sediment | Anti-EV71 activity (IC50 = 19.8 µM) | [68] | |
| Spiromastilactone D (67) (127026306) 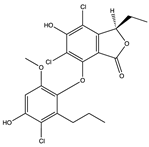 | Spiromastix sp. MCCC3A00308 | Deep sea sediment from South Atlantic Ocean | Antiviral activity against WSN influenza virus (IC50 = 6.0 µM, CC50 > 100 µM) | [69] | |
| Pyrone | Methyl-(4-chloro-l,6-dihydroxy-3-methylxanthone)-8-carboxylate (68) (156581832) 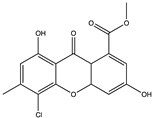 | Aspergillus iizukae KL33 | Coastal saline soil in Kenli, Shandong Province of China | Anti-H1N1 activity (IC50 = 44.6 µM) Anti-HSV-1 (IC50 = 21.4 µM) Anti-HSV-2 (IC50 = 76.7 µM) | [70] |
| Sterol | 3α-hydroxy-pregn-7-ene-6,20-dione (cladosporisteroid B) (69) 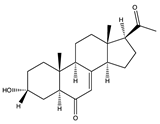 | Cladosporium sp. WZ-2008-0042 Cladosporium sp. SCSIO41007 | Gorgonian Dichotella gemmacea collected at the Meizhou Island coral reef, from the South China Sea and Callyspongia sp. sponge collected from the sea area near Xuwen County, Guangdong Province, China | Antiviral activity against RSV (IC50 = 0.12 mM) and H3N2 (IC50 = 16.2 μM) and a TC50 value of 1.19 mM | [71,72] |
| 3β-hydroxyergosta- 8,14,24(28)-trien-7-one (70) 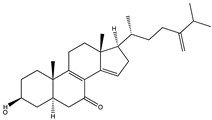 | Penicillium sp. IMB17-046 | Marine sediments collected from a mangrove swamp in Sanya, Hainan province, China | Antiviral activities against HIV (IC50 =3.5 µM, CC50 = 51.2 ± 3.5 µM) and IAV (IC50 = 0.5 µM, CC50 > 100 μM) | [54] | |
| Terpenoid | Talaromyolide D (71) (146683236)  | Talaromyces sp. CX11 | NA | Antiviral activity against pseudorabies virus at a concentration of 1.56–25 μM (CC50 =3.4 μM) | [73] |
| 3-hydroxypentacecilide A (18) (154573703) 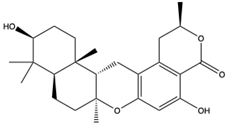 Chrodrimanin N (17) (139589677) 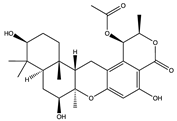 | Penicillium sp. SCS-KFD09 | Marine worm of Haikou Bay, China | Anti-H1N1 activity (IC50 values of 34 and 58 µM) | [47] | |
| Stachybonoid A (72) (156581564) 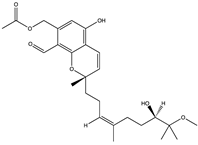 | Stachybotrys chartarum 952 | Crinoid (Himerometra magnipinna) isolated from Xuwen Coral Reef Nature Reserve, Zhanjiang City, Guangdong Province, China | Inhibitory effect on dengue viral protein (prM) at concentrations of 5–50 µM | [74] | |
| Stachybogrisephenone B (73) (139583964) 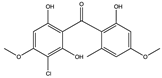 | Stachybotry sp. HH1 ZSDS1F1-2 | Sponge at Xisha Island China | Antiviral activity against EV71 (IC50 = 30.1 μM) | [75] | |
| Spirocyclic γ - lactam | Spirostaphylotrichin X (74) (153210908) 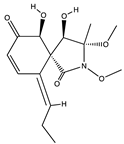 | Cochliobolus lunatus SCSIO41401 | Marine alga at Yongxing Island South China Sea | Antiviral activity against influenza virus strains with IC50 values from 1.2 to 5.5 µM and CC50 > 200 μM | [76] |
| Xanthone | Norlichexanthone (75) (5281657) 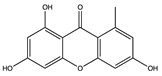 Griseophenone A (76) (3083749) 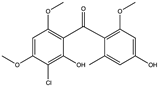 | Stachybotry sp. HH1 ZSDS1F1-2 | Sponge at Xisha Island China | Antiviral activity against EV71 (IC50 values of 50.0 and 40.3 μM) | [75] |
| Group of Compounds | Compound Name and Structural Formula (PubChem CID) | Type of Marine Macroorganism(s) | Marine Macroorganism(s) | Source of Macroorganism(s) | Antiviral Activity | Refs. |
|---|---|---|---|---|---|---|
| Peptides | Bengamide A (133) (10077016)  | Sponge | Jaspis cf. coriacea | NA | HIV-1 inhibitor (EC50 = 0.015 ± 0.009 μM and CC50 = 2.5 ± 1.0 μM) | [92] |
| Stellattapeptins A (134) and B (135) (See Figure S7 in Supplementary File) | Sponge | Stellatta sp. | North-western Australia | Anti-HIV-1 activity (EC50 of 23 and 27 nM) | [93] | |
| Macrodiolide | Pateamine A (136) (10053416) 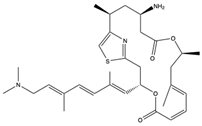 | Sponge | Mycale sp. | NA | Inhibition of sindbis virus genomic mRNA at concentrations of 50–400 nM | [96] |
| Nortopsentin alkaloids | Nortopsentin indole-imidazole derivative (137)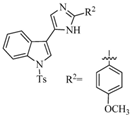 | Sponge | Spongosorites ruetzleri | Deepwater | Anti-TMV activity (50% and 18% inactivation inhibitory effect in vivo at 500 and 100 μg/mL) | [97] |
| Alkaloids | Polycarpine bis(2,2,2-trifluoroacetate) (138)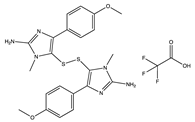 | Ascidian | Polycarpa aurata | NA | Anti-TMV activity (57% and 19% inactivation inhibitory effect in vivo at 500 and 100 μg/ml | [98] |
| Diterpenoids | Ehrenbergol C (139) (163116389) 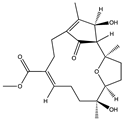 Acetyl ehrenberoxide B (140)  | Soft coral | Sarcophyton ehrenbergi | Collected at San-Hsian-Tai Taitong County, Taiwan | Anti-HCMV activity (EC50 of 52.8 and 22 μM) | [99] |
| Cembrane-type diterpenoids | Secocrassumol (141) (129905909)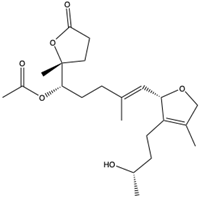 | Soft coral | Lobophytum crassum | Collected from the coral reefs, at Dongsha Atoll off Taiwan | Anti-HCMV activity (IC50 = 12.7 μM) | [100] |
| Steroids | Echrebsteroid C (142) (90680710) | Coral | Echinogorgia rebekka | Collected from the South China Sea | Anti-RSV activity (IC50 = 0.19 μM; TC50 = 24.4 μM) | [101] |
| Polyhydroxylated steroids | (24R)-methylcholest-7-en-3β,5α,6β-triol (143)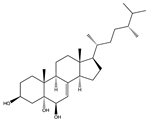 (24S)-ergost-3β,5α,6β, 11α-tetraol (sarcoaldesterol B) (144) (10718409)  | Cnidarian (Soft coral) | Sarcophyton sp. | South Sea (Weizhou Islands Sea area) | Anti-H1N1 IAV activity (IC50 of 19.6 μg/mL and 81.4 μM) | [102] |
| Peptide | LvSWD3 (SWD gene) (145) | Crustacean (Pacific white shrimp) | Litopenaeus vannamei, | Shrimp farm in Zhanjiang, Guangdong Province | Anti-WSSV activity at 5 μg/shrimp | [94] |
| Polysaccharide | Acidic mucopolysaccharide (146) | Echinoderm | Stichopus japonicus selenka | NA | Anti-HBV activity at concentrations of 30–50 mg kg−1) | [133] |
| Enzyme | Phospholipase A2 (AP-PLA-2) (132) (See Figure S6 in Supplementary File) | Echinoderm (starfish) | Acanthaster planci | Moluccas Islands, eastern Indonesia | Anti-HIV1 activity (LC50 of 1.6 mg/mL) | [105] |
| Naphthopyrones | Comaparvin (147) (324099) 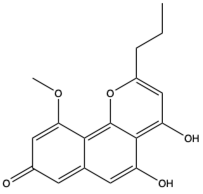 | Echinoderms (crinoids) | Capillaster multiradiatus | Collected from the Torres Strait, Queensland, Australia | Anti-HIV-1 activity with EC50 of 7.5 ± 1.7 µm | [106] |
| Proteins/ peptides | Papain hydrolysate (>100 kDa fraction) (148) | Echinoderms (sea cucumbers) | aquapharyngeal bulb of Cucumaria frondosa | Collected in Passamaquoddy Bay, Bay of Fundy, New Brunswick, Canada | Anti-HSV-1 activity (EC50 of 18.2 µg/mL; CC50 > 500 µg/mL) | [95] |
| Sulfated steroids | Disodium 2β,3α-dihydroxy-6E-hydroximine-5α-cholestane-2,3-disulfate (149)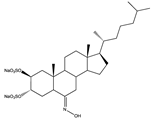 | Echinoderms | Echinoderms from cold waters | South Atlantic Ocean | Antiviral activity against HSV-1 (EC50 = 16.5 ± 1.4 µg/mL; CC50 > 100 µg/mL) | [103] |
| Polysaccharide | Water-soluble polysaccharides (150) | Worm | Sipunculus nudus | Collected from the Xiamen market, China | Inhibition of the HBV DNA and HBsAg mRNA synthesis at concentrations of 1, 0.5, 0.25, and 0.13 mg/mL) | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okechukwu, Q.N.; Adepoju, F.O.; Kanwugu, O.N.; Adadi, P.; Serrano-Aroca, Á.; Uversky, V.N.; Okpala, C.O.R. Marine-Derived Bioactive Metabolites as a Potential Therapeutic Intervention in Managing Viral Diseases: Insights from the SARS-CoV-2 In Silico and Pre-Clinical Studies. Pharmaceuticals 2024, 17, 328. https://doi.org/10.3390/ph17030328
Okechukwu QN, Adepoju FO, Kanwugu ON, Adadi P, Serrano-Aroca Á, Uversky VN, Okpala COR. Marine-Derived Bioactive Metabolites as a Potential Therapeutic Intervention in Managing Viral Diseases: Insights from the SARS-CoV-2 In Silico and Pre-Clinical Studies. Pharmaceuticals. 2024; 17(3):328. https://doi.org/10.3390/ph17030328
Chicago/Turabian StyleOkechukwu, Queency N., Feyisayo O. Adepoju, Osman N. Kanwugu, Parise Adadi, Ángel Serrano-Aroca, Vladimir N. Uversky, and Charles Odilichukwu R. Okpala. 2024. "Marine-Derived Bioactive Metabolites as a Potential Therapeutic Intervention in Managing Viral Diseases: Insights from the SARS-CoV-2 In Silico and Pre-Clinical Studies" Pharmaceuticals 17, no. 3: 328. https://doi.org/10.3390/ph17030328
APA StyleOkechukwu, Q. N., Adepoju, F. O., Kanwugu, O. N., Adadi, P., Serrano-Aroca, Á., Uversky, V. N., & Okpala, C. O. R. (2024). Marine-Derived Bioactive Metabolites as a Potential Therapeutic Intervention in Managing Viral Diseases: Insights from the SARS-CoV-2 In Silico and Pre-Clinical Studies. Pharmaceuticals, 17(3), 328. https://doi.org/10.3390/ph17030328










