Exploring the Antimicrobial, Antioxidant, and Antiviral Potential of Eco-Friendly Synthesized Silver Nanoparticles Using Leaf Aqueous Extract of Portulaca oleracea L.
Abstract
1. Introduction
2. Results and Discussion
2.1. Portulaca oleracea-Mediated Biosynthesis of Ag-NPs
2.2. Characterization
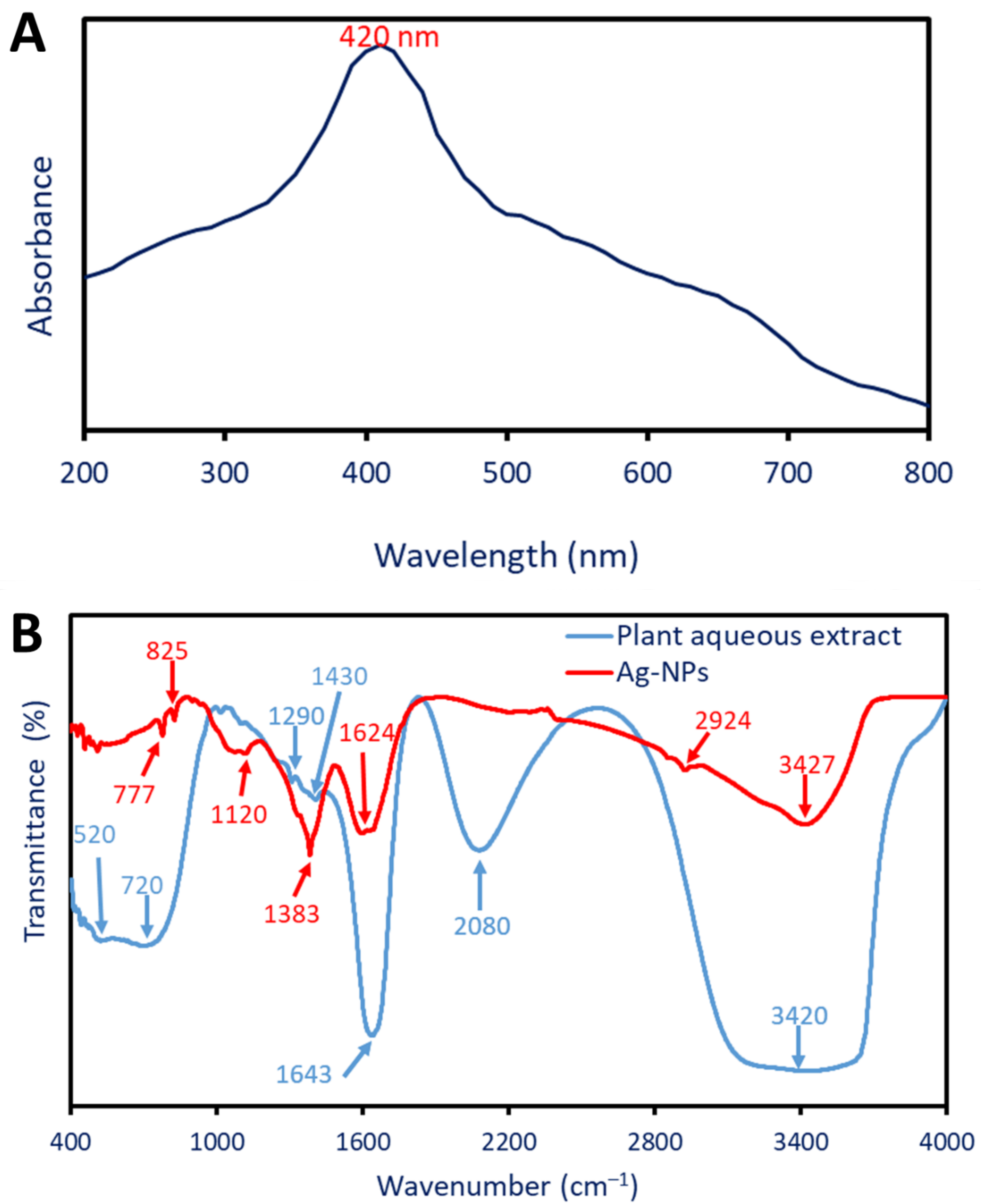
2.2.1. Optical Properties Using UV-Vis Spectroscopy
2.2.2. Fourier Transform Infrared (FT-IR)
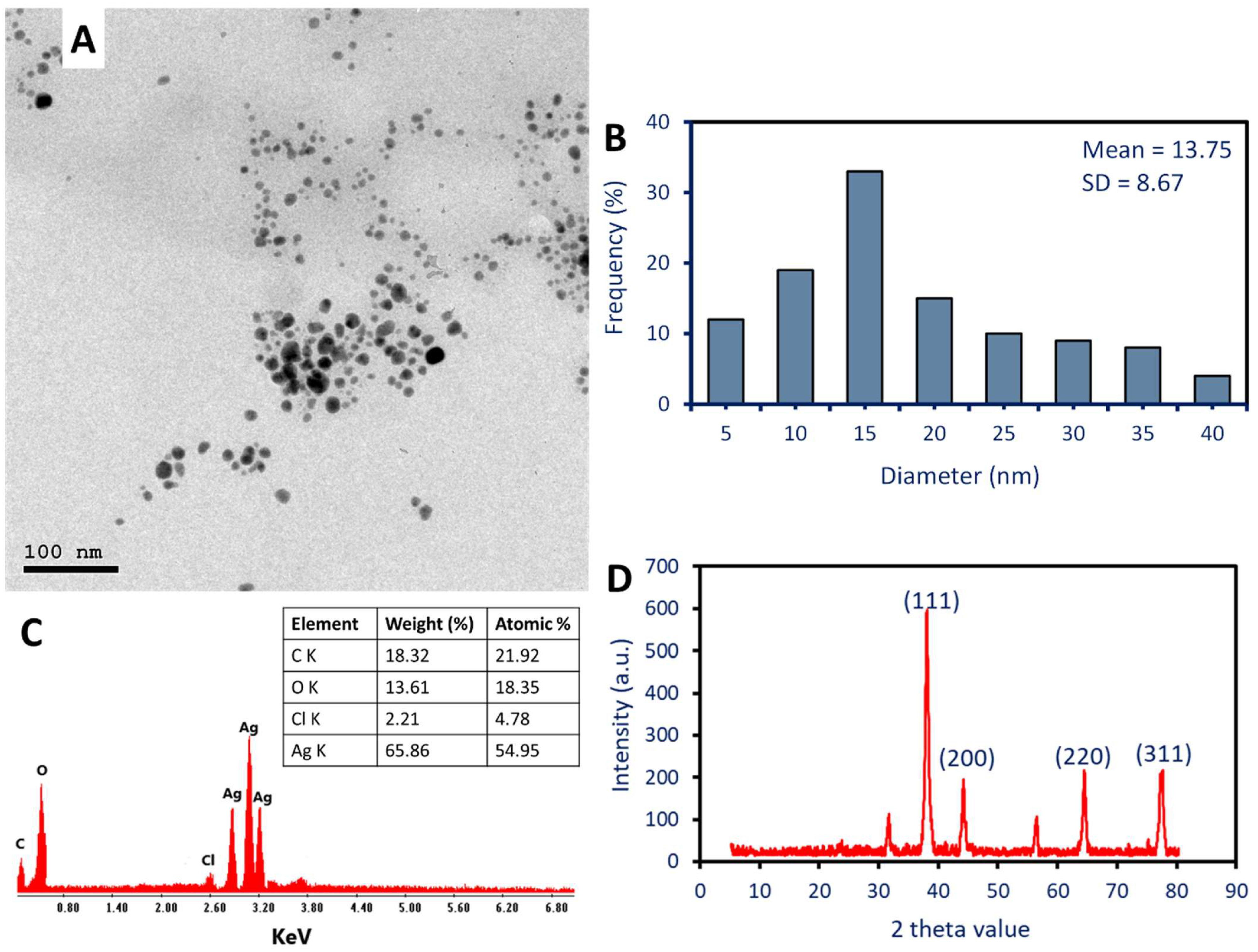
2.2.3. Morphological and Elemental Composition Investigation
2.2.4. X-ray Diffraction (XRD)
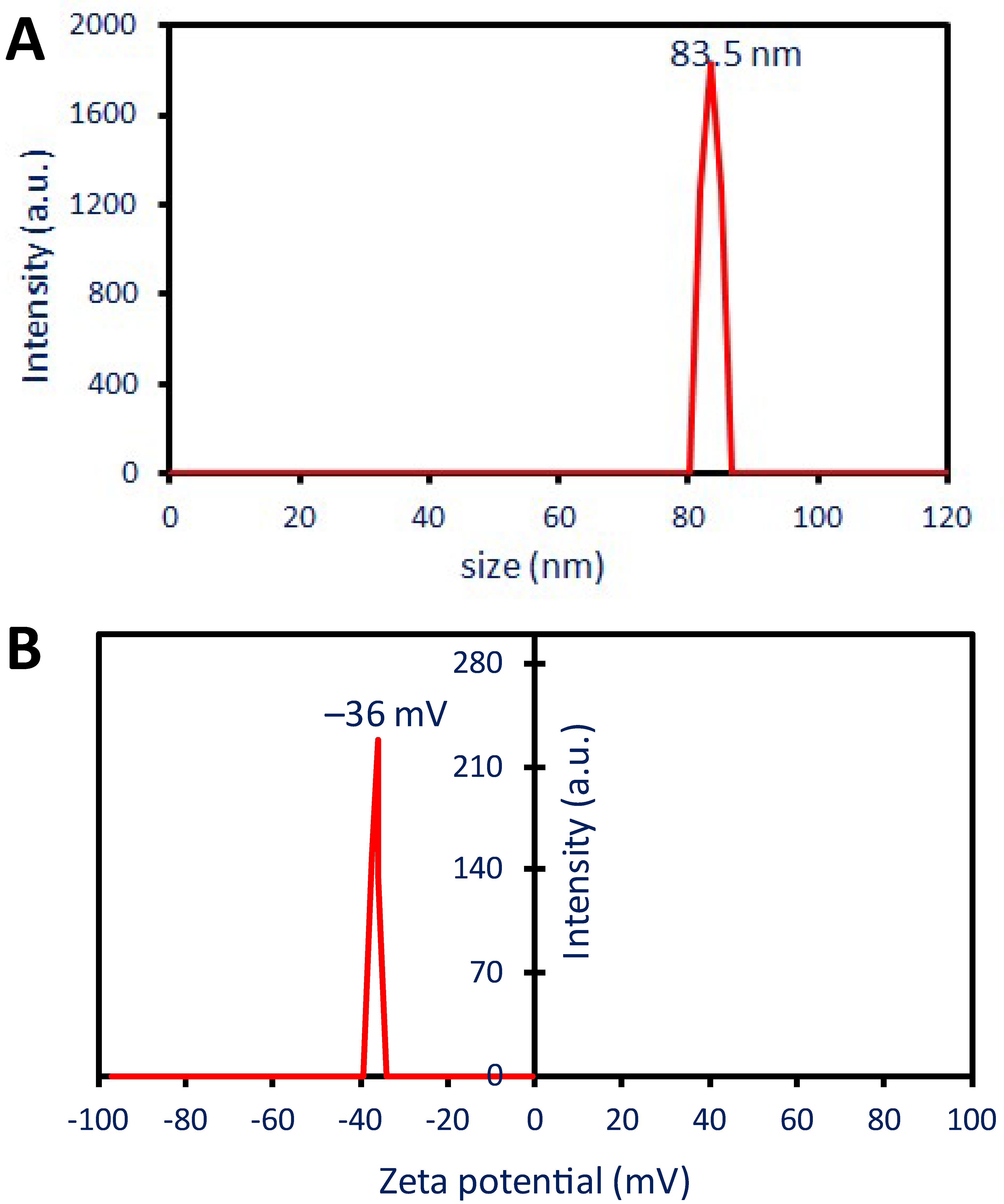
2.2.5. Dynamic Light Scattering (DLS)
2.2.6. Zeta Potential (ζ)
2.3. Antimicrobial Activity
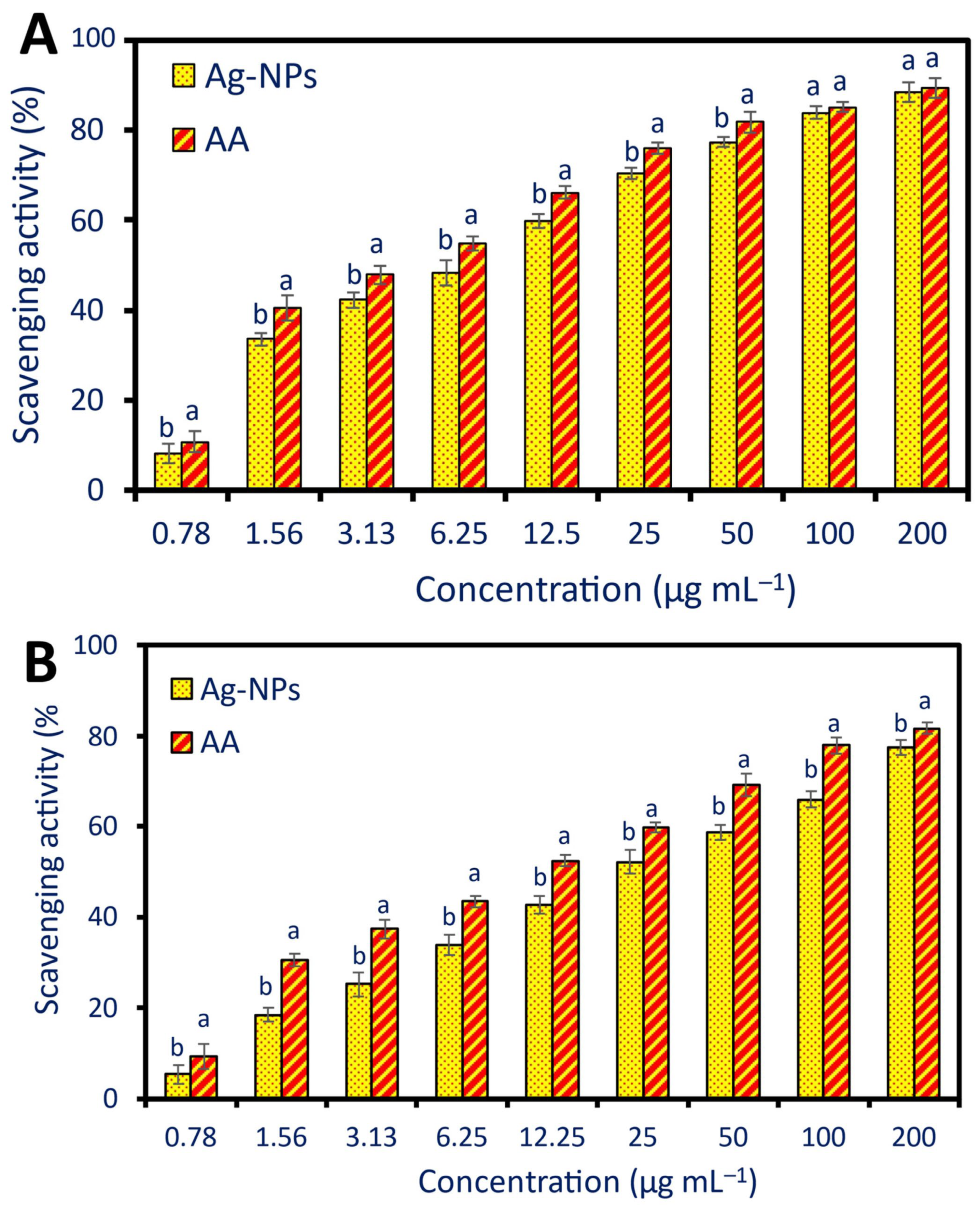
2.4. Antioxidant Activity
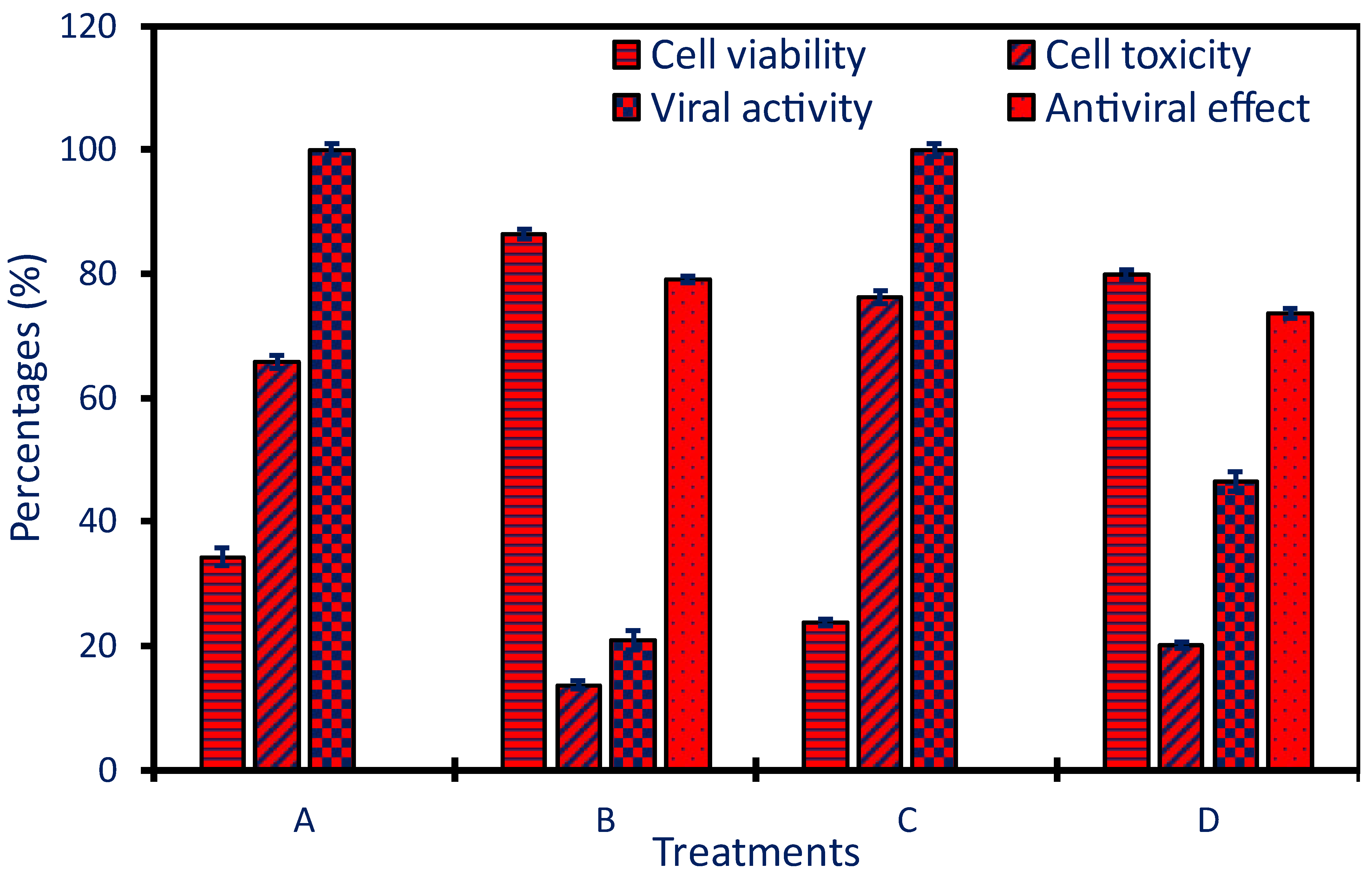
2.5. Antiviral Activity
3. Materials and Methods
3.1. Materials
3.2. Plant-Synthesized Ag-NPs
3.3. Characterization
3.4. Pharmacological Activities
3.4.1. Antimicrobial Activity
3.4.2. Antioxidant Activity
DPPH Assay Method
H2O2 Assay Method
3.4.3. Antiviral Activity
Detection of Maximum Non-Toxic Concentration (MNTC) of Biosynthesized Ag-NPs
3.5. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Luna, A.R.; Cruz-Martínez, H.; Vásquez-López, A.; Medina, D.I. Metal nanoparticles as novel antifungal agents for sustainable agriculture: Current advances and future directions. J. Fungi 2021, 7, 1033. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Nanotechnology: A Revolution in Modern Industry. Molecules 2023, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Kulkarni, A.G.; De Britto, S.; Jogaiah, S. 18—Economic considerations and limitations of green synthesis vs chemical synthesis of nanomaterials. In Advances in Nano-Fertilizers and Nano-Pesticides in Agriculture; Jogaiah, S., Singh, H.B., Fraceto, L.F., Lima, R.D., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 459–468. [Google Scholar]
- Abdo, A.M.; Fouda, A.; Eid, A.M.; Fahmy, N.M.; Elsayed, A.M.; Khalil, A.M.A.; Alzahrani, O.M.; Ahmed, A.F.; Soliman, A.M. Green synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) by Pseudomonas aeruginosa and their activity against pathogenic microbes and common house mosquito, Culex pipiens. Materials 2021, 14, 6983. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.K.; Jena, B.; Biswal, B.; Pradhan, A.K.; Arakha, M.; Acharya, S.; Acharya, L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci. Rep. 2022, 12, 8383. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 2018, 16, 84. [Google Scholar] [CrossRef]
- Amin, M.A.; Abu-Elsaoud, A.M.; Nowwar, A.I.; Abdelwahab, A.T.; Awad, M.A.; Hassan, S.E.-D.; Boufahja, F.; Fouda, A.; Elkelish, A. Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L. Green Process. Synth. 2024, 13, 20230215. [Google Scholar] [CrossRef]
- Radulescu, D.M.; Surdu, V.A.; Ficai, A.; Ficai, D.; Grumezescu, A.M.; Andronescu, E. Green Synthesis of Metal and Metal Oxide Nanoparticles: A Review of the Principles and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 15397. [Google Scholar] [CrossRef]
- Salari, S.; Esmaeilzadeh Bahabadi, S.; Samzadeh-Kermani, A.; Yosefzaei, F. In-vitro Evaluation of Antioxidant and Antibacterial Potential of GreenSynthesized Silver Nanoparticles Using Prosopis farcta Fruit Extract. Iran. J. Pharm. Res. IJPR 2019, 18, 430–455. [Google Scholar]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.; Un, A.; Ali, M.E.; Rahman, M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef] [PubMed]
- Ghorani, V.; Saadat, S.; Khazdair, M.R.; Gholamnezhad, Z.; El-Seedi, H.; Boskabady, M.H. Phytochemical Characteristics and Anti-Inflammatory, Immunoregulatory, and Antioxidant Effects of Portulaca oleracea L.: A Comprehensive Review. Evid.-Based Complement. Altern. Med. 2023, 2023, 2075444. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Fouda, A.; Hassan, S.E.; Hamza, M.F.; Alharbi, N.K.; Elkelish, A.; Alharthi, A.; Salem, W.M. Plant-Based Copper Oxide Nanoparticles; Biosynthesis, Characterization, Antibacterial Activity, Tanning Wastewater Treatment, and Heavy Metals Sorption. Catalysts 2023, 13, 348. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; Omar, N.I.; Hasan, E.A.; Ahmed, K.A.; Youssef, A.M. Assessment of the Biological Activities of Egyptian Purslane (Portulaca oleracea) Extract after Incorporating Metal Nanoparticles, in Vitro and in Vivo Study. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 287–310. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Singh, N.; Singh, A.; Singh, H.; Singh, S. Green synthesis of nanoparticles and its potential application. Biotechnol. Lett. 2016, 38, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Govindappa, M.; Hemashekhar, B.; Arthikala, M.-K.; Rai, V.R.; Ramachandra, Y. Characterization, antibacterial, antioxidant, antidiabetic, anti-inflammatory and antityrosinase activity of green synthesized silver nanoparticles using Calophyllum tomentosum leaves extract. Results Phys. 2018, 9, 400–408. [Google Scholar] [CrossRef]
- Ege, E.; Kurtay, G.; Karaca, B.; Büyük, İ.; Gökdemir, F.Ş.; Sumer, A. Green synthesis of silver nanoparticles from Phaseolus vulgaris L. extracts and investigation of their antifungal activities. Hacet. J. Biol. Chem. 2020, 49, 11–23. [Google Scholar]
- Haggag, E.G.; Elshamy, A.M.; Rabeh, M.A.; Gabr, N.M.; Salem, M.; Youssif, K.A.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019, 14, 6217–6229. [Google Scholar] [CrossRef]
- Kambale, E.K.; Nkanga, C.I.; Mutonkole, B.-P.I.; Bapolisi, A.M.; Tassa, D.O.; Liesse, J.-M.I.; Krause, R.W.M.; Memvanga, P.B. Green synthesis of antimicrobial silver nanoparticles using aqueous leaf extracts from three Congolese plant species (Brillantaisia patula, Crossopteryx febrifuga and Senna siamea). Heliyon 2020, 6, e04493. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Panda, S.K.; Jayabalan, R.; Sharma, N.; Bastia, A.K.; Mohanta, T.K. Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Ravichandran, V.; Vasanthi, S.; Shalini, S.; Shah, S.A.A.; Harish, R. Green synthesis of silver nanoparticles using Atrocarpus altilis leaf extract and the study of their antimicrobial and antioxidant activity. Mater. Lett. 2016, 180, 264–267. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Y.; Xie, J.; Wu, S.; Wu, Z. Characterization, antioxidant and antimicrobial activities of green synthesized silver nanoparticles from Psidium guajava L. leaf aqueous extracts. Mater. Sci. Eng. C 2018, 86, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, N.; Jahan, N.; Khalil Ur, R.; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef]
- Fernández-Poyatos, M.D.P.; Llorent-Martínez, E.J.; Ruiz-Medina, A. Phytochemical Composition and Antioxidant Activity of Portulaca oleracea: Influence of the Steaming Cooking Process. Foods 2021, 10, 94. [Google Scholar] [CrossRef]
- Kate, S.; Sahasrabudhe, M.; Pethe, A. Biogenic Silver Nanoparticle Synthesis, Characterization and its Antibacterial activity against Leather Deteriorates. Jordan J. Biol. Sci. 2020, 13, 493–498. [Google Scholar]
- Lee, Y.J.; Park, Y. Graphene oxide grafted gold nanoparticles and silver/silver chloride nanoparticles green-synthesized by a Portulaca oleracea extract: Assessment of catalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125527. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Q.; Hou, W.; Qin, L. Silver-Based Surface Plasmon Sensors: Fabrication and Applications. Int. J. Mol. Sci. 2023, 24, 4142. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Narsing Rao, M.P.; Xiao, M.; Wang, H.-F.; Hozzein, W.N.; Chen, W.; Li, W.-J. Antibacterial activity of silver nanoparticles against Staphylococcus warneri synthesized using endophytic bacteria by photo-irradiation. Front. Microbiol. 2017, 8, 1090. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lin, S.-J.; Lin, C.-H.; Tsai, C.-S.; Lu, Y.-J. Size effect of Ag nanoparticles on surface plasmon resonance. Surf. Coat. Technol. 2008, 202, 5339–5342. [Google Scholar] [CrossRef]
- Taha, Z.K.; Hawar, S.N.; Sulaiman, G.M. Extracellular biosynthesis of silver nanoparticles from Penicillium italicum and its antioxidant, antimicrobial and cytotoxicity activities. Biotechnol. Lett. 2019, 41, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Mira, H.; Wei, Y.; Ning, S.; Guibal, E.; El-Etrawy, A.-A.S.; El Dakkony, S.R. Chitosan functionalized with pyrazolinone derivative for water treatment: Application to Hg (II) removal. J. Water Process Eng. 2023, 56, 104258. [Google Scholar] [CrossRef]
- Khademi-Azandehi, P.; Moghaddam, J. Green synthesis, characterization and physiological stability of gold nanoparticles from Stachys lavandulifolia Vahl extract. Particuology 2015, 19, 22–26. [Google Scholar] [CrossRef]
- Hamza, M.F.; Guibal, E.; Wei, Y.; Fouda, A.; Althumayri, K.; Khoziem, H.A.A.; Mashaal, N.M. Functionalization of thiosemicarbazide/gellan gum for the enhancement of cadmium removal from aqueous solutions–Grafting of tributyl phosphate derivative. J. Water Process Eng. 2023, 54, 103928. [Google Scholar] [CrossRef]
- Coury, C.; Dillner, A.M. A method to quantify organic functional groups and inorganic compounds in ambient aerosols using attenuated total reflectance FTIR spectroscopy and multivariate chemometric techniques. Atmos. Environ. 2008, 42, 5923–5932. [Google Scholar] [CrossRef]
- Ghoshal, G.; Kaur, M. Optimization of extraction of starch from sweet potato and its application in making edible film. Food Chem. Adv. 2023, 3, 100356. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry: Applications, Theory, and Instrumentation; Wiley: Hoboken, NJ, USA, 2000; pp. 10881–10882. [Google Scholar]
- Awwad, M.A.; Salem, M.N.; Abdeen, O.A. Biosynthesis of silver nanoparticles using Loquat leaf extract and its antibacterial activity. Adv. Mater. Lett. 2013, 4, 338–342. [Google Scholar] [CrossRef]
- Zhao, M.; Salih, K.A.; Wei, Y.; Guibal, E.; Ning, S.; Goda, A.E.-S.; Hamza, M.F. Novel method for synthesizing high S-bearing hybrid sorbent for efficient silver binding–Characterization, testing, and application to metal recovery from X-ray films. Chem. Eng. J. 2023, 477, 147010. [Google Scholar] [CrossRef]
- Murillo-Rábago, E.I.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Garcia-Marin, L.E.; Quester, K.; Castro-Longoria, E. Optimized Synthesis of Small and Stable Silver Nanoparticles Using Intracellular and Extracellular Components of Fungi: An Alternative for Bacterial Inhibition. Antibiotics 2022, 11, 800. [Google Scholar] [CrossRef]
- Fouda, A.; Awad, M.A.; Al-Faifi, Z.E.; Gad, M.E.; Al-Khalaf, A.A.; Yahya, R.; Hamza, M.F. Aspergillus flavus-mediated green synthesis of silver nanoparticles and evaluation of their antibacterial, anti-candida, acaricides, and photocatalytic activities. Catalysts 2022, 12, 462. [Google Scholar] [CrossRef]
- Moreno-Martin, G.; León-González, M.E.; Madrid, Y. Simultaneous determination of the size and concentration of AgNPs in water samples by UV–vis spectrophotometry and chemometrics tools. Talanta 2018, 188, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Acharya, D.; Singha, K.M.; Pandey, P.; Mohanta, B.; Rajkumari, J.; Singha, L.P. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci. Rep. 2018, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Bagur, H.; Poojari, C.C.; Melappa, G.; Rangappa, R.; Chandrasekhar, N.; Somu, P. Biogenically synthesized silver nanoparticles using endophyte fungal extract of Ocimum tenuiflorum and evaluation of biomedical properties. J. Clust. Sci. 2020, 31, 1241–1255. [Google Scholar] [CrossRef]
- Rónavári, A.; Kovács, D.; Igaz, N.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: A comprehensive study. Int. J. Nanomed. 2017, 12, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 313081. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop. J. Pharm. Res. 2013, 12, 7–11. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Lashin, I.; Fouda, A.; Gobouri, A.A.; Azab, E.; Mohammedsaleh, Z.M.; Makharita, R.R. Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-NPs) fabricated by callus extract of Solanum incanum L. Biomolecules 2021, 11, 341. [Google Scholar] [CrossRef]
- Salem, S.S.; El-Belely, E.F.; Niedbała, G.; Alnoman, M.M.; Hassan, S.E.-D.; Eid, A.M.; Shaheen, T.I.; Elkelish, A.; Fouda, A. Bactericidal and in-vitro cytotoxic efficacy of silver nanoparticles (Ag-NPs) fabricated by endophytic actinomycetes and their use as coating for the textile fabrics. Nanomaterials 2020, 10, 2082. [Google Scholar] [CrossRef]
- More, P.R.; Pandit, S.; Filippis, A.; Franci, G.; Mijakovic, I.; Galdiero, M. Silver Nanoparticles: Bactericidal and Mechanistic Approach against Drug Resistant Pathogens. Microorganisms 2023, 11, 369. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.C.; Gupta, P.; Saini, S.; Mohiyuddin, S.; Pruthi, V.; Prasad, R. Plausible mechanistic insights in biofilm eradication potential against Candida spp. using in situ-synthesized tyrosol-functionalized chitosan gold nanoparticles as a versatile antifouling coating on implant surfaces. ACS Omega 2022, 7, 8350–8363. [Google Scholar] [CrossRef] [PubMed]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.-R.A.; Atta, H.M.; Abdel-Rahman, M.A.; El Naghy, W.S.; Fouda, A. Myco-synthesized copper oxide nanoparticles using harnessing metabolites of endophytic fungal strain Aspergillus terreus: An insight into antibacterial, anti-Candida, biocompatibility, anticancer, and antioxidant activities. BMC Complement. Med. Ther. 2023, 23, 261. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A brief overview on antioxidant activity determination of silver nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.; DeLong, R.K. Nanoscale Interaction Mechanisms of Antiviral Activity. ACS Pharmacol. Transl. Sci. 2023, 6, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, S.E.-D.; Eid, A.M.; Abdel-Rahman, M.A.; Hamza, M.F. Light enhanced the antimicrobial, anticancer, and catalytic activities of selenium nanoparticles fabricated by endophytic fungal strain, Penicillium crustosum EP-1. Sci. Rep. 2022, 12, 11834. [Google Scholar] [CrossRef]
- Louten, J. Virus Replication. In Essential Human Virology; Academic Press: Cambridge, MA, USA, 2016; pp. 49–70. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Khatun, S.; Putta, C.L.; Hak, A.; Rengan, A.K. Immunomodulatory nanosystems: An emerging strategy to combat viral infections. Biomater. Biosyst. 2023, 9, 100073. [Google Scholar] [CrossRef]
- Kamli, M.R.; Srivastava, V.; Hajrah, N.H.; Sabir, J.S.; Ali, A.; Malik, M.A.; Ahmad, A. Phytogenic fabrication of Ag–Fe Bimetallic nanoparticles for cell cycle arrest and apoptosis signaling pathways in Candida auris by generating oxidative stress. Antioxidants 2021, 10, 182. [Google Scholar] [CrossRef]
- Hamza, M.F.; Mira, H.; Khalafalla, M.S.; Wang, J.; Wei, Y.; Yin, X.; Ning, S.; Althumayri, K.; Fouda, A. Photocatalytic Performance of Functionalized Biopolymer for Neodymium (III) Sorption and the Recovery from Leachate Solution. Catalysts 2023, 13, 672. [Google Scholar] [CrossRef]
- Humphries, R.M.; Ambler, J.; Mitchell, S.L.; Castanheira, M.; Dingle, T.; Hindler, J.A.; Koeth, L.; Sei, K. CLSI Methods Development and Standardization Working Group Best Practices for Evaluation of Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2018, 56, e01934-17. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Mashrur, F.R.; Chhoan, A.P.; Shahriar, S.M.; Haidere, M.F.; Runa, N.J.; Kim, S.; Kweon, D.H.; Hosseinzadeh, H.; Cho, J.Y. Silver Nanoparticles as Potential Antiviral Agents. Pharmaceutics 2021, 13, 2034. [Google Scholar] [CrossRef]

| Test Organism | MIC Value (µg mL−1) | Clear Zone (mm) |
|---|---|---|
| B. subtilis | 12.5 | 9.3 ± 0.5 |
| S. aureus | 6.25 | 10.7 ± 0.6 |
| P. aeruginosa | 6.25 | 11.3 ± 0.5 |
| E. coli | 6.25 | 9.7 ± 0.5 |
| C. albicans | 12.5 | 11.0 ± 0.5 |
| A. brasiliensis | 12.5 | 10.3 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Rahman, M.A.; Alshallash, K.S.; Eid, A.M.; Hassan, S.E.-D.; Salih, M.; Hamza, M.F.; Fouda, A. Exploring the Antimicrobial, Antioxidant, and Antiviral Potential of Eco-Friendly Synthesized Silver Nanoparticles Using Leaf Aqueous Extract of Portulaca oleracea L. Pharmaceuticals 2024, 17, 317. https://doi.org/10.3390/ph17030317
Abdel-Rahman MA, Alshallash KS, Eid AM, Hassan SE-D, Salih M, Hamza MF, Fouda A. Exploring the Antimicrobial, Antioxidant, and Antiviral Potential of Eco-Friendly Synthesized Silver Nanoparticles Using Leaf Aqueous Extract of Portulaca oleracea L. Pharmaceuticals. 2024; 17(3):317. https://doi.org/10.3390/ph17030317
Chicago/Turabian StyleAbdel-Rahman, Mohammed Ali, Khalid S. Alshallash, Ahmed M. Eid, Saad El-Din Hassan, Mutaz Salih, Mohammed F. Hamza, and Amr Fouda. 2024. "Exploring the Antimicrobial, Antioxidant, and Antiviral Potential of Eco-Friendly Synthesized Silver Nanoparticles Using Leaf Aqueous Extract of Portulaca oleracea L." Pharmaceuticals 17, no. 3: 317. https://doi.org/10.3390/ph17030317
APA StyleAbdel-Rahman, M. A., Alshallash, K. S., Eid, A. M., Hassan, S. E.-D., Salih, M., Hamza, M. F., & Fouda, A. (2024). Exploring the Antimicrobial, Antioxidant, and Antiviral Potential of Eco-Friendly Synthesized Silver Nanoparticles Using Leaf Aqueous Extract of Portulaca oleracea L. Pharmaceuticals, 17(3), 317. https://doi.org/10.3390/ph17030317










