Graft-Versus-Host Disease: Can Biomarkers Assist in Differential Diagnosis, Prognosis, and Therapeutic Strategy?
Abstract
1. Introduction
2. Protein Biomarkers for Acute and Chronic GVHD
3. GVHD and Transplant-Associated Thrombotic Microangiopathy (TA-TMA)
4. Genetic Biomarkers for aGVHD and cGVHD
5. SNPs as Biomarkers in GVHD
6. Oral Biomarkers for GVHD
7. Potential Role of Biomarkers in Disease Detection
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Haverman, T.M.; Raber-Durlacher, J.E.; Raghoebar, I.I.; Rademacher, W.M.H.; Rozema, F.R.; Hazenberg, M.D.; Epstein, J.B.; Treister, N.S. Oral Chronic Graft-versus-Host Disease: What the General Dental Practitioner Needs to Know. J. Am. Dent. Assoc. 2020, 151, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.B.; Oh, U.; Rothen, M.; Sroussi, H.Y.; Dean, D.R.; Lloid, C.M.; Cintron, K.; Lee, S.J.; Cutler, C.S.; Treister, N.S. A Review of Oral Chronic Graft-Versus-Host Disease: Considerations for Dental Hygiene Practice. J. Dent. Hyg. 2022, 96, 6–17. [Google Scholar] [PubMed]
- Goker, H.; Aladag, E.; Buyukasik, Y.; Chao, N.J.; Akman, U.; Demiroglu, H. ST2 and Rg3a As a Biomarker for Predicting of Acute Graft-Versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Blood 2019, 134, 5684. [Google Scholar] [CrossRef]
- Riccardi, G.; Bellizzi, M.G.; Fatuzzo, I.; Zoccali, F.; Cavalcanti, L.; Greco, A.; Vincentiis, M.D.; Ralli, M.; Fiore, M.; Petrella, C.; et al. Salivary Biomarkers in Oral Squamous Cell Carcinoma: A Proteomic Overview. Proteomes 2022, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, G.K.; Mathews, L.R.; Villegas, J.A.; Lucas, A.; Gonzalez de Peredo, A.; Blazar, B.R.; Girard, J.-P.; Poholek, A.C.; Luther, S.A.; Shlomchik, W.; et al. IL-33 Acts as a Costimulatory Signal to Generate Alloreactive Th1 Cells in Graft-versus-Host Disease. J. Clin. Investig. 2022, 132, e150927. [Google Scholar] [CrossRef] [PubMed]
- Dattagupta, A.; Immaneni, S. ST2: Current Status. Indian Heart J. 2018, 70 (Suppl. S1), S96–S101. [Google Scholar] [CrossRef]
- Jacobsohn, D.A.; Vogelsang, G.B. Acute Graft versus Host Disease. Orphanet J. Rare Dis. 2007, 2, 35. [Google Scholar] [CrossRef]
- Paczesny, S.; Raiker, N.; Brooks, S.; Mumaw, C. Graft-versus-Host Disease Biomarkers: Omics and Personalized Medicine. Int. J. Hematol. 2013, 98, 275–292. [Google Scholar] [CrossRef]
- Bidgoli, A.; DePriest, B.P.; Saatloo, M.V.; Jiang, H.; Fu, D.; Paczesny, S. Current Definitions and Clinical Implications of Biomarkers in Graft-versus-Host Disease. Transplant. Cell. Ther. 2022, 28, 657–666. [Google Scholar] [CrossRef]
- Paczesny, S.; Krijanovski, O.I.; Braun, T.M.; Choi, S.W.; Clouthier, S.G.; Kuick, R.; Misek, D.E.; Cooke, K.R.; Kitko, C.L.; Weyand, A.; et al. A Biomarker Panel for Acute Graft-versus-Host Disease. Blood 2009, 113, 273–278. [Google Scholar] [CrossRef]
- Sarantopoulos, S.; Stevenson, K.E.; Kim, H.T.; Bhuiya, N.S.; Cutler, C.S.; Soiffer, R.J.; Antin, J.H.; Ritz, J. High Levels of B-Cell Activating Factor in Patients with Active Chronic Graft-versus-Host Disease. Clin. Cancer Res. 2007, 13, 6107–6114. [Google Scholar] [CrossRef]
- Rozmus, J.; Kariminia, A.; Abdossamadi, S.; Storer, B.E.; Martin, P.J.; Lee, S.J.; Wolff, D.; Arora, M.; Cutler, C.; Schultz, K.R. Comprehensive B Cell Phenotyping Profile for Chronic Graft-versus-Host Disease Diagnosis. Biol. Blood Marrow Transplant. 2019, 25, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.S.; Wang, X.N.; Norden, J.; Pearce, K.; El-Gezawy, E.; Atarod, S.; Hromadnikova, I.; Collin, M.; Holler, E.; Dickinson, A.M. Identification and Validation of Biomarkers Associated with Acute and Chronic Graft versus Host Disease. Bone Marrow Transplant. 2015, 50, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Kariminia, A.; Holtan, S.G.; Ivison, S.; Rozmus, J.; Hebert, M.J.; Martin, P.J.; Lee, S.J.; Wolff, D.; Subrt, P.; Abdossamadi, S.; et al. Heterogeneity of Chronic Graft-versus-Host Disease Biomarkers: Association with CXCL10 and CXCR3+ NK Cells. Blood 2016, 127, 3082–3091. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.L.M.; Harris, A.C.; Greenson, J.K.; Braun, T.M.; Holler, E.; Teshima, T.; Levine, J.E.; Choi, S.W.J.; Huber, E.; Landfried, K.; et al. Regenerating Islet-Derived 3-Alpha Is a Biomarker of Gastrointestinal Graft-versus-Host Disease. Blood 2011, 118, 6702–6708. [Google Scholar] [CrossRef] [PubMed]
- Kitko, C.L.; Pidala, J.; Schoemans, H.M.; Lawitschka, A.; Flowers, M.E.; Cowen, E.W.; Tkaczyk, E.; Farhadfar, N.; Jain, S.; Steven, P.; et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: IIa. The 2020 Clinical Implementation and Early Diagnosis Working Group Report. Transplant. Cell. Ther. 2021, 27, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.A.; Hanash, S.M.; Tabellini, L.; Baik, C.; Lawler, R.L.; Grogan, B.M.; Storer, B.; Chin, A.; Johnson, M.; Wong, C.H.; et al. A Novel Soluble Form of Tim-3 Associated with Severe Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2013, 19, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yue, Z.; Yu, J.; Daguindau, E.; Kushekhar, K.; Zhang, Q.; Ogata, Y.; Gafken, P.R.; Inamoto, Y.; Gracon, A.; et al. Proteomic Characterization Reveals That MMP-3 Correlates With Bronchiolitis Obliterans Syndrome Following Allogeneic Hematopoietic Cell and Lung Transplantation. Am. J. Transplant. 2016, 16, 2342–2351. [Google Scholar] [CrossRef]
- Solán, L.; Carbonell, D.; Muñiz, P.; Dorado, N.; Landete, E.; Chicano-Lavilla, M.; Anguita, J.; Gayoso, J.; Kwon, M.; Díez-Martín, J.L.; et al. Elafin as a Predictive Biomarker of Acute Skin Graft- Versus-Host Disease After Haploidentical Stem Cell Transplantation Using Post-Transplant High-Dose Cyclophosphamide. Front. Immunol. 2021, 12, 516078. [Google Scholar] [CrossRef]
- Vander Lugt, M.T.; Braun, T.M.; Hanash, S.; Ritz, J.; Ho, V.T.; Antin, J.H.; Zhang, Q.; Wong, C.-H.; Wang, H.; Chin, A.; et al. ST2 as a Marker for Risk of Therapy-Resistant Graft-versus-Host Disease and Death. N. Engl. J. Med. 2013, 369, 529–539. [Google Scholar] [CrossRef]
- McDonald, G.B.; Tabellini, L.; Storer, B.E.; Martin, P.J.; Lawler, R.L.; Rosinski, S.L.; Schoch, H.G.; Hansen, J.A. Predictive Value of Clinical Findings and Plasma Biomarkers after Fourteen Days of Prednisone Treatment for Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Whittle, R.; Taylor, P.C. Circulating B-Cell Activating Factor Level Predicts Clinical Response of Chronic Graft-versus-Host Disease to Extracorporeal Photopheresis. Blood 2011, 118, 6446–6449. [Google Scholar] [CrossRef]
- Giesen, N.; Schwarzbich, M.A.; Dischinger, K.; Becker, N.; Hummel, M.; Benner, A.; Radujkovic, A.; Müller-Tidow, C.; Dreger, P.; Luft, T. CXCL9 Predicts Severity at the Onset of Chronic Graft-versus-Host Disease. Transplantation 2020, 104, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Martin, P.J.; Lee, S.J.; Momin, A.A.; Tabellini, L.; Onstad, L.E.; Pidala, J.; Flowers, M.E.D.; Lawler, R.L.; Katayama, H.; et al. Dickkopf-Related Protein 3 Is a Novel Biomarker for Chronic GVHD after Allogeneic Hematopoietic Cell Transplantation. Blood Adv. 2020, 4, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Martin, P.J.; Onstad, L.E.; Cheng, G.S.; Williams, K.M.; Pusic, I.; Ho, V.T.; Arora, M.; Pidala, J.; Flowers, M.E.D.; et al. Relevance of Plasma Matrix Metalloproteinase-9 for Bronchiolitis Obliterans Syndrome after Allogeneic Hematopoietic Cell Transplantation. Transplant. Cell. Ther. 2021, 27, 759.e1–759.e8. [Google Scholar] [CrossRef] [PubMed]
- DePriest, B.P.; Li, H.; Bidgoli, A.; Onstad, L.; Couriel, D.; Lee, S.J.; Paczesny, S. Regenerating Islet-Derived Protein 3-α Is a Prognostic Biomarker for Gastrointestinal Chronic Graft-versus-Host Disease. Blood Adv. 2022, 6, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, Y.; Martin, P.J.; Paczesny, S.; Tabellini, L.; Momin, A.A.; Mumaw, C.L.; Flowers, M.E.D.; Lee, S.J.; Carpenter, P.A.; Storer, B.E.; et al. Association of Plasma CD163 Concentration with De Novo-Onset Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2017, 23, 1250–1256. [Google Scholar] [CrossRef]
- Srinagesh, H.K.; Özbek, U.; Kapoor, U.; Ayuk, F.; Aziz, M.; Ben-David, K.; Choe, H.K.; DeFilipp, Z.; Etra, A.; Grupp, S.A.; et al. The MAGIC Algorithm Probability Is a Validated Response Biomarker of Treatment of Acute Graft-versus-Host Disease. Blood Adv. 2019, 3, 4034–4042. [Google Scholar] [CrossRef]
- Major-Monfried, H.; Renteria, A.S.; Pawarode, A.; Reddy, P.; Ayuk, F.; Holler, E.; Efebera, Y.A.; Hogan, W.J.; Wölfl, M.; Qayed, M.; et al. MAGIC Biomarkers Predict Long-Term Outcomes for Steroid-Resistant Acute GVHD. Blood 2018, 131, 2846–2855. [Google Scholar] [CrossRef]
- Naymagon, S.; Naymagon, L.; Wong, S.Y.; Ko, H.M.; Renteria, A.; Levine, J.; Colombel, J.F.; Ferrara, J. Acute Graft-versus-Host Disease of the Gut: Considerations for the Gastroenterologist. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 711–726. [Google Scholar] [CrossRef]
- Scott, I.C.; Houslay, K.F.; Cohen, E.S. Prospects to Translate the Biology of IL-33 and ST2 during Organ Transplantation into Therapeutics to Treat Graft-versus-Host Disease. Ann. Transl. Med. 2016, 4, 500. [Google Scholar] [CrossRef] [PubMed]
- Paczesny, S. Post-Haematopoietic Cell Transplantation Outcomes: Why ST2 Became a “golden Nugget” Biomarker. Br. J. Haematol. 2021, 192, 951–967. [Google Scholar] [CrossRef] [PubMed]
- 33 Piper, C.; Drobyski, W.R. Inflammatory Cytokine Networks in Gastrointestinal Tract Graft vs. Host Disease. Front. Immunol. 2019, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, J.; Prado-Acosta, M. Graft-versus-Host Disease: Establishing IL-33 as an Important Costimulatory Molecule. J. Clin. Investig. 2022, 132, e160692. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Barrett, A.J. ST2: The Biomarker at the Heart of GVHD Severity. Blood 2015, 125, 10–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reichenbach, D.K.; Schwarze, V.; Matta, B.M.; Tkachev, V.; Lieberknecht, E.; Liu, Q.; Koehn, B.H.; Pfeifer, D.; Taylor, P.A.; Prinz, G.; et al. The IL-33/ST2 Axis Augments Effector T-Cell Responses during Acute GVHD. Blood 2015, 125, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Storer, B.E.; Kushekhar, K.; Zaid, M.A.; Zhang, Q.; Gafken, P.R.; Ogata, Y.; Martin, P.J.; Flowers, M.E.; Hansen, J.A.; et al. Biomarker Panel for Chronic Graft-Versus-Host Disease. J. Clin. Oncol. 2016, 34, 2583–2590. [Google Scholar] [CrossRef]
- Giaccone, L.; Faraci, D.G.; Butera, S.; Lia, G.; Di Vito, C.; Gabrielli, G.; Cerrano, M.; Mariotti, J.; Dellacasa, C.; Felicetti, F.; et al. Biomarkers for Acute and Chronic Graft versus Host Disease: State of the Art. Expert Rev. Hematol. 2021, 14, 79–96. [Google Scholar] [CrossRef]
- Zhao, D.; Kim, Y.H.; Jeong, S.; Greenson, J.K.; Chaudhry, M.S.; Hoepting, M.; Anderson, E.R.; Van Den Brink, M.R.M.; Peled, J.U.; Gomes, A.L.C.; et al. Survival Signal REG3α Prevents Crypt Apoptosis to Control Acute Gastrointestinal Graft-versus-Host Disease. J. Clin. Investig. 2018, 128, 4970–4979. [Google Scholar] [CrossRef]
- Nagasawa, M. Biomarkers of Graft-vs-Host Disease: Understanding and Applications for the Future. World J. Transplant. 2021, 11, 335. [Google Scholar] [CrossRef]
- Presland, R.B. Application of Proteomics to Graft-versus-Host Disease: From Biomarker Discovery to Potential Clinical Applications. Expert Rev. Proteom. 2017, 14, 997–1006. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Sakellari, I.; Chatzikonstantinou, T.; Mallouri, D.; Batsis, I.; Vardi, A.; Bousiou, Z.; Koravou, E.E.; Masmanidou, M.; Touloumenidou, T.; et al. Endothelial and Complement Activation As Predictors of Survival in Adult Allogeneic Hematopoietic Cell Transplantation. Hemasphere 2020, 5, E487. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Sakellari, I.; Anyfanti, P.; Batsis, I.; Vardi, A.; Bousiou, Z.; Lazaridis, A.; Nikolaidou, B.; Zarifis, I.; Masmanidou, M.; et al. Assessment of Endothelial Injury and Pro-Coagulant Activity Using Circulating Microvesicles in Survivors of Allogeneic Hematopoietic Cell Transplantation. Int. J. Mol. Sci. 2020, 21, 9768. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Touloumenidou, T.; Sakellari, I.; Batsis, I.; Mallouri, D.; Psomopoulos, F.; Tsagiopoulou, M.; Koutra, M.; Yannaki, E.; Papalexandri, A.; et al. Pretransplant Genetic Susceptibility: Clinical Relevance in Transplant-Associated Thrombotic Microangiopathy. Thromb. Haemost. 2020, 120, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Ajit, S.K. Circulating MicroRNAs as Biomarkers, Therapeutic Targets, and Signaling Molecules. Sensors 2012, 12, 3359. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Wohlan, K.; Dallmann, I.; Förster, R.; Ganser, A.; Krueger, A.; Scherr, M.; Eder, M.; Koenecke, C. MiR-181a Expression in Donor T Cells Modulates Graft-versus-Host Disease after Allogeneic Bone Marrow Transplantation. J. Immunol. 2016, 196, 3927–3934. [Google Scholar] [CrossRef] [PubMed]
- Sang, W.; Zhang, C.; Zhang, D.; Wang, Y.; Sun, C.; Niu, M.; Sun, X.; Zhou, C.; Zeng, L.; Pan, B.; et al. MicroRNA-181a, a Potential Diagnosis Marker, Alleviates Acute Graft versus Host Disease by Regulating IFN-γ Production. Am. J. Hematol. 2015, 90, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.A.; Fattahi, F.; Bosmann, M. New Insights into Molecular Mechanisms of Immune Complex-Induced Injury in Lung. Front. Immunol. 2016, 7, 86. [Google Scholar] [CrossRef]
- Crossland, R.E.; Norden, J.; Juric, M.K.; Pearce, K.F.; Lendrem, C.; Bibby, L.A.; Collin, M.; Greinix, H.T.; Dickinson, A.M. Serum and Extracellular Vesicle MicroRNAs MiR-423, MiR-199, and MiR-93* As Biomarkers for Acute Graft-versus-Host Disease. Front. Immunol. 2017, 8, 1446. [Google Scholar] [CrossRef]
- Earls, M.R.; Coleman, D.C.; Brennan, G.I.; Fleming, T.; Monecke, S.; Slickers, P.; Ehricht, R.; Shore, A.C. Differential MicroRNA Expression Levels in Cutaneous Acute Graft-Versus-Host Disease. Front. Immunol. 2018, 9, 1485. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, Y.; Li, W.; Baker, M.; Guo, J.; Corbet, K.; Tsalik, E.L.; Li, Q.J.; Palmer, S.M.; Woods, C.W.; et al. Plasma MicroRNA Signature as a Noninvasive Biomarker for Acute Graft-versus-Host Disease. Blood 2013, 122, 3365. [Google Scholar] [CrossRef] [PubMed]
- Reikvam, H.; Vo, A.K.; Johansen, S.; Hemsing, A.L.; Solheim, M.H.; Mosevoll, K.A.; Tvedt, T.H.A.; Hatfield, K.J. MicroRNA Serum Profiles and Chronic Graft-versus-Host Disease. Blood Adv. 2022, 6, 5295–5306. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Schutt, S.; Paz, K.; Zhang, M.; Flynn, R.P.; Bastian, D.; Hanief Sofi, M.; Nguyen, H.; Dai, M.; Liu, C.; et al. MicroRNA-17-92 Is Required for T-Cell and B-Cell Pathogenicity in Chronic Graft-versus-Host Disease in Mice. Blood 2018, 131, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.C.; Ferrara, J.L.M.; Levine, J.E. Advances in Predicting Acute GVHD. Br. J. Haematol. 2013, 160, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.A.; Chien, J.W.; Warren, E.H.; Zhao, L.P.; Martin, P.J. Defining Genetic Risk for Graft-versus-Host Disease and Mortality Following Allogeneic Hematopoietic Stem Cell Transplantation. Curr. Opin. Hematol. 2010, 17, 483–492. [Google Scholar] [CrossRef]
- Gruhn, B.; Intek, J.; Pfaffendorf, N.; Zell, R.; Corbacioglu, S.; Zintl, F.; Beck, J.F.; Debatin, K.M.; Steinbach, D. Polymorphism of Interleukin-23 Receptor Gene but Not of NOD2/CARD15 Is Associated with Graft-versus-Host Disease after Hematopoietic Stem Cell Transplantation in Children. Biol. Blood Marrow Transplant. 2009, 15, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Elmaagacli, A.H.; Koldehoff, M.; Landt, O.; Beelen, D.W. Relation of an Interleukin-23 Receptor Gene Polymorphism to Graft-versus-Host Disease after Hematopoietic-Cell Transplantation. Bone Marrow Transplant. 2008, 41, 821–826. [Google Scholar] [CrossRef]
- Sarin, R.; Wu, X.; Abraham, C. Inflammatory Disease Protective R381Q IL23 Receptor Polymorphism Results in Decreased Primary CD4+ and CD8+ Human T-Cell Functional Responses. Proc. Natl. Acad. Sci. USA 2011, 108, 9560–9565. [Google Scholar] [CrossRef]
- Hanash, A.M.; Dudakov, J.A.; Hua, G.; O’Connor, M.H.; Young, L.F.; Singer, N.V.; West, M.L.; Jenq, R.R.; Holland, A.M.; Kappel, L.W.; et al. Interleukin-22 Protects Intestinal Stem Cells from Immune-Mediated Tissue Damage and Regulates Sensitivity to Graft versus Host Disease. Immunity 2012, 37, 339–350. [Google Scholar] [CrossRef]
- Mougeot, J.L.C.; Beckman, M.F.; Hovan, A.J.; Hasséus, B.; Legert, K.G.; Johansson, J.E.; von Bültzingslöwen, I.; Brennan, M.T.; Bahrani Mougeot, F. Identification of Single Nucleotide Polymorphisms (SNPs) Associated with Chronic Graft-versus-Host Disease in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Support. Care Cancer 2023, 31, 587. [Google Scholar] [CrossRef]
- Bassim, C.W.; Fassil, H.; Mays, J.W.; Edwards, D.; Baird, K.; Steinberg, S.M.; Cowen, E.W.; Naik, H.; Datiles, M.; Stratton, P.; et al. Oral Disease Profiles in Chronic Graft versus Host Disease. J. Dent. Res. 2015, 94, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Ion, D.; Stevenson, K.; Woo, S.B.; Ho, V.T.; Soiffer, R.; Antin, J.H.; Treister, N.S. Characterization of Oral Involvement in Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2014, 20, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Socié, G.; Curtis, R.E.; Deeg, H.J.; Sobocinski, K.A.; Filipovich, A.H.; Travis, L.B.; Sullivan, K.M.; Rowlings, P.A.; Kingma, D.W.; Banks, P.M.; et al. New Malignant Diseases after Allogeneic Marrow Transplantation for Childhood Acute Leukemia. J. Clin. Oncol. 2000, 18, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Adès, L.; Guardiola, P.; Sociè, G. Second Malignancies after Allogeneic Hematopoietic Stem Cell Transplantation: New Insight and Current Problems. Blood Rev. 2002, 16, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N. Engl. J. Med. 2017, 377, 2565–2579. [Google Scholar] [CrossRef] [PubMed]
- Crossland, R.E.; Perutelli, F.; Bogunia-Kubik, K.; Mooney, N.; Milutin Gašperov, N.; Pučić-Baković, M.; Greinix, H.; Weber, D.; Holler, E.; Pulanić, D.; et al. Potential Novel Biomarkers in Chronic Graft-Versus-Host Disease. Front. Immunol. 2020, 11, 602547. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Chen, H.; Zhou, M.; Liu, Y.; Hou, Y.; Nie, M.; Liu, X. Identification and Validation of Potential Novel Biomarkers for Oral Squamous Cell Carcinoma. Bioengineered 2021, 12, 8845–8862. [Google Scholar] [CrossRef]
- Zhao, X.S.; Huang, X.J. Seeking Biomarkers for Acute Graft-versus-Host Disease: Where We Are and Where We Are Heading? Biomark. Res. 2019, 7, 17. [Google Scholar] [CrossRef]
- Chiusolo, P.; Giammarco, S.; Fanali, C.; Bellesi, S.; Metafuni, E.; Sica, S.; Iavarone, F.; Cabras, T.; Messana, I.; Leone, G.; et al. Salivary Proteomic Analysis and Acute Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2013, 19, 888–892. [Google Scholar] [CrossRef]
- Costa-da-Silva, A.C.; Aure, M.H.; Dodge, J.; Martin, D.; Dhamala, S.; Cho, M.; Rose, J.J.; Bassim, C.W.; Ambatipudi, K.; Hakim, F.T.; et al. Salivary ZG16B Expression Loss Follows Exocrine Gland Dysfunction Related to Oral Chronic Graft-versus-Host Disease. iScience 2022, 25, 103592. [Google Scholar] [CrossRef]
- Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; Bravo, S.B.; López-Jornet, P.; García-Vence, M.; Alonso-Sampedro, M.; Carballo, J.; García-García, A. Protein-Based Salivary Profiles as Novel Biomarkers for Oral Diseases. Dis. Markers 2018, 2018, 6141845. [Google Scholar] [CrossRef] [PubMed]
- Uma Maheswari, T.N.; Venugopal, A.; Sureshbabu, N.M.; Ramani, P. Salivary Micro RNA as a Potential Biomarker in Oral Potentially Malignant Disorders: A Systematic Review. Ci Ji Yi Xue Za Zhi 2018, 30, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Srinagesh, H.K.; Levine, J.E.; Ferrara, J.L.M. Biomarkers in Acute Graft- versus-Host Disease: New Insights. Ther. Adv. Hematol. 2019, 10, 204062071989135. [Google Scholar] [CrossRef] [PubMed]
- Pietraszkiewicz, A.A.; Payne, D.; Abraham, M.; Garced, A.; Devarasetty, K.C.; Wall, M.; Menezes, S.M.; Ugarte, S.; Pirsl, F.; Goklemez, S.; et al. Ocular Surface Indicators and Biomarkers in Chronic Ocular Graft-versus-Host Disease: A Prospective Cohort Study. Bone Marrow Transplant. 2021, 56, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Rozmus, J.; Schultz, K.R. Biomarkers in Chronic Graft-versus-Host Disease. Expert Rev. Hematol. 2011, 4, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Aghdaei, H.A.; Jasemi, S.; Gazouli, M.; Dovrolis, N.; Sadeghi, A.; Schlüter, H.; Zali, M.R.; Sechi, L.A.; Feizabadi, M.M. Oral Microbiota as Novel Biomarkers for Colorectal Cancer Screening. Cancers 2022, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.; Chincholkar, T.; Dixit, R.; Pandey, M. A Systematic Review of Proteomic Biomarkers in Oral Squamous Cell Cancer. World J. Surg. Oncol. 2021, 19, 315. [Google Scholar] [CrossRef]
 : Increased levels;
: Increased levels;  : decreased levels.
: decreased levels.
 : Increased levels;
: Increased levels;  : decreased levels.
: decreased levels.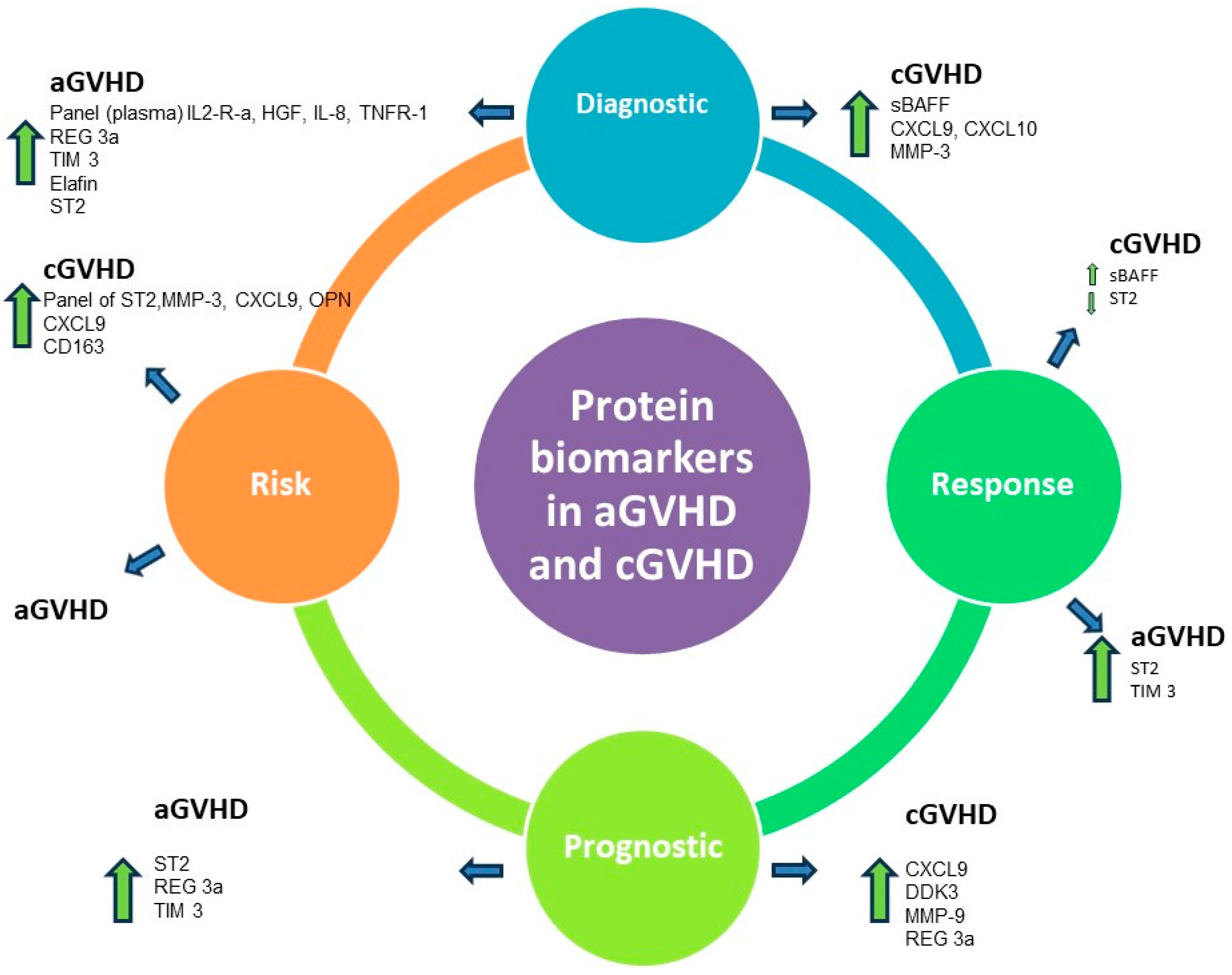
| - | aGVHD | cGVHD |
|---|---|---|
| - | Protein Biomarkers | |
| Diagnostic | Panel (plasma) IL2-R-a, HGF, IL-8, TNFR-1 [10]  | sBAFF [11,12,13,14]  |
| - | Reg-3a [15]  | CXCL9, CXCL10 [14,16]  |
| - | TIM 3 [17]  | MMP-3 [18]  |
| - | Elafin [19]  | - |
| - | ST2 [20]  | - |
| Response | ST2 [21]  | sBAFF [22]  |
| - | TIM3 [21]  | ST2 [9]  |
| Prognostic | ST2 [20]  | CXCL9 [23]  |
| - | REG3a [15]  | DDK3 [24]  |
| - | TIM3 [9]  | MMP-9 [25]  |
| - | - | Reg-3a [26]  |
| Risk | - | Panel of ST2, MMP-3, CXCL9, OPN [11]  |
| - | - | CXCL9 [9]  |
| - | - | CD163 [27]  |
 : Increased levels;
: Increased levels;  : decreased levels.
: decreased levels.| aGVHD | cGVHD |
|---|---|
| Genetic Biomarkers | |
miR-155 [46,47]  | miR17-92 [52,53]  |
miR181a [46,47]  | miR-365-3p, miR-148-3p, and miR-378-3p [52,53] (proposed as a panel)  |
miR20a [49]  | |
miR15a [49]  | |
miR146a [48,49]  | |
miR30b-5p [49]  | |
miR374-5p [49]  | |
miR34a-3p and miR503-5p [50]  | |
 : Upregulation;
: Upregulation;  : downregulation.
: downregulation.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexoudi, V.-A.; Gavriilaki, E.; Cheva, A.; Sakellari, I.; Papadopoulou, S.; Paraskevopoulos, K.; Vahtsevanos, K. Graft-Versus-Host Disease: Can Biomarkers Assist in Differential Diagnosis, Prognosis, and Therapeutic Strategy? Pharmaceuticals 2024, 17, 298. https://doi.org/10.3390/ph17030298
Alexoudi V-A, Gavriilaki E, Cheva A, Sakellari I, Papadopoulou S, Paraskevopoulos K, Vahtsevanos K. Graft-Versus-Host Disease: Can Biomarkers Assist in Differential Diagnosis, Prognosis, and Therapeutic Strategy? Pharmaceuticals. 2024; 17(3):298. https://doi.org/10.3390/ph17030298
Chicago/Turabian StyleAlexoudi, Vaia-Aikaterini, Eleni Gavriilaki, Angeliki Cheva, Ioanna Sakellari, Stavroula Papadopoulou, Konstantinos Paraskevopoulos, and Konstantinos Vahtsevanos. 2024. "Graft-Versus-Host Disease: Can Biomarkers Assist in Differential Diagnosis, Prognosis, and Therapeutic Strategy?" Pharmaceuticals 17, no. 3: 298. https://doi.org/10.3390/ph17030298
APA StyleAlexoudi, V.-A., Gavriilaki, E., Cheva, A., Sakellari, I., Papadopoulou, S., Paraskevopoulos, K., & Vahtsevanos, K. (2024). Graft-Versus-Host Disease: Can Biomarkers Assist in Differential Diagnosis, Prognosis, and Therapeutic Strategy? Pharmaceuticals, 17(3), 298. https://doi.org/10.3390/ph17030298








