A Comprehensive Physiologically Based Pharmacokinetic Model of Nadolol in Adults with Renal Disease and Pediatrics with Supraventricular Tachycardia
Abstract
1. Introduction
2. Results
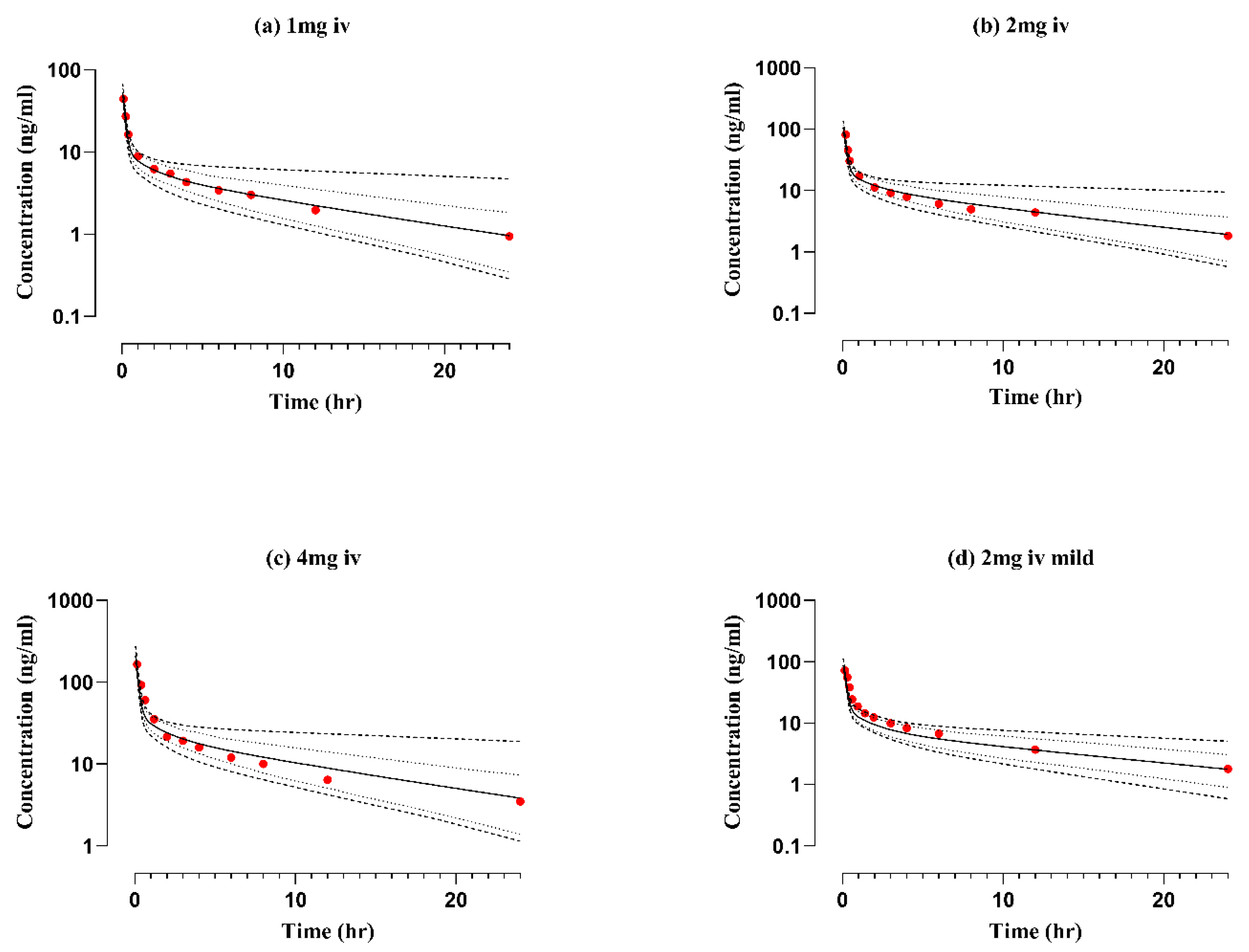
2.1. Healthy Adults after Intravenous Application
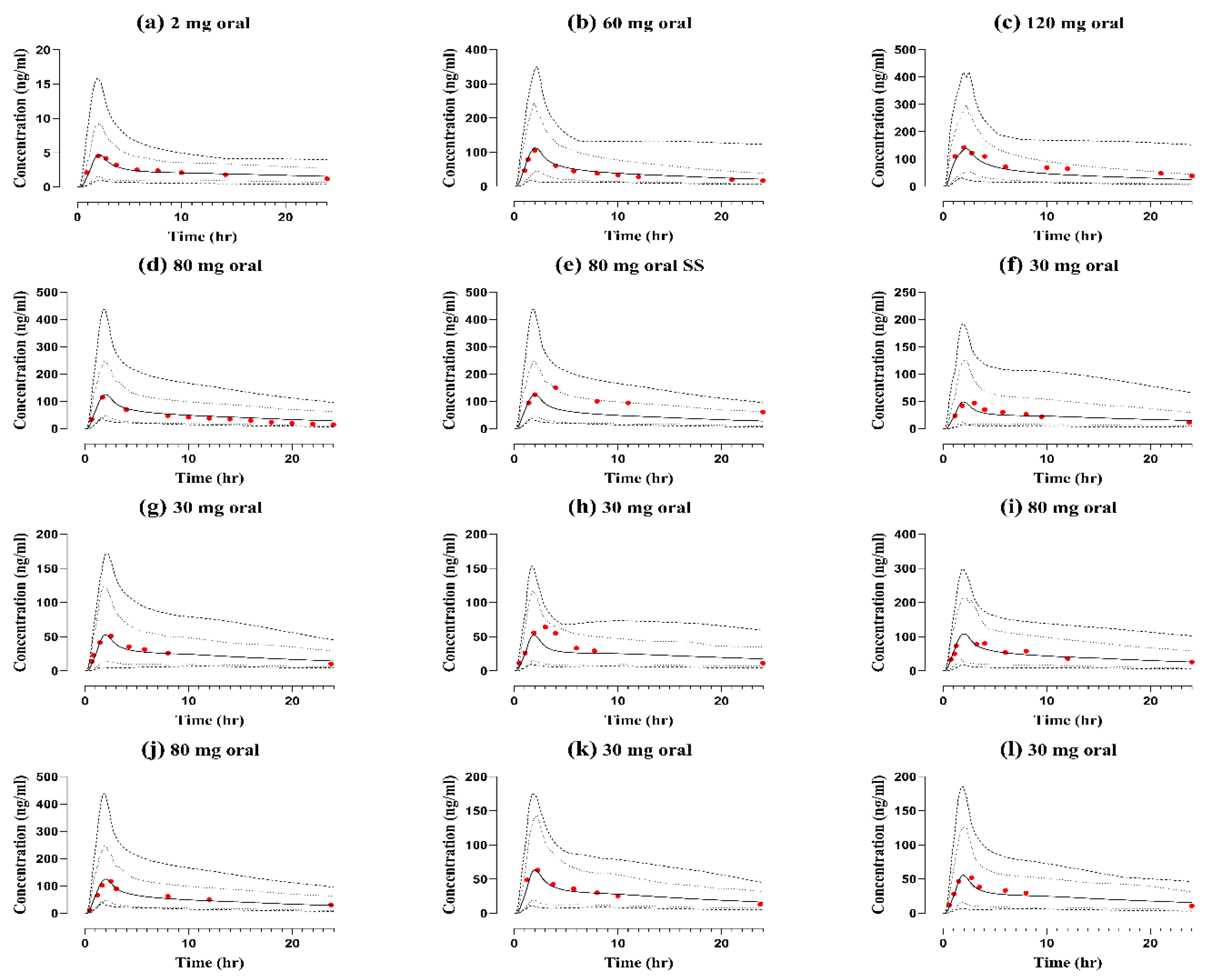
2.2. Healthy Adults after Oral Application
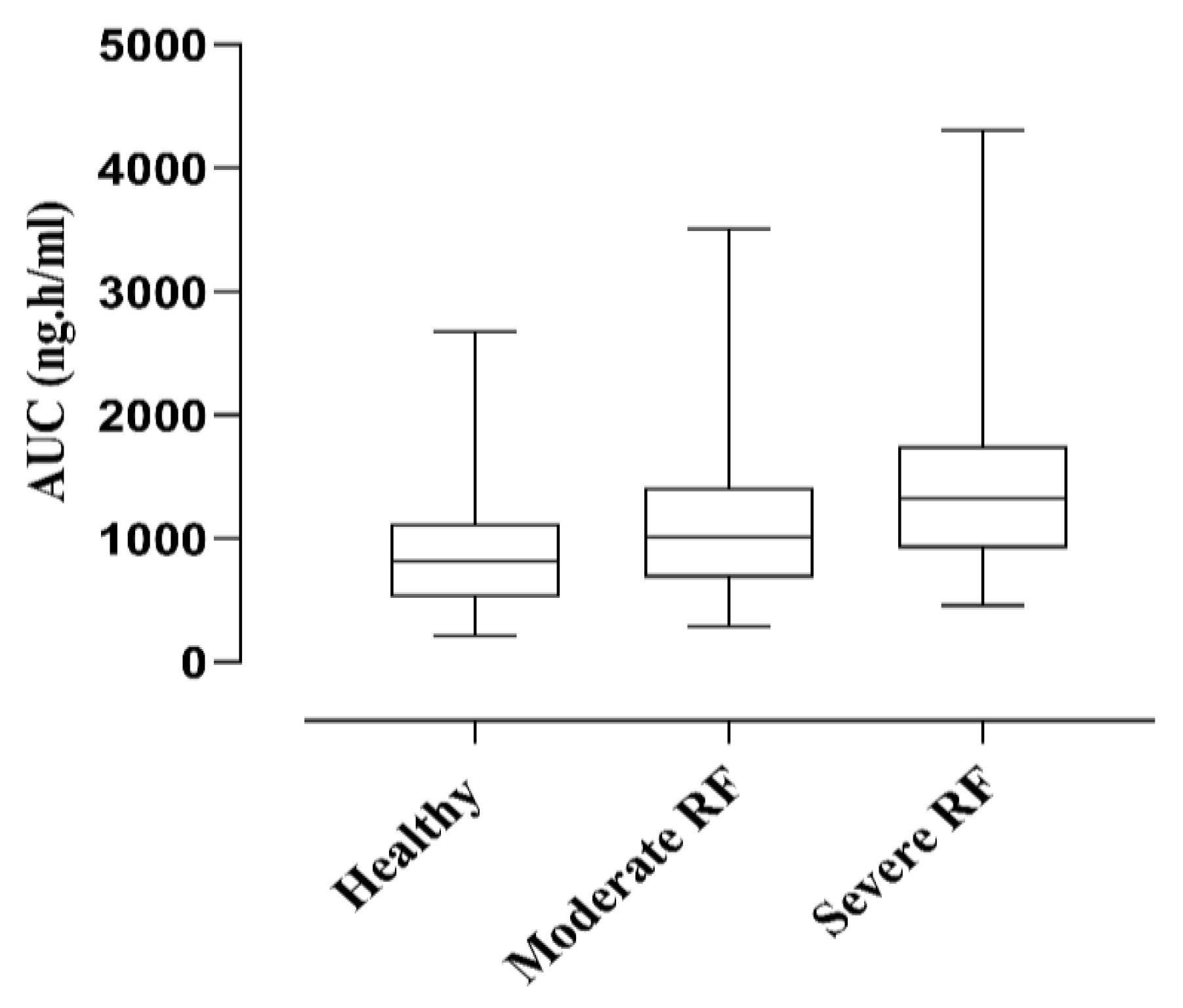
2.3. Renal Failure Population
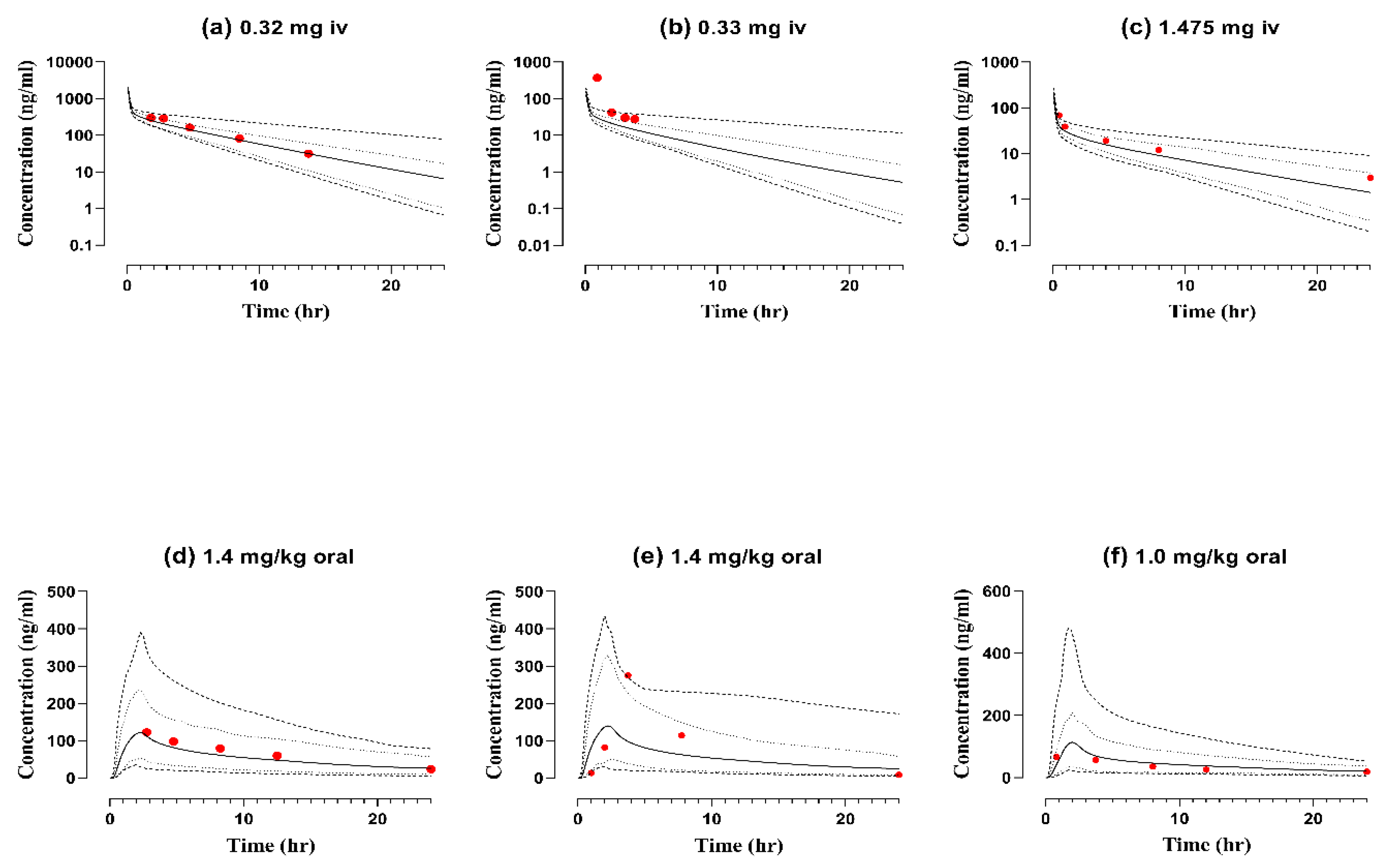
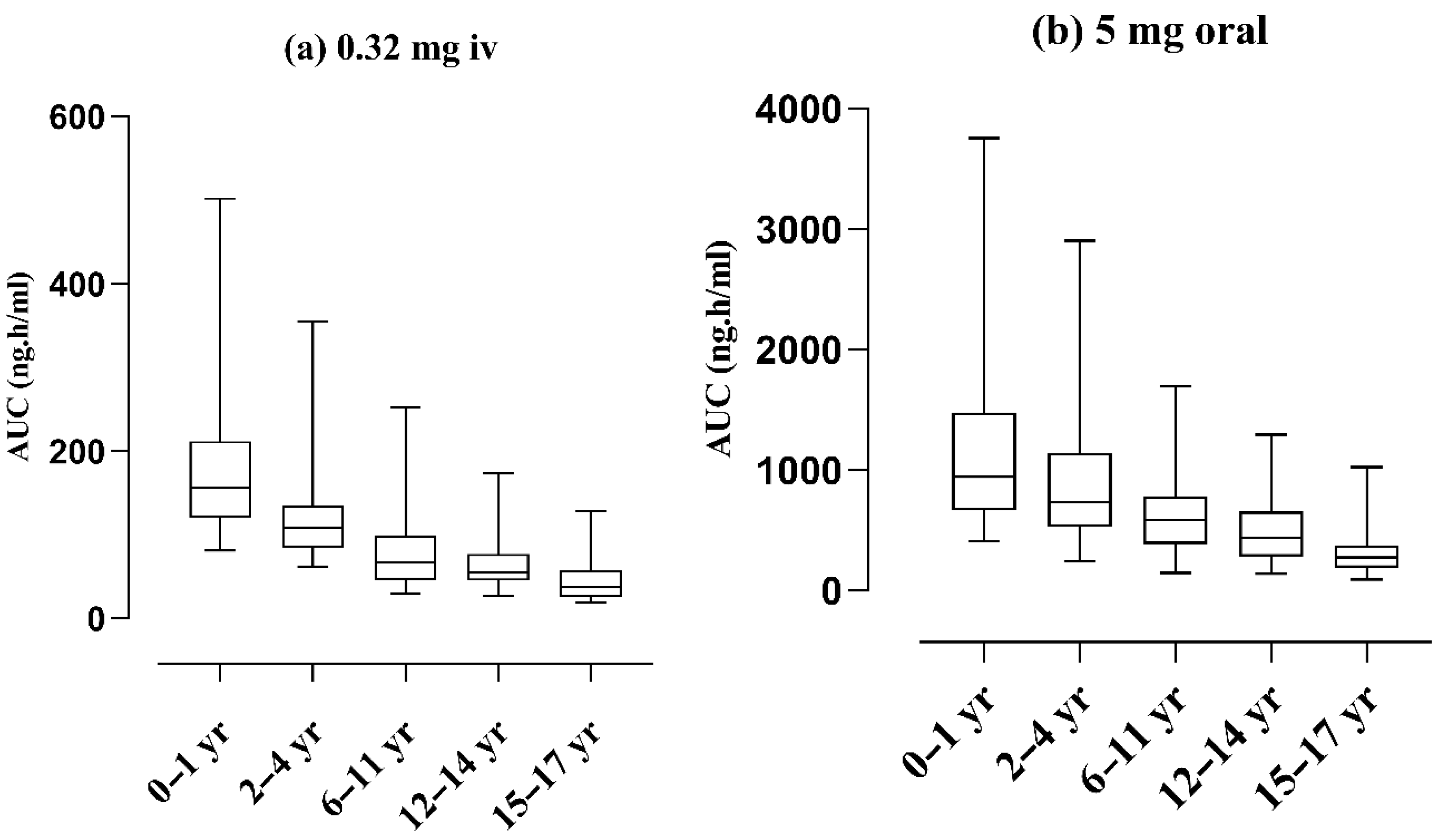
2.4. Pediatric Population
2.5. Age-Related Changes in Exposure
3. Discussion
4. Methods
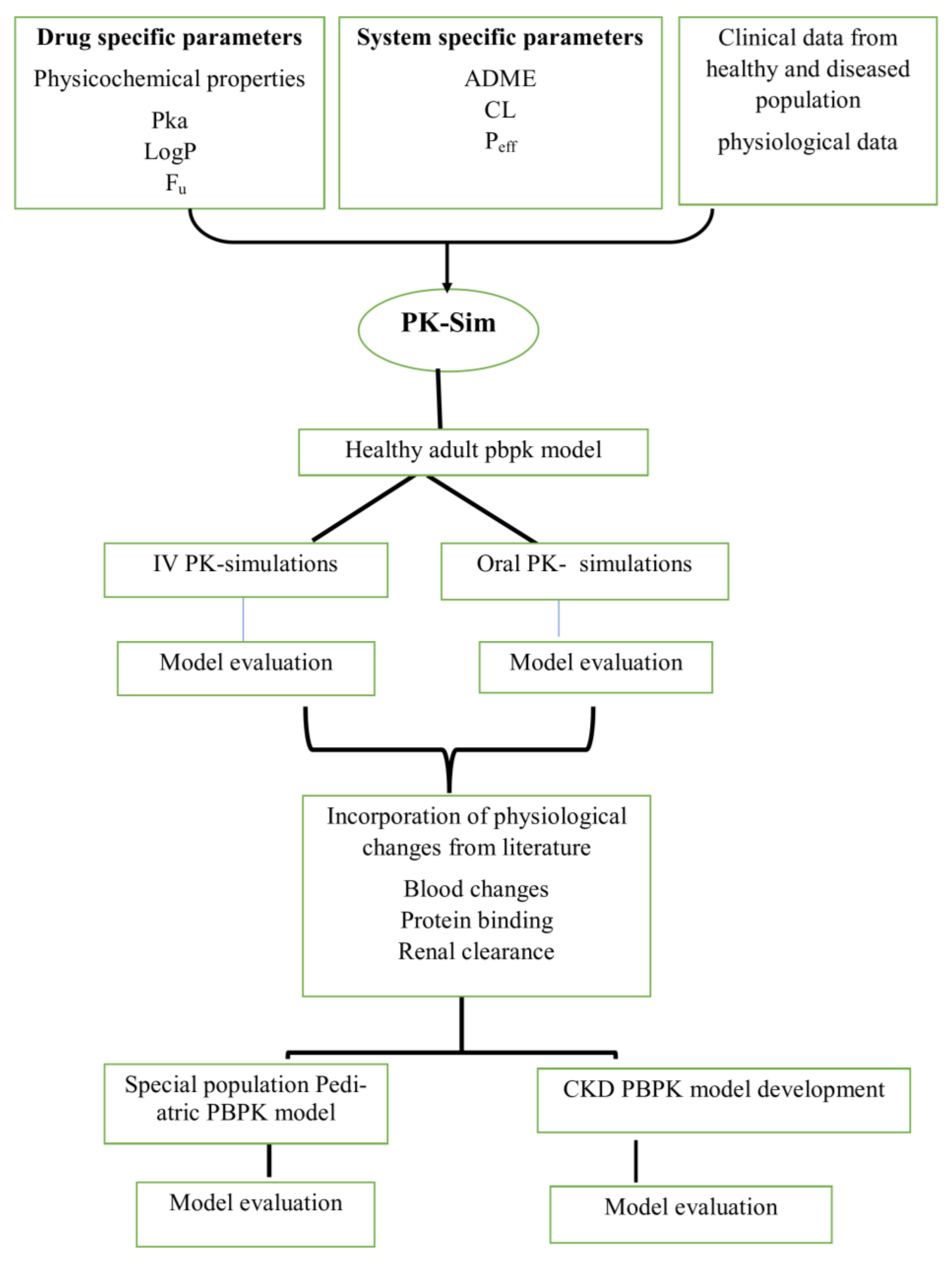
4.1. Modeling Software
4.2. PBPK Modeling Strategy
4.3. Building Blocks for PBPK Model Development
4.4. Clinical Pharmacokinetic Data
4.5. Renal Impairment Population
4.6. Pediatric Model
4.7. Model Verification
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, D.B.; Peschka, M.T.; Lee, R.J.; Laffan, R.J. Anti-arrhythmic action of nadolol, a β-adrenergic receptor blocking agent. Eur. J. Pharmacol. 1976, 35, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Dreyfuss, J.; Brannick, L.; Vukovich, R.; Shaw, J.; Willard, D. Metabolic studies in patients with nadolol: Oral and intravenous administration. J. Clin. Pharmacol. 1977, 17, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.; Gould, B.; Kieso, H.; Raftery, E. A study of nadolol to determine its effect on ambulatory blood pressure over 24 hour, and during exercise testing. Br. J. Clin. Pharmacol. 1982, 14, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Gopal, S.; Mandiga, P. Nadolol. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Frishman, W.H. Nadolol: A new β-adrenoceptor antagonist. N. Engl. J. Med. 1981, 305, 678–682. [Google Scholar]
- Kalsoom, S.; Zamir, A.; Rehman, A.U.; Ashraf, W.; Imran, I.; Saeed, H.; Majeed, A.; Alqahtani, F.; Rasool, M.F. Clinical pharmacokinetics of nadolol: A systematic review. J. Clin. Pharm. Ther. 2022, 47, 1506–1516. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Faustino, P.J.; Volpe, D.A.; Ellison, C.D.; Lyon, R.C.; Yu, L.X. Biopharmaceutics classification of selected beta-blockers: Solubility and permeability class membership. Mol. Pharm. 2007, 4, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.; Duchin, K.; Fleiss, P. Nadolol in human serum and breast milk. Br. J. Clin. Pharmacol. 1981, 12, 393–396. [Google Scholar] [CrossRef] [PubMed]
- King-Mallory, R.; Barker, S.; McGrogan, K.; Franks, A.M. Nadolol in pregnancy: A medical student’s reflection on her pregnancy. Marshall J. Med. 2018, 4, 16. [Google Scholar] [CrossRef]
- Dreyfuss, J.; Griffith, D.; Singhvi, S.; Shaw, J.; Ross Jr, J.; Vukovich, R.; Willard, D. Pharmacokinetics of Nadolol, a Beta-Receptor Antagonist: Administration of Therapeutic Single-and Multiple-Dosage Regimens to Hypertensive Patients. J. Clin. Pharmacol. 1979, 19, 712–720. [Google Scholar] [CrossRef]
- Patel, L.; Johnston, A.; Turner, P. Nadolol binding to human serum proteins. J. Pharm. Pharmacol. 1984, 36, 414–415. [Google Scholar] [CrossRef]
- Heel, R.; Brogden, R.; Pakes, G.; Speight, T.; Avery, G. Nadolol: A review of its pharmacological properties and therapeutic efficacy in hypertension and angina pectoris. Drugs 1980, 20, 1–23. [Google Scholar] [CrossRef]
- Höcht, C.; Bertera, F.M.; Mayer, M.A.; Taira, C.A. Issues in drug metabolism of major antihypertensive drugs: Beta-blockers, calcium channel antagonists and angiotensin receptor blockers. Expert Opin. Drug Metab. Toxicol. 2010, 6, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Schmoldt, A.; Andresen-Streichert, H.; Iwersen-Bergmann, S. Revisited: Therapeutic and toxic blood concentrations of more than 1100 drugs and other xenobiotics. Crit. Care 2020, 24, 195. [Google Scholar] [CrossRef]
- Repetto, M.R.; Repetto, M. Therapeutic, toxic, and lethal concentrations in human fluids of 90 drugs affecting the cardiovascular and hematopoietic systems. J. Toxicol. Clin. Toxicol. 1997, 35, 345–351. [Google Scholar] [CrossRef]
- Abdelmawla, A.H.; Langley, R.W.; Szabadi, E.; Bradshaw, C.M. Comparison of the effects of nadolol and bisoprolol on the isoprenaline-evoked dilatation of the dorsal hand vein in man. Br. J. Clin. Pharmacol. 2001, 51, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Ranade, V.V.; Somberg, J.C. Chiral cardiovascular drugs: An overview. Am. J. Ther. 2005, 12, 439–459. [Google Scholar] [CrossRef] [PubMed]
- Morganroth, J. Antiarrhythmic effects of beta-adrenergic blocking agents in benign or potentially lethal ventricular arrhythmias. Am. J. Cardiol. 1987, 60, 10–14. [Google Scholar] [CrossRef]
- Waal-Manning, H.; Hobson, C. Renal function in patients with essential hypertension receiving nadolol. Br. Med. J. 1980, 281, 423. [Google Scholar] [CrossRef][Green Version]
- Herrera, J.; Vukovich, R.; Griffith, D. Elimination of nadolol by patients with renal impairment. Br. J. Clin. Pharmacol. 1979, 7, 227S–231S. [Google Scholar] [CrossRef]
- Malik, P.R.; Yeung, C.H.; Ismaeil, S.; Advani, U.; Djie, S.; Edginton, A.N. A physiological approach to pharmacokinetics in chronic kidney disease. J. Clin. Pharmacol. 2020, 60, S52–S62. [Google Scholar] [CrossRef]
- Johnson, T.N.; Boussery, K.; Rowland-Yeo, K.; Tucker, G.T.; Rostami-Hodjegan, A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin. Pharmacokinet. 2010, 49, 189–206. [Google Scholar] [CrossRef] [PubMed]
- Rowland Yeo, K.; Aarabi, M.; Jamei, M.; Rostami-Hodjegan, A. Modeling and predicting drug pharmacokinetics in patients with renal impairment. Expert Rev. Clin. Pharmacol. 2011, 4, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Rowland, M. The role of physiologically based pharmacokinetic modeling in regulatory review. Clin. Pharmacol. Ther. 2012, 91, 542–549. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, L.; Grillo, J.; Liu, Q.; Bullock, J.; Moon, Y.; Song, P.; Brar, S.; Madabushi, R.; Wu, T. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin. Pharmacol. Ther. 2011, 89, 259–267. [Google Scholar] [CrossRef]
- Teorell, T. Studies on the diffusion effect upon ionic distribution: II. Experiments on ionic accumulation. J. Gen. Physiol. 1937, 21, 107. [Google Scholar] [CrossRef]
- Leong, R.; Vieira, M.; Zhao, P.; Mulugeta, Y.; Lee, C.; Huang, S.M.; Burckart, G. Regulatory experience with physiologically based pharmacokinetic modeling for pediatric drug trials. Clin. Pharmacol. Ther. 2012, 91, 926–931. [Google Scholar] [CrossRef]
- Roberts, R.; Rodriguez, W.; Murphy, D.; Crescenzi, T. Pediatric drug labeling: Improving the safety and efficacy of pediatric therapies. JAMA 2003, 290, 905–911. [Google Scholar] [CrossRef]
- Choonara, I. Unlicensed and off-label drug use in children: Implications for safety. Expert Opin. Drug Saf. 2004, 3, 81–83. [Google Scholar] [CrossRef]
- Edginton, A.N.; Schmitt, W.; Willmann, S. Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin. Pharmacokinet. 2006, 45, 1013–1034. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.V.; Chidambaram, B.; Rice, P.J. Pharmacokinetics of nadolol in children with supraventricular tachycardia. J. Clin. Pharmacol. 1992, 32, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Franchetti, Y.; Nolin, T.D. Dose optimization in kidney disease: Opportunities for PBPK modeling and simulation. J. Clin. Pharmacol. 2020, 60, S36–S51. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.F.; Khalil, F.; Läer, S. Optimizing the clinical use of carvedilol in liver cirrhosis using a physiologically based pharmacokinetic modeling approach. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Björkman, S. Prediction of drug disposition in infants and children by means of physiologically based pharmacokinetic (PBPK) modelling: Theophylline and midazolam as model drugs. Br. J. Clin. Pharmacol. 2005, 59, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Gaohua, L.; Abduljalil, K.; Jamei, M.; Johnson, T.N.; Rostami-Hodjegan, A. A pregnancy physiologically based pharmacokinetic (p-PBPK) model for disposition of drugs metabolized by CYP1A2, CYP2D6 and CYP3A4. Br. J. Clin. Pharmacol. 2012, 74, 873–885. [Google Scholar] [CrossRef]
- Morrison, R.; Singhvi, S.; Creasey, W.; Willard, D. Dose proportionality of nadolol pharmacokinetics after intravenous administration to healthy subjects. Eur. J. Clin. Pharmacol. 1988, 33, 625–628. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Bach, N.; Knauf, H.; Mutschler, E. Pharmacokinetics of nadolol in healthy subjects. Eur. J. Clin. Pharmacol. 1984, 26, 125–127. [Google Scholar] [CrossRef]
- Krukemyer, J.J.; Boudoulas, H.; Binkley, P.F.; Lima, J.J. Comparison of single-dose and steady-state nadolol plasma concentrations. Pharm. Res. 1990, 7, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Misaka, S.; Miyazaki, N.; Yatabe, M.S.; Ono, T.; Shikama, Y.; Fukushima, T.; Kimura, J. Pharmacokinetic and pharmacodynamic interaction of nadolol with itraconazole, rifampicin and grapefruit juice in healthy volunteers. J. Clin. Pharmacol. 2013, 53, 738–745. [Google Scholar] [CrossRef]
- Misaka, S.; Yatabe, J.; Müller, F.; Takano, K.; Kawabe, K.; Glaeser, H.; Yatabe, M.; Onoue, S.; Werba, J.; Watanabe, H. Green tea ingestion greatly reduces plasma concentrations of nadolol in healthy subjects. Clin. Pharmacol. Ther. 2014, 95, 432–438. [Google Scholar] [CrossRef]
- Misaka, S.; Abe, O.; Ono, T.; Ono, Y.; Ogata, H.; Miura, I.; Shikama, Y.; Fromm, M.F.; Yabe, H.; Shimomura, K. Effects of single green tea ingestion on pharmacokinetics of nadolol in healthy volunteers. Br. J. Clin. Pharmacol. 2020, 86, 2314–2318. [Google Scholar] [CrossRef]
- Jack, D.B.; Kendall, M.J.; Dean, S.; Laugher, S.J.; Zaman, R.; Tenneson, M.E. The effect of hydralazine on the pharmacokinetics of three different beta adrenoceptor antagonists: Metoprolol, nadolol, and acebutolol. Biopharm. Drug Dispos. 1982, 3, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Souich, P.d.; Caille, G.; Larochelle, P. Enhancement of nadolol elimination by activated charcoal and antibiotics. Clin. Pharmacol. Ther. 1983, 33, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Yoshida, K.; Murano, M.; Naruto, S. Determination of nadolol in serum by high-performance liquid chromatography with fluorimetric detection. J. Chromatogr. B Biomed. Sci. Appl. 1992, 573, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Abe, O.; Ono, T.; Sato, H.; Müller, F.; Ogata, H.; Miura, I.; Shikama, Y.; Yabe, H.; Onoue, S.; Fromm, M.F. Role of (−)-epigallocatechin gallate in the pharmacokinetic interaction between nadolol and green tea in healthy volunteers. Eur. J. Clin. Pharmacol. 2018, 74, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Child Development. 22 February 2021. Available online: https://www.cdc.gov/ncbddd/childdevelopment/positiveparenting/preschoolers.html (accessed on 17 July 2022).
- Michaels, R.; Duchin, K.; Akbar, S.; Meister, J.; Levin, N. Nadolol in hypertensive patients maintained on long-term hemodialysis. Am. Heart J. 1984, 108, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zajicek, A. Review of the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act: What can the obstetric community learn from the pediatric experience? Semin. Perinatol. 2015, 39, 530–531. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Sun, J.; He, Z. Exploring the Feasibility of Biowaiver Extension of BCS Class III Drugs with Site-Specific Absorption Using Gastrointestinal Simulation Technology. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Tatsuoka, H.; Tsuda, M.; Sumi, T.; Eguchi, Y.; So, K.; Higuchi, Y.; Takayama, K.; Torisawa, Y.; Yamashita, F. Intestinal Permeability of Drugs in Caco-2 Cells Cultured in Microfluidic Devices. Biol. Pharm. Bull. 2022, 45, 1246–1253. [Google Scholar] [CrossRef]
- Slusarek, L.; Florey, K. Nadolol. In Analytical Profiles of Drug Substances; Elsevier: Amsterdam, The Netherlands, 1981; pp. 455–485. [Google Scholar]
- DrugBank Online. Nadolol: Uses, Interactions, Mechanism of Action. Available online: https://go.drugbank.com/drugs/DB01203 (accessed on 1 November 2022).
- Lombardo, F.; Obach, R.S.; Shalaeva, M.Y.; Gao, F. Prediction of human volume of distribution values for neutral and basic drugs. 2. Extended data set and leave-class-out statistics. J. Med. Chem. 2004, 47, 1242–1250. [Google Scholar] [CrossRef]
- Duchin, K.L.; Stern, M.A.; Willard, D.A.; McKinstry, D.N. Comparison of kinetic interactions of nadolol and propranolol with cimetidine. Am. Heart J. 1984, 108, 1084–1086. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Xie, S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 2010, 99, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; You, X.; Ke, M.; Jiao, Z.; Wu, H.; Huang, P.; Lin, C. Prediction of ticagrelor and its active metabolite in liver cirrhosis populations using a physiologically based pharmacokinetic model involving pharmacodynamics. J. Pharm. Sci. 2019, 108, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.L.; Gerhart, J.G.; Edginton, A.N.; Yanovski, J.A.; Hon, Y.Y.; Gonzalez, D. Physiologically Based Pharmacokinetic Modeling of Metformin in Children and Adolescents with Obesity. J. Clin. Pharmacol. 2022, 62, 960–969. [Google Scholar] [CrossRef] [PubMed]
| Sr. No | Dose (mg) | Cmax (ng/mL) | Ratio | AUC0-t (ng.h/mL) | Ratio | CL (L/h) | Ratio | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Predicted | Observed | Predicted | Observed | Predicted | Observed | ||||||
| IV Healthy | |||||||||||
| 1 | 52 | 44.6 | 1.16 | 78 | 82 | 0.95 | 10.5 | 10.6 | 0.99 | [36] | |
| 2 | 104 | 81 | 1.28 | 157 | 172 | 0.91 | 19.3 | 20.4 | 0.94 | [36] |
| 4 | 209 | 165 | 1.26 | 314 | 306 | 1.02 | 46.2 | 44.4 | 1.04 | [36] |
| 2 | 86 | 71 | 1.19 | 128 | 179 | 0.71 | 13.5 | 14.1 | 0.96 | [2] |
| Oral Healthy | |||||||||||
| 2 | 4.7 | 4.4 | 1.07 | 71.9 | 68.6 | 1.04 | 40 | 56 | 0.71 | [2] | |
| 60 | 112 | 105 | 1.06 | 973.5 | 972.7 | 1.00 | 2280 | 2820 | 0.80 | [37] |
| 120 | 138.4 | 143.3 | 0.96 | 1215 | 1992.5 | 0.60 | 7524 | 6240 | 1.20 | [37] |
| 80 | 125.2 | 115 | 1.08 | 1202.9 | 1011.3 | 1.18 | 3300 | 5360 | 0.61 | [38] |
| 80 (SS) | 125 | 151 | 0.82 | 1205 | 2224 | 0.54 | 1126 | 1440 | 0.78 | [38] |
| 30 | 125.7 | 117.8 | 1.05 | 1583 | 1830.7 | 0.79 | 1050 | 1020 | 1.02 | [39] |
| 30 | 53 | 51 | 1.03 | 722 | 716 | 1.00 | 1080 | 1140 | 0.94 | [40] |
| 30 | 52 | 64 | 0.81 | 802 | 838 | 0.95 | 930 | 960 | 0.96 | [41] |
| 80 | 108 | 80 | 1.35 | 1063 | 1071 | 0.99 | 3520 | 3840 | 0.91 | [42] |
| 80 | 125 | 117 | 1.07 | 1583 | 1813 | 0.87 | 3824 | 3040 | 1.25 | [43] |
| 30 | 64.4 | 63.2 | 1.01 | 830 | 840.4 | 0.98 | 960 | 990 | 0.96 | [44] |
| 30 | 55 | 52 | 1.05 | 759.7 | 765.5 | 0.99 | 1050 | 1020 | 1.02 | [45] |
| IV Pediatrics Population | |||||||||||
| 0.32 | 360 | 346 | 1.04 | 2255 | 2288 | 0.98 | 0.22 | 0.20 | 1.13 | [31] | |
| 0.33 | 191 | 1312 | 0.14 | 163 | 351 | 0.46 | 0.07 | 2.7 | 0.25 | [31] |
| 1.475 | 198 | 99.5 | 1.98 | 244 | 337 | 0.72 | 57.5 | 47.2 | 1.21 | [31] |
| Oral Pediatrics Population | |||||||||||
| 1.4 ∞ | 122 | 122.4 | 1.00 | 1281 | 1479.3 | 0.86 | 1.09 | 0.99 | 1.10 | [31] | |
| 1.4 ∞ | 1306 | 2148 | 0.60 | 140 | 275 | 0.51 | 1.82 | 3.40 | 0.53 | [31] |
| 1.0 ∞ | 113 | 66.8 | 1.68 | 1013.6 | 799 | 1.26 | 0.66 | 0.87 | 0.75 | [31] |
| PK Parameters | AFE (Average Fold Error) |
|---|---|
| IV Healthy | |
| Cmax | 0.87 |
| AUC | 0.56 |
| Cl | 0.98 |
| Oral Healthy | |
| Cmax | 1.06 |
| AUC | 0.99 |
| Cl | 0.76 |
| IV Pediatrics | |
| Cmax | 1.10 |
| AUC | 0.98 |
| Cl | 0.85 |
| Oral Pediatrics | |
| Cmax | 1.03 |
| AUC | 0.76 |
| Cl | 0.86 |
| Input Parameters | Value/Model | References |
|---|---|---|
| Physico-chemical Characteristics | ||
| Molecular Weight (g/mol) | 309.4 | [51] |
| Water Solubility (mg/mL) | 8.33 | [49] |
| pKa | 9.17 | [7] |
| LogP o:w | 0.81 | [52] |
| Absorption | ||
| Peff, man (cm/min) | 1.03 × 10−6 | Optimized by Visual Predictive Checks |
| Distribution | ||
| fu | 0.7 | [53] |
| Distribution Prediction Method | PK-Sim Standard | |
| Excretion | ||
| CLR (ml/min) | 131 | [36] |
| CLT (ml/min) | 219 | [36] |
| Sr. No | Population | No. of Participants | Dose (mg) | Route | Females Proportion | Age (Years) | Weight (kg) Ranges | References |
|---|---|---|---|---|---|---|---|---|
| Healthy | 9 | 1, 2, 4 | IV ᵜ | 0 | 20–27 | 63.6–96.6 | [36] | |
| Mild hypertensive | 4 | 2 | IV ᵜ | 0 | 43–54 | 76–111 | [2] |
| Oral | ||||||||
| Healthy | 8 | 80 | Oral | 0 | 21–24 | 73–84 | [38] |
| Healthy | 7 | 60, 120 | Oral | 4 | 24–42 | 42–70 | [37] |
| Healthy | 11 | 30 | Oral | 0 | 21–29 | 47–98.6 | [39] |
| Healthy | 8 | 30 | Oral | 2 | 20–30 | 47.5–57.7 | [40] |
| Healthy | 12 | 30 | Oral | 6 | 20–63 | 48–98.9 | [41] |
| Healthy | 7 | 80 | Oral | 0 | 19–22 | N/R * | [42] |
| Healthy | 8 | 80 | Oral | 4 | 18–23 | 51.8–74.9 | [43] |
| Healthy | 8 | 30 | Oral | 0 | N/R * | N/R * | [44] |
| Healthy | 12 | 80 | Oral | 0 | 18–30 | 34–76 | [54] |
| Healthy | 13 | 30 | Oral | 7 | 21–63 | 51.3–88.5 | [45] |
| CKD ⱷ | 24 | 80 | Oral | 11 | 22–74 | N/R * | [20] |
| Pediatrics | 6 | 0.05–5 ∞ | IV ᵜ | 1 | 3 months–14 years | 6.4–50 | [31] |
| 0.5–2 ∞ | Oral |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalsoom, S.; Rasool, M.F.; Imran, I.; Saeed, H.; Ahmad, T.; Alqahtani, F. A Comprehensive Physiologically Based Pharmacokinetic Model of Nadolol in Adults with Renal Disease and Pediatrics with Supraventricular Tachycardia. Pharmaceuticals 2024, 17, 265. https://doi.org/10.3390/ph17020265
Kalsoom S, Rasool MF, Imran I, Saeed H, Ahmad T, Alqahtani F. A Comprehensive Physiologically Based Pharmacokinetic Model of Nadolol in Adults with Renal Disease and Pediatrics with Supraventricular Tachycardia. Pharmaceuticals. 2024; 17(2):265. https://doi.org/10.3390/ph17020265
Chicago/Turabian StyleKalsoom, Samia, Muhammad Fawad Rasool, Imran Imran, Hamid Saeed, Tanveer Ahmad, and Faleh Alqahtani. 2024. "A Comprehensive Physiologically Based Pharmacokinetic Model of Nadolol in Adults with Renal Disease and Pediatrics with Supraventricular Tachycardia" Pharmaceuticals 17, no. 2: 265. https://doi.org/10.3390/ph17020265
APA StyleKalsoom, S., Rasool, M. F., Imran, I., Saeed, H., Ahmad, T., & Alqahtani, F. (2024). A Comprehensive Physiologically Based Pharmacokinetic Model of Nadolol in Adults with Renal Disease and Pediatrics with Supraventricular Tachycardia. Pharmaceuticals, 17(2), 265. https://doi.org/10.3390/ph17020265









