Pharmacological Topical Therapy for Intra-Oral Post Traumatic Trigeminal Neuropathic Pain: A Comprehensive Review
Abstract
1. Introduction
2. Orofacial Neuropathic Pain Types
Post-Traumatic Trigeminal Neuropathic Pain and Its Management
3. Neuropathic Pain Topical Management
3.1. Oral Neuropathic Pain Topical Management
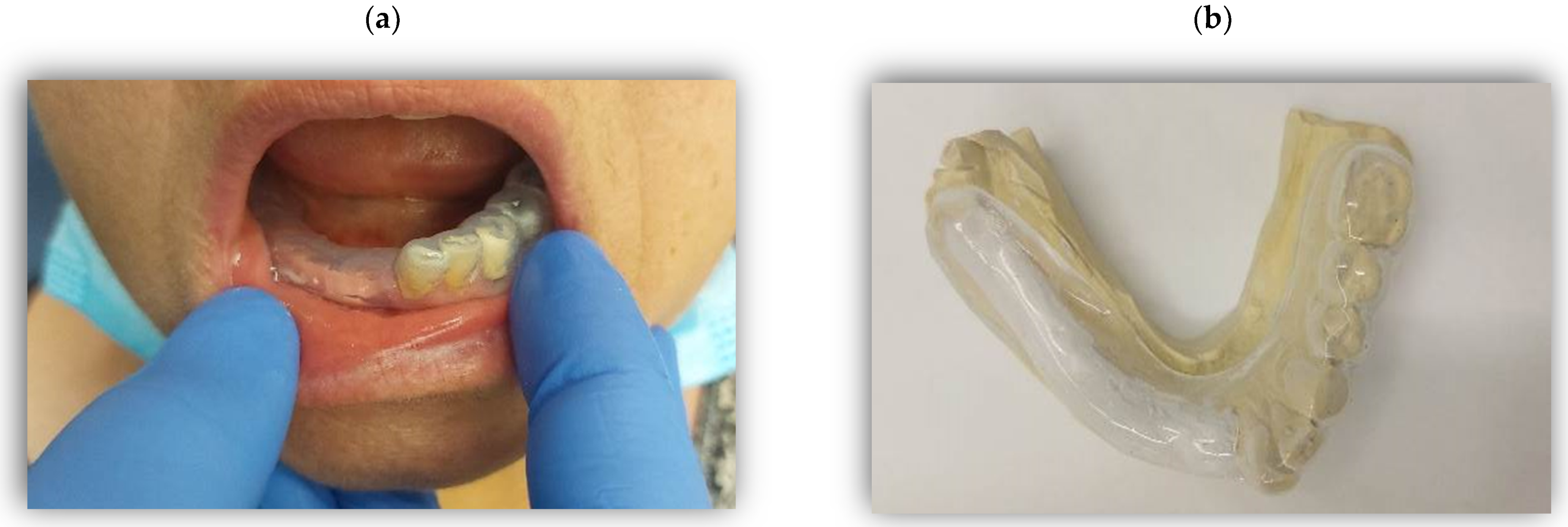
3.1.1. The Transmucosal Drug-Delivery Systems
3.1.2. Proposed Intra-Oral Topical Compounds for Neuropathic Pain
4. Conclusions
- Topical treatments for oral neuropathic pain show potential but require stronger evidence for efficacy;
- Synergistic benefits might exist in combined topical formulations, warranting further investigation into their underlying mechanisms;
- While stents improve drug delivery to affected areas, their independent advantages remain unclear.
Funding
Conflicts of Interest
References
- Padilla, M.; Clark, G.T.; Merrill, R.L. Topical medications for orofacial neuropathic pain: A review. J. Am. Dent. Assoc. 2000, 131, 184–195. [Google Scholar] [CrossRef]
- Ness, T.J.; Jones, L.; Smith, H. Use of compounded topical analgesics—Results of an Internet survey. Reg. Anesth. Pain Med. 2002, 27, 309–312. [Google Scholar] [CrossRef]
- Jensen, T.S.; Baron, R.; Haanpää, M.; Kalso, E.; Loeser, J.D.; Rice, A.S.; Treede, R.-D.J.P. A new definition of neuropathic pain. PAIN® 2011, 152, 2204–2205. [Google Scholar] [CrossRef]
- Haanpää, M.; Attal, N.; Backonja, M.; Baron, R.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Haythornthwaite, J.A.; Iannetti, G.D.J.P. NeuPSIG guidelines on neuropathic pain assessment. PAIN® 2011, 152, 14–27. [Google Scholar] [CrossRef]
- Bouhassira, D.J.R.N. Neuropathic pain: Definition, assessment and epidemiology. Rev. Neurol. 2019, 175, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Haviv, Y.; Khan, J.; Zini, A.; Almoznino, G.; Sharav, Y.; Benoliel, R. Trigeminal neuralgia (part I): Revisiting the clinical phenotype. Cephalalgia Int. J. Headache 2016, 36, 730–746. [Google Scholar] [CrossRef]
- Haviv, Y.; Zadik, Y.; Sharav, Y.; Benoliel, R. Painful traumatic trigeminal neuropathy: An open study on the pharmacotherapeutic response to stepped treatment. J. Oral Facial Pain Headache 2014, 28, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Findler, M.; Michaeli, E.; Haviv, Y. Burning Mouth Syndrome—A Flame Hard to Extinguish. Harefuah 2016, 155, 506–509. [Google Scholar] [PubMed]
- Benoliel, R.; Gaul, C. Persistent idiopathic facial pain. Cephalalgia Int. J. Headache 2017, 37, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Baad-Hansen, L.; Benoliel, R. Neuropathic orofacial pain: Facts and fiction. Cephalalgia Int. J. Headache 2017, 37, 670–679. [Google Scholar] [CrossRef]
- Hooten, W.M. Chronic Pain and Mental Health Disorders: Shared Neural Mechanisms, Epidemiology, and Treatment. Mayo Clin. Proc. 2016, 91, 955–970. [Google Scholar] [CrossRef] [PubMed]
- International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia Int. J. Headache 2020, 40, 129–221. [CrossRef]
- Tinastepe, N.; Oral, K. Neuropathic pain after dental treatment. Agri 2013, 25, 1–6. [Google Scholar] [CrossRef]
- Korczeniewska, O.A.; Kohli, D.; Benoliel, R.; Baddireddy, S.M.; Eliav, E. Pathophysiology of Post-Traumatic Trigeminal Neuropathic Pain. Biomolecules 2022, 12, 1753. [Google Scholar] [CrossRef] [PubMed]
- Okeson, J.P. Bell’s Oral and Facial Pain, 7th ed.; Quintessence Publishing Company: Chicago, IL, USA, 2014; pp. 435–501. [Google Scholar]
- Markman, J.D.; Dworkin, R.H. Ion channel targets and treatment efficacy in neuropathic pain. J. Pain 2006, 7, S38–S47. [Google Scholar] [CrossRef]
- Benoliel, R.; Birenboim, R.; Regev, E.; Eliav, E. Neurosensory changes in the infraorbital nerve following zygomatic fractures. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Jaaskelainen, S.K.; Teerijoki-Oksa, T.; Virtanen, A.; Tenovuo, O.; Forssell, H. Sensory regeneration following intraoperatively verified trigeminal nerve injury. Neurology 2004, 62, 1951–1957. [Google Scholar] [CrossRef]
- Benoliel, R.; Kahn, J.; Eliav, E. Peripheral painful traumatic trigeminal neuropathies. Oral Dis. 2012, 18, 317–332. [Google Scholar] [CrossRef]
- Farrar, J.T.; Young, J.P., Jr.; LaMoreaux, L.; Werth, J.L.; Poole, M.R. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001, 94, 149–158. [Google Scholar] [CrossRef]
- Sindrup, S.H.; Otto, M.; Finnerup, N.B.; Jensen, T.S. Antidepressants in the treatment of neuropathic pain. Basic Clin. Pharmacol. Toxicol. 2005, 96, 399–409. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [PubMed]
- Heir, G.M.; Katzmann, G.; Covalesky, B.; Cammarata, J.; Mangal, J.; Kalladka, M.; Nasri-Heir, C. Use of compounded topical medications for treatment of orofacial pain: A narrative review. J. Oral Maxillofac. Anesth. 2022, 1, 12. [Google Scholar] [CrossRef]
- Sharav, Y.B.R. Orofacial Pain and Headache, 2nd ed.; Quintessence Publishing: Chicago, IL, USA, 2015. [Google Scholar]
- Kocot-Kepska, M.; Zajaczkowska, R.; Mika, J.; Kopsky, D.J.; Wordliczek, J.; Dobrogowski, J.; Przeklasa-Muszynska, A. Topical Treatments and Their Molecular/Cellular Mechanisms in Patients with Peripheral Neuropathic Pain-Narrative Review. Pharmaceutics 2021, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Wiffen, P.J.; Xia, J. Systematic review of topical diclofenac for the treatment of acute and chronic musculoskeletal pain. Curr. Med. Res. Opin. 2020, 36, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Stanos, S.P.; Galluzzi, K.E. Topical therapies in the management of chronic pain. Postgrad. Med. 2013, 125, 25–33. [Google Scholar] [CrossRef]
- Sommer, C.; Cruccu, G. Topical Treatment of Peripheral Neuropathic Pain: Applying the Evidence. J. Pain Symptom Manag. 2017, 53, 614–629. [Google Scholar] [CrossRef]
- Maloney, J.; Pew, S.; Wie, C.; Gupta, R.; Freeman, J.; Strand, N. Comprehensive Review of Topical Analgesics for Chronic Pain. Curr. Pain Headache Rep. 2021, 25, 7. [Google Scholar] [CrossRef]
- Derry, S.; Wiffen, P.J.; Kalso, E.A.; Bell, R.F.; Aldington, D.; Phillips, T.; Gaskell, H.; Moore, R.A. Topical analgesics for acute and chronic pain in adults—An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 5, CD008609. [Google Scholar] [CrossRef]
- Derry, S.; Rice, A.S.; Cole, P.; Tan, T.; Moore, R.A. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 1, CD007393. [Google Scholar] [CrossRef]
- Baron, R.; Allegri, M.; Correa-Illanes, G.; Hans, G.; Serpell, M.; Mick, G.; Mayoral, V. The 5% Lidocaine-Medicated Plaster: Its Inclusion in International Treatment Guidelines for Treating Localized Neuropathic Pain, and Clinical Evidence Supporting its Use. Pain Ther. 2016, 5, 149–169. [Google Scholar] [CrossRef]
- Guilherme, V.A.; Ribeiro, L.N.M.; Tofoli, G.R.; Franz-Montan, M.; de Paula, E.; de Jesus, M.B. Current Challenges and Future of Lipid nanoparticles formulations for topical drug application to oral mucosa, skin, and eye. Curr. Pharm. Des. 2017, 23, 6659–6675. [Google Scholar] [CrossRef] [PubMed]
- Haribabu, P.K.; Eliav, E.; Heir, G.M. Topical medications for the effective management of neuropathic orofacial pain. J. Am. Dent. Assoc. 2013, 144, 612–614. [Google Scholar] [CrossRef]
- Nasri-Heir, C.; Khan, J.; Heir, G.M. Topical medications as treatment of neuropathic orofacial pain. Dent. Clin. N. Am. 2013, 57, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Kienzler, J.L.; Gold, M.; Nollevaux, F. Systemic bioavailability of topical diclofenac sodium gel 1% versus oral diclofenac sodium in healthy volunteers. J. Clin. Pharmacol. 2010, 50, 50–61. [Google Scholar] [CrossRef]
- Heir, G.; Karolchek, S.; Kalladka, M.; Vishwanath, A.; Gomes, J.; Khatri, R.; Nasri, C.; Eliav, E.; Ananthan, S. Use of topical medication in orofacial neuropathic pain: A retrospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 466–469. [Google Scholar] [CrossRef]
- Dworkin, R.H.; O’Connor, A.B.; Backonja, M.; Farrar, J.T.; Finnerup, N.B.; Jensen, T.S.; Kalso, E.A.; Loeser, J.D.; Miaskowski, C.; Nurmikko, T.J.; et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain 2007, 132, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W.; Malec-Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Haviv, Y.; Merimsky, B.; Kay, Z.; Sharav, Y.; Czerninski, R.; Brotman, Y.; Klutstein, M.; Aframian, D.J. Topical tretinoin treatment for burning mouth syndrome: A pilot study. Quintessence Int. 2022, 53, 860–867. [Google Scholar] [CrossRef]
- Casale, R.; Romanenko, Y.; Allegri, M. 5% lidocaine medicated plaster double effect in a case of orofacial localized neuropathic pain. J. Pain Res. 2014, 7, 639–643. [Google Scholar] [CrossRef][Green Version]
- Fusco, B.M.; Alessandri, M. Analgesic effect of capsaicin in idiopathic trigeminal neuralgia. Anesth. Analg. 1992, 74, 375–377. [Google Scholar] [CrossRef]
- Chinna Reddy, P.; Chaitanya, K.S.; Madhusudan Rao, Y. A review on bioadhesive buccal drug delivery systems: Current status of formulation and evaluation methods. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2011, 19, 385–403. [Google Scholar]
- Ivarsson, D.; Wahlgren, M. Comparison of in vitro methods of measuring mucoadhesion: Ellipsometry, tensile strength and rheological measurements. Colloids Surf. B Biointerfaces 2012, 92, 353–359. [Google Scholar] [CrossRef]
- Bavarian, R.; Khawaja, S.N.; Treister, N.S. Oral appliances in the management of neuropathic orofacial pain: A retrospective case series. Oral Dis. 2022, 28, 805–812. [Google Scholar] [CrossRef]
- Axell, T. Treatment of smarting symptoms in the oral mucosa by appliance of lingual acrylic splints. Swed. Dent. J. 2008, 32, 165–169. [Google Scholar]
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Zur, E. Topical treatment of neuropathic pain using compounded medications. Clin. J. Pain 2014, 30, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Mauro, A.; Goldstein, L. Topical Medications for Common Orofacial Pain Conditions. Pract. Pain Manag. 2018, 18. [Google Scholar]
- Vickers, E.R.; Cousins, M.J.; Walker, S.; Chisholm, K. Analysis of 50 patients with atypical odontalgia. A preliminary report on pharmacological procedures for diagnosis and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Villegas, F.; Heir, G.; Markman, S.; Khan, J.; Noma, N.; Benoliel, R.; Patel, J.; Eliav, E. Topical pregabalin and diclofenac for the treatment of neuropathic orofacial pain in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Argoff, C.E. A review of the use of topical analgesics for myofascial pain. Curr. Pain Headache Rep. 2002, 6, 375–378. [Google Scholar] [CrossRef] [PubMed]

| Drug | Direct/Indirect Mechanism of Action | Cellular Targets | Medical Considerations (with Systemic Use) | Common Side Effects (with Systemic Use) |
|---|---|---|---|---|
| Systemic/topical route Carbamazepine | GABA system modulation, Sodium-channel blockade, NMDA-receptor blockade | Neurons Keratinocytes | Bone marrow problems, cardiac problems, blood disorders, glaucoma | Hypertension, hypotension, lightheadedness, rash, pruritus, erythematous condition, nausea, vomiting, confusion, dizziness, nystagmus, somnolence, blurred vision, diplopia |
| Gabapentinoids: Gabapentin Pregabalin | Calcium-channel blockade, NMDA blockade, Potassium-channel activation, Anti-inflammatory effect | Neurons Keratinocytes | Renal problems | Edema, myalgia, ataxia, dizziness, somnolence, tremor, mood swings, hostility, fatigue, weight gain, constipation, xerostomia, blurred vision, diplopia |
| Amitriptyline | Sodium-channel blockade, Calcium-channel blockade, NMDA receptor antagonism, Potassium-channel activation, Adrenergic receptor down-regulation, TRPA1 desensitization, GABA receptor modulation, 5-HT receptor blockade, Histamine receptor blockade, Anticholinergic, Reduction in nitric oxide, prostaglandin E2, Noradrenaline, 5-HT, dopamine and adenosine reuptake inhibition, Opioid system modulation | Neurons Keratinocytes | Cardiac problems, diabetes, epilepsy, glaucoma, urinary retention, thyroid problems, hepatic problems, psychiatric problems | Weight gain, somnolence, blurred vision, fatigue, urinary retention, headache, asthenia, dizziness, constipation, xerostomia |
| Anti-inflammatory: Diclofenac Ketoprofen | Cox-2 inhibition, NMDA receptor antagonism, TRPV1, TRPA1, TRPM3 ligand, Sodium-channel blockade, Calcium-channel inhibition, Potassium channels modulation, Adrenergic receptor interaction, Opioid receptor modulation | Immune cells Neurons Keratinocytes Schwann cells | Cardiovascular problems, gastrointestinal disease, asthma | Edema, gastrointestinal complaints, rashes or pruritus, tinnitus, dizziness, somnolence, headache, increased liver function tests |
| Topical route Lidocaine | Sodium-channel blockade, Cholinergic-receptor blockade, TRPA1desensitization, NMDA receptor antagonism, Acid-sensing ion channel blockade, Potassium-channel blockade, Calcium-channel blockade, P2X7 inhibition, Nerve growth factor modulation, Glycinergic pathways modulation, Anti-inflammatory effect | Neurons Keratinocytes Immune cells Schwann cells | None (with topical use) | Local irritation, erythema |
| Capsaicin | TRPV1 activation | Neurons Keratinocytes | None | Local burning sensation, erythema |
| Reference | Summary | Drugs Mentioned | Method of Use |
|---|---|---|---|
| [34]. Haribabu et al., 2013 | Case report on topical medications for managing neuropathic orofacial pain, including various drug combinations. | 4% carbamazepine, 1% lidocaine, 4% gabapentin | Several weeks of topical treatment |
| [35]. Nasri-Heir et al., 2013 | Overview of topical medications as a treatment for neuropathic orofacial pain, highlighting their application and benefits. | N/A | N/A |
| [1]. Padilla et al., 2000 | Review of topical medications for orofacial neuropathic pain, discussing their applications and potential effectiveness. | N/A | N/A |
| [38]. Dworkin et al., 2007 | Evidence-based recommendations for pharmacologic management of neuropathic pain, including topical options. | N/A | N/A |
| [10]. Baad-Hansen and Benoliel, 2017 | Discussion of neuropathic orofacial pain, highlighting facts and fiction in its treatment approaches. | N/A | N/A |
| [23]. Heir et al., 2022 | A narrative review discussing the use of compounded topical medications for orofacial pain treatment. | ketamine 4%, carbamazepine 4%, lidocaine 1%, ketoprofen 4%, gabapentin 4%, and especially pregabalin 5–10% within a lipoderm inert vehicle | Application beneath neurosensory stent 3 to 4 times per day |
| capsaicin 0.025–0.075% paste or 8% patch | Up to 4 times a day for 8 weeks 1–4 patches for 30–60 min, once in 3 months | ||
| [41]. Casale et al., 2014 | Case report on the use of 5% lidocaine-medicated plaster for localized neuropathic orofacial pain. | 5% lidocaine-medicated plaster | Application to affected area |
| [42]. Fusco and Alessandri, 1992 | Discussion of the analgesic effect of capsaicin in trigeminal neuralgia. | capsaicin | Application to affected area |
| [45]. Bavarian et al., 2022 | Retrospective case series on the use of oral appliances in the management of neuropathic orofacial pain. | N/A | N/A |
| [46]. Axell, T., 2008 | Exploration of the treatment of painful symptoms in the oral mucosa using lingual acrylic splints. | N/A | N/A |
| [49]. Patel S et al., 2018 | Discussion of topical medications for common orofacial pain conditions, emphasizing practical applications. | benzocaine, lidocaine | N/A |
| [50]. Vickers et al., 1998 | Analysis of patients with atypical odontalgia and pharmacological procedures for diagnosis and treatment. | 0.025% capsaicin for 3 min twice a day after local anesthetic application | Local application for 3 min twice a day after local anesthetic application |
| [37]. Heir et al., 2008 | Retrospective study on the use of topical medications in orofacial neuropathic pain treatment. | 4% carbamazepine, 1% lidocaine, 4% ketoprofen, 4% ketamine, and 4% gabapentin | Topical application |
| [51]. Plaza-Villegas et al., 2012 | The only study on an animal model—rats with local neuropathic pain, applying a topical regimen of pregabalin and diclofenac—showing a reduction in pain intensity. | Topical regimen of pregabalin 10% and diclofenac 5%. | Topical application |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharav, Y.; Heiliczer, S.; Benoliel, R.; Haviv, Y. Pharmacological Topical Therapy for Intra-Oral Post Traumatic Trigeminal Neuropathic Pain: A Comprehensive Review. Pharmaceuticals 2024, 17, 264. https://doi.org/10.3390/ph17020264
Sharav Y, Heiliczer S, Benoliel R, Haviv Y. Pharmacological Topical Therapy for Intra-Oral Post Traumatic Trigeminal Neuropathic Pain: A Comprehensive Review. Pharmaceuticals. 2024; 17(2):264. https://doi.org/10.3390/ph17020264
Chicago/Turabian StyleSharav, Yair, Shimrit Heiliczer, Rafael Benoliel, and Yaron Haviv. 2024. "Pharmacological Topical Therapy for Intra-Oral Post Traumatic Trigeminal Neuropathic Pain: A Comprehensive Review" Pharmaceuticals 17, no. 2: 264. https://doi.org/10.3390/ph17020264
APA StyleSharav, Y., Heiliczer, S., Benoliel, R., & Haviv, Y. (2024). Pharmacological Topical Therapy for Intra-Oral Post Traumatic Trigeminal Neuropathic Pain: A Comprehensive Review. Pharmaceuticals, 17(2), 264. https://doi.org/10.3390/ph17020264





