In Vitro and Ex Vivo Investigation of the Antibacterial Effects of Methylene Blue against Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. Bacterial Isolates
2.2. Agar Dilution Method
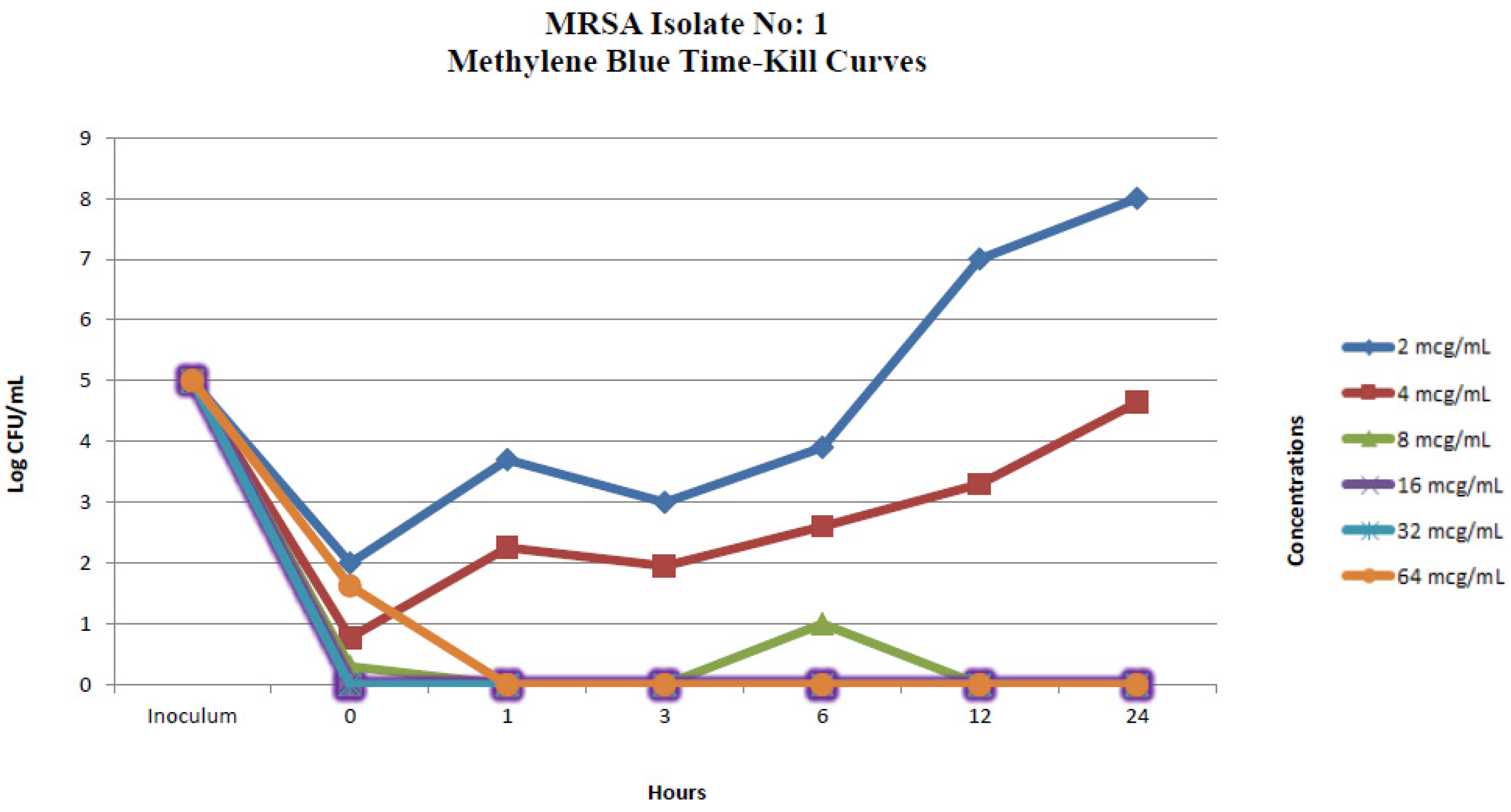
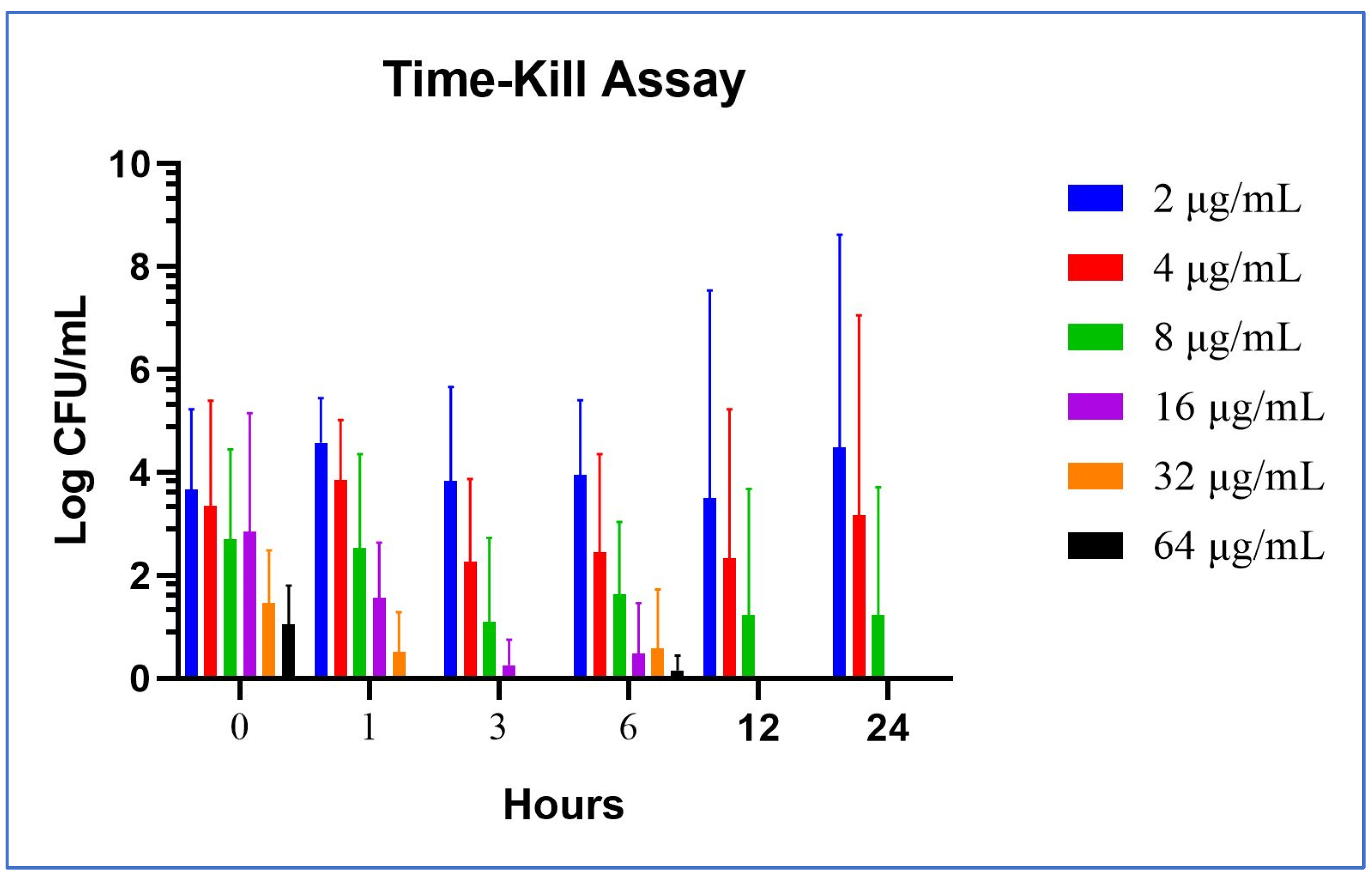
2.2.1. In Vitro Time-Kill Method
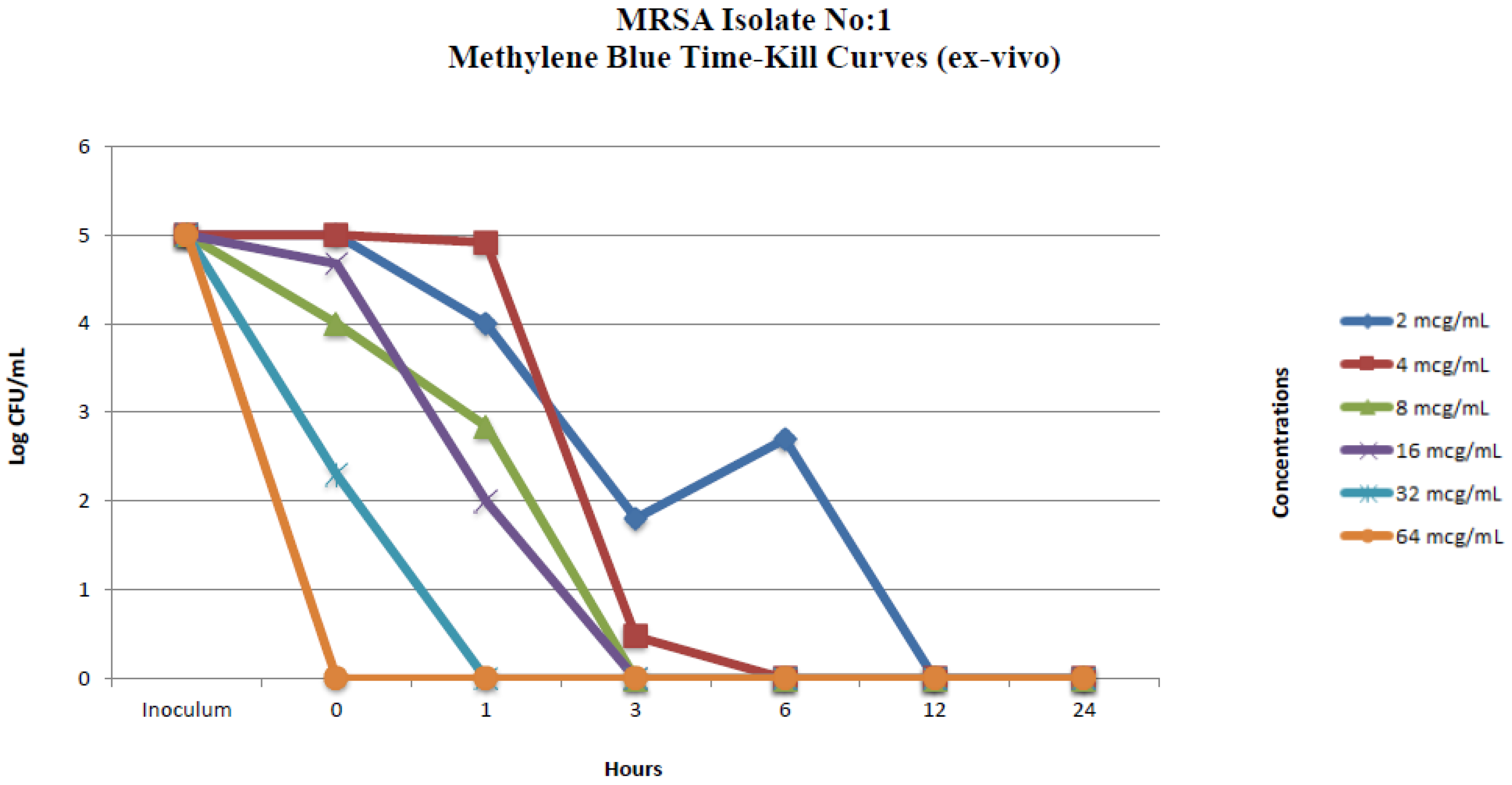
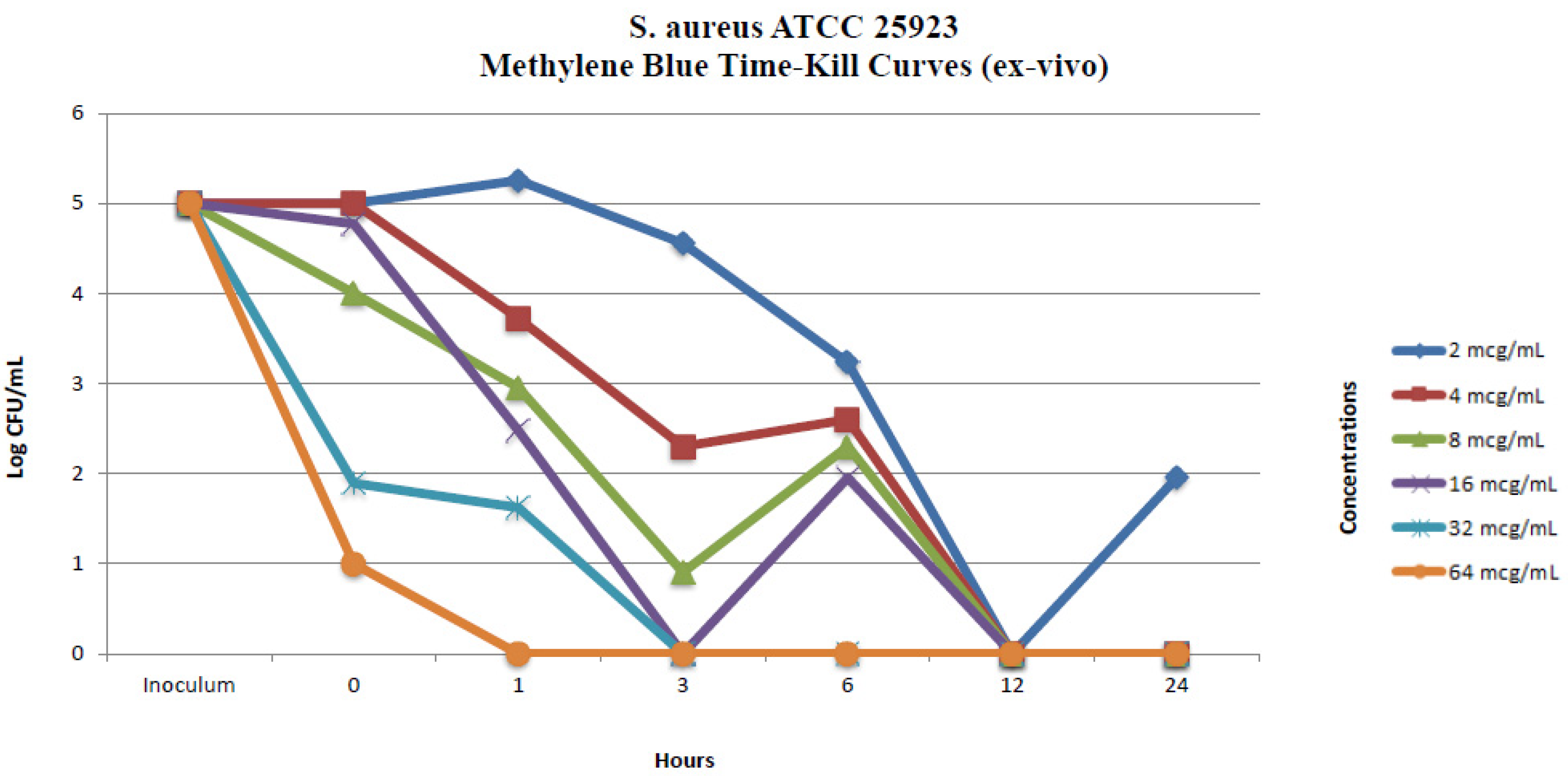
2.2.2. Ex Vivo Time-Kill Method
2.2.3. Cumulative Analysis of the Mean and Standard Deviation Values in Time-Kill Studies
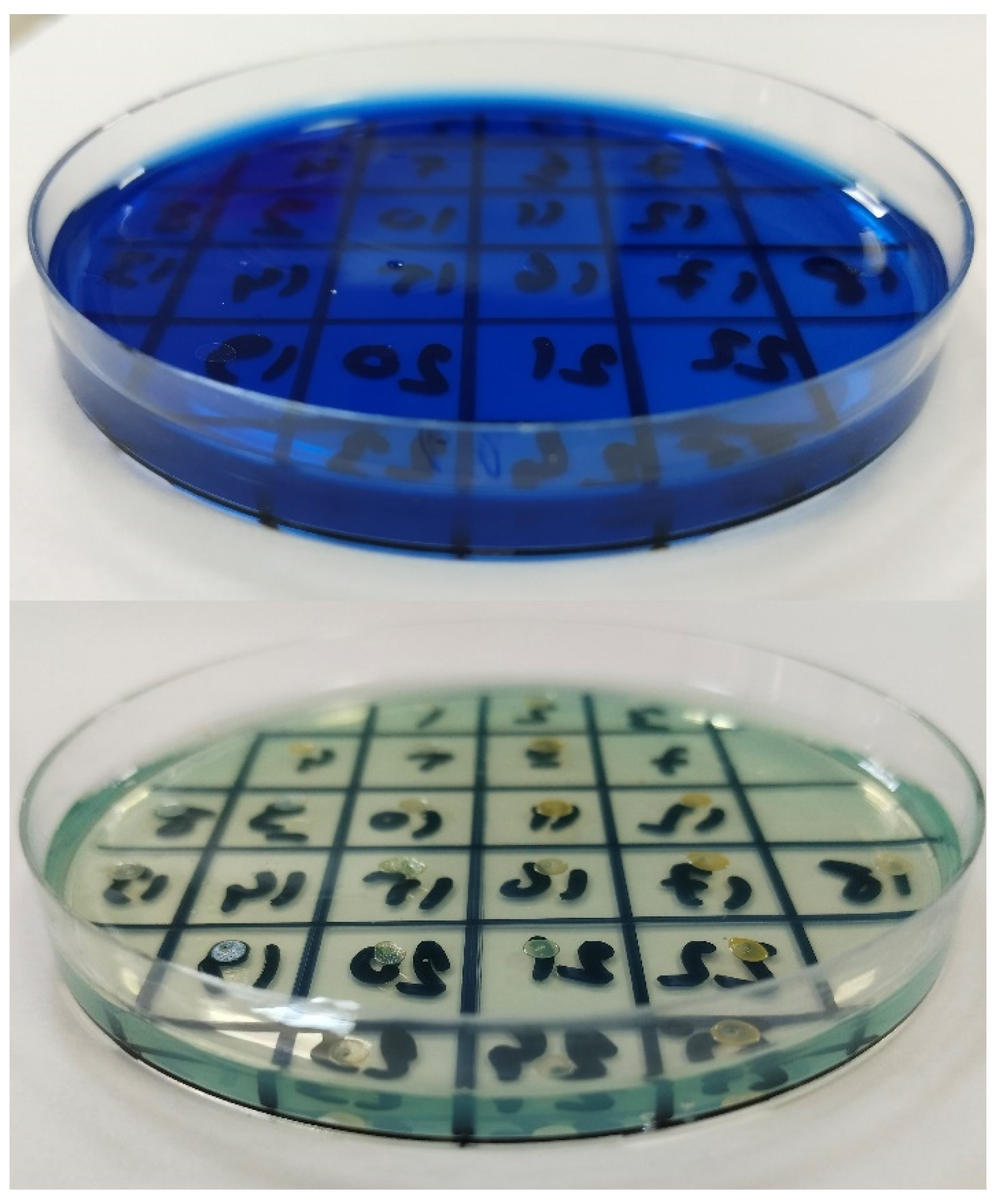
2.3. Methylene Blue and Antibiotic Synergy Testing via Disc Diffusion Method
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Determination of Minimum Inhibitory Concentrations
4.2.1. In Vitro Time-Kill Assay
4.2.2. Ex Vivo Time-Kill Assay
4.3. Methylene Blue and Antibiotic Synergy Testing via Disc Diffusion Method
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misclescu, A.; Wiklund, L. Methylene blue, an old drug with new indications? Rom. J. Anaesth. Intensive Care 2010, 17, 35–41. [Google Scholar]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you–methylene blue. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.D.; Coleman, N.A. Drug-induced methaemoglobinaemia. Treatment issues. Drug Saf. 1996, 14, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Zulian, G.B.; Tullen, E.; Maton, B. Methylene blue for ifosfamide-associated encephalopathy. N. Engl. J. Med. 1995, 332, 1239–1240. [Google Scholar] [PubMed]
- Orth, K.; Ruck, A.; Stanescu, A.; Beger, H.G. Intraluminal treatment of inoperable oesophagealtumours by intralesional photodynamic therapy with methylene blue. Lancet 1995, 345, 519–520. [Google Scholar] [CrossRef] [PubMed]
- Williamson, L.M.; Cardigan, R.; Prowse, C.V. Methylene blue-treated fresh-frozen plasma: What is its contribution to blood safety? Transfusion 2003, 43, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Gazel, D.; Zanapalıoğlu Gazel, Ö. In Vitro activity of methylene blue on Mycobacterium tuberculosis complex isolates. Metilen mavisinin Mycobacterium tuberculosis kompleksizolatlarına in vitro etkisi. Tuberk. Toraks 2021, 69, 279–284. [Google Scholar] [CrossRef]
- Pascual, A.; Henry, M.; Briolant, S.; Charras, S.; Baret, E.; Amalvict, R.; Etages, E.H.D.; Feraud, M.; Rogier, C.; Pradines, B. In Vitro activity of Proveblue (methylene blue) on Plasmodium falciparum strains resistant to standard antimalarial drugs. Antimicrob. Agents Chemother. 2011, 55, 2472–2474. [Google Scholar] [CrossRef][Green Version]
- Steczko, J.; Ash, S.R.; Nivens, D.E.; Brewer, L.; Winger, R.K. Microbial inactivation properties of a new antimicrobial/antithrombotic catheter lock solution (citrate/methylene blue/parabens). Nephrol. Dial. Transplant. 2009, 24, 1937–1945. [Google Scholar] [CrossRef]
- Li, R.; Chen, J.; Cesario, T.C.; Wang, X.; Yuan, J.S.; Rentzepis, P.M. Synergistic reaction of silver nitrate, silver nanoparticles, and methylene blue against bacteria. Proc. Natl. Acad. Sci. USA 2016, 113, 13612–13617. [Google Scholar] [CrossRef]
- Piccirillo, C.; Perni, S.; Gil-Thomas, J.; Prokopovich, P.; Wilson, M.; Pratten, J.; Parkin, I.P. Antimicrobial activity of methylene blue and toluidine blue O covalently bound to a modified silicone polymer surface. J. Mater. Chem. 2009, 19, 6167–6171. [Google Scholar] [CrossRef][Green Version]
- Gazel, D.; Tatman Otkun, M.; Akçalı, A. In Vitro activity of methylene blue and eosin methylene blue agar on colistin-resistant A. baumannii: An experimental study. J. Med. Microbiol. 2019, 68, 1607–1613. [Google Scholar] [CrossRef]
- Kuriloff, D.B.; Sanborn, K.V. Rapid intraoperative localization of parathyroid glands utilizing methylene blue infusion. Otolaryngol. Neck Surg. 2004, 131, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Walter-Sack, I.; Rengelshausen, J.; Oberwittler, H.; Burhenne, J.; Mueller, O.; Meissner, P.; Mikus, G. High absolute bioavailability of methylene blue given as an aqueous oral formulation. Eur. J. Clin. Pharmacol. 2009, 65, 179–189. [Google Scholar] [CrossRef]
- Warth, A.; Goeppert, B.; Bopp, C.; Schirmacher, P.; Flechtenmacher, C.; Burhenne, J. Turquoise to dark green organs at autopsy. Virchows Arch. 2009, 454, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.; de Araujo, F.P.; Cruciani, M.; Coccia, E.M.; Pantosti, A. Worldwide epidemiology and antibiotic resistance of Staphylococcus aureus. In Staphylococcus aureus. Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2016; pp. 1–56. [Google Scholar]
- Horn, J.; Stelzner, K.; Rudel, T.; Fraunholz, M. Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 2018, 308, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, D.; Harper, L.; Shopsin, B.; Torres, V.J. Staphylococcus aureus pathogenesis in diverse host environments. Pathog. Dis. 2017, 75, ftx005. [Google Scholar] [CrossRef] [PubMed]
- Von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. New. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef]
- Lakhundi, S.; Zhang, K. Methicillin-resistant Staphylococcus aureus: Molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018, 31, e00020-18. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 14.0. 2024. Available online: http://www.eucast.org (accessed on 11 January 2024).
- de Kraker, M.E.; Davey, P.G.; Grundmann, H.; BURDEN Study Group. Mortality and hospital stay associated with resistant Staphylococcus aureus and Escherichia coli bacteremia: Estimating the burden of antibiotic resistance in Europe. PLoS Med. 2011, 8, e1001104. [Google Scholar] [CrossRef]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- García-Álvarez, L.; Holden, M.T.; Lindsay, H.; Webb, C.R.; Brown, D.F.; Curran, M.D.; Walpole, E.; Brooks, K.; Pickard, D.J.; Teale, C.; et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: A descriptive study. Lancet Infect. Dis. 2011, 11, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- M100-S21; Performance Standards for Antimicrobial Susceptibility Testing. 21st Informational Supplement. Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012. Available online: https://clsi.org/media/2663/m100ed29_sample.pdf (accessed on 11 December 2022).
- Dubos, R. The Relation of The Bacteriostatic Action of Certain Dyes to Oxidation-Reduction Processes. J. Exp. Med. 1929, 49, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K. New Twist on an Old Favorite: Gentian Violet and Methylene Blue Antibacterial Foams. Adv. Wound Care 2016, 5, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Heil, J. A prospective evaluation of methylene blue and gentian violet dressing for management of chronic wounds with local infection. Int. Wound J. 2017, 14, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Viveiros, M.; Molnar, J. Antimicrobial activity of phenothiazines. In Vivo 2004, 18, 725–731. [Google Scholar] [PubMed]
- Pal, R.; Ansari, M.A.; Saibabu, V.; Das, S.; Fatima, Z.; Hameed, S. Nonphotodynamic roles of methylene blue: Display of dis-tinct antimycobacterial and anticandidal mode of actions. J. Pathog. 2018, 2018, 3759704. [Google Scholar] [CrossRef]
- Thesnaar, L.; Bezuidenhout, J.J.; Petzer, A.; Petzer, J.P.; Cloete, T.T. Methylene blue analogs: In-vitro antimicrobial mini-mum inhibitory concentrations and in silico pharmacophore modeling. Eur. J. Pharm. Sci. 2021, 157, 105603. [Google Scholar] [CrossRef]
- Ronqui, M.R.; de Aguiar Coletti, T.M.; de Freitas, L.M.; Miranda, E.T.; Fontana, C.R. Synergistic antimicrobial effect of photodynamic therapy and ciprofloxacin. J. Photochem. Photobiol. B 2016, 158, 122–129. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed]
- Shatti, Z.A.; Authman, S.H. Effect of methylene blue on the growth of bacteria isolated from patients with Effect of methylene blue on the growth of bacteria isolated from patients with atopic dermatitis. J. Coll. Educ. 2015, 21, 77–88. [Google Scholar]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, A.F.D.; Radicioni, M.M.; Vaccani, A.; Fransioli, A.; Longo, L.; Moro, L.; Repici, A. Methylene blue MMX® tablets for chromoendoscopy. Bioavailability, colon staining and safety in healthy volunteers undergoing a full colonoscopy. Contemp. Clin. Trials 2018, 71, 96–102. [Google Scholar] [CrossRef]
- Repici, A.; Di Stefano, A.F.D.; Radicioni, M.M.; Jas, V.; Moro, L.; Danese, S. Methylene blue MMX® tablets for chromoendoscopy. Safety tolerability and bioavailability in healthy volunteers. Contemp. Clin. Trials 2012, 33, 260–267. [Google Scholar] [CrossRef]
- Teichert, M.C.; Jones, J.W.; Usacheva, M.N.; Biel, M.A. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 155–160. [Google Scholar] [CrossRef]
- Buco Bleu. Tab İlaç. Available online: https://www.tabilac.com/en/category/drugs/buco-bleu (accessed on 14 April 2023).
- Fadel, M.; Salah, M.; Samy, N.; Mona, S. Liposomal methylene blue hydrogel for selective photodynamic therapy of acne vulgaris. J. Drugs Dermatol. 2009, 8, 983–990. [Google Scholar]
- Coates, T.; Bax, R.; Coates, A. Nasal decolonization of Staphylococcus aureus with mupirocin: Strengths, weaknesses and future prospects. J. Antimicrob. Chemother. 2009, 64, 9–15. [Google Scholar] [CrossRef]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, 9. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Scholz, M.T.; Parks, P.J.; Peterson, M.L. Ex Vivo porcine vaginal mucosal model of infection for determining effectiveness and toxicity of antiseptics. J. Appl. Microbiol. 2013, 115, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Gagandeep; Kumar, P.; Kandi, S.K.; Mukhopadhyay, K.; Rawat, D.S. Synthesis of novel monocarbonyl curcuminoids, evaluation of their efficacy against MRSA, including ex vivo infection model and their mechanistic studies. Eur. J. Med. Chem. 2020, 195, 112276. [Google Scholar] [CrossRef] [PubMed]
- Garzoni, C.; Kelley, W.L. Staphylococcus aureus: New evidence for intracellular persistence. Trends Microbiol. 2009, 17, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Methicillin-Resistant Staphylococcus aureus (MRSA): Cleaning and Disinfection. Available online: https://www.cdc.gov/mrsa/community/environment/index.html (accessed on 3 February 2024).
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Bistas, E.; Sanghavi, D.K. Methylene Blue. [Updated 2023 Jun 26]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK557593/ (accessed on 1 December 2023).
- CLSI M100-ED31:2021; Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI: Annapolis Junction, MD, USA, 2021. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 25 June 2021).
- Gómez-Junyent, J.; Benavent, E.; Sierra, Y.; El Haj, C.; Soldevila, L.; Torrejón, B.; Rigo-Bonnin, R.; Tubau, F.; Ariza, J.; Murillo, O. Efficacy of ceftolozane/tazobactam, alone and in combination with colistin, against multidrug-resistant Pseudomonas aeruginosa in an in vitro biofilm pharmacodynamic model. Int. J. Antimicrob. Agents 2019, 53, 612–619. [Google Scholar] [CrossRef]


| MRSA Isolate No. 1 Colony Counts (Log CFU/mL) * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methylene Blue Concentrations | ||||||||||||
| 2 μg/mL | 4 μg/mL | 8 μg/mL | 16 μg/mL | 32 μg/mL | 64 μg/mL | |||||||
| Time (h) | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo |
| 0 | 2 | 5 | 0.77 | 5 | 0.30 | 4 | 0 | 4.67 | 0 | 2.30 | 1.62 | 0 |
| 1 | 3.69 | 4 | 2.25 | 4.91 | 0 | 2.84 | 0 | 2 | 0 | 0 | 0 | 0 |
| 3 | 3 | 1.80 | 1.95 | 0.47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | 3.90 | 2.69 | 2.60 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | 7 | 0 | 3.30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 8 | 0 | 4.64 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| S. aureus ATCC 25923 Colony Counts (Log CFU/mL) * | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methylene Blue Concentrations | ||||||||||||
| 2 μg/mL | 4 μg/mL | 8 μg/mL | 16 μg/mL | 32 μg/mL | 64 μg/mL | |||||||
| Time (h) | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo | in vitro | ex vivo |
| 0 | 2.69 | 5 | 2.60 | 5 | 2.47 | 4 | 1.96 | 4.77 | 1.70 | 1.89 | 1.57 | 1 |
| 1 | 5.39 | 5.25 | 4.50 | 3.72 | 4.34 | 2.95 | 1.77 | 2.47 | 0.47 | 1.62 | 0 | 0 |
| 3 | 6 | 4.55 | 4.36 | 2.30 | 3.47 | 0.90 | 1 | 0 | 0 | 0 | 0 | 0 |
| 6 | 6 | 3.23 | 4.63 | 2.60 | 3.20 | 2.30 | 0 | 1.95 | 2.30 | 0 | 0.60 | 0 |
| 12 | 7 | 0 | 6 | 0 | 4.90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 8 | 1.95 | 8 | 0 | 4.95 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Isolate No | MB [C] | 0 µg/mL | 1 µg/mL | 2 µg/mL | 4 µg/mL | 8 µg/mL | 16 µg/mL | 32 µg/mL | 64 µg/mL |
|---|---|---|---|---|---|---|---|---|---|
| 31 | P: 21 FOX: 19 | P: 21 FOX: 22 a | P: 24 FOX: 26 | P: 24 FOX: 26 | P: 25 FOX: 26 | P: 26 a FOX: 26 | P: 27 FOX: 26 | NO GROWTH c | |
| 33 | P: 10 FOX: 10 | P: 10 FOX: 10 | P: 10 FOX: 11 | P: 11 FOX: 15 | P: 11 FOX: 15 | P: 12 FOX: 16 | P: 14 FOX: 17 | NO GROWTH c | |
| 37 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | NO GROWTH c | |
| 47 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 10 FOX: 7 | P: 12 FOX: 7 | NO GROWTH c | |
| 50 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 10 FOX: 7 | P: 10 FOX: 10 | NO GROWTH c | |
| 75 | P: 10 FOX: 10 | P: 10 FOX: 10 | P: 10 FOX: 10 | P: 11 FOX: 11 | P: 12 FOX: 12 | P: 12 FOX: 12 | P: 16 FOX: 16 | NO GROWTH c | |
| 80 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | NO GROWTH b | NO GROWTH | |
| 89 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 9 FOX: 7 | NO GROWTH b | NO GROWTH | |
| 93 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 7 FOX: 7 | P: 9 FOX: 7 | NO GROWTH b | NO GROWTH | |
| 99 | P: 12 FOX: 16 | P: 13 FOX: 16 | P: 13 FOX: 27 a | P: 14 FOX: 27 | P: 15 FOX: 27 | P: 15 FOX: 27 | P: 19 FOX: 28 | NO GROWTH c | |
| MEAN ± SD, P: MEAN ± SD, FOX: | 9.5 ± 4.42 9.7 ± 4.35 | 9.6 ± 4.50 10 ± 5.09 | 9.9 ± 5.36 11.6 ± 7.98 | 10.2 ± 5.45 12.2 ± 8.02 | 10.5 ± 5.83 12.2 ± 8.02 | 11.7 ± 5.57 12.3 ± 8.06 | 15 ± 6.58 15.8 ± 8.59 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazel, D.; Erinmez, M.; Çalışkantürk, G.; Saadat, K.A.S.M. In Vitro and Ex Vivo Investigation of the Antibacterial Effects of Methylene Blue against Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals 2024, 17, 241. https://doi.org/10.3390/ph17020241
Gazel D, Erinmez M, Çalışkantürk G, Saadat KASM. In Vitro and Ex Vivo Investigation of the Antibacterial Effects of Methylene Blue against Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals. 2024; 17(2):241. https://doi.org/10.3390/ph17020241
Chicago/Turabian StyleGazel, Deniz, Mehmet Erinmez, Gönenç Çalışkantürk, and Khandakar A. S. M. Saadat. 2024. "In Vitro and Ex Vivo Investigation of the Antibacterial Effects of Methylene Blue against Methicillin-Resistant Staphylococcus aureus" Pharmaceuticals 17, no. 2: 241. https://doi.org/10.3390/ph17020241
APA StyleGazel, D., Erinmez, M., Çalışkantürk, G., & Saadat, K. A. S. M. (2024). In Vitro and Ex Vivo Investigation of the Antibacterial Effects of Methylene Blue against Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals, 17(2), 241. https://doi.org/10.3390/ph17020241