Proportion of Over-The-Counter Medicines Containing a Plant Component and Those with Synthetic Substances Administered among Children in a Bulgarian Population
Abstract
1. Introduction
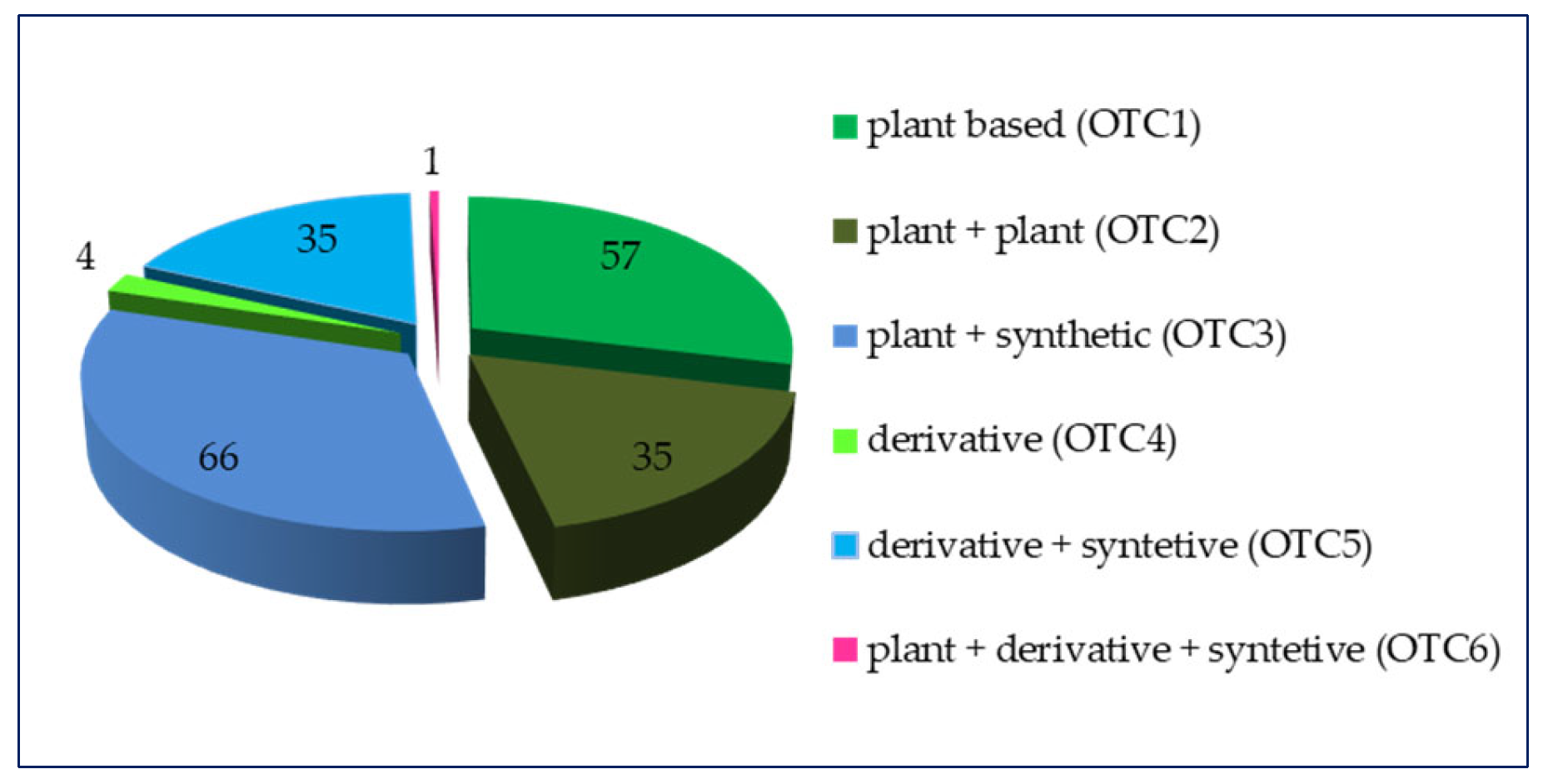
2. Results
2.1. Upper Respiratory Tract
- -
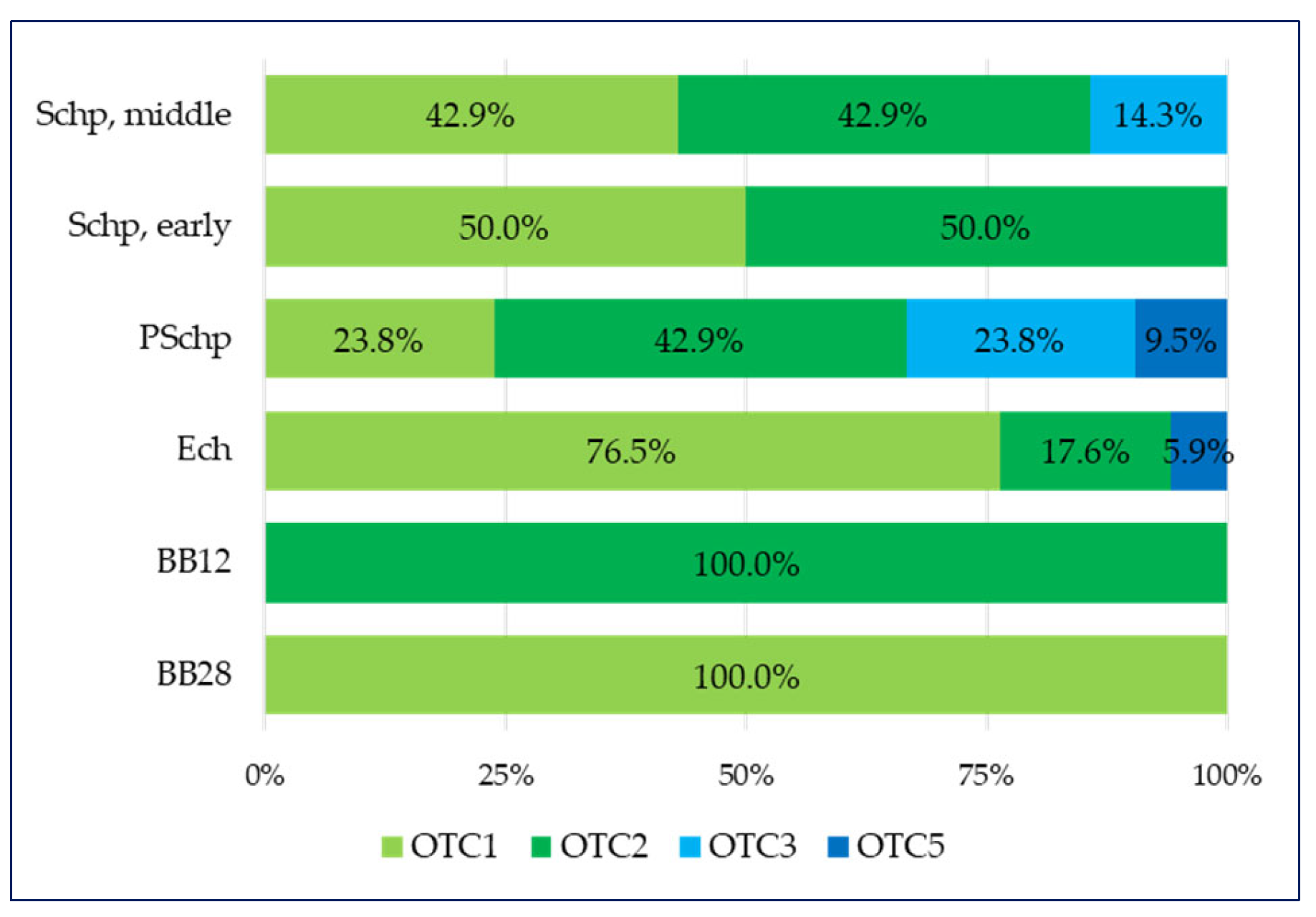
- over-the-counter medicines containing only a plant component (OTC1) and over-the-counter medicines containing a combination of a plant substance + a synthetic substance (OTC3) entirely dominate the lowest age groups such as breastfeeding babies by the 28th day (BB28) and breastfeeding babies by the 12th month (BB12);
- -
- three types of OTCs are applied in the Ech group, of which those that dominate are over-the-counter medicines containing only a plant component (OTC1) at 76.5%, and over-the-counter medicines containing a combination of a derivative + a synthetic substance (OTC5) have the smallest share (5.9%);
- -
- in the fourth age group (PSchp), there are four types of OTCs, the largest share (42.9%) of which is over-the-counter medicines containing a combination of a plant components (OTC2) and the smallest share (9.5%) of which is over-the-counter medicines containing a combination of a derivative + a synthetic substance (OTC5);
- -
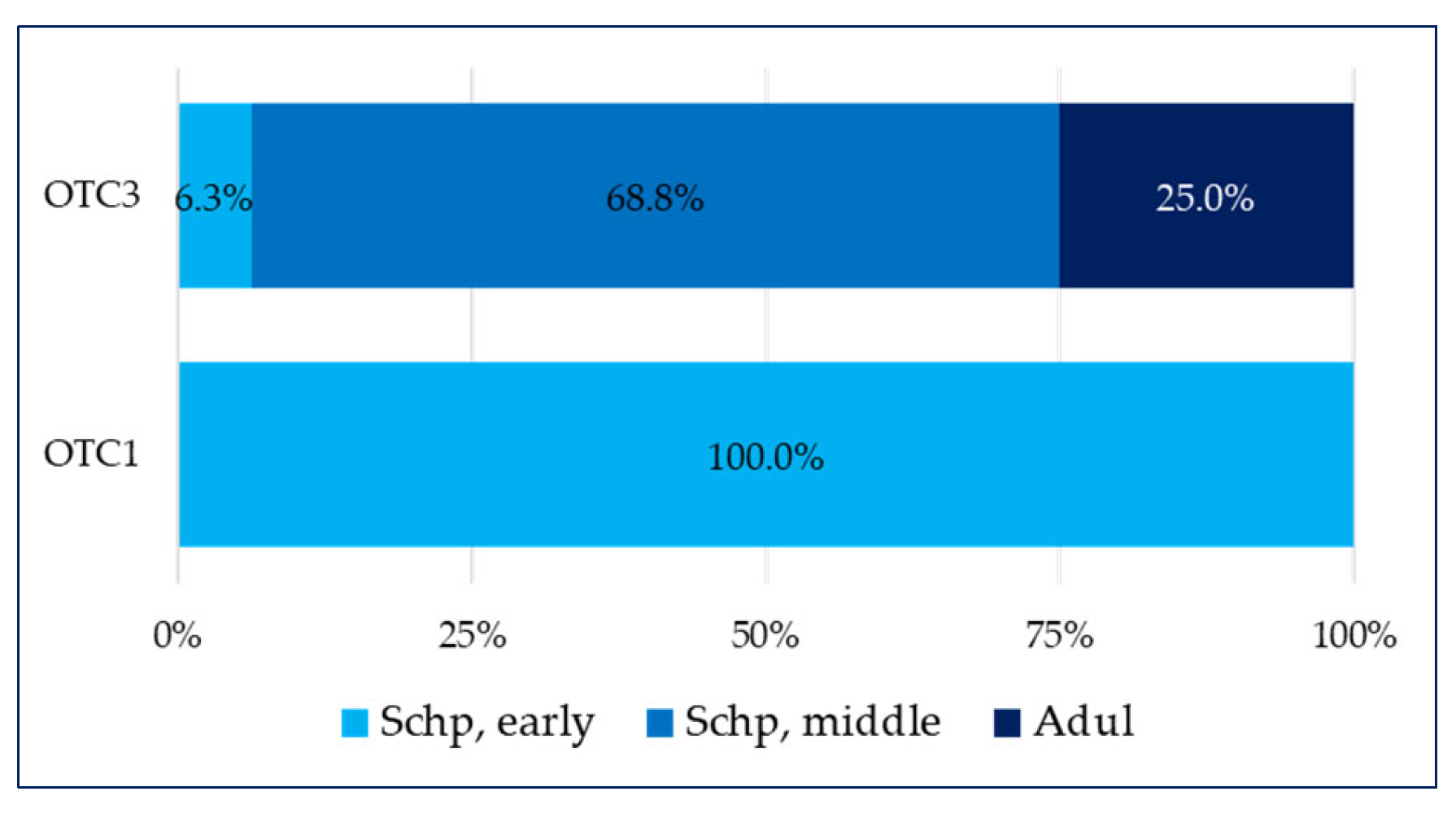
- in the school period, which includes the early school age (Schp early) group, two types are present—over-the-counter medicines containing only a plant component (OTC1) and over-the-counter medicines containing a combination of a plant components (OTC2), which have equal shares (50% each);
- -
- in the last school period age group that includes the secondary school age (Schp, middle), there are three types of OTC, wherein over-the-counter medicines containing only a plant component (OTC1) and over-the-counter medicines containing a combination of a plant components (OTC2) have equal shares (42.9% each), and the smallest share is comprises over-the-counter medicines containing a combination of a plant substance + a synthetic substance (OTC3) (14.3%).
- -
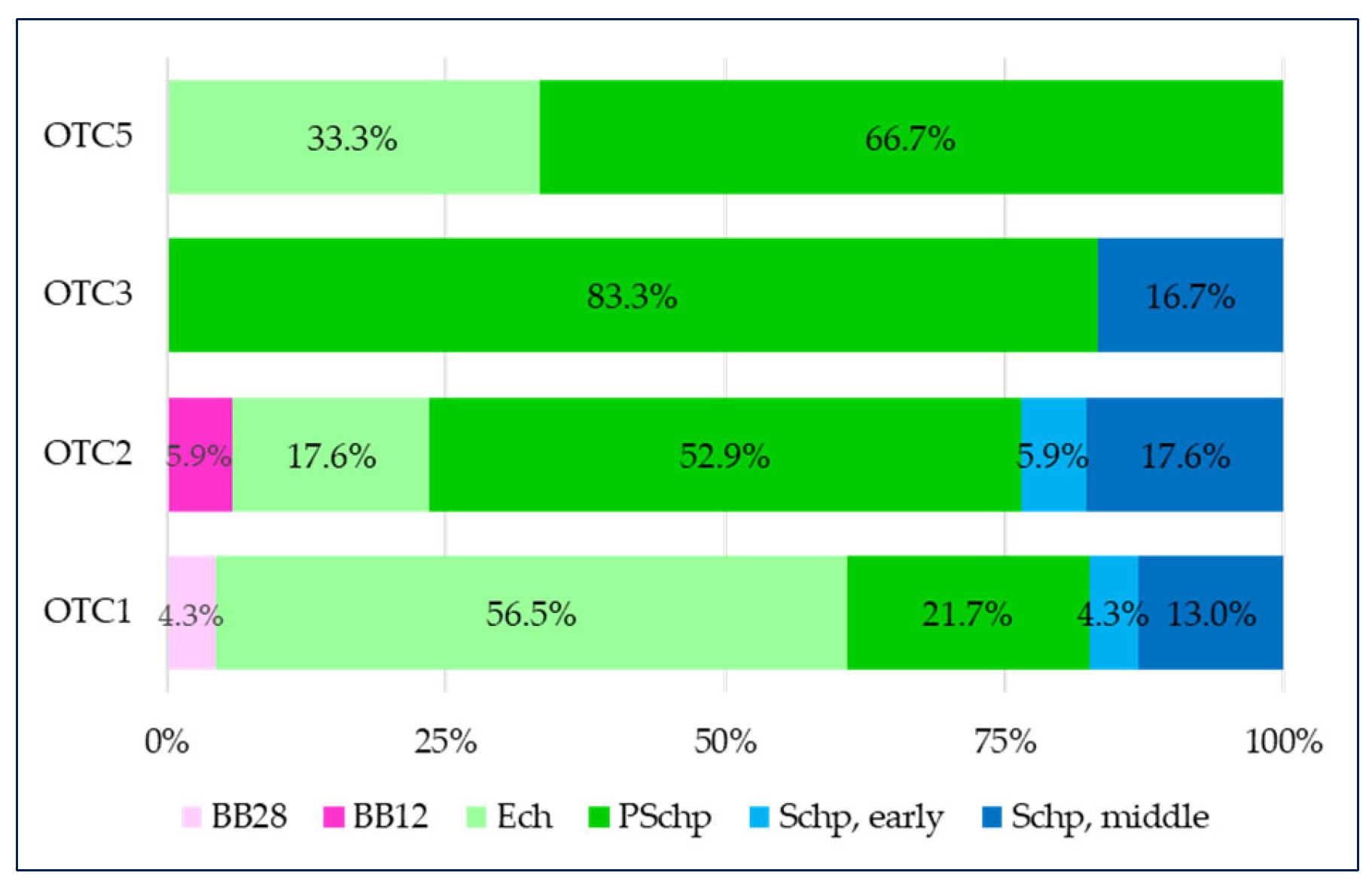
- in the first group of over-the-counter medicines containing only a plant component (OTC1), there are medicines for five of all age groups (with the exception of breastfeeding babies by the 12th month (BB12)), but over half of them (56.5%) are intended for children in the pre-school period (PSchp);
- -
- there is a similar distribution in the over-the-counter medicines containing a combination of a plant component (OTC2) group where the medicines intended for children in the pre-school period (PSchp) dominate (52.9%), as in this group there are also medicines intended for the youngest children—breastfeeding babies by the 12th month (BB12);
- -
- in the over-the-counter medicines containing a combination of a plant substance + a synthetic substance (OTC3) group, over 80% of all medicines are intended for children in the pre-school period (PSchp) and 16.7% are intended for children in the secondary school period (Schp, middle);
- -
- over-the-counter medicines containing a combination of a derivative + a synthetic substance (OTC5) are divided in a 33% to 66% proportion in two age groups—early childhood (Ech) and pre-school period (PSchp).
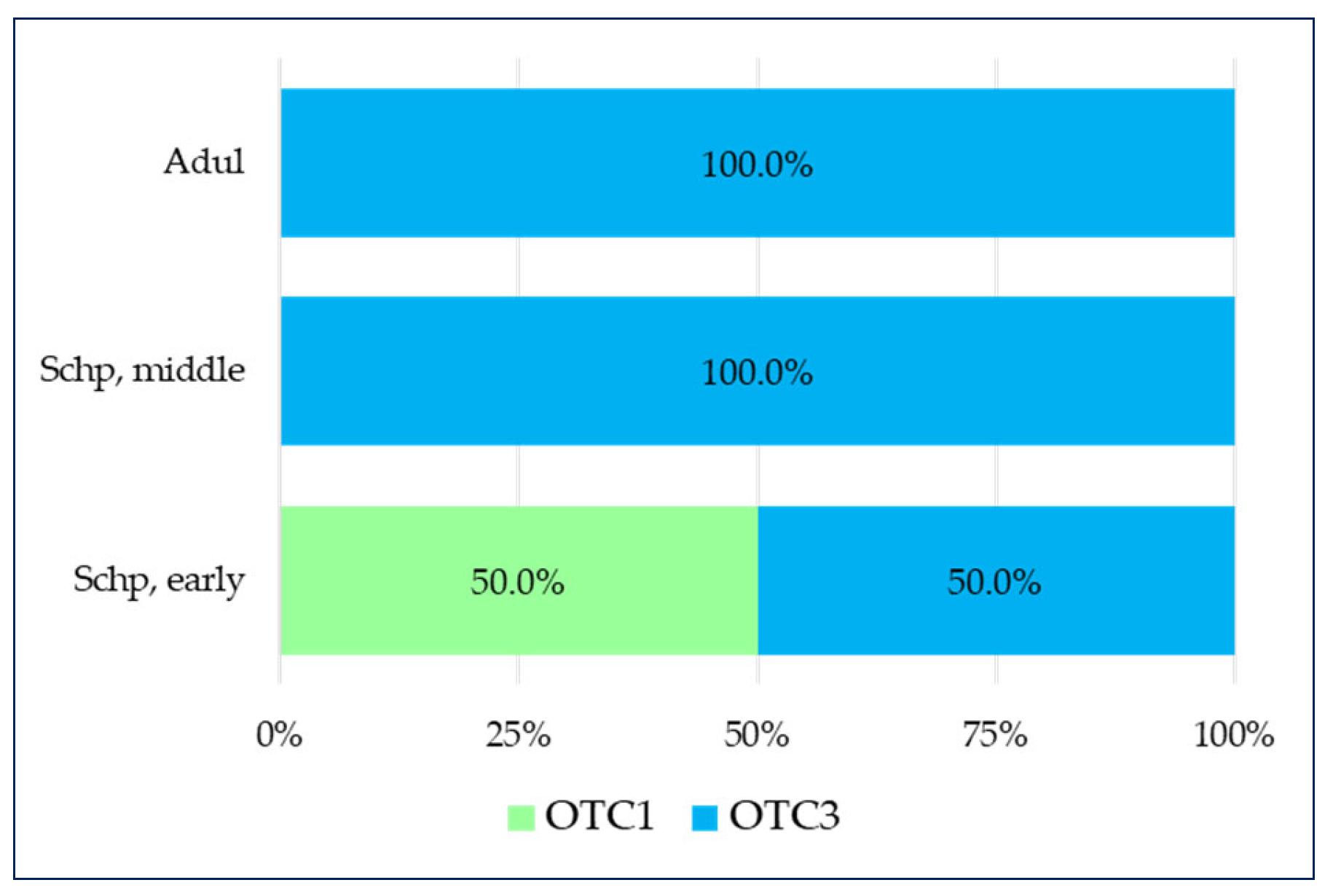
2.2. Pain
3. Discussion
4. Materials and Methods
4.1. A Documentary Analysis of the List of Medicines Registered as Nonprescription Medicines
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OTC1 | Over-the-counter medicines containing only a plant component |
| OTC2 | Over-the-counter medicines containing a combination of plant components |
| OTC3 | Over-the-counter medicines containing a combination of a plant substance + a synthetic substance |
| OTC4 | Over-the-counter medicines containing a derivative |
| OTC5 | Over-the-counter medicines containing a combination of a derivative + a synthetic substance |
| OTC6 | Over-the-counter medicines containing a combination of a plant substance + a derivative + a synthetic substance |
| BB12 | Breastfeeding babies by the 28th day |
| BB28 | Breastfeeding babies by the 12th month |
| Ech | Early childhood |
| PSchp | Pre-school period |
| Schp, early | School period which includes the early school age |
| Schp, middle | School period which includes the secondary school age |
| Adul | Adolescence |
References
- Petrovska, B.B. Historical review of medicinal plants usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Aboelsoud, N.H. Herbal medicine in ancient Egypt. J. Med. Plants Res. 2010, 4, 82–86. [Google Scholar]
- Šantić, Ž.; Pravdić, N.; Bevanda, M.; Galić, K. The historical use of medicinal plants in traditional and scientific medicine. Psychiatr. Danub. 2017, 29, 787–792. [Google Scholar]
- Hardy, K.; Buckley, S.; Collins, M.J.; Estalrrich, A.; Brothwell, D.; Copeland, L.; García-Tabernero, A.; García-Vargas, S.; de la Rasilla, M.; Lalueza-Fox, C.; et al. Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschafte 2012, 99, 617–626. [Google Scholar] [CrossRef]
- Weyrich, L.S.; Duchene, S.; Soubrier, J.; Arriola, A.; Llamas, B.; Breen, J.; Morris, A.G.; Alt, K.W.; Caramelli, D.; Dresely, V.; et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature 2017, 544, 357–361. [Google Scholar] [CrossRef]
- Kuchta, K.; Cameron, S. Tradition to Pathogenesis: A Novel Hypothesis for Elucidating the Pathogenesis of Diseases Based on the Traditional use of Medicinal Plants. Front. Pharmacol. 2021, 12, 70507. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Miraldi, E.; Baini, G. Medicinal plants and health in human history: From empirical use to modern phytotherapy. J. Siena Acad. Sci. 2019, 10, 7–12. [Google Scholar] [CrossRef]
- Eddouks, M.; Chattopadhyay, D.; De Feo, V.; Cho, W.C. Medicinal Plants in the Prevention and Treatment of Chronic Diseases. Evid. Based Complement. Altern. Med. 2012, 2012, 1–2. [Google Scholar] [CrossRef]
- Ernst, E. Herbal medicine: Buy one, get two free. Postgrad. Med. J. 2007, 83, 615–616. [Google Scholar] [CrossRef]
- Mohamed, I.; Shuid, A.; Borhanuddin, B.; Fozi, N. The Application of Phytomedicine in Modern Drug. Internet J. Herbal. Plant Med. 2012, 1, 1–9. [Google Scholar]
- Firenzuoli, F.; Gori, L. Herbal medicine today: Clinical and research issues. Evid. Based Complement. Altern. Med. 2007, 4, 37–40. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A. Nutraceuticals: Efficacy, Safety and Toxicity, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2021; p. 1396. [Google Scholar]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The role and place of medicinal plants in the strategies for disease prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Sachan, A.K.; Vishnoi, G.; Kumar, R. Need of standardization of herbal medicines in Modern era. Int. J. Phytomedicine 2016, 8, 300–307. [Google Scholar] [CrossRef]
- Pan, S.Y.; Zhou, S.F.; Gao, S.H.; Yu, Z.L.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ma, D.L.; Han, Y.F.; Fong, W.F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid.-Based Complement. Altern. Med. 2013, 2013, 627375. [Google Scholar] [CrossRef]
- Ahmed, E.; Arshad, M.N.; Khan, M.Z.; Amjad, M.; Sadaf, H.M.; Riaz, I.; Sabir, S.; Ahmad, N.; Saboon. Secondary metabolites and their multidimensional prospective in plant life. J. Pharmacogn. Phytochem. 2017, 6, 205–214. [Google Scholar]
- Freire, C.J.; Barbosa, L.R.; Costa, J.G.; Santos, R.; Santos, A.F. Phytotherapy in pediatrics: The production of knowledge and practices in Primary Care. Rev. Bras. Enferm. 2018, 71, 637–645. [Google Scholar] [CrossRef]
- Cohen, M.H.; Kemper, K.J.; Stevens, L.; Hashimoto, D.; Gilmour, J. Pediatric use of complementary therapies: Ethical and policy choices. Pediatrics 2005, 116, e568–e575. [Google Scholar] [CrossRef]
- Cohen, M.H.; Kemper, K.J. Complementary Therapies in Pediatrics: A Legal Perspective. Pediatrics 2005, 115, 774–780. [Google Scholar] [CrossRef]
- Catlin, J.R.; Brass, E.P. The Effectiveness of Nonprescription Drug Labels in the United States: Insights from Recent Research and Opportunities for the Future. Pharmacy 2018, 6, 119. [Google Scholar] [CrossRef]
- El-Khatib, F.M.; Yafi, N.R.; Yafi, F.A. Over-the-Counter supplements and men’s health. In Effects of Lifestyle on Men’s Health; Academic Press: Cambridge, MA, USA, 2019; pp. 281–300. [Google Scholar] [CrossRef]
- European Union. Directive 2004/24/EC of the European Parliament and of the Council of 31 March 2004 amending, as Regards Traditional Herbal Medicinal Products, Directive 2001/83/EC on the Community Code Relating to Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/eli/dir/2004/24/oj (accessed on 1 December 2020).
- Minghetti, P.; Franzè, S.; Zaccara, V.; Raso, F.; Morazzoni, P. Innovation in Phytotherapy: Is a New Regulation the Feasible Perspective in Europe? Planta Med. 2016, 82, 591–595. [Google Scholar] [CrossRef]
- Bilia, A.R. Herbal medicinal products versus botanical-food supplements in the European market: State of art and perspectives. Nat. Prod. Commun. 2015, 10, 125–131. [Google Scholar] [CrossRef]
- Sansgiry, S.S.; Bhansali, A.H.; Bapat, S.S.; Xu, Q. Abuse of Over-the-Counter Medicines: A Pharmacist’s Perspective. Integr. Pharm. Res. Pract. 2017, 6, 1–6. [Google Scholar] [CrossRef]
- Kulevanova, S. Modern herbal medicine. In Phytotherapy; Max Zeller Söhne AG: Romanshorn, Switzerland, 2014; p. 584. [Google Scholar]
- Wijesundara, N.M.; Sekhon-Loodu, S.; Rupasinghe, H.V. Phytochemical-rich medicinal plant extracts suppress bacterial antigens-induced inflammation in human tonsil epithelial cells. PeerJ 2017, 5, e3469. [Google Scholar] [CrossRef]
- Sherazi, B.A.; Mahmood, K.T.; Amin, F.; Zaka, M.; Riaz, M.; Javed, A. Prevalence and Measure of Self Medication: A Review. J. Pharm. Sci. Res. 2012, 4, 1774–1778. [Google Scholar]
- Marathe, P.A.; Kamat, S.K.; Tripathi, R.K.; Raut, S.B.; Khatri, N.P. Over-the-counter medicines: Global perspective and Indian scenario. J. Postgrad. Med. 2020, 66, 28–34. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, E.; Fernández-Cerezo, F.L.; Díaz-Jimenez, J.; Rosety-Rodriguez, M.; Díaz, A.J.; Ordonez, F.J.; Rosety, M.Á.; Rosety, I. Consumption of over-the-Counter Drugs: Prevalence and Type of Drugs. Int. J. Environ. Res. Public Health 2021, 18, 5530. [Google Scholar] [CrossRef]
- Dalton, K.; Byrne, S. Role of the pharmacist in reducing healthcare costs: Current insights. Integr. Pharm. Res. Pract. 2017, 6, 37–46. [Google Scholar] [CrossRef]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic Immune System Development in Newborn Children. Cell 2018, 174, 1277–1292.e14. [Google Scholar] [CrossRef]
- Kollmann, T.R.; Kampmann, B.; Mazmanian, S.K.; Marchant, A.; Levy, O. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 2017, 46, 350–363. [Google Scholar] [CrossRef]
- Worrall, G. Common cold. Can. Fam. Physician 2011, 57, 1289–1290. [Google Scholar]
- Arroll, B. Common cold. BMJ Clin. Evid. 2011, 2011, 1510. [Google Scholar] [PubMed]
- Delfan, B.; Kazemeini, H.; Bahmani, M. Identifying Effective Medicinal Plants for Cold in Lorestan Province, West of Iran. J. Evid. Based Complement. Altern. Med. 2015, 20, 173–179. [Google Scholar] [CrossRef]
- Abate, L.; Bachheti, R.; Tadesse, M.; Bachheti, A. Ethnobotanical Uses, Chemical Constituents, and Application of Plantago lanceolata L. J. Chem. 2022, 2022, 1532031. [Google Scholar] [CrossRef]
- Chitra, S.; Sebareze, L. Antibacterial Activity of Thymus Vulgaris L. (Thyme) From Leaf Extract—A Medicinal Plant. Int. J. Ecol. Dev. 2011, 20, 60–68. [Google Scholar]
- Lissiman, E.; Bhasale, A.L.; Cohen, M. Garlic for the common cold. Cochrane Database Syst. Rev. 2014, 11, CD006206. [Google Scholar] [CrossRef]
- Csikós, E.; Csekő, K.; Ashraf, A.R.; Kemény, Á.; Kereskai, L.; Kocsis, B.; Böszörményi, A.; Helyes, Z.; Horváth, G. Effects of Thymus vulgaris L., Cinnamomum verum J.Presl and Cymbopogon nardus (L.) Rendle Essential Oils in the Endotoxin-induced Acute Airway Inflammation. Molecules 2020, 25, 3553. [Google Scholar] [CrossRef]
- Hong, E.-H.; Song, J.-H.; Shim, A.; Lee, B.-R.; Kwon, B.-E.; Song, H.-H.; Kim, Y.-J.; Chang, S.-Y.; Jeong, H.G.; Kim, J.G.; et al. Coadministration of Hedera helix L. Extract Enabled Mice to Overcome Insufficient Protection against Influenza A/PR/8 Virus Infection under Suboptimal Treatment with Oseltamivir. PLoS ONE 2015, 10, e0131089. [Google Scholar] [CrossRef][Green Version]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil—New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef]
- Anheyer, D.; Cramer, H.; Lauche, R.; Saha, F.J.; Dobos, G. Herbal Medicine in Children with Respiratory Tract Infection: Systematic Review and Meta-Analysis. Acad. Pediatr. 2018, 18, 8–19. [Google Scholar] [CrossRef]
- Polat, S.; Gürol, A. Safety of Herbal Medicines in Children. Altern. Med. 2020. [Google Scholar] [CrossRef]
- Snodgrass, W.R. Herbal products: Risks and benefits of use in children. Curr. Thera Res. 2001, 62, 724–737. [Google Scholar] [CrossRef]
- Lokker, N.; Sanders, L.; Perrin, E.M.; Kumar, D.; Finkle, J.; Franco, V.; Choi, L.; Johnston, P.E.; Rothman, R.L. Parental Misinterpretations of over-the-Counter Pediatric Cough and Cold Medication Labels. Pediatrics 2009, 123, 1464–1471. [Google Scholar] [CrossRef]
- Shefrin, A.E.; Goldman, R.D. Use of over-the-counter cough and cold medications in children. Can. Fam. Physician 2009, 55, 1081–1083. [Google Scholar]
- Tittarelli, R.; Pellegrini, M.; Scarpellini, M.G.; Marinelli, E.; Bruti, V.; Di Luca, N.M.; Busardo, F.P.; Zaami, S. Hepatotoxicity of paracetamol and related fatalities. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 95–101. [Google Scholar]
- Cranswick, N.; Coghlan, D. Paracetamol Efficacy and Safety in Children: The First 40 Years. Am. J. Ther. 2000, 7, 135–141. [Google Scholar] [CrossRef]
- Lowe, A.J.; Carlin, J.B.; Bennett, C.M.; Hosking, C.S.; Allen, K.J.; Robertson, C.F.; Axelrad, C.; Abramson, M.J.; Hill, D.J.; Dharmage, S.C. Paracetamol use in early life and asthma: Prospective birth cohort study. BMJ 2010, 341, c4616. [Google Scholar] [CrossRef]
- Cendejas-Hernandez, J.; Sarafian, J.T.; Lawton, V.G.; Palkar, A.; Anderson, L.G.; Larivière, V.; Parker, W. Paracetamol (acetaminophen) use in infants and children was never shown to be safe for neurodevelopment: A systematic review with citation tracking. Eur. J. Pediatr. 2022, 181, 1835–1857. [Google Scholar] [CrossRef]
- Schifano, F.; Chiappini, S.; Miuli, A.; Mosca, A.; Santovito, M.C.; Corkery, J.M.; Guirguis, A.; Pettorruso, M.; Di Giannantonio, M.; Martinotti, G. Focus on Over-the-Counter Drugs’ Misuse: A Systematic Review on Antihistamines, Cough Medicines, and Decongestants. Front. Psychiatry 2021, 12, 657397. [Google Scholar] [CrossRef]
- Piątek, A.; Koziarska-Rościszewska, M.; Zawilska, J.B. Recreational use of over-the counter drugs: The doping of the brain. Alcohol. Drug Addict. 2015, 28, 65–77. [Google Scholar] [CrossRef]
- Głowacka, K.; Wiela-Hojeńska, A. Pseudoephedrine-Benefits and Risks. Int. J. Mol. Sci. 2021, 22, 5146. [Google Scholar] [CrossRef]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/news/prac-starts-safety-review-pseudoephedrine-containing-medicines (accessed on 7 April 2023).
- Du, Y.; Knopf, H. Self-medication among children and adolescents in Germany: Results of the National Health Survey for Children and Adolescents (KiGGS). Br. J. Clin. Pharmacol. 2009, 68, 599–608. [Google Scholar] [CrossRef]
| № | Freely Chosen Name | Medicinal Form and Quantity of the Active Substance in a Dose Unit | INN | ATC Code | Content | Use among Pediatric Patients | Plant-Based OTC Depending on the Active Substance | Use of OTC in the Period of (in Accordance with the Age Differentiation among the Pediatric Population) | Therapeutic Indications |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Bronchicum | 100 mg compressed lozenges × 20; × 50 | Thyme fluid extract | R05CA00 | Each tablet contains:
| Over 4 years of age | OTC1 | Pre-school period | Upper respiratory tract |
| 2. | Bronchicum Elixir S | 5.0 g/2.5 g/100 g syrup—130 g | Thymi herbae tincture//Grindelii herbae tincture/Quebrachi corticis tincture/Primulae radicis tincture/Pimpinellae radicis tincture | R05CA10 | 100 mL of the syrup contains:
| Over 4 years of age | OTC2 | Pre-school period | Upper respiratory tract, cough |
| 3. | Broncholytin | 5.75 mg/4.6 mg/5 ml syrup × 125 g | Glaucine bromide/Basilici oil/Ephedrine hydrochloride | R05FA02 | B 5 mL of the syrup contains:
| Over 3 years of age | OTC2 | Pre-school period | Upper respiratory tract, cough |
| 4. | Bronchostop | 59.5 mg pastilles × 20; × 40 | Plant medicinal product | R05CA | 1 pastille contains: 59.5 mg Thymus vulg./Thymus zyg. L.) dry extract from blades (7–13:1) | Over 12 years of age | OTC1 | School period (middle school age) | Upper respiratory tract, throat |
| 5. | Bronchostop | 0.77 g/0.66 g/5 ml—syrup × 150 ml | Plant medicinal product | R05CA10 | 5 ml (approximately 5.7 g) of syrup contains:
| Over 4 years of age | OTC2 | Pre-school period | Upper respiratory tract, cough |
| 6. | Bronchostop Duo | 51.1 mg/4.5 mg pastilles × 10; × 20; × 30; × 40 | Plant medicinal product | R05CA | 1 pastille contains:
| Over 6 years of age | OTC2 | Pre-school period | Upper respiratory tract, cough |
| 7. | Bronchostop Sine | 0.12 g/1.28 g/15 ml oral solution—100 ml; × 120 ml; × 150 ml; × 200 ml | Plant medicinal product | R05CA10 | 15 ml (=15.45 g) of the solution contains:
| Over 2 years of age (not recommended for children under 2 years of age) | OTC2 | Early childhood | Upper respiratory tract, cough |
| 8. | Bronchoton | 4.6 mg/5.75 mg/5 ml syrup × 125 g | Glaucine hydrochloride/Ephedrine hydrochloride/Basilici oil | R05FA02 | 5 ml of syrup contains:
| Over 3 years of age | OTC2 | Pre-school period | Upper respiratory tract, cough |
| 9. | Carmolis | Oral drops, solution/cutaneous solution/inhalation vapor, solution—20 ml; × 40 ml, × 80 ml; × 160 ml | Anise oil/Clove oil/Citronella oil/Lavander oil/Lemon oil/Spirit of lemon balm/Menthol racemic/Nutmeg oil/Sage oil/Spiciae aetheroleum/Thyme oil | Plant medicinal product | 100 g Carmolis contains:
| Not recommended for children under 12 years of age | OTC2 | School period (middle school age) | Upper respiratory tract, cough |
| 10. | Cefabronchin | 50 g/21 g/100 g oral drops, solution × 20 ml, × 50 ml, × 100 ml | Plant medicinal product | 100 g of drops contains:
| Over 4 years of age | OTC2 | Pre-school period | Upper respiratory tract, cough | |
| 11. | Hedelix | 0.8 g/100 ml syrup | Hederae helicis folium extractum spissum | R05CA12 | 100 ml of syrup contains: 0.8 g Hederae helicis folium extractum spissum with a proportion of the active substance and the extract (2.2–2.9: 1). | Yes 0 + | OTC1 | Breastfeeding period | Upper respiratory tract, cough |
| 12. | Hedespan | 7 mg/ml syrup ×100 ml | Plant medicinal product | R05CA 0 | 1 ml contains: 7 mg extr. siccum Hedera helix L., folium | Over 2 years of age | OTC1 | Early childhood | Upper respiratory tract, cough |
| 13. | Herbion iceland Moss | 6 mg/ml syrup × 150 ml | Plant medicinal product | 1 ml of syrup contains: 6 mg Cetraria islandica (L.) Ach, tallus (16–18:1) | Yes (for children aged 4–6 after being diagnosed) | OTC1 | Pre-school period | Upper respiratory tract, cough | |
| 14. | Herbion Ivy | 7 mg/ml syrup × 150 ml | Plant medicinal product | R05CA 12 | 1 ml of syrup contains: 7 mg Hederae helicis folium extractum spirituosum siccum (5–7.5:1) | Over 2 years of age (contraindicated for children under 2 years of age) | OTC1 | Early childhood | Upper respiratory tract, cough |
| 15. | Herbion Ivy | lozengez × 8; × 16; × 24; × 32; × 40 | Plant medicinal product | R05CA 12 | 1 tablet for sucking contains: 35 mg Hedera helix L., folium extractum siccum (5–7.5:1) | Over 6 years of age | OTC1 | Pre-school period | Upper respiratory tract, cough |
| 16. | Hustagil Thyme Cough Syrup | syrup × 150 ml | Thyme fluid extract | R05CA | 6 g (= 5 ml) contains medicinal substance: 480 mg Extr. Thymi fluidum (DAB) standardized over min. 0.03% Tymol | Over 1 year of age | OTC1 | Early childhood | Upper respiratory tract, cough |
| 17. | Ivitus | 7 mg/ml oral solution— × 100 ml; × 150 ml; × 200 ml | Expetorants | R05CA 0 | 1 ml of solution contains: Hedera helix L. folium (5–8:1) | Over 2 years of age | OTC1 | Early childhood | Upper respiratory tract, cough |
| 18. | Mucohelix | syrup × 100 ml | Hederae helicis folii extractium siccum | R05CA12 | 1 ml of syrup contains: 8.25 mg Hederae helicis folium extractum siccum | Over 2 years of age | OTC1 | Early childhood | Upper respiratory tract, cough |
| 19. | Mucoplant Spitzwegerich Hustensaft | syrup 5 g/100 g × 100 ml; × 250 ml | Plant medicinal product | R05FB02 | 1 g (0.8 ml) of syrup contains: 50 mg Plantaginis lanceolatae folium extractum fluidum (Plantago lanceolata L.) (1:1) | Over 2 years of age | OTC1 | Early childhood | Upper respiratory tract, cough |
| 20. | Mucoplant Anisol | Capsules 100 mg × 30 | Anise oil/Anisi aetheroleum | R05CA | 1 capsule contains: Anisi stellati aetheroleum—100 mg | Over 12 years of age | OTC1 | School period (middle school age) | Upper respiratory tract, cough |
| 21. | Mucoplant Cough Syrup Ivy | 154 mg/100 ml syrup × 100 ml; × 200 ml; × 250 ml | Ivy leaf dry extract (Hedera helix L.) Extraction solvent: Ethanol | R05C | Each ml contains:1.54 mg Hedera helix L. (DER 4–8:1) | Over 2 years of age (contraindicated for children under 2 years of age owing to a risk of deterioration of the respective symptoms) | OTC1 | Early childhood | Upper respiratory tract, cough |
| 22. | Pinosol | nasal drops, solution 10 ml | Pine oil/Eucalyptus oil/Tocopherol acetate/Thymol/Guaizulene/Peppermint oil | R01AX00 | Medicines contain:
| Over 6 years of age for inhalation among children over 12 years of age | OTC2 | Pre-school period | Upper respiratory tract, inflammation of the nasal and nasopharyngeal mucous membrane |
| 23. | Prospan | 20 mg/ml oral drops, solution × 20 ml | Hedera helix extract | R05FB00 | 1 ml of solution contains:
| Over 1 year of age | OTC1 | Early childhood | Upper respiratory tract, cough |
| 24. | Prospan | 65 mg eff. tablets × 10; × 20 | Hedera helix extract | R05FB00 | 1 effervescent tablet contains: 65 mg Hedera helix folium extractum siccum (5–7.5:1) | Over 12 years of age (children over 6 years of age take ½ of an effervescent tablet 2 times a day) | OTC1 | Pre-school period | Upper respiratory tract, cough |
| 25. | Prospan | 26 mg lozenges × 10; × 20 | Hedera helix extract | R05CA | 1 tablet for sucking contains: 26 mg Hedera helix folium extractum siccum (5–7.5): 1) | Over 6 years of age | OTC1 | Pre-school period | Upper respiratory tract, cough |
| 26. | Prospan | 7 mg/ml syrup × 5 ml; × 100 ml; × 200 ml | Hedera helix extract | R05FB00 | 1 ml of syrup contains: 7 mg Hedera helix folium extractum siccum (5–7.5:1) | Over 1 year of age. For children under 1 year of age it must be prescribed by a doctor | OTC1 | Early childhood | Upper respiratory tract, cough |
| 27. | Prospan Liquid | 7 mg/ml oral liquid—5 ml × 21 | Hedera helix extract | R05FB00 | 1 ml of syrup contains: 7 mg Hedera helix folium extractum siccum (5–7.5:1) | Over 6 years of age | OTC1 | Pre-school period | Upper respiratory tract, cough |
| 28. | Sinupret | syrup × 50 ml, × 100 ml, × 200 ml, × 500 ml | Plant medicinal product | R05CA10 | 100 g of syrup contains: extract (1:11) from:
| Over 2 years of age | OTC2 | Early childhood | Upper respiratory tract, inflammation of the paranasal sinuses |
| 29. | Sinupret | coated tablets × 50; × 100; × 200; × 500 | Rumicis herbae pulvis/Sambuci flos pulvis/Gentianae radix pulvis/Primulae flos pulvis/Verbenae herbae pulvis | R05CA10 | 1 coated tablet contains:
| Over 6 years of age (not recommended for children under 6 years of age owing to the lack of sufficient evidence) | OTC2 | School period (early school age) | Upper respiratory tract, inflammation of the paranasal sinuses |
| 30. | Sinupret Forte | coated tablets × 20; × 50; × 100; × 500 | Rumicis herbae pulvis/Sambuci flos pulvis/Gentianae radicis pulvis/Primulae flos pulvis/Verbenae herbae pulvis | R05CA10 | 1 coated tablet contains:
| Not recommended for children under 12 years of age owing to the lack of sufficient data | OTC2 | School period (middle school age) | Upper respiratory tract, inflammation of the paranasal sinuses |
| 31. | Tavipec | 150 mg gastro-resistant capsules, soft × 20; × 30 | Spicae aetheroleum | R05CA00 | 1 capsule contains: Spicae aetheroleum—150 mg | Over 12 years of age | OTC1 | School period (middle school age) | Upper respiratory tract, expectorant |
| 32. | Tuspan | 7 mg/ml syrup × 120 ml | Ivy leaf dry extract | R05CA12 | 1 ml of syrup contains: 7 mg extract from Hedera helix L. (5–7.5:1) | Over 2 years of age (not recommended for children under 2 years of age owing to their oversensitivity to the active substance) | OTC1 | Early childhood | Upper respiratory tract, expectorant |
| 33. | Tussavit | 7.53 g/7.53 g/100 g syrup 125 g; 250 g | Thymi folium extr./Plantaginis folium | R05CA00 | 100 g of syrup contains:
| Over 2 years of age (not recommended for children under 2 years of age owing to the content of alcohol) | OTC2 | Early childhood | Upper respiratory tract, cough |
| 34. | Umckalor | 20 mg film-coated tablets × 15; × 30 | Plant medicinal product | R05 | 1 coated tablet contains: 20 mg. dried liquid extract from Pelargonii sidoidi radix (1:8–10) (EPs 7630) | Over 6 years of age | OTC1 | School period (early school age) | Respiratory tract, acute infections, bronchitis, sinusitis |
| 35. | Umckalor | 8 g/10 g oral drops, solution × 20 ml; 50 ml; × 100 ml | Pelargonii sidoidi radicis extractum liquidum | R05 | 10 g (= 9.75 ml) contains Pelargonii sidoidi radix extr. fliudum (1:8–10) (EPs 7630) | Over 1 year of age | OTC1 | Early childhood | Respiratory tract, acute infections, bronchitis, sinusitis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadzhieva, B.; Petkova-Dimitrova, V. Proportion of Over-The-Counter Medicines Containing a Plant Component and Those with Synthetic Substances Administered among Children in a Bulgarian Population. Pharmaceuticals 2024, 17, 192. https://doi.org/10.3390/ph17020192
Hadzhieva B, Petkova-Dimitrova V. Proportion of Over-The-Counter Medicines Containing a Plant Component and Those with Synthetic Substances Administered among Children in a Bulgarian Population. Pharmaceuticals. 2024; 17(2):192. https://doi.org/10.3390/ph17020192
Chicago/Turabian StyleHadzhieva, Bozhidarka, and Valentina Petkova-Dimitrova. 2024. "Proportion of Over-The-Counter Medicines Containing a Plant Component and Those with Synthetic Substances Administered among Children in a Bulgarian Population" Pharmaceuticals 17, no. 2: 192. https://doi.org/10.3390/ph17020192
APA StyleHadzhieva, B., & Petkova-Dimitrova, V. (2024). Proportion of Over-The-Counter Medicines Containing a Plant Component and Those with Synthetic Substances Administered among Children in a Bulgarian Population. Pharmaceuticals, 17(2), 192. https://doi.org/10.3390/ph17020192









