Abstract
Plant extracts are in the focus of the pharmaceutical industry as potential antimicrobials for oral care due to their high antimicrobial activity coupled with low production costs and safety for eukaryotic cells. Here, we show that the extract from Hop (Humulus lupulus L.) exhibits antimicrobial activity against Staphylococcus aureus and Streptococci in both planktonic and biofilm-embedded forms. An extract was prepared by acetone extraction from hop infructescences, followed by purification and solubilization of the remaining fraction in ethanol. The effect of the extract on S. aureus (MSSA and MRSA) was comparable with the reference antibiotics (amikacin, ciprofloxacin, and ceftriaxone) and did not depend on the bacterial resistance to methicillin. The extract also demonstrated synergy with amikacin on six S. aureus clinical isolates, on four of six isolates with ciprofloxacin, and on three of six isolates with ceftriaxone. On various Streptococci, while demonstrating lower antimicrobial activity, an extract exhibited a considerable synergistic effect in combination with two of three of these antibiotics, decreasing their MIC up to 512-fold. Moreover, the extract was able to penetrate S. aureus and S. mutans biofilms, leading to almost complete bacterial death within them. The thin-layer chromatography and LC-MS of the extract revealed the presence of prenylated flavonoids (2′,4′,6′,4-tetrahydroxy-3′-geranylchalcone) and acylphloroglucides (cohumulone, colupulone, humulone, and lupulone), apparently responsible for the observed antimicrobial activity and ability to increase the efficiency of antibiotics. Taken together, these data suggest an extract from H. lupulus as a promising antimicrobial agent for use both as a solely antiseptic and to potentiate conventional antimicrobials.
1. Introduction
The human oral cavity is a habitat for many microorganisms, including bacteria, archaea, fungi, viruses, and protozoa. Various internal and external environmental factors affect the oral microbiota, leading to dynamic changes in its composition, from insignificant alteration to dysbiosis. Consequently, various diseases develop, such as caries, periodontal disease, oral candidiasis, and systemic infections [1,2,3]. Furthermore, biofilm formation significantly complicates the treatment of oral infections with common antimicrobials. Due to the inability of antimicrobials to penetrate the extracellular matrix, the effect of antimicrobials on bacteria in the biofilm is significantly lower compared to free-floating planktonic cells. Therefore, the development of new antiseptics with low toxicity for the mucosa and a low risk of resistance development that are thus acceptable for daily use, while being efficient against the oral pathogenic flora, including biofilms, is challenging.
The primary colonizers of the oral cavity are Streptococcus sanguinis, S. oralis, S. intermedius, S. gordonii, Peptostreptococcus micros, Gemella morbillorum, and Actinomyces species [4,5,6]. Streptococci and Actinomyces colonize the oral mucosa of a baby from the first days of life and come from the microbiota of the mother [7]. However, over the lifecycle, environmental pressure leads to an imbalance of microorganisms or an abundance of pathogenic ones [8,9,10]. As a consequence, dramatic changes in the production of acid and alkali by bacteria occur, which, in turn, lead to tooth surface damage and caries development. The development of caries is closely related to the formation of polymicrobial biofilms by the oral microbiota on teeth and soft tissues [11]. Lactobacilli and Streptococcus mutans play a main role in the occurrence and progression of caries due to the fermentation of sucrose and other sugars with the formation of lactic acid, which plays a major role in the local acidification of the caries environment [12,13,14,15,16]. In addition, the symbiotic interaction of Porphyromonas gingivalis with S. gordonii or S. oralis has been shown to lead to significant complications in the development of disease and bone loss [17,18]. In addition to Streptococci, Staphyloococcus aureus is one of the main microorganisms involved in the development of periodontitis [19]. Thus, S. aureus forms mixed biofilms with Streptococci and causes inflammation [20,21]. Therefore, the search for substances that can penetrate through the biofilm and have a bactericidal effect on bacteria embedded in the extracellular matrix is an important challenge.
Phytoextracts are widely known for their therapeutic effects due to their high antimicrobial and antibiofilm activity [22,23,24]. Their natural origin, low toxicity, and low economic costs make them attractive for pharmaceuticals [25,26]. Moreover, the use of chemicals from phytoextracts as antimicrobials has a low probability of bacterial resistance development [27,28,29]. The beneficial use of phytoextracts for antimicrobial treatment is also due to the synergistic effects of active components in their composition. Synergism is provided by various effects, like the multitargency of substances, the ability to suppress intracellular resistance mechanisms, and the increased bioavailability of substances [30]. Thus, the use of bearberry and cranberry juice contributed to the effective treatment of urinary tract infections. Extracts of lemon balm (Melissa officinalis), garlic (Allium sativum), and tea tree (Melaleuca alternifolia) have been described as broad-spectrum antimicrobial agents [31]. Also, high antibacterial activity against S. aureus, Escherichia coli, Pseudomonas aeruginosa, and Salmonella typhimurium has been described for Myrtus communis and Verbena officinalis [32]. Extracts of the bark and leaves of Eucalyptus camaldulensis showed high antimicrobial activity against a wide range of Gram-positive and Gram-negative bacteria, while the effective concentration varied from 0.08 μg/mL to 200 μg/mL and depended on the extraction procedure [33]. In vitro studies have shown that catechins found in green tea extracts are able to inhibit the growth of Vibrio cholerae, S. mutans, and Shigella [34,35,36]. Soursop leaf extract [37] had antimicrobial activity similar to that of chlorhexidine against the most significant cariogenic microorganisms, such as S. mutans, S. mitis, and C. albicans. In vivo studies of garlic extract have shown antimicrobial activity against pathogenic Streptococci [38,39,40,41].
In addition to their antimicrobial activity, phytoextracts are also able to increase the effectiveness of systemic antibiotics. Thus, mangosteen extract increased the effectiveness of β-lactam antibiotics against several resistant strains [42]. Camellia sinensis dry leaf extract increased the effectiveness of nalidixic acid against Salmonella typhi by eight times [43]. Also, the combination of individual components of extracts from Jatropha elliptica demonstrated a synergistic effect with fluoroquinolones against S. aureus [44]. The use of carnosic acid isolated from Rosmarinus officinalis L. extract potentiated the effect of erythromycin against multidrug-resistant bacteria [45]. Myrtenol was able to increase the efficiency of various antimicrobials against S. aureus and C. albicans [46].
The antimicrobial properties of hops (Humulus lupulus L.) have been well known for a long time and have been used for beer production since the 11th century, mainly to repress bacteria that spoil the beer [47]. Since then, the antimicrobial activity of hop infructescence extracts against a wide range of bacteria, as well as its antiviral and antifungal activity, has also been reported [48,49]. Furthermore, hop extracts do not have a toxic effect on the human body [50,51,52], which makes them suitable for use as an antiseptic. The antimicrobial activity of hop essential oil has been also shown against a number of pathogens, such as Yersinia enterocolitica, Salmonella enteritidis, S. typhimurium, Proteus mirabilis, Escherichia coli, Klebsiella oxytoca Enterobacteriaceae, Enterococci, and anaerobic bacteria [48,53,54,55]. However, only a small number of studies have been devoted to evaluating its effect on oral microflora, both alone and in combination with other antimicrobials [56].
Here, we report that the ethanol extract from hop infructescence demonstrates high antimicrobial activity and a synergetic effect with various conventional antimicrobials against S. aureus (both MSSA and MRSA), S. gordonii, S. mutans, S. sobrinus, and S. salivarius, including the biofilm-embedded forms, thus being an attractive phytosubstance for oral care.
2. Results
2.1. Metabolite Profile of Hop Extracts
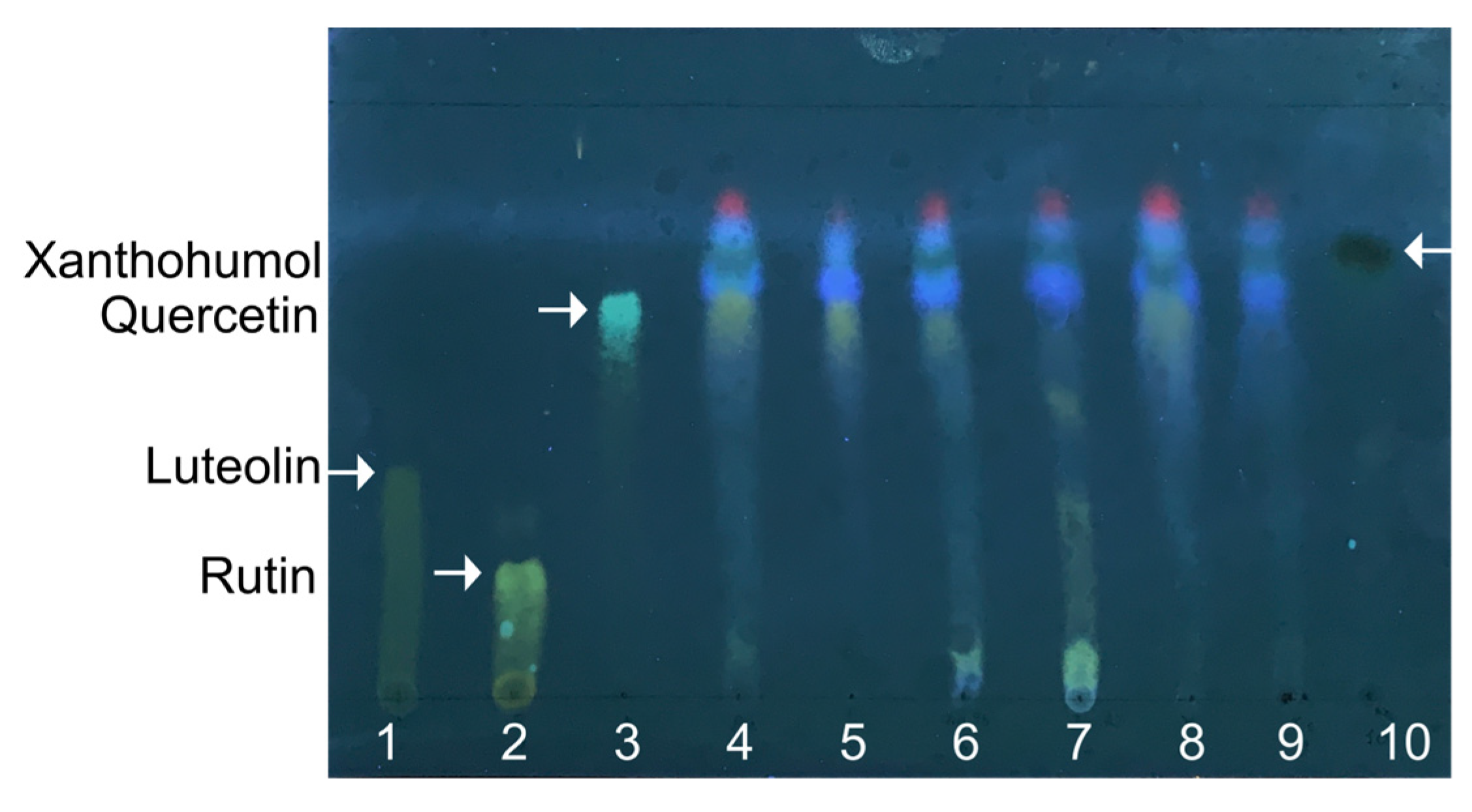
In the first step, the extraction of the active compounds from hops was performed by the reflux technique using an eluotropic variety of solvents: hexane, chloroform, acetone, 95% ethanol, and 70% ethanol. The extracts obtained were then separated with TLC, and the extraction efficiency was evaluated by the yield of hop flavonoids with each solvent (Figure 1, lanes 4–8) in comparison with luteolin, rutin, quercetin, and xanthohumol (Figure 1, lanes 1–3 and 10, respectively). All extracts seem to contain xanthohumol and quercetin. Xanthohumol, the prenylated flavonoid, is located on the top of the TLC plate, higher than other less polar flavonoids, and appears as a spot with dark-green fluorescence. Furthermore, the highest yield of total flavonoids was observed after acetone extraction (lane 4); therefore, this solvent was used for further preparation of extracts on a preparative scale.
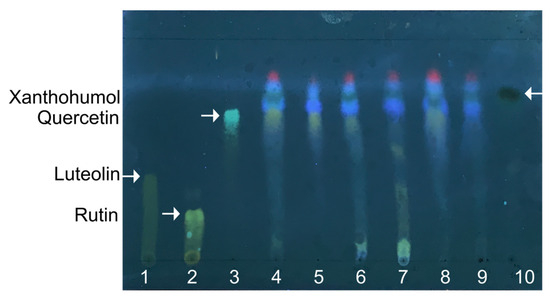
Figure 1.
The representative thin-layer chromatography of hops extracts in chloroform, ethanol, and water (25:18:2). Extracts with acetone (4), hexane (5), 95% ethanol (6), 70% ethanol (7), and chloroform (8) were loaded with 5 µL each. Lane (9) corresponds to acetone extract prepared on a preparative scale and dissolved in 95% ethanol. Commercially available pure luteolin (1), rutin (2), quercetin (3), and xanthohumol (10) were used as references, and the corresponding spots are shown by arrows.
The final extract obtained by acetone extraction appeared as a dark, viscous orange liquid. It was dissolved in 95% ethanol at the final concentration of 40 mg/mL and used for other tests as a pharmaceutical substance from H. lupulus. The TLC analysis of the extract (Figure 1, lane 9) revealed the presence of xanthohumol, while other polar flavonoids were not detected. Furthermore, the Beer–Lambert law-based UV-vis-spectrophotometric qualitative analysis of the obtained extract revealed the maximum optical absorbance at λ = 285 nm and λ = 334 nm (Figure S1) that correspond to the characteristic absorption lines of APs.
For a deeper analysis of the compounds in the extract, LC-MS was performed. Among the 10 most abundant compounds identified with LC-MS (see Figure S2 for chromatogram), prenylated flavonoids (2′,4′,6′,4-tetrahydroxy-3′-geranylchalcone) and acylphloroglucides (cohumulone, colupulone, humulone, lupulone, and others) were detected (see Table 1 and Table S1 for structures), suggesting potential antimicrobial activity of the obtained substance.

Table 1.
The prenylated flavonoids and acylphloroglucides identified by LC-MS in the hop infructescence extract.
2.2. Antimicrobial Effect of an Extract from H. lupulus
The antimicrobial effect of an extract from H. lupulus was tested on methicillin-sensitive S. aureus ATCC 29213 and six clinical isolates of S. aureus (three MSSA and three MRSA), as well as on four different strains of Streptococci (S. mutans, S. sobrinus, S. salivarius, and S. gordonii) (Table 2). The fraction of H. lupulus infructescence demonstrated high antimicrobial activity against all studied S. aureus strains. The minimum inhibitory concentration (MIC) varied within 10–40 µg/mL of total compounds in the extract and did not depend on bacterial sensitivity to methicillin. At the same time, the effective concentrations of the extract in relation to individual strains were comparable to or significantly lower than the MIC of the reference antibiotics, excepting S. aureus 68 isolate, which was sensitive to all antimicrobials (Table 2). On the contrary, the extract demonstrated moderate activity against S. mutans, S. sobrinus, S. salivarius, and S. gordonii (MIC for all Streptococci was 625 µg/mL) compared to reference antibiotics.

Table 2.
Minimal inhibitory concentrations of the extract from H. lupulus, amikacin, ciprofloxacin, and ceftriaxone on bacterial cells. Median values from four biological replicates are shown.
2.3. Potentiation of Antimicrobial Agents by the H. lupulus Extract
The combined effect of the hop extract and antibiotics was evaluated by the checkerboard assay. For both S. aureus and Streptococci, the final concentration of the studied antimicrobials was in the range of 0.06–2×MIC. After 24 h of incubation, the fractional inhibitory concentration index was calculated.
The combined use of an extract and amikacin increased the efficiency of the antibiotic against four of six S. aureus strains by 8–1024-fold, suggesting considerable synergy (Table 3). The synergism was less pronounced when the extract was combined with ciprofloxacin and ceftriaxone. In relation to S. aureus ATCC 29213, the combination of these antimicrobials with an extract from the hop demonstrated an additive effect with FICI of 0.75 and 1.25, respectively. Nevertheless, for half of clinical isolates, in the presence of extract, the bacterial susceptibility to antimicrobials increased up to 128-fold; however, this effect was strain-specific and did not depend on resistance to methicillin (Table 3). Of note, an extract was unable to increase the bacterial susceptibility to those antimicrobials that were inefficient for the given isolate, apparently because of their genetically determined resistance.

Table 3.
Fractional inhibitory concentration and FICI (index of fractional inhibitory concentration) values of antimicrobials in the presence of an extract from H. lupulus on bacterial cells. Median values of FICI from four biological replicates are shown.
Next, the synergism of the hop extract and antibiotics was evaluated on four different isolates of Streptococci. Despite the low antimicrobial activity on these bacteria, the combined use of the extract with antibiotics increased the efficiency of the latter considerably. Thus, the MIC of amikacin and ceftriaxone in the presence of the extract against S. mutans decreased 64- and 8-fold, respectively, while the combination with ciprofloxacin showed an additive effect (Table 3). In relation to S. sobrinus and S. salivarius, the MIC of all three antibiotics (except amikacin for S. salivarius) in the presence of hop extract decreased 256–512-fold (FICI = 0.25). However, in relation to S. gordonii, the synergism of the extract was observed only when it was combined with amikacin (FICI = 0.26), while for ciprofloxacin and ceftriaxone, either antagonism (FICI = 8.6) or an additive effect (FICI = 1.25) was detected, respectively (Table 3).
Thus, these data clearly show that the combined use of the extract from hop infructescence is capable of significantly increasing the efficiency of antimicrobial therapy for the most significant pathogenic microorganisms of the oral cavity, including those with antimicrobial resistance.
2.4. Extract from H. lupulus Kills Bacterial Cells in Biofilm
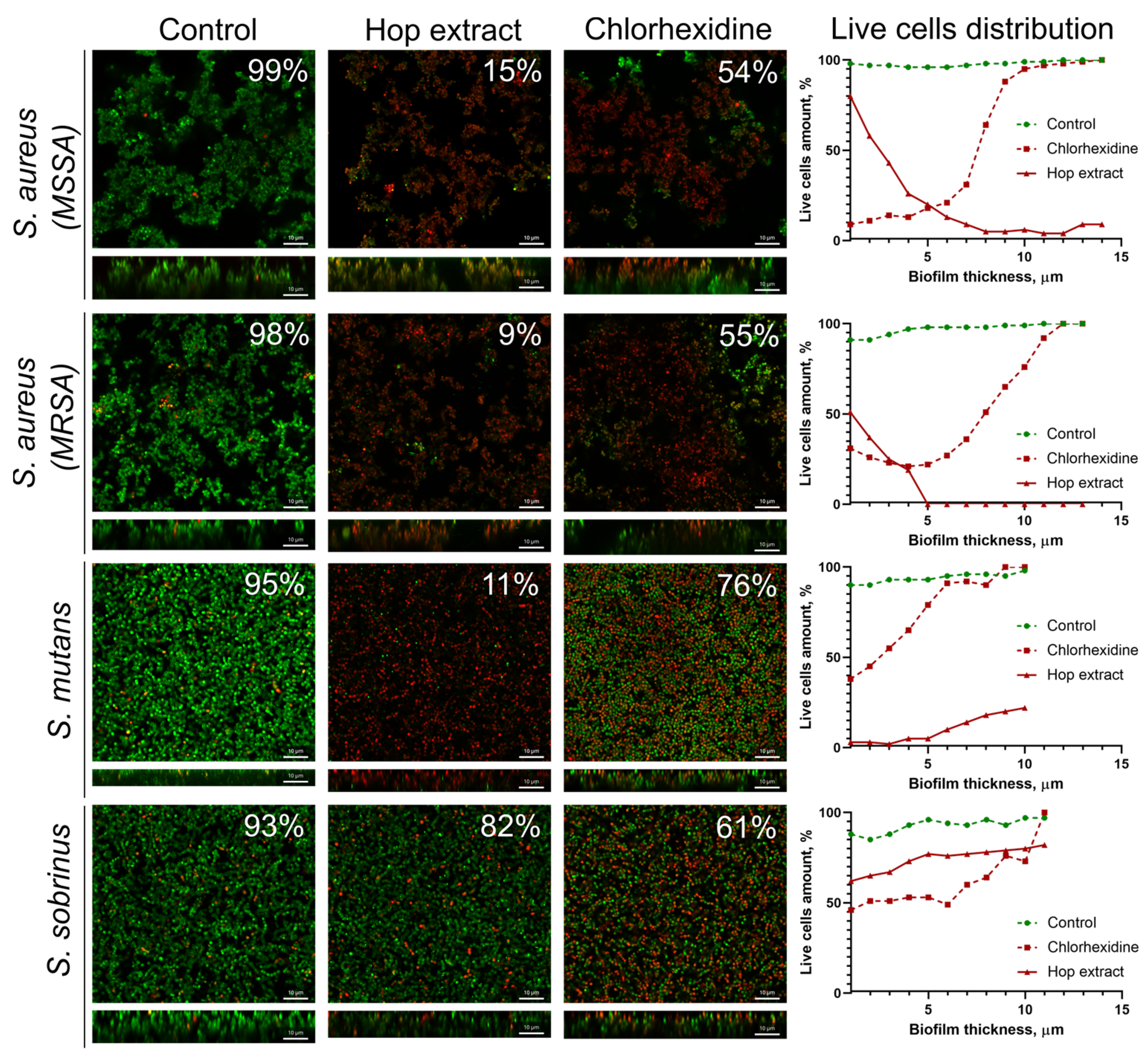
It has been reported in several works that extracts of medicinal plants are able to target biofilm-embedded bacteria, which makes them attractive agents for oral care [57]. We tested the effect of the extract from H. lupulus infructescence on mature biofilms of S. aureus (MSSA and MRSA), S. mutans, and S. sobrinus, common pathogens causing oral diseases. A 0.05% solution of chlorhexidine, an antiseptic widely used as a mouthwash [58], was used as a reference. For that, 48-hour-old biofilms were treated for 3 h and then analyzed with confocal laser scanning microscopy (Figure 2). CLSM data showed that the treatment of S. aureus biofilms, formed by both MSSA and MRSA isolates with an extract solution at a concentration equal to 2×MIC, led to the death of almost all cells after 3 h of treatment. The fraction of viable bacteria in the presence of hop extract was 9–15%, while the treatment of biofilms with chlorhexidine caused the death of only 55–56% of cells (Figure 2). Also, the extract was effective against S. mutans cells in the biofilm since the average fraction of viable cells in the biofilm was 11%, while the treatment with chlorhexidine led to the death of 24% of the cells. On the contrary, the cells in the S. sobrinus biofilm were not sensitive to extract under the conditions tested (the fraction of viable cells consisted 82% of the entire biofilm), whereas chlorhexidine treatment led to 61% of residual viability (Figure 2).
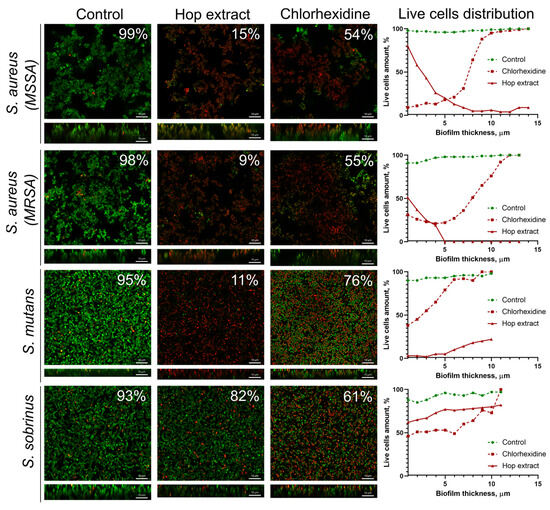
Figure 2.
The effect of the hop extract on bacterial cells in biofilms. An extract of H. lupulus was added to mature 48 h biofilms of S. aureus (MSSA and MRSA), S. mutans, and S. sobrinus at a concentration equal to respective 2×MIC, incubated for 3 h, and the viability of the bacteria was evaluated using confocal laser scanning microscopy. Living cells are shown in green, and dead cells are shown in red. The fraction of viable cells in each Z-stack was calculated using the BioFilmAnalyzer software (version 1.2) and estimated as the relative fraction of the red cells among all cells in the combined images obtained by overlaying the green and red fluorescence microphotographs. The scale bars indicate 10 μm.
A deeper analysis of the distribution of viable cells in biofilms revealed that an extract has worse penetration into the S. aureus biofilms, and a significant decrease in viable cells (more than twice) was already observed in the middle layers of the biofilm. By contrast, the extract was able to penetrate the S. mutans biofilm, which was confirmed by only a few viable cells in the bottom layers. At the same time, the S. sobrinus biofilm was the least permeable for an extract, and the number of viable cells throughout the entire biofilm layers varied between 70 and 80% (Figure 2). In turn, chlorhexidine penetrated the biofilms of all studied strains. However, only S. aureus MSSA cells were sensitive to the antiseptic, where almost complete cell death could be observed in the bottom layers of the biofilm. At the same time, in the biofilms of MRSA and both Streptococci, cell viability in the presence of chlorhexidine in the bottom layers varied within 40–50%, which confirms the low efficiency of this antimicrobial against biofilms (Figure 2).
3. Discussion
Dental caries and periodontal diseases are considered the most common chronic diseases of the oral cavity worldwide, and currently, there are only a few approved antimicrobials for their treatment [8]. To date, chlorhexidine remains the most prevalent treatment agent widely used in oral care due to its broad spectrum of antimicrobial activity and prolonged action. However, the prolonged use of chlorhexidine in sublethal concentrations induces resistance development by oral bacteria [59] and has various side effects [58,60]. Plant extracts with antimicrobial properties seem like a promising alternative to synthetic antibacterial agents, and the search for new sources of plant-derived antimicrobial agents that are generally non-toxic and do not induce resistance remains an urgent task to enhance the efficiency of infectious disease treatment.
In this study, we investigated the antimicrobial activity of an extract from H. lupulus against some oral pathogens. A specific component of hop extracts, a bitter acid xanthohumol, has been reported as targeting the membrane of Gram-positive bacteria and thus exhibiting high antimicrobial activity [56,61,62], and its presence in the extract has been confirmed by TLC (Figure 1). Indeed, the hop extract demonstrated significant antimicrobial activity against S. aureus, and this activity was not dependent on the bacterial resistance to methicillin (Table 2), that fits with other studies [63]. By contrast, the activity of the extract from hop was low on Streptococci (S. mutans, S. sobrinus, S. salivarius, and S. gordonii). This may be associated with the ability of Streptococci to alter the acid-base balance of the environment during their lifecycle [64,65,66], and the effectiveness of hop extracts has been reported to be directly dependent on the pH of the medium. Nevertheless, the presence of antimicrobial activity against both S. aureus and various strains of Streptococci opens possibilities for treatment of mixed infections, which are dominant in periodontal diseases [67].
Bacteria associated with the development of dental caries and periodontal diseases are often present in the form of polymicrobial communities [19]. Therefore, the development of a universal antiseptic agent active against a wide range of bacteria embedded in biofilms remains an urgent task for pharmaceuticals. Extract from H. lupulus demonstrated the ability to penetrate into S. aureus (MSSA and MRSA) and S. mutans biofilms, which fits with earlier data [68]. Thus, the fraction of viable cells in treated biofilms decreased up to 9–15%, including the bottom layers of the biofilm (see viable cell distribution on Figure 2). At the same time, the effect of chlorhexidine was less pronounced, especially against S. mutans biofilm, which seems to be a consequence of the low permeability of the antiseptic through the biofilm matrix. Surprisingly, the cells in the S. sobrinus biofilm were insensitive to both chlorhexidine and the extract, apparently because of the low permeability of S. sobrinus biofilms to antimicrobials: the fraction of viable cells in all layers of the biofilm varied in the range of 60–90% after 3 h of treatment with either chlorhexidine or the extract. The extracellular matrix of biofilms of different bacteria differs in biochemical composition as well as physico-chemical properties, which could affect the biofilm’s permeability to antimicrobial agents and their activity [69,70,71]. Nevertheless, the high antimicrobial activity of the hop extract against biofilm-embedded cells of three of the four studied strains suggests its potential as a promising antibiofilm agent.
Currently, to overcome microbial resistance to antimicrobial agents, complex therapy with several compounds is used. For that, essential oils, phytoextracts, and active antimicrobial components of medicinal plants are increasingly considered as antibiotic enhancers for both susceptible and resistant microorganisms [42,72]. The hop extract demonstrated high synergistic potential with antibiotics from three different groups used as model ones. Thus, the efficiency of amikacin increased against five out of the seven S. aureus strains used in this study, with a 1024-fold increase for S. aureus 73 (Table 3). Additionally, the introduction of the extract increased the susceptibility of 67% and 50% of S. aureus clinical isolates to ciprofloxacin and ceftriaxone, respectively (Table 3). The combination of the extract with antimicrobials also led to an increase in the effectiveness of the latter against Streptococci. A synergetic effect on three out of four strains (S. mutans, S. sobrinus, and S. gordonii) was shown when the extract was combined with amikacin. In the case of ciprofloxacin and ceftriaxone, the synergism of the extract was shown in relation to two (S. sobrinus, S. salivarius) and three (S. mutans, S. sobrinus, S. salivarius) species of Streptococci out of four, respectively (Table 3). Natarajan et al. showed in their study that the synergistic effect of the active components of hops and synthetic antibiotics (polymyxin B, ciprofloxacin, and tobramycin) does not depend on the structure of the bacterial cell wall [73]. It is probable that the different degree of synergy between antibiotics and the extract of hops used in our study may have been due to the physicochemical interactions of molecules, as well as the different degree of sensitivity of bacteria to the components of the extract. For example, a comparative evaluation of the synergism of the crude hop extract and xanthohumol with cefepime, ceftriaxone, ciprofloxacin, and sparfloxacin showed a synergistic or additive effect against Gram-positive bacteria when antibiotics were combined with the crude extract, while xanthohumol did not show similar results [64]. Thus, it is likely that the synergy of the extract of hops is provided by other secondary metabolites present in the phytoextract, and the leading compound remains to be elucidated.
Taken together, our data allow us to suggest the hop extract as either a solely antibacterial substance or an enhancer of various antimicrobials for oral care. It could be speculated that the observed synergistic effect of the extract with aminoglycoside, fluroquinolone, and cephalosporin antibiotics could be an indicator of a putatively similar effect with other classes of antimicrobials for systemic and topical applications. The increase in their efficiency would make it possible to decrease the required concentration of antimicrobials and thus reduce the risk of both side effects and bacterial resistance development.
4. Materials and Methods
4.1. Plant Material and Sample Preparation
The raw plant material (hops of H. lupulus from the Cannabaceae family) was harvested in Yutazy district of the Republic of Tatarstan (54°35′28.0″ N 53°16′52.5″ E) on 17 August 2022, in accordance with World Health Organization (WHO) guidelines on good agricultural and collection practices (GACP) for medicinal plants. The hops were dried at 25 °C and used for the extraction of common bioactive compounds (BAC): acylphloroglucides (APs) and prenylated flavonoids (PFs). The samples of hops corresponded to the requirements of official pharmaceutical documents (European Pharmacopoeia, 11th edition) on morphological and microscopical characteristics. The hop cones were milled using the laboratory grain mill LGM-1 (OLIS LLC, Moscow, Russia) until the particle size became less than 2 mm. The analytical samples for extraction contained crushed hops and lupulin glands.
4.2. Solvents, Chemicals, and Apparatus
Acetone and hydrochloric acid were analytical grade and purchased from JSC «EKOS-1» (Moscow, Russia). The pure deionized water was prepared using an automatic purification system (Merck Millipore, Darmstadt, Germany). Xanthohumol (purity ≥ 96% for HPLC) was purchased from Merck (Darmstadt, Germany), and its solution in ethanol (final concentration of 0.1 mg/mL) was prepared as a reference. Other reference standards for flavonoids (rutin, quercetin, and luteolin) were commercial standards from the Institute of Pharmacy of Samara State Medical University, which were authenticated by standard analytical chemistry techniques (1H, 13C NMR). Solutions of rutin, quercetin, and luteolin had a final concentration of 5 mg/mL and were stored at 4 °C, protected from light. Amikacin, ciprofloxacin, and ceftriaxone were purchased from Sigma (St. Louis, MO, USA). Stock solutions were prepared at a concentration of 20 mg/mL in deionized water.
4.3. Extraction, Isolation, and Purification of Bioactive Compounds from Hop
On the first step, hop extracts were obtained using various solvents (acetone, hexane, 95% ethanol, 70% ethanol, and chloroform) using the reflux extraction technique. Briefly, milled hop infructescence (1 g) was subjected to 15 min of reflux at boiling temperature of the solvent (10 mL) on Biosan-Grant SUB Aqua Pro, Royston, UK.
As we learned from our investigations, the best extraction efficiency has been observed when using acetone. Therefore, the next extraction procedures were performed with pure acetone. The bioactive compounds from H. lupulus were isolated and purified using preparative methods described in [74] with modifications. Briefly, the dry milled hop cones (10 g) were mixed with 150 mL of acetone and incubated at room temperature for 1 h with stirring at 200 rpm. An extract was filtered through a paper filter (5 µm), and one volume of pure water was added. Then, the pH of the mixture was adjusted to 6.0 with 1% HCl, and the solution was concentrated on a rotary evaporator (Rotavapor® R-300, Lausanne, Switzerland) at a temperature not exceeding 56 °C with a vacuum of 5 mbar and a stirring speed of about 100 rpm. The residual organic fraction was kept.
4.4. Thin-Layer Chromatography
Thin-layer chromatography (TLC) was performed on TLC plates with a size of 10 × 15 cm and a thickness of about 90–120 µm (Aluminum TLC Silica Gel Sorbfil, Krasnodar, Russia). Before TLC, a TLC chamber was pre-equilibrated for 30 min with solvent vapors by lining the chamber with filter paper moistened with mobile-phase solution (chloroform-ethanol-water; 25:18:2; v/v/v). Drops of each extract solution and working standard solutions were loaded onto the baseline of the TLC plate by syringe, and the plates were placed into the pre-equilibrated TLC chamber. After separation, TLC plates were dried and observed at 366 nm in a UV Cabinet 4 (CAMAG, Muttenz, Switzerland). The extracted compounds were visualized using derivatization by a 2% solution of aluminum chloride in ethanol, the basic chemical revelator for flavonoids.
4.5. LC-MS Analysis
Initially, 1 mL of the final extract was dissolved in 10 mL of pure water and loaded onto Hypersep C18 (Thermo Electron Corporation, 100 mg/1 mL/100 pg, Waltham, MA, USA), and after being washed by 20 mL of pure water, bound compounds were eluted in 1 mL of acetonitrile:pure water (containing 10% formic acid) with a ratio of 1:1 (v/v). The samples were analyzed by HPLC-MS on a Thermo Finnigan LCQ fleet (Themo Fisher Scientific, Waltham, MA, USA) equipped with a BDS Hypersil C 18 column (2 × 150 mm, 5 µm). Then, 1 µL of the sample was loaded, and the chromatography was performed in an isocratic mode with a flow rate of 0.8 mL/min, in a solvent system of acetonitrile-water (10% formic acid and 0.01% i-propanol) in a ratio of 1:1 (v/v) and detection on a PDA detector at 335 nm.
The mass spectrometry (ESI) was performed at 275 °C with a capillary voltage of 5 kV in helium flow (7 L/min) and the detection of positive ions (M+) in the mass range of 100–2000 (m/z). Data acquisition was carried out with the Xcalibur data system (Thermo Finnigan, Themo Fisher Scientific, Waltham, MA, USA). The compounds were identified by comparing the obtained ESI/MS spectra (retention time and molecular ion weight m/z) with those for authentic standards described in [75,76,77,78] and additionally obtained for the pure humulone and lupulone (Merck, Lebanon, NJ, USA).
4.6. Bacterial Strains and Growth Conditions
The antimicrobial activity of the extract from H. lupulus was tested on Staphylococcus aureus ATCC 29213 and six clinical isolates of S. aureus (3 MSSA and 3 MRSA) provided by the Pharmaceuticals Research Center of Kazan Federal University. To evaluate the antimicrobial activity of the extract against oral microbiota, isolates of Streptococcus mutans, Streptococcus sobrinus, Streptococcus salivarius, and Streptococcus gordonii obtained at Kazan Federal University from tooth plaques of healthy volunteers were used. S. aureus strains were cultivated in the LB medium. The LB medium supplemented with FBS (5% v/v) and glucose (2% w/v) was used for the maintenance of Streptococci. The basal medium (BM) (peptone 7g, MgSO4 × 7H2O 2.0 g and CaCl2 × 2H2O 0.05g in 1.0 L of tap water) or supplemented with FBS (5% v/v) and glucose (2% w/v) was used to obtain S. aureus or Streptococci biofilms, respectively [79,80].
4.7. Determination of Minimal Inhibitory Concentration (MIC)
The minimum inhibitory concentration (MIC) of antimicrobials was determined by serial 2-fold microdilution in 96-well plates according to the EUCAST rules for antimicrobial susceptibility testing [81] with some modifications. The final concentrations of compounds in wells were 0.05–80 µg/mL for the extract and 0.06–2048 µg/mL for the antimicrobials. The wells were seeded with microbial culture with a final density of 106 CFU/mL in LB medium, and plates were incubated at 37 °C for 24 h under static conditions. The concentration of compounds providing no bacterial growth assessed by the Alamar-blue cell viability test, with 0.1% resazurine (Applichem, Darmstadt, Germany) considered as the MIC.
4.8. Assessment of Synergy between Hop Extract and Antimicrobials
To assess the ability of extract to potentiate the activity of antimicrobials, a checkerboard assay was performed as described previously [82]. Each plate contained serial dilutions of extract and various antimicrobials in a checkerboard pattern. One of the antimicrobials was diluted horizontally and the extract vertically on a 96-well plate. The last lines and columns contained only one of the considered compounds to determine their MICs in each experiment. The initial concentration of each of the studied compounds was 2×MIC. All wells contained bacterial cultures with a final density of 106 CFU/mL in LB medium. The plates were incubated at 37 °C for 24 h. The experiments were performed in triplicate, and a growth control without the addition of any antimicrobial agent was included in each plate. After incubation, the optical density OD600 was measured on an Infinite 200 PRO plate spectrophotometer (Tecan, Männedorf, Switzerland), and the fractional inhibitory concentration index (FICI) for each double combination was calculated as described earlier [83]. Interpretation of the obtained FICI values was carried out according to Odds [84].
4.9. Biofilm Formation
Bacterial biofilms were obtained by cultivation of bacteria in BM broth in a cell imaging chambered coverslip with 8 wells (Ibidi, Gräfelfing, Germany). The wells were seeded with an 18 h-old culture of bacteria until the final concentration of 106 CFU/mL and grown for 24 h under static conditions at 37 °C.
4.10. Confocal Laser Scanning Microscopy
To assess the ability of the extract to kill bacterial cells in biofilms, confocal laser scanning microscopy was performed. For that, bacteria were grown in BM medium in a cell imaging chambered coverslip with 8 wells (Ibidi, Gräfelfing, Germany) under static conditions. After 24 h of incubation, fresh broth supplemented with extract with a final concentration equal to 2 × MIC was added. Chlorhexidine was used as the reference antiseptic. After 3 h of incubation, biofilms were stained for 15 min with 3,3′-Dihexyloxacarbocyanine iodide (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 0.02 µg/mL (green fluorescence) and propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 3 µg/mL (red fluorescence) to differentiate live and dead cells. CLSM was performed using an Olympus IX83 (Olympus Europa, Hamburg, Germany) inverted microscope supplemented with a STEDYCON ultrawide extension platform.
4.11. Data Analysis
All experiments were performed in four independent biological repeats, with three technical repeats in each run. The number of viable cells was estimated as the relative fraction of the green cells among all cells in the overlayed images obtained by confocal laser scanning microscopy. For each sample, Z-stackes were individually uploaded and analyzed by BioFilmAnalyzer software (version 1.2) [85].
5. Conclusions
Taken together, our results indicate that the extract from H. lupulus can serve as a universal antimicrobial agent and be used as both a solely substance highly active against S. aureus (both MSSA and MRSA) and an enhancer of the efficiency of various conventional antimicrobials against oral pathogenic cocci (S. aureus and S. mutans). Thus, the MIC of amikacin decreased 64-, 512-, and 1024-fold against MSSA and the cariogenic Streptococci S. sobrinus and S. mutans, respectively, and the 256-fold potentiation of ciprofloxacin and ceftriaxone was observed against S. sobrinus and S. salivarilus. Moreover, the expressed anti-biofilm activity against S. aureus (MSSA and MRSA) and S. mutans makes the hop extract a promising active component for dental products, providing treatment and prevention of caries and other periodontal diseases associated with biofilm formation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17020162/s1, Figure S1: UV-Vis spectra of the working solution of the final extract at a concentration of 40 mg/mL after purification. The absorbance (cm−c) has been measured in the wavelength range of 200–500 nm. Arrows show absorbance peaks at λ = 285 nm and λ = 334 nm that correspond to the characteristic absorption lines of APs, Figure S2: The typical LC-MS chromatogram of the extract of H. lupulus. The LC-MS was performed on the Thermo finnigan LCQ fleet equipped with a BDS hypersil C 18 2 × 150 mm column. The chromatography has been performed at isocratic flow at 0.8 mL/min in a solvent system of acetonitrile-water (10% formic acid and 0.01% i-propanol) in a ratio of 1:1 (v/v) and detection on a PDA detector at 335 nm, Table S1: The chemical structures of the main active compounds (2′,4′,6′,4-tetrahydroxy-3′-geranylchalcone and acylphloroglucides) identified in the hop extract by LC-MS.
Author Contributions
Conceptualization—A.K. (Airat Kayumov), D.S.; methodology—E.T., A.K. (Alyona Khaliullina); investigation—A.K. (Alyona Kolesnikova), E.T., A.K. (Alyona Khaliullina), L.K., S.P., O.M., P.N.; formal analysis—E.T., A.K. (Alyona Khaliullina), D.S., M.B., A.K. (Airat Kayumov); resources—E.T.; visualization—A.K. (Alyona Kolesnikova), A.K. (Alyona Khaliullina), E.T.; project administration—D.S., A.K. (Airat Kayumov); supervision—D.S., V.K., A.K. (Airat Kayumov); funding acquisition—E.T.; writing—original draft preparation, review and editing—E.T., A.K. (Alyona Khaliullina), M.B., A.K. (Airat Kayumov). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the subsidy allocated to Kazan Federal University for the state assignment in the sphere of scientific activities, project No. FZSM-2022-0017 to ET.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Local Ethics Committee of the Kazan (Volga Region) Federal University (protocol code №39 approved on 22 December 2022).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was performed in the framework of the Strategic Academic Leadership Program (PRIORITY-2030) at Kazan Federal University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, B.; González-Febles, J.; Garnier-Rodríguez, J.L.; Dadlani, S.; Bustabad-Reyes, S.; Sanz, M.; Sánchez-Alonso, F.; Sánchez-Piedra, C.; González-Dávila, E.; Díaz-González, F. Association between severity of periodontitis and clinical activity in rheumatoid arthritis patients: A case-control study. Arthritis Res. Ther. 2019, 21, 27. [Google Scholar] [CrossRef]
- Moore, W.E.; Moore, L.V. The bacteria of periodontal diseases. Periodontology 2000 1994, 5, 66–77. [Google Scholar] [CrossRef]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R.J.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Palmer, R.J.; Rickard, A.H.; Jakubovics, N.S.; Chalmers, N.I.; Diaz, P.I. Bacterial interactions and successions during plaque development. Periodontology 2000 2006, 42, 47–79. [Google Scholar] [CrossRef] [PubMed]
- Al-Shehri, S.S.; Sweeney, E.L.; Cowley, D.M.; Liley, H.G.; Ranasinghe, P.D.; Charles, B.G.; Shaw, P.N.; Vagenas, D.; Duley, J.A.; Knox, C.L. Deep sequencing of the 16S ribosomal RNA of the neonatal oral microbiome: A comparison of breast-fed and formula-fed infants. Sci. Rep. 2016, 6, 38309. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; He, X.; Shi, W. Precision Reengineering of the Oral Microbiome for Caries Management. Adv. Dent. Res. 2019, 30, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Grier, A.; Faustoferri, R.; Alzoubi, S.; Gill, A.L.; Feng, C.; Liu, Y.; Quivey, R.; Kopycka-Kedzierawski, D.; Koo, H. Association between oral candida and bacteriome in children with severe ECC. J. Dent. Res. 2018, 97, 1468–1476. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, F.; Qiu, L.; Gao, M.; Xu, P.; Zhang, L.; Liao, X.; Wang, M.; Hu, X.; Sun, Y. Ecological influence by colonization of fluoride-resistant Streptococcus mutans in oral biofilm. Front. Cell. Infect. Microbiol. 2023, 12, 1942. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Chu, C.-H.; Yu, O.Y. Oral microbiome and dental caries development. Dent. J. 2022, 10, 184. [Google Scholar] [CrossRef]
- Vasconcelos, L.C.; Sampaio, F.C.; Sampaio, M.C.; Pereira, M.O.S.; Higino, J.S.; Peixoto, M.H. Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz. Dent. J. 2006, 17, 223–227. [Google Scholar] [CrossRef]
- Zeng, L.; Burne, R.A. Sucrose- and Fructose-Specific Effects on the Transcriptome of Streptococcus mutans, as Determined by RNA Sequencing. Appl. Environ. Microbiol. 2016, 82, 146–156. [Google Scholar] [CrossRef]
- Guo, L.; Hu, W.; He, X.; Lux, R.; McLean, J.; Shi, W. Investigating acid production by Streptococcus mutans with a surface-displayed pH-sensitive green fluorescent protein. PLoS ONE 2013, 8, e57182. [Google Scholar] [CrossRef]
- Hwang, G.; Liu, Y.; Kim, D.; Sun, V.; Aviles-Reyes, A.; Kajfasz, J.K.; Lemos, J.A.; Koo, H. Simultaneous spatiotemporal mapping of in situ pH and bacterial activity within an intact 3D microcolony structure. Sci. Rep. 2016, 6, 32841. [Google Scholar] [CrossRef]
- Tanner, A.C.R.; Kressirer, C.A.; Rothmiller, S.; Johansson, I.; Chalmers, N.I. The Caries Microbiome: Implications for Reversing Dysbiosis. Adv. Dent. Res. 2018, 29, 78–85. [Google Scholar] [CrossRef]
- Daep, C.A.; Novak, E.A.; Lamont, R.J.; Demuth, D.R. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect. Immun. 2011, 79, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Nagata, H.; Yamamoto, Y.; Tanaka, M.; Tanaka, J.; Minamino, N.; Shizukuishi, S. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect. Immun. 2004, 72, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.Y.; Furtado Araujo, M.V.; Strausbaugh, L.D.; Terzi, E.; Ioannidou, E.; Diaz, P.I. Microbiome profiles in periodontitis in relation to host and disease characteristics. PLoS ONE 2015, 10, e0127077. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.; Ibrahim, S.A.; Cliver, D. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Pandey, A.; Kumar, S. Perspective on plant products as antimicrobials agents: A review. Pharmacologia 2013, 4, 469–480. [Google Scholar] [CrossRef]
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.-J.; Zhao, F.; Chrolavicius, S. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef]
- Hurtuková, K.; Fajstavrová, K.; Rimpelová, S.; Vokatá, B.; Fajstavr, D.; Kasálková, N.S.; Siegel, J.; Švorčík, V.; Slepička, P. Antibacterial Properties of a Honeycomb-like Pattern with Cellulose Acetate and Silver Nanoparticles. Materials 2021, 14, 4051. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef]
- Lewis, K.; Ausubel, F.M. Prospects for plant-derived antibacterials. Nat. Biotechnol. 2006, 24, 1504–1507. [Google Scholar] [CrossRef]
- Ody, P. The Complete Medicinal Herbal: A Practical Guide to the Healing Properties of Herbs; Simon and Schuster: New York, NY, USA, 2017. [Google Scholar]
- Ruddaraju, L.K.; Pammi, S.V.N.; Guntuku, G.S.; Padavala, V.S.; Kolapalli, V.R.M. A review on anti-bacterials to combat resistance: From ancient era of plants and metals to present and future perspectives of green nano technological combinations. Asian J. Pharm. Sci. 2020, 15, 42–59. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Joshi, R.K. A perspective on the phytopharmaceuticals responsible for the therapeutic applications. In Recent Advances in Drug Delivery Technology; IGI Global: Hershey, PA, USA, 2017; pp. 229–262. [Google Scholar]
- Wangchuk, P.; Keller, P.A.; Pyne, S.G.; Taweechotipatr, M.; Tonsomboon, A.; Rattanajak, R.; Kamchonwongpaisan, S. Evaluation of an ethnopharmacologically selected Bhutanese medicinal plants for their major classes of phytochemicals and biological activities. J. Ethnopharmacol. 2011, 137, 730–742. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Ghirga, F.; Quaglio, D.; Mori, M.; Cammarone, S.; Iazzetti, A.; Goggiamani, A.; Ingallina, C.; Botta, B.; Calcaterra, A. A unique high-diversity natural product collection as a reservoir of new therapeutic leads. Org. Chem. Front. 2021, 8, 996–1025. [Google Scholar] [CrossRef]
- Liu, L.; Yu, J.; Shen, X.; Cao, X.; Zhan, Q.; Guo, Y.; Yu, F. Resveratrol enhances the antimicrobial effect of polymyxin B on Klebsiella pneumoniae and Escherichia coli isolates with polymyxin B resistance. BMC Microbiol. 2020, 20, 306. [Google Scholar] [CrossRef]
- Kang, J.; Liu, L.; Liu, M.; Wu, X.; Li, J. Antibacterial activity of gallic acid against Shigella flexneri and its effect on biofilm formation by repressing mdoH gene expression. Food Control 2018, 94, 147–154. [Google Scholar] [CrossRef]
- Pai, B.M.; Rajesh, G.; Shenoy, R.; Rao, A. Anti-microbial efficacy of soursop leaf extract (Annona muricata) on oral pathogens: An in-vitro study. J. Clin. Diagn. Res. 2016, 10, ZC01–ZC04. [Google Scholar] [CrossRef] [PubMed]
- Hoglund, K.B.; Barnett, B.K.; Watson, S.A.; Melgarejo, M.B.; Kang, Y. Activity of bioactive garlic compounds on the oral microbiome: A literature review. Gen. Dent. 2020, 68, 27–33. [Google Scholar] [PubMed]
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A. Plant-based proteins and their multifaceted industrial applications. LWT 2022, 154, 112620. [Google Scholar] [CrossRef]
- Mitra, S.; Anand, U.; Sanyal, R.; Jha, N.K.; Behl, T.; Mundhra, A.; Ghosh, A.; Kumar, M.; Proćków, J.; Dey, A. Neoechinulins: Molecular, cellular, and functional attributes as promising therapeutics against cancer and other human diseases. Biomed. Pharmacother. 2022, 145, 112378. [Google Scholar] [CrossRef]
- Sasi, M.; Kumar, S.; Kumar, M.; Thapa, S.; Prajapati, U.; Tak, Y.; Changan, S.; Saurabh, V.; Kumari, S.; Kumar, A. Garlic (Allium sativum L.) bioactives and its role in alleviating oral pathologies. Antioxidants 2021, 10, 1847. [Google Scholar] [CrossRef]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 195. [Google Scholar] [CrossRef]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic antimicrobial activity of Camellia sinensis and Juglans regia against multidrug-resistant bacteria. PLoS ONE 2015, 10, e0118431. [Google Scholar] [CrossRef] [PubMed]
- Marquez, B.; Neuville, L.; Moreau, N.J.; Genet, J.-P.; Dos Santos, A.F.; De Andrade, M.C.C.; Sant’Ana, A.E.G. Multidrug resistance reversal agent from Jatropha elliptica. Phytochemistry 2005, 66, 1804–1811. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.Y.; Trizna, E.Y.; Sulaiman, R.K.; Pavelyev, R.S.; Gilfanov, I.R.; Lisovskaya, S.A.; Ostolopovskaya, O.V.; Frolova, L.L.; Kutchin, A.V.; Guseva, G.B. Increasing the Efficacy of Treatment of Staphylococcus aureus–Candida albicans Mixed Infections with Myrtenol. Antibiotics 2022, 11, 1743. [Google Scholar] [CrossRef]
- Moir, M. Hops—A millennium review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G. Empirical prediction and validation of antibacterial inhibitory effects of various plant essential oils on common pathogenic bacteria. Int. J. Food Microbiol. 2015, 202, 35–41. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. Antifungal activities of hop bitter resins and related compounds. Agric. Biol. Chem. 1985, 49, 399–403. [Google Scholar]
- Alonso-Esteban, J.I.; Pinela, J.; Barros, L.; Ćirić, A.; Soković, M.; Calhelha, R.C.; Torija-Isasa, E.; de Cortes Sánchez-Mata, M.; Ferreira, I.C. Phenolic composition and antioxidant, antimicrobial and cytotoxic properties of hop (Humulus lupulus L.) Seeds. Ind. Crops Prod. 2019, 134, 154–159. [Google Scholar] [CrossRef]
- Gerhäuser, C. Beer constituents as potential cancer chemopreventive agents. Eur. J. Cancer 2005, 41, 1941–1954. [Google Scholar] [CrossRef]
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.L.; Zhang, R.; et al. Xanthohumol isolated from Humulus lupulus prevents thrombosis without increased bleeding risk by inhibiting platelet activation and mtDNA release. Free Radic. Biol. Med. 2017, 108, 247–257. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Satoh-Yamaguchi, K.; Ono, M. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Miyajima, F.; Roberts, P.; Ellison, L.; Pickard, D.J.; Martin, M.J.; Connor, T.R.; Harris, S.R.; Fairley, D.; Bamford, K.B.; et al. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat. Genet. 2013, 45, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, C.; Menten, J.F.M.; Pereira, R.; Fagundes, N.S.; Napty, G.; Pedroso, A.; Bigaton, A.D.; Andreote, F.D. Hops β-acids and zinc bacitracin affect the performance and intestinal microbiota of broilers challenged with Eimeria acervulina and Eimeria tenella. Anim. Feed Sci. Technol. 2015, 207, 181–189. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Virani, S.; Zavro, M.; Haas, G.J. Inhibition of Streptococcus mutans and Other Oral streptococci by hop (Humulus lupulus L.) constituents. Econ. Bot. 2003, 57, 118–125. [Google Scholar] [CrossRef]
- Mani, A.; Mahalingam, G. Effect of anti-biofilm potential of different medicinal plants: Review. Asian J. Pharm. Clin. Res. 2017, 10, 24–32. [Google Scholar]
- Brookes, Z.L.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current uses of chlorhexidine for management of oral disease: A narrative review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Verspecht, T.; Rodriguez Herrero, E.; Khodaparast, L.; Khodaparast, L.; Boon, N.; Bernaerts, K.; Quirynen, M.; Teughels, W. Development of antiseptic adaptation and cross-adapatation in selected oral pathogens in vitro. Sci. Rep. 2019, 9, 8326. [Google Scholar] [CrossRef]
- Deschepper, M.; Waegeman, W.; Eeckloo, K.; Vogelaers, D.; Blot, S. Effects of chlorhexidine gluconate oral care on hospital mortality: A hospital-wide, observational cohort study. Intensive Care Med. 2018, 44, 1017–1026. [Google Scholar] [CrossRef]
- Čermák, P.; Palečková, V.; Houška, M.; Strohalm, J.; Novotna, P.; Mikyška, A.; Jurkova, M.; Sikorova, M. Inhibitory effects of fresh hops on Helicobacter pylori strains. Czech J. Food Sci. 2015, 33, 302–307. [Google Scholar] [CrossRef]
- Stompor, M.; Żarowska, B. Antimicrobial Activity of Xanthohumol and Its Selected Structural Analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef]
- Di Lodovico, S.; Menghini, L.; Ferrante, C.; Recchia, E.; Castro-Amorim, J.; Gameiro, P.; Cellini, L.; Bessa, L.J. Hop Extract: An Efficacious Antimicrobial and Anti-biofilm Agent against Multidrug-Resistant Staphylococci Strains and. Front. Microbiol. 2020, 11, 1852. [Google Scholar] [CrossRef]
- Chin, Y.C.; Chang, N.C.; Anderson, H.H. Factors Influencing the Antibiotic Activity of Lupulon. J. Clin. Investig. 1949, 28 Pt 1, 909–915. [Google Scholar] [CrossRef]
- Roehrer, S.; Behr, J.; Stork, V.; Ramires, M.; Médard, G.; Frank, O.; Kleigrewe, K.; Hofmann, T.; Minceva, M. Xanthohumol C, a minor bioactive hop compound: Production, purification strategies and antimicrobial test. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1095, 39–49. [Google Scholar] [CrossRef]
- Simpson, W.J.; Smith, A.R. Factors affecting antibacterial activity of hop compounds and their derivatives. J. Appl. Bacteriol. 1992, 72, 327–334. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Palmer, R.J.; Periasamy, S.; Jakubovics, N.S. Oral multispecies biofilm development and the key role of cell-cell distance. Nat. Rev. Microbiol. 2010, 8, 471–480. [Google Scholar] [CrossRef]
- Bogdanova, K.; Röderova, M.; Kolar, M.; Langova, K.; Dusek, M.; Jost, P.; Kubelkova, K.; Bostik, P.; Olsovska, J. Antibiofilm activity of bioactive hop compounds humulone, lupulone and xanthohumol toward susceptible and resistant staphylococci. Res. Microbiol. 2018, 169, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Mironova, A.V.; Karimova, A.V.; Bogachev, M.I.; Kayumov, A.R.; Trizna, E.Y. Alterations in Antibiotic Susceptibility of Staphylococcus aureus and Klebsiella pneumoniae in Dual Species Biofilms. Int. J. Mol. Sci. 2023, 24, 8475. [Google Scholar] [CrossRef] [PubMed]
- Cendra, M.D.M.; Blanco-Cabra, N.; Pedraz, L.; Torrents, E. Optimal environmental and culture conditions allow the in vitro coexistence of Pseudomonas aeruginosa and Staphylococcus aureus in stable biofilms. Sci. Rep. 2019, 9, 16284. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.C.; Rice, S.A. Influence of interspecies interactions on the spatial organization of dual species bacterial communities. Biofilm 2020, 2, 100035. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, S.; Oluwatuyi, M.; Veitch, N.C.; Gray, A.I. Bacterial resistance modifying agents from Lycopus europaeus. Phytochemistry 2003, 62, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, P.; Katta, S.; Andrei, I.; Babu Rao Ambati, V.; Leonida, M.; Haas, G.J. Positive antibacterial co-action between hop (Humulus lupulus) constituents and selected antibiotics. Phytomedicine 2008, 15, 194–201. [Google Scholar] [CrossRef]
- Gagos, M. Process for Preparation of Xanthohumol. Patent US9556097B2, 31 January 2017. [Google Scholar]
- Zhang, X.; Liang, X.; Xiao, H.; Xu, Q. Direct characterization of bitter acids in a crude hop extract by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2004, 15, 180–187. [Google Scholar] [CrossRef]
- Santos, S.A.; Freire, C.S.; Domingues, M.R.M.; Silvestre, A.J.; Neto, C.P. Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography–mass spectrometry. J. Agric. Food Chem. 2011, 59, 9386–9393. [Google Scholar] [CrossRef]
- Negri, G.; Di Santi, D.; Tabach, R. Bitter acids from hydroethanolic extracts of Humulus lupulus L., Cannabaceae, used as anxiolytic. Rev. Bras. Farmacogn. 2010, 20, 850–859. [Google Scholar] [CrossRef]
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus–a story that begs to be told. A review. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar]
- Kayumov, A.R.; Khakimullina, E.N.; Sharafutdinov, I.S.; Trizna, E.Y.; Latypova, L.Z.; Lien, H.T.; Margulis, A.B.; Bogachev, M.I.; Kurbangalieva, A.R. Inhibition of biofilm formation in Bacillus subtilis by new halogenated furanones. J. Antibiot. 2015, 68, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Trizna, E.Y.; Khakimullina, E.N.; Latypova, L.Z.; Kurbangalieva, A.R.; Sharafutdinov, I.S.; Evtyugin, V.G.; Babynin, E.V.; Bogachev, M.I.; Kayumov, A.R. Thio Derivatives of 2(5H)-Furanone As Inhibitors against Bacillus subtilis Biofilms. Acta Naturae 2015, 7, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Canton, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Sharafutdinov, I.S.; Trizna, E.Y.; Baidamshina, D.R.; Ryzhikova, M.N.; Sibgatullina, R.R.; Khabibrakhmanova, A.M.; Latypova, L.Z.; Kurbangalieva, A.R.; Rozhina, E.V.; Klinger-Strobel, M.; et al. Antimicrobial Effects of Sulfonyl Derivative of 2(5H)-Furanone against Planktonic and Biofilm Associated Methicillin-Resistant and -Susceptible Staphylococcus aureus. Front. Microbiol. 2017, 8, 2246. [Google Scholar] [CrossRef]
- Sulaiman, R.; Trizna, E.; Kolesnikova, A.; Khabibrakhmanova, A.; Kurbangalieva, A.; Bogachev, M.; Kayumov, A. Antimicrobial and Biofilm-Preventing Activity of l-Borneol Possessing 2 (5 H)-Furanone Derivative F131 against S. aureus—C. albicans Mixed Cultures. Pathogens 2022, 12, 26. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Bogachev, M.I.; Volkov, V.Y.; Markelov, O.A.; Trizna, E.Y.; Baydamshina, D.R.; Melnikov, V.; Murtazina, R.R.; Zelenikhin, P.V.; Sharafutdinov, I.S.; Kayumov, A.R. Fast and simple tool for the quantification of biofilm-embedded cells sub-populations from fluorescent microscopic images. PLoS ONE 2018, 13, e0193267. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).