Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database
Abstract
1. Introduction
2. Results
2.1. Descriptive Characteristics of Individual Case Safety Reports
2.2. Descriptive Characteristics of Suicidal Events
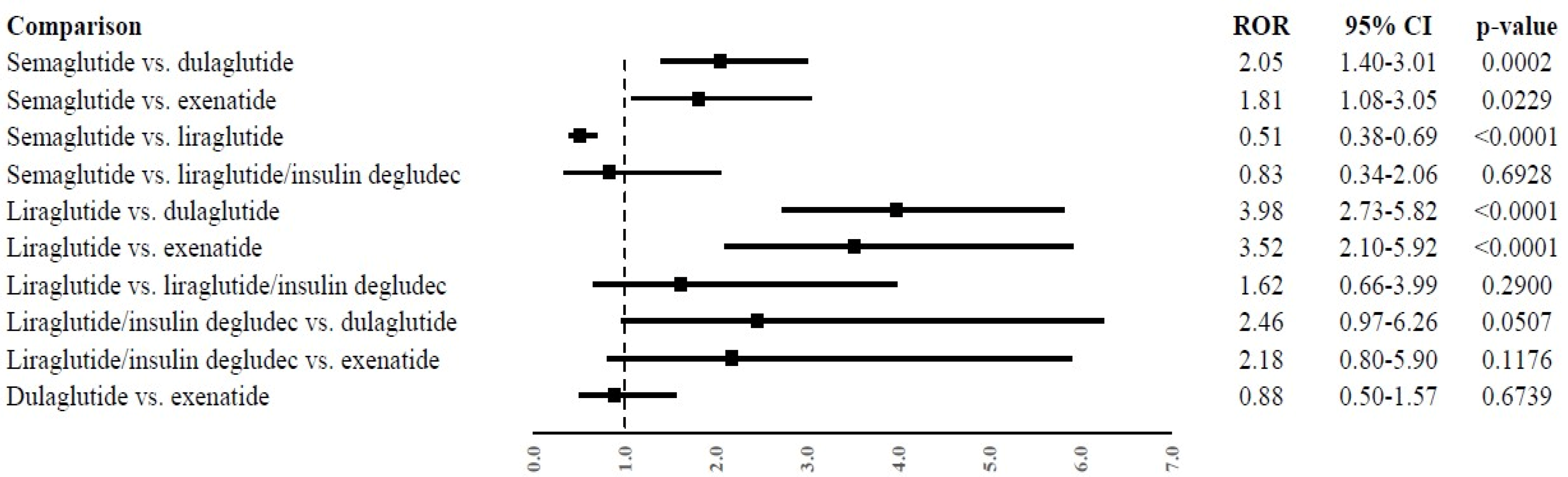
2.3. Disproportionality Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Data Source
4.3. Data Retrieval
4.4. Data Management
4.5. Descriptive Analysis
4.6. Disproportionality Analysis
4.7. Ethical Statement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Medicine Agency. EMA Statement on Ongoing Review of GLP-1 Receptor Agonists. Available online: https://www.ema.europa.eu/en/news/ema-statement-ongoing-review-glp-1-receptor-agonists (accessed on 31 July 2023).
- Górriz, J.L.; Romera, I.; Cobo, A.; O’Brien, P.D.; Merino-Torres, J.F. Glucagon-like Peptide-1 Receptor Agonist Use in People Living with Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Narrative Review of the Key Evidence with Practical Considerations. Diabetes Ther. 2022, 13, 389–421. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C. Management of Hyperglycaemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes—State-of-the-Art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. GLP-1 Physiology Informs the Pharmacotherapy of Obesity. Mol. Metab. 2022, 57, 101351. [Google Scholar] [CrossRef]
- Chiappini, S.; Vickers-Smith, R.; Harris, D.; Pelletier, G.D.P.; Corkery, J.M.; Guirguis, A.; Martinotti, G.; Sensi, S.L.; Schifano, F. Is There a Risk for Semaglutide Misuse? Focus on the Food and Drug Administration’s FDA Adverse Events Reporting System (FAERS) Pharmacovigilance Dataset. Pharmaceuticals 2023, 16, 994. [Google Scholar] [CrossRef]
- Ozempic: French Authorities. Issue Alert for Anti-Diabetic Drug Misused for Weight Loss. Available online: https://www.lemonde.fr/en/health/article/2023/03/02/ozempic-french-authorities-issue-alert-for-anti-diabetic-drug-misused-for-weight-loss_6017913_14.html (accessed on 1 August 2023).
- Italian Medicines Agency. Direct Healthcare Professional Communications Regarding Ozempic® (Semaglutide). 2023. Available online: https://aifa.gov.it/-/nota-informativa-importante-su-ozempic®-semaglutide- (accessed on 1 January 2024).
- McIntyre, R.S.; Mansur, R.B.; Rosenblat, J.D.; Kwan, A.T.H. The Association between Glucagon-like Peptide-1 Receptor Agonists (GLP-1 RAs) and Suicidality: Reports to the Food and Drug Administration Adverse Event Reporting System (FAERS). Expert. Opin. Drug Saf. 2023, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Volkow, N.D.; Berger, N.A.; Davis, P.B.; Kaelber, D.C.; Xu, R. Association of Semaglutide with Risk of Suicidal Ideation in a Real-World Cohort. Nat. Med. 2024. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, R.; Fu, F.; Xiao, J. Postmarket Safety Profile of Suicide/Self-Injury for GLP-1 Receptor Agonist: A Real-World Pharmacovigilance Analysis. Eur. Psychiatry 2023, 66, e99. [Google Scholar] [CrossRef]
- Lucas, S.; Ailani, J.; Smith, T.R.; Abdrabboh, A.; Xue, F.; Navetta, M.S. Pharmacovigilance: Reporting Requirements throughout a Product’s Lifecycle. Ther. Adv. Drug Saf. 2022, 13. [Google Scholar] [CrossRef]
- European Medicines Agency. Saxenda. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda (accessed on 2 August 2023).
- European Medicines Agency. Wegovy. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/wegovy (accessed on 2 August 2023).
- Kang, J.G.; Park, C.Y. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab. J. 2012, 36, 13–25. [Google Scholar] [CrossRef]
- Rodgers, R.J.; Tschöp, M.H.; Wilding, J.P.H. Anti-Obesity Drugs: Past, Present and Future. Dis. Model. Mech. 2012, 5, 621–626. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, P.M.; Aroda, V.R.; Astrup, A.; Kushner, R.; Lau, D.C.W.; Wadden, T.A.; Brett, J.; Cancino, A.P.; Wilding, J.P.H. Neuropsychiatric Safety with Liraglutide 3.0 Mg for Weight Management: Results from Randomized Controlled Phase 2 and 3a Trials. Diabetes Obes. Metab. 2017, 19, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Blonde, L.; Russell-Jones, D. The Safety and Efficacy of Liraglutide with or without Oral Antidiabetic Drug Therapy in Type 2 Diabetes: An Overview of the LEAD 1-5 Studies. Diabetes Obes. Metab. 2009, 11 (Suppl. S3), 26–34. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. Drug Ther. Bull. 2016, 54, 101. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Admistration. Saxenda—Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/206321s016lbl.pdf (accessed on 12 January 2024).
- Food and Drug Administration. Wegovy—Highlights of Prescribing Information. 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215256s005lbl.pdf (accessed on 12 January 2024).
- Wisłowska-Stanek, A.; Kołosowska, K.; Maciejak, P. Neurobiological Basis of Increased Risk for Suicidal Behaviour. Cells 2021, 10, 2519. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, O.Y.; Song, J. Alleviation of Depression by Glucagon-Like Peptide 1 Through the Regulation of Neuroinflammation, Neurotransmitters, Neurogenesis, and Synaptic Function. Front. Pharmacol. 2020, 11, 1270. [Google Scholar] [CrossRef]
- Anderberg, R.H.; Richard, J.E.; Eerola, K.; López-Ferreras, L.; Banke, E.; Hansson, C.; Nissbrandt, H.; Berqquist, F.; Gribble, F.M.; Reimann, F.; et al. Glucagon-like Peptide 1 and Its Analogs Act in the Dorsal Raphe and Modulate Central Serotonin to Reduce Appetite and Body Weight. Diabetes 2017, 66, 1062–1073. [Google Scholar] [CrossRef]
- Chivite, M.; Naderi, F.; Conde-Sieira, M.; Soengas, J.L.; Lopez-Patiño, M.A.; Míguez, J.M. Central Serotonin Participates in the Anorexigenic Effect of GLP-1 in Rainbow Trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2021, 304, 113716. [Google Scholar] [CrossRef]
- Suicide. Available online: https://www.who.int/news-room/fact-sheets/detail/suicide (accessed on 2 August 2023).
- Rossi, C.; Ruggiero, R.; Sportiello, L.; Pentella, C.; Gaio, M.; Pinto, A.; Rafaniello, C. Did the COVID-19 Pandemic Affect Contrast Media-Induced Adverse Drug Reaction’s Reporting? A Pharmacovigilance Study in Southern Italy. J. Clin. Med. 2022, 11, 5104. [Google Scholar] [CrossRef]
- Ruggiero, R.; Balzano, N.; Di Napoli, R.; Mascolo, A.; Berrino, P.M.; Rafaniello, C.; Sportiello, L.; Rossi, F.; Capuano, A. Capillary Leak Syndrome Following COVID-19 Vaccination: Data from the European Pharmacovigilance Database Eudravigilance. Front. Immunol. 2022, 13, 956825. [Google Scholar] [CrossRef]
- Sher, L.; Oquendo, M.A. Suicide: An Overview for Clinicians. Med. Clin. N. Am. 2023, 107, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Iwatate, E.; Atem, F.D.; Jones, E.C.; Hughes, J.L.; Yokoo, T.; Messiah, S.E. Association of Obesity, Suicide Behaviors, and Psychosocial Wellness Among Adolescents in the United States. J. Adolesc. Health 2023, 72, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Balhara, Y.P.S. Diabetes Mellitus and Suicide. Indian. J. Endocrinol. Metab. 2014, 18, 468. [Google Scholar] [CrossRef] [PubMed]
- Conti, C.; Mennitto, C.; Di Francesco, G.; Fraticelli, F.; Vitacolonna, E.; Fulcheri, M. Clinical Characteristics of Diabetes Mellitus and Suicide Risk. Front. Psychiatry 2017, 8, 248999. [Google Scholar] [CrossRef]
- Amiri, S.; Behnezhad, S. Body Mass Index and Risk of Suicide: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2018, 238, 615–625. [Google Scholar] [CrossRef]
- Klinitzke, G.; Steinig, J.; Blüher, M.; Kersting, A.; Wagner, B. Obesity and Suicide Risk in Adults—A Systematic Review. J. Affect. Disord. 2013, 145, 277–284. [Google Scholar] [CrossRef]
- Branco, J.C.; Motta, J.; Wiener, C.; Oses, J.P.; Pedrotti Moreira, F.; Spessato, B.; Dias, L.; da Silva, R. Association between Obesity and Suicide in Woman, but Not in Man: A Population-Based Study of Young Adults. Psychol. Health Med. 2016, 22, 275–281. [Google Scholar] [CrossRef]
- Ju, Y.J.; Han, K.T.; Lee, T.H.; Kim, W.; Park, J.H.; Park, E.C. Association between Weight Control Failure and Suicidal Ideation in Overweight and Obese Adults: A Cross-Sectional Study. BMC Public. Health 2016, 16, 259. [Google Scholar] [CrossRef]
- Klein, P.; Devinsky, O.; French, J.; Harden, C.; Krauss, G.L.; McCarter, R.; Sperling, M.R. Suicidality Risk of Newer Antiseizure Medications: A Meta-Analysis. JAMA Neurol. 2021, 78, 1118–1127. [Google Scholar] [CrossRef]
- Mula, M.; Kanner, A.M.; Schmitz, B.; Schachter, S. Antiepileptic Drugs and Suicidality: An Expert Consensus Statement from the Task Force on Therapeutic Strategies of the ILAE Commission on Neuropsychobiology. Epilepsia 2013, 54, 199–203. [Google Scholar] [CrossRef]
- Bell, G.S.; Mula, M.; Sander, J.W. Suicidality in People Taking Antiepileptic Drugs What Is the Evidence? CNS Drugs 2009, 23, 281–292. [Google Scholar] [CrossRef]
- Mula, M. Suicidality and Antiepileptic Drugs in People with Epilepsy: An Update. Expert Rev. Neurother. 2022, 22, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Pop, G.; Farcaș, A.; Butucă, A.; Morgovan, C.; Arseniu, A.M.; Pumnea, M.; Teodoru, M.; Gligor, F.G. Post-Marketing Surveillance of Statins—A Descriptive Analysis of Psychiatric Adverse Reactions in EudraVigilance. Pharmaceuticals 2022, 15, 1536. [Google Scholar] [CrossRef] [PubMed]
- Fong, P.; Tong Chan, S.; Nap Lei, P.; Ian Cheong, H.; Man Cheong, I.; Lam Hoe, W. Association of Suicidal Ideation and Depression with the Use of Proton Pump Inhibitors in Adults: A Cross-Sectional Study. Sci. Rep. 2022, 12, 19539. [Google Scholar] [CrossRef] [PubMed]
- Mamdani, M.; Gomes, T.; Greaves, S.; Manji, S.; Juurlink, D.N.; Tadrous, M.; Kennedy, S.H.; Antoniou, T. Association Between Angiotensin-Converting Enzyme Inhibitors, Angiotensin Receptor Blockers, and Suicide. JAMA Netw. Open 2019, 2, e1913304. [Google Scholar] [CrossRef] [PubMed]
- Herdeiro, M.T.; Figueiras, A.; Polónia, J.; Gestal-Otero, J.J. Physicians’ Attitudes and Adverse Drug Reaction Reporting A Case-Control Study in Portugal. Drug Saf. 2005, 28, 825–833. [Google Scholar] [CrossRef]
- Lopez-Gonzalez, E.; Herdeiro, M.T.; Figueiras, A. Determinants of Under-Reporting of Adverse Drug Reactions A Systematic Review. Drug Saf. 2009, 32, 19–31. [Google Scholar] [CrossRef]
- García-Abeijon, P.; Costa, C.; Taracido, M.; Herdeiro, M.T.; Torre, C.; Figueiras, A. Factors Associated with Underreporting of Adverse Drug Reactions by Health Care Professionals: A Systematic Review Update. Drug Saf. 2023, 46, 625–636. [Google Scholar] [CrossRef]
- Goldman, S.A. Limitations and Strengths of Spontaneous Reports Data. Clin. Ther. 1998, 20, C40–C44. [Google Scholar] [CrossRef]
- Ferrajolo, C.; Capuano, A.; Trifirò, G.; Moretti, U.; Rossi, F.; Santuccio, C. Pediatric Drug Safety Surveillance in Italian Pharmacovigilance Network: An Overview of Adverse Drug Reactions in the Years 2001–2012. Expert Opin. Drug Saf. 2014, 13, 939581. [Google Scholar] [CrossRef]
- Ferrajolo, C.; Arcoraci, V.; Sullo, M.G.; Rafaniello, C.; Sportiello, L.; Ferrara, R.; Cannata, A.; Pagliaro, C.; Tari, M.G.; Patrizio Caputi, A.; et al. Pattern of Statin Use in Southern Italian Primary Care: Can Prescription Databases Be Used for Monitoring Long-Term Adherence to the Treatment? PLoS ONE 2014, 9, e102146. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Eperzan. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/eperzan (accessed on 2 August 2023).
- Zhang, F.; Chen, Z.; Wu, D.; Zhou, Z.; Du, Z.; Hu, F. Recombinant Human GLP-1 Beinaglutide Regulates Lipid Metabolism of Adipose Tissues in Diet-Induced Obese Mice. iScience 2021, 24, 103382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhou, C.; Li, X.F.; Yang, M.N.; Tao, L.; Zheng, X.Y.; Jia, Y.S. Beinaglutide Showed Significant Weight-Loss Benefit and Effective Glycaemic Control for the Treatment of Type 2 Diabetes in a Real-World Setting: A 3-Month, Multicentre, Observational, Retrospective, Open-Label Study. Obes. Sci. Pract. 2019, 5, 366–375. [Google Scholar] [CrossRef] [PubMed]
| Dulaglutide (n = 37) | Exenatide (n = 16) | Liraglutide (n = 88) | Liraglutide/Insulin Degludec (n = 5) | Semaglutide (n = 84) | Overall (n = 230) | |
|---|---|---|---|---|---|---|
| Age group | ||||||
| 12–17 years | 0 (0%) | 1 (6.3%) | 5 (5.7%) | 0 (0%) | 0 (0%) | 6 (2.6%) |
| 18–64 years | 19 (51.4%) | 5 (31.3%) | 54 (61.4%) | 4 (80.0%) | 52 (61.9%) | 134 (58.3%) |
| 65–85 years | 10 (27.0%) | 2 (12.5%) | 9 (10.2%) | 1 (20.0%) | 4 (4.8%) | 26 (11.3%) |
| Not specified | 8 (21.6%) | 8 (50.0%) | 20 (22.7%) | 0 (0%) | 28 (33.3%) | 64 (27.8%) |
| Sex | ||||||
| Female | 18 (48.6%) | 7 (43.8%) | 60 (68.2%) | 0 (0%) | 48 (57.1%) | 133 (57.8%) |
| Male | 17 (45.9%) | 9 (56.3%) | 28 (31.8%) | 5 (100%) | 29 (34.5%) | 88 (38.3%) |
| Not specified | 2 (5.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (8.3%) | 9 (3.9%) |
| Reporter | ||||||
| Healthcare professional | 26 (70.3%) | 5 (31.3%) | 64 (72.7%) | 5 (100%) | 51 (60.7%) | 151 (65.7%) |
| Non-healthcare professional | 11 (29.7%) | 11 (68.8%) | 24 (27.3%) | 0 (0%) | 33 (39.3%) | 79 (34.3%) |
| Country | ||||||
| European Economic Area | 6 (16.2%) | 0 (0%) | 14 (15.9%) | 3 (60.0%) | 16 (19.0%) | 39 (17.0%) |
| Non-European Economic Area | 31 (83.8%) | 16 (100%) | 74 (84.1%) | 2 (40.0%) | 68 (81.0%) | 191 (83.0%) |
| Concomitant drugs | ||||||
| 0 | 22 (59.5%) | 2 (12.5%) | 62 (70.5%) | 2 (40.0%) | 64 (76.2%) | 152 (66.1%) |
| 1 | 7 (18.9%) | 0 (0%) | 5 (5.7%) | 1 (20.0%) | 5 (6.0%) | 18 (7.8%) |
| 2 | 3 (8.1%) | 0 (0%) | 10 (11.4%) | 0 (0%) | 5 (6.0%) | 18 (7.8%) |
| 3 | 3 (8.1%) | 3 (18.8%) | 5 (5.7%) | 0 (0%) | 1 (1.2%) | 12 (5.2%) |
| 4 | 0 (0%) | 6 (37.5%) | 0 (0%) | 0 (0%) | 2 (2.4%) | 8 (3.5%) |
| 5 | 2 (5.4%) | 5 (31.3%) | 6 (6.8%) | 2 (40.0%) | 7 (8.3%) | 22 (9.6%) |
| Suspected drugs | ||||||
| 1 | 21 (56.8%) | 7 (43.8%) | 62 (70.5%) | 1 (20.0%) | 75 (89.3%) | 166 (72.2%) |
| 2 | 11 (29.7%) | 5 (31.3%) | 18 (20.5%) | 4 (80.0%) | 7 (8.3%) | 45 (19.6%) |
| 3 | 3 (8.1%) | 0 (0%) | 2 (2.3%) | 0 (0%) | 2 (2.4%) | 7 (3.0%) |
| 4 | 2 (5.4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.9%) |
| 5 | 0 (0%) | 4 (25.0%) | 6 (6.8%) | 0 (0%) | 0 (0%) | 10 (4.3%) |
| GLP1 RA 1 therapeutic indication | ||||||
| Diabetes mellitus | 24 (64.9%) | 7 (43.8%) | 9 (10.2%) | 3 (60.0%) | 20 (23.8%) | 63 (27.4%) |
| Unknown | 13 (35.1%) | 5 (31.3%) | 47 (53.4%) | 1 (20.0%) | 46 (54.8%) | 112 (48.7%) |
| Blood glucose control | 0 (0%) | 3 (18.8%) | 1 (1.1%) | 0 (0%) | 0 (0%) | 4 (1.7%) |
| Weight control | 0 (0%) | 1 (6.3%) | 19 (21.6%) | 0 (0%) | 12 (14.3%) | 32 (13.9%) |
| Obesity | 0 (0%) | 0 (0%) | 10 (11.4%) | 0 (0%) | 2 (2.4%) | 12 (5.2%) |
| Polycystic ovaries, weight control | 0 (0%) | 0 (0%) | 1 (1.1%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Suicide attempt | 0 (0%) | 0 (0%) | 1 (1.1%) | 1 (20.0%) | 0 (0%) | 2 (0.9%) |
| Diabetes mellitus, overweight/Obesity | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (3.6%) | 3 (1.3%) |
| Polycystic ovaries | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.2%) | 1 (0.4%) |
| Dulaglutide (n = 38) | Exenatide (n = 17) | Liraglutide (n = 90) | Liraglutide/Insulin Degludec (n = 5) | Semaglutide (n = 86) | Overall (n = 236) | Female (n = 137) | Male (n = 90) | Not Specified (n = 9) | |
|---|---|---|---|---|---|---|---|---|---|
| Events | |||||||||
| Completed suicide | 2 (5.3%) | 1 (5.9%) | 5 (5.6%) | 0 (0%) | 3 (3.5%) | 11 (4.7%) | 2 (1.5%) | 9 (10.0%) | 0 (0%) |
| Depression suicidal | 2 (5.3%) | 1 (5.9%) | 5 (5.6%) | 0 (0%) | 9 (10.5%) | 17 (7.2%) | 11 (8.0%) | 6 (6.7%) | 0 (0%) |
| Suicidal behaviour | 2 (5.3%) | 2 (11.8%) | 1 (1.1%) | 0 (0%) | 0 (0%) | 5 (2.1%) | 2 (1.5%) | 3 (3.3%) | 0 (0%) |
| Suicidal ideation | 17 (44.7%) | 10 (58.8%) | 60 (66.7%) | 0 (0%) | 67 (77.9%) | 154 (65.3%) | 99 (72.3%) | 47 (52.2%) | 8 (88.9%) |
| Suicide attempt | 15 (39.5%) | 3 (17.6%) | 16 (17.8%) | 5 (100%) | 7 (8.1%) | 46 (19.5%) | 22 (16.1%) | 23 (25.6%) | 1 (11.1%) |
| Suspected suicide | 0 (0%) | 0 (0%) | 3 (3.3%) | 0 (0%) | 0 (0%) | 3 (1.3%) | 1 (0.7%) | 2 (2.2%) | 0 (0%) |
| Dulaglutide (n = 38) | Exenatide (n = 17) | Liraglutide (n = 90) | Liraglutide/Insulin Degludec (n = 5) | Semaglutide (n = 86) | Overall (n = 236) | |
|---|---|---|---|---|---|---|
| Seriousness Criteria | ||||||
| Caused/prolonged hospitalization | 10 (26.3%) | 5 (29.4%) | 10 (11.1%) | 4 (80.0%) | 8 (9.3%) | 37 (15.7%) |
| Life-threatening | 3 (7.9%) | 4 (23.5%) | 6 (6.7%) | 1 (20.0%) | 6 (7.0%) | 20 (8.5%) |
| Other medically important condition | 23 (60.5%) | 7 (41.2%) | 63 (70.0%) | 0 (0%) | 68 (79.1%) | 161 (68.2%) |
| Results in death | 2 (5.3%) | 1 (5.9%) | 8 (8.9%) | 0 (0%) | 3 (3.5%) | 14 (5.9%) |
| Not reported | 0 (0%) | 0 (0%) | 1 (1.1%) | 0 (0%) | 0 (0%) | 1 (0.4%) |
| Disabling | 0 (0%) | 0 (0%) | 2 (2.2%) | 0 (0%) | 1 (1.2%) | 3 (1.3%) |
| Outcome | ||||||
| Fatal | 2 (5.3%) | 1 (5.9%) | 8 (8.9%) | 0 (0%) | 3 (3.5%) | 14 (5.9%) |
| Not Recovered/not resolved | 1 (2.6%) | 1 (5.9%) | 9 (10.0%) | 0 (0%) | 13 (15.1%) | 24 (10.2%) |
| Recovered/resolved | 11 (28.9%) | 2 (11.8%) | 26 (28.9%) | 2 (40.0%) | 33 (38.4%) | 74 (31.4%) |
| Recovering/resolving | 3 (7.9%) | 0 (0%) | 8 (8.9%) | 0 (0%) | 6 (7.0%) | 17 (7.2%) |
| Unknown | 21 (55.3%) | 13 (76.5%) | 39 (43.3%) | 3 (60.0%) | 30 (34.9%) | 106 (44.9%) |
| Recovered/resolved with sequelae | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.2%) | 1 (0.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruggiero, R.; Mascolo, A.; Spezzaferri, A.; Carpentieri, C.; Torella, D.; Sportiello, L.; Rossi, F.; Paolisso, G.; Capuano, A. Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database. Pharmaceuticals 2024, 17, 147. https://doi.org/10.3390/ph17020147
Ruggiero R, Mascolo A, Spezzaferri A, Carpentieri C, Torella D, Sportiello L, Rossi F, Paolisso G, Capuano A. Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database. Pharmaceuticals. 2024; 17(2):147. https://doi.org/10.3390/ph17020147
Chicago/Turabian StyleRuggiero, Rosanna, Annamaria Mascolo, Angela Spezzaferri, Claudia Carpentieri, Daniele Torella, Liberata Sportiello, Francesco Rossi, Giuseppe Paolisso, and Annalisa Capuano. 2024. "Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database" Pharmaceuticals 17, no. 2: 147. https://doi.org/10.3390/ph17020147
APA StyleRuggiero, R., Mascolo, A., Spezzaferri, A., Carpentieri, C., Torella, D., Sportiello, L., Rossi, F., Paolisso, G., & Capuano, A. (2024). Glucagon-like Peptide-1 Receptor Agonists and Suicidal Ideation: Analysis of Real-Word Data Collected in the European Pharmacovigilance Database. Pharmaceuticals, 17(2), 147. https://doi.org/10.3390/ph17020147







