Abstract
Tenofovir disoproxil fumarate (TDF) is a widely used pharmacological agent for the treatment of human immunodeficiency virus infection. While prolonged exposure to TDF has been associated with a decrease in bone mineral density (BMD) and increased fracture risk, limited discussion exists on its effects on various aspects of bone quality. This scoping review aims to provide a comprehensive overview of the impact of TDF on bone quality beyond BMD. A literature search was conducted using the PubMed and Scopus databases to identify studies investigating the effects of TDF on bone quality. Original research articles written in English, irrespective of study type or publication year, were included in the review. Seven articles met the inclusion criteria. Findings indicate that prolonged exposure to TDF adversely affects bone microarchitecture and strength, impeding fracture healing and skeletal microdamage repair. The observed effects suggest a complex interplay involving bone cell signalling, cytokines and bone remodelling processes as potential mechanisms underlying TDF’s impact on bone quality. As a conclusion, TDF impairs bone remodelling and microarchitecture by influencing dynamic bone cell behaviour and signalling pathways. Future studies should delve deeper into understanding the intricate negative effects of TDF on bone and explore strategies for reversing these effects.
1. Introduction
The human immunodeficiency virus (HIV), belonging to the Lentivirus genus within the Retroviridae family, is primarily recognised for its consequential immunologic effects in humans due to its replication in CD4+ T lymphocytes and monocytes/macrophages, ultimately resulting in severe lymphopenia and immunodeficiency if left untreated. The fatal effects of this virus, if left untreated, are not merely restricted to the cellular level but are widespread throughout various bodily organs and systems such as the heart, kidney, lungs, liver and musculoskeletal and central nervous system [1]. The Global Burden of Disease report indicated that there were 36.85 million cases of HIV/AIDS in 2019, which shows an increment of 307.26/100,000 cases compared with 1990. Similarly, there were 863.84 thousand deaths in 2019 due to HIV/AIDS, which showed an increase of 10.72 deaths/100,000 cases compared with 1990 [2].
Given the devastating effects of HIV, a therapy regimen known as combination antiretroviral therapy was introduced in the mid-1990s. It currently boasts more than thirty drugs that have been approved for the treatment of HIV-positive individuals [3]. The current combination of antiretroviral drugs can be further divided into five main classes that target distinct steps in the HIV cycle. These classes include HIV entry inhibitors such as enfuviritide/ENF (Fuzeon), nucleoside reverse transcriptase inhibitors (NRTIs) such as tenofovir disoproxil fumarate/TDF (Viread), non-nucleoside reverse transcription inhibitors (NNRTIs) such as efavirenz/EFV (Sustiva), integrase strand transfer inhibitors (INSTIs) such as raltegrivir (Isentress) and protease inhibitors such as ritonavir (Norvir) [4]. Despite the wide array of drugs available for the treatment of HIV, current guidelines strongly support the use of a combination regimen of INSTIs and NRTIs as initial therapy for most people with HIV [5].
TDF is one of the most widely used antiretroviral agents in the world [6]. TDF is a NRTI that competitively inhibits HIV reverse transcriptase, which consequently prevents the transcription of viral ribonucleic acid (RNA) to viral deoxyribonucleic acid (DNA). This then halts a cascade of reactions which include the following, in order: integration of viral DNA into the host’s DNA following translocation of viral DNA to the nucleus, transcription of mRNA coding for viral proteins, translation to proteins and post-translational cleavage by HIV protease and lastly viral maturation, budding and replication [6]. The widespread use of TDF can be attributed to its extended half-life in plasma and intracellularly, its low risk of developing viral resistance mutations and its favourable tolerability by patients, which improves patients’ compliance [7]. Apart from that, tenofovir (or entecavir) is also indicated for patients with hepatitis B virus infection, which substantially increases the number of users of this drug [8].
Despite the impressive efficacy and tolerability of TDF, there are a plethora of undesirable side effects that are associated with its use. TDF has been shown to induce cellular toxicity and damage to the proximal tubule of the kidney, which, if left untreated and progresses, ultimately results in the decline of kidney function, osteomalacia and pathological fractures [6,9,10]. The use of TDF has also been positively associated with low bone mineral density (BMD) [11,12,13]. Individuals exposed only to TDF exhibited a more prominent loss in BMD as compared with those who were exposed to other antiretroviral drugs or a multi-drug regimen [11,12,13,14,15,16,17]. A randomised trial of TDF and emtricitabine as pre-exposure prophylaxis (PrEP) in 498 HIV-seronegative men who have sex with men (MSM) and transgender women from Thailand, South and North America showed a significant decrease in spine and hip BMD amongst participants randomised to emtricitabine/TDF PrEP after 24 weeks of treatment [18]. A significant decrease in lumbar spine, total hip and total body BMD Z-scores was observed in another clinical trial in the United States involving 101 MSM (15–22 years old), whereby participants with the highest exposure to TDF exhibited the greatest decline in total hip and femoral neck BMD [19]. Similarly, in patients with chronic hepatitis B infection, treatment with TDF was associated with lower BMD compared with controls and a more rapid decline in TDF compared with patients on entecavir [20,21]. In addition, switching patients with chronic hepatitis B infection and normal baseline BMD from TDF to entecavir or tenofovir alafenamide improved their BMD after 96 weeks [22]. These observations are especially worrying for older adults on TDF because they are already suffering from osteoporosis or are at high risk for fractures [23].
Although there is extensive research and evidence available regarding the detrimental effects of TDF use on BMD, its effects on other aspects of bone quality remain elusive. It is not possible to develop an effective agent or regimen to combat the destructive effects of TDF on bone if the mechanisms that cause these effects are unknown. Therefore, this paper aims to analyse findings from studies on these mechanisms to further understand the mechanism of bone loss induced by TDF. Ultimately, this paper hopes to propel the efforts in developing an agent that may reduce the destructive effects of TDF on bone and amplify the understanding of the scientific community on the mechanisms of TDF-induced bone loss to further enhance prescribing guidelines of TDF to maintain the quality of life of patients.
2. Results
The literature search, encompassing the PubMed and Scopus databases, identified 2442 unique articles, with 481 duplicates removed. Subsequent screening of titles and abstracts led to the exclusion of 2435 articles due to various reasons, including not being original research articles (n = 113), being conference abstracts (n = 15) or falling outside the scope of the current review (n = 2307). The full texts of the remaining seven articles were then retrieved and analysed, meeting the criteria for inclusion in the review (Figure 1).
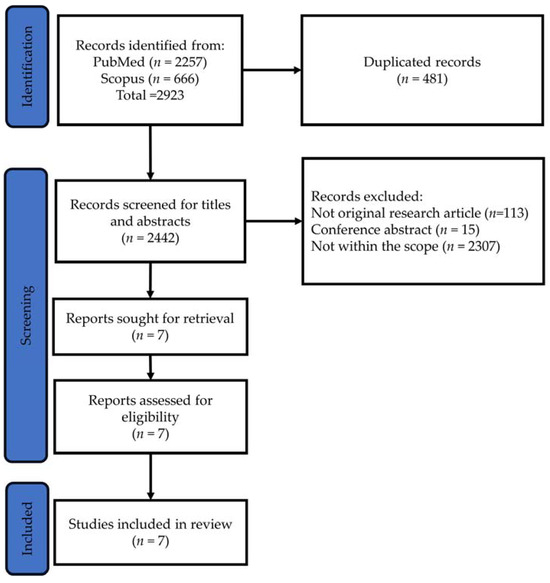
Figure 1.
PRISMA flow chart for article search, screening and identification.
Aligning with the objective of examining bone quality and understanding the mechanisms of bone loss induced by TDF or in combination with other drugs, the seven selected studies employed diverse animal models: rhesus monkeys (n = 1), rats (n = 2), mice (n = 2) and zebrafish (n = 1). Additionally, one study focused on patients taking TDF. Notably, one investigation explored the impact of antiretroviral therapy (ART), including TDF, on fracture healing, while the others administered TDF to intact animals. The assessment of bone quality and remodelling processes was predominantly conducted through techniques such as skeletal micro-computed tomography (µCT), bone histomorphometry, immunohistology, bone biomechanical strength and bone remodelling markers, providing a comprehensive understanding of TDF-induced effects on skeletal health.
Carnovali et al. (2016) explored the effects of TDF on zebrafish osteogenesis and scale remodelling. While TDF did not significantly impact the mineralisation rate in embryos, it modulated bone metabolism markers in adult fish, blocking scale growth and increasing the resorption area. This implies potential catabolic effects that span developmental stages [24].
Conesa-Buendía et al. (2019) conducted in vitro and in vivo studies involving bone marrow cells and mice treated with TDF. TDF exhibited complex effects, inhibiting osteoblast differentiation and increasing osteoclast activity in vitro and in vivo. The effects of TDF on osteoclasts could be mediated by enhanced nuclear factor kappa B (NFκB) signalling and pannexin-1 or connexin-43 function, in conjunction with increased levels of receptor activator of nuclear factor kappa beta (RANKL) and decreased osteoprotegerin (OPG) by osteoblasts. TDF also decreased cortical and trabecular bone volume and trabecular number and increased trabecular separation in mice, coincidentally with the decreased mineral apposition rate. The effects of TDF on osteoblasts could be mediated by the decreased nuclear translocation of β-catenin. These effects translated to decreased BMD and altered bone markers in TDF-treated mice, emphasising the need for a comprehensive understanding of its mechanisms [25].
In a study by Conradie et al. (2017), 12- to 14-week-old male Wistar rats were orally treated with TDF, stavudine or a control for 9 weeks. While TDF did not show significant differences in BMD compared with the control group, it reduced bending stress and mineralising surface area, indicating potential effects on bone mechanical strength and mineralisation activity. Although bone cellular histomorphometry was not impacted, lipid droplets in bone marrow increased significantly with TDF treatment. Compared with stavudine, the negative impact of TDF on the skeleton was less severe [26].
Matuszewska et al. (2020) investigated 8-week-old male albino Wistar rats treated with TDF, efavirenz, or a control for 24 weeks. The TDF group displayed reductions in femoral indices, femoral weight, mid-femoral diameter and total BMD after 24 weeks. Microscopically, the femoral trabecular number increased and trabecular separation decreased in the TDF group. Biomechanically, Young’s modulus also decreased in the TDF group. These findings suggest potential adverse effects on bone integrity and strength with prolonged TDF exposure, highlighting the importance of considering the duration of treatment. However, the bone remodelling markers did not change with TDF treatment [27].
Castillo et al. (2002) investigated growing rhesus monkeys treated with TDF and infected with simian immunodeficiency virus (SIV). TDF increased tibial osteoid seam width, a marker of bone microdamage, regardless of SIV infection. Additionally, SIV infection itself increased resorption cavity density, indicating potential combined effects on bone health that warrant further investigation [28].
Graham et al. (2022) explored the effects of ART containing TDF, lamivudine and efavirenz on fracture healing in Wistar rats. While an initial decrease in fracture healing union rate was observed at week 4, no significant differences in biomechanical strength were noted between the ART and control groups at week 8. This underscores the importance of studying the temporal dynamics of bone healing under ART regimens [29].
Ramalho et al. (2019) conducted a study on ART-naive male individuals with HIV initiated on TDF-based therapy. Post-treatment, there was a decrease in BMD at various sites, alterations in bone turnover markers and cytokine expression, improved cortical thickness and changes in osteoblast and osteoclast parameters. These multifaceted effects highlight the complexity of TDF’s impact on bone health [30].
The included studies are summarised in Table 1. In summary, the studies collectively reveal the diverse effects of TDF on bone health across species and models, impacting various aspects, such as structure, mechanical strength and turnover markers. The findings underscore the importance of considering factors like treatment duration and the temporal dynamics of bone healing in understanding TDF-related bone effects and their potential clinical implications.

Table 1.
Summary of findings from selected studies.
3. Discussion
Bones are perpetually remodelling tissues that serve essential roles in sustaining human life such as providing mechanical support, permitting locomotion, protecting vital organs and being the main haematopoietic organ for adults. Therefore, the continuous remodelling of bone to preserve its strength and avoid the accumulation of damage is vital for optimal functioning [31,32]. Bone remodelling is essentially a process where osteoclasts resorb old or damaged bone and osteoblasts form new bone [33]. In this review, the effects of TDF on various aspects of bone quality through modulation of bone remodelling are discussed.
3.1. Effects on Osteoblasts
Osteoblasts derive from mesenchymal stem cells and mineralised the skeleton [33,34,35]. TDF was shown to induce a significant age- and dose-dependent decrease in ALP, an osteoblastic marker, in a study conducted by Carnovali et al. using a zebrafish model [24]. TDF treatment was also shown to significantly increase the bone marrow adiposity in the femurs of rats, which indicates the potential ability of TDF to skew progenitor cell differentiation away from osteoblastogenesis towards adipogenesis, thus decreasing osteoblasts [26,36]. A histomorphometry analysis in a study conducted by Conesa-Buendía et al., in which a mouse model was used, also showed a significant decrease in the number of osteoblast cells per surface. Furthermore, this study also indicated that TDF treatment inhibited osteoblast differentiation in a dose-dependent manner [25]. These data suggest that TDF treatment results in a decrease in osteoblast activity, which tallies with a previous in vitro study that demonstrated an alteration in the expression of genes involved in various vital cellular signalling pathways and in amino acid biosynthesis and metabolism that ultimately inhibited osteoblast differentiation, growth and activity in primary osteoblasts that were treated with TDF [37].
3.2. Effects on Osteoclasts
Osteoclasts originated from myeloid/monocyte precursors are the cells primarily responsible for bone resorption. Their differentiation is stimulated by RANKL and suppressed by OPG secreted by osteoblasts [38,39,40,41,42,43]. TDF treatment results in increased activity of osteoclasts, as evidenced by the significant elevation in the expression of TRAP, an osteoclastic activity marker, in various studies [24,25]. A study conducted by Conesa-Buendía et al. demonstrated the various mechanisms through which TDF treatment significantly enhanced osteoclast differentiation. In this study, TDF treatment significantly increased the following: RANKL mRNA expression, NFATc1 expression, ERK1/2 phosphorylation, ERK1/2, p38 and NFkB nuclear translocation and RAW264.7 macrophage differentiation. The enhancement of these gene expressions and cellular signalling pathways ultimately resulted in an increased rate of osteoclast differentiation [25]. Various studies have highlighted the ability of TDF to cause a significant elevation in CD4+ and CD68+ macrophages, which serve as precursor cells to osteoclasts [25,30]. TDF also increased the expression of cathepsin K, a lytic enzyme secreted by osteoclasts during the degradation of bone [25]. OPG mRNA expression was also decreased by TDF treatment, which resulted in a net increase in osteoclast differentiation and activation that increases bone resorption [25].
3.3. Effects on Bone Remodelling and Quality
A tightly regulated balance between osteoblastic bone formation and osteoclastic bone resorption exists in normal bone physiology. This ensures the establishment of a homeostatic environment that prevents major alteration in net bone mass and mechanical strength. Alternation in bone remodelling skewing towards the bone resorption process will result in net bone loss and osteoporosis [44]. Two major types of bone exist, i.e., cortical bone, which provides mechanical strength and protection, and trabecular bone, in which most of the metabolic functions of the bone occur. The trabecular bone is therefore primarily affected by pathologies in bone remodelling [32]. An imbalance between bone formation and bone resorption will result in abnormal bone remodelling, which may result in the development of an osteoporotic phenotype which is characterised by low bone mass, structural deterioration of bone, increased bone fragility and increased vulnerability to fractures [45].
TDF treatment results in a dysregulation of physiological bone remodelling by promoting osteoclast function while simultaneously depressing osteoblast activity, which results in a net increase in bone resorption and bone loss. This was made further evident by a study by Conradie et al. which showed that TDF treatment significantly lowered the percentage of mineralising surfaces and resulted in a slight tendency towards a lower bone formation rate in the femurs of rats [26]. Moreover, bone loss was also observed in another study utilising Wistar rats where all the rats receiving TDF treatment showed significantly lower total body BMD, femoral indices, femoral weights, mid femoral and tibial diameters, bone surface/tissue volume and number of trabeculae when compared with the control group [27]. When compared with the control, the femurs of the rats treated with TDF in this study also exhibited significantly increased trabecular separation, which is indicative of an osteoporotic phenotype and consequently a lower biomechanical strength and increased risk of fracture, as demonstrated by a significantly lower Young’s modulus [46]. The scales of old zebrafish were not spared from an osteoporotic phenotype during TDF treatment wherein TDF completely blocked the physiological scale formation of 6-month and 12-month-old zebrafish [24]. The effects of TDF treatment on individuals with developing bones are also dire; a study by Castillo et al. showed that growing rhesus monkeys treated with TDF showed a significantly increased osteoid seam width as compared with the control group [28]. Osteoid is unmineralised and softer than bone which has been mineralised. An increased amount of osteoid may result in microdamage to the bone and ultimately bone fragility [47]. This evidence suggests that TDF treatment may result in the alteration of the mineralisation of bone, termed osteomalacia [48]. This puts younger patients on TDF treatment at risk of orthopaedic complications such as kyphoscoliosis and insufficiency fractures known as looser zones in the femoral neck, pubic and ischial rami [49,50]. It is also important to note that TDF treatment delays the process of bone healing due to its mechanism of inhibiting osteoblast-mediated bone formation and promoting osteoclast-mediated bone resorption, as evidenced by a study by Graham et.al, where the fractured tibias of Wistar rats treated with TDF, lamivudine and efavirenz for four weeks showed a significantly lower union rate as compared with the rats in the control group. These ununited fracture sites also revealed a clear gap at the fracture ends filled with fibrous tissue where no woven bone nor cartilaginous callus was found in the inter-fragmentary area, which further highlights TDF’s ability to suppress osteoblast-mediated bone formation [29].
The effects of TDF on the bone remodelling process and various aspects of bone quality are summarised in Figure 2.
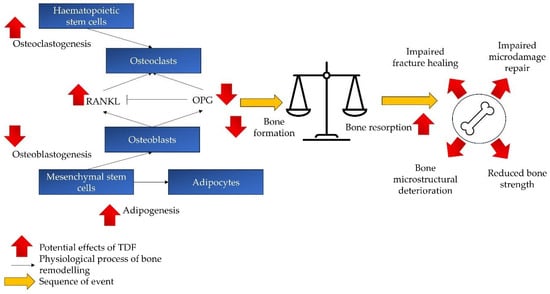
Figure 2.
The effects of TDF on bone remodelling and quality. TDF skews the bone remodelling towards the catabolic process (bone resorption > bone formation), thereby compromising bone microstructure and strength. It also impairs bone fracture healing and microdamage repair. Abbreviations: RANKL: receptor activator of nuclear factor kappa beta; OPG: osteoprotegerin; TDF: tenofovir disoproxil fumarate.
3.4. Limitations
The current research field is not without limitations. As individuals with HIV live longer with proper treatment, prolonged exposure to TDF may elevate their risk of osteoporosis in their later years. This concern is particularly relevant in the context of age-related hormone deficiencies resulting from menopause and testosterone deficiency syndrome [51,52]. To enhance our understanding of the skeletal effects of TDF, investigating castrated animal models is recommended. The existing literature explores bone regulatory mechanisms, such as the NFκB and Wnt signalling pathways [25]. TDF is also known to induce oxidative stress in patients, a phenomenon detrimental to bone health [53,54]. Exploring the involvement of nuclear factor erythroid 2-related factor 2, a major regulator of antioxidant defence mechanisms [55], and assessing mitochondrial redox status in bone cells exposed to TDF could provide valuable insights. Additionally, HIV or hepatitis B infection could cause bone loss independently of treatment [56,57]. Therefore, the bone loss caused by TDF could be compounded by the infections and should be examined with proper study design to delineate the effects between the drug and the infection.
Despite the establishment of TDF-induced bone loss models in the literature, limitations exist. This review primarily utilised two major databases, potentially overlooking relevant literature, and only included English articles, introducing a language bias. The preponderance of preclinical studies is due to the invasive nature of procedures required for assessing bone quality indices. While advances in skeletal assessment methods like µCT and bone indentation techniques exist, their application in studying the effects of TDF on human bone health is limited. Nevertheless, this scoping review offers a distinctive overview of TDF’s impact on bone quality, a critical component of bone strength beyond BMD.
4. Methodology
This scoping review was formulated utilising Arksey and O’Malley’s framework [58] and in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for scoping reviews (Table S1) [59]. The following steps were adopted: (1) identifying the research question; (2) identifying the relevant studies; (3) study selection; (4) charting the data; (5) collating, summarising and reporting the results.
4.1. Identifying the Research Question
The research question was: what are the effects of TDF on bone quality? Bone quality is defined as the structural and material properties of bone that contribute to its overall strength, durability and ability to function. Its determinants include bone density, microarchitecture, mineralisation and the composition of the bone matrix [60]. This review looked into components other than bone density because this aspect has been reviewed extensively elsewhere.
4.2. Identifying Relevant Studies
A literature search was performed on electronic databases (PubMed and Scopus) in October 2023 using the following search string: tenofovir AND (osteoblast OR osteoclast OR osteocyte OR osteoporosis OR bone). The inclusion criteria for papers were as follows: primary studies involving in vitro and animal models or humans and investigating the effects of TDF on bone quality. Articles without primary results, such as reviews, perspectives, commentary, letters to the editor, books and book chapters, were not considered. Conference abstracts and proceedings were not included due to incomplete data and possible overlap with research articles. Articles not written in English were excluded. No additional filters were applied during the search.
4.3. Study Selection
Endnote (version 20.4, Clarivate, London, UK) was used to organise the literature. The search results from the three electronic bases were downloaded. Duplicated items were removed automatically using Endnote and checked manually. The titles and abstracts were screened by K.-Y.C. and T.S.J.S. The inclusion and exclusion criteria were then applied to obtain the full texts of the selected articles. The reference list of the included articles was screened to identify literature that was missed during the search. Any discrepancies were resolved by discussion, and opinions were sought from the other authors.
4.4. Charting the Data
Relevant information from selected studies were extracted by T.S.H.S. and T.S.J.S., which included researchers, publication years, study design (subjects or disease models used, dosage, treatment period) and major findings using a standard Excel table.
4.5. Collating, Summarising and Reporting the Results
Instead of synthesising any variables, the scoping review approach was selected due to the heterogeneity of the studies involved and the variables of interest reported. The aim of a scoping review is mainly to provide an overview of the current understanding of the matter. In this regard, the study types, disease models, TDF (dose and treatment period) and major outcomes were summarised and reported. The role of TDF in damaging bone and the research gaps identified are discussed.
5. Conclusions
In conclusion, the selected literature suggests that TDF may exert multifaceted effects on bone remodelling, thus altering various bone quality aspects beyond BMD. While rodent studies indicated potential implications for bone histomorphometric indices and biomechanical strength, the findings from zebrafish and monkey models emphasised the complexity of TDF’s influence on bone remodelling and microdamage. Human studies among ART-naive individuals with HIV revealed significant changes in BMD, remodelling markers and cytokine levels, showcasing the clinical relevance of TDF-induced skeletal alterations. Of note, the temporal effects of ART containing TDF, as evidenced by the varying outcomes in fracture healing observed in rat models over different time points, highlight the need for longitudinal assessments in understanding the evolving impact of TDF on bone health.
The evidence summarised underscores the importance of a context-dependent consideration of TDF’s negative skeletal effects. Further research is warranted to elucidate the underlying molecular mechanisms, identify potential mitigating factors and inform clinical strategies for managing bone health in individuals undergoing TDF-based antiretroviral therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17020146/s1, Table S1: PRISMA checklist for scoping review.
Author Contributions
Conceptualisation, K.-Y.C.; methodology, T.S.H.S., T.S.J.S. and K.-Y.C.; validation, K.-Y.C.; formal analysis, T.S.H.S. and T.S.J.S.; investigation, T.S.H.S. and T.S.J.S.; resources, K.-Y.C.; data curation, T.S.H.S. and T.S.J.S.; writing—original draft preparation, T.S.H.S. and T.S.J.S.; writing—review and editing, K.-Y.C.; visualisation, T.S.J.S. and K.-Y.C.; supervision, K.-Y.C.; project administration, K.-Y.C.; funding acquisition, K.-Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
The researchers are supported by Universiti Kebangsaan Malaysia through the Fundamental Research Grant (FF-2022-312).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Acknowledgments
We thank Muhammad Rafie Hamzah for inspiring this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Erlandson, K.M.; Allshouse, A.A.; Jankowski, C.M.; MaWhinney, S.; Kohrt, W.M.; Campbell, T.B. Functional impairment is associated with low bone and muscle mass among persons aging with HIV infection. J. Acquir. Immune Defic. Syndr. 2013, 63, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, J.; Wang, X.; Xie, Y.; Zhang, X.; Han, D.; Fu, H.; Yin, W.; Wu, N. Global, regional, and national HIV/AIDS disease burden levels and trends in 1990–2019: A systematic analysis for the global burden of disease 2019 study. Front. Public Health 2023, 11, 1068664. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Allergy and Infectious Disease. Antiretroviral Drug Discovery and Development. Available online: https://www.niaid.nih.gov/diseases-conditions/antiretroviral-drug-development (accessed on 28 December 2023).
- Pau, A.K.; George, J.M. Antiretroviral therapy: Current drugs. Infect. Dis. Clin. N. Am. 2014, 28, 371–402. [Google Scholar] [CrossRef] [PubMed]
- Clinicalinfo.HIV.gov. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/what-start-initial-combination-regimens (accessed on 28 December 2023).
- Atta, M.G.; De Seigneux, S.; Lucas, G.M. Clinical Pharmacology in HIV Therapy. Clin. J. Am. Soc. Nephrol. 2019, 14, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kearney, B.P.; Flaherty, J.F.; Shah, J. Tenofovir disoproxil fumarate: Clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 2004, 43, 595–612. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 16 January 2024).
- Liborio, A.B.; Andrade, L.; Pereira, L.V.; Sanches, T.R.; Shimizu, M.H.; Seguro, A.C. Rosiglitazone reverses tenofovir-induced nephrotoxicity. Kidney Int. 2008, 74, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.J.; Hosseini, S.H.; Green, E.; Abuin, A.; Ludaway, T.; Russ, R.; Santoianni, R.; Lewis, W. Tenofovir renal proximal tubular toxicity is regulated by OAT1 and MRP4 transporters. Lab. Invest. 2011, 91, 852–858. [Google Scholar] [CrossRef]
- Gallant, J.E.; Staszewski, S.; Pozniak, A.L.; DeJesus, E.; Suleiman, J.M.; Miller, M.D.; Coakley, D.F.; Lu, B.; Toole, J.J.; Cheng, A.K.; et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: A 3-year randomized trial. JAMA 2004, 292, 191–201. [Google Scholar] [CrossRef]
- Stellbrink, H.J.; Orkin, C.; Arribas, J.R.; Compston, J.; Gerstoft, J.; Van Wijngaerden, E.; Lazzarin, A.; Rizzardini, G.; Sprenger, H.G.; Lambert, J.; et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010, 51, 963–972. [Google Scholar] [CrossRef]
- McComsey, G.A.; Kitch, D.; Daar, E.S.; Tierney, C.; Jahed, N.C.; Tebas, P.; Myers, L.; Melbourne, K.; Ha, B.; Sax, P.E. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: Aids Clinical Trials Group A5224s, a substudy of ACTG A5202. J. Infect. Dis. 2011, 203, 1791–1801. [Google Scholar] [CrossRef]
- Aurpibul, L.; Puthanakit, T. Review of tenofovir use in HIV-infected children. Pediatr. Infect. Dis. J. 2015, 34, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Bloch, M.; Tong, W.W.; Hoy, J.; Baker, D.; Lee, F.J.; Richardson, R.; Carr, A.; for the TROP (Switch from Tenofovir to Raltegravir for Low Bone Density) study team. Switch from tenofovir to raltegravir increases low bone mineral density and decreases markers of bone turnover over 48 weeks. HIV Med. 2014, 15, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Maggiolo, F.; Rizzardini, G.; Raffi, F.; Pulido, F.; Mateo-Garcia, M.G.; Molina, J.M.; Ong, E.; Shao, Y.; Piontkowsky, D.; Das, M.; et al. Bone mineral density in virologically suppressed people aged 60 years or older with HIV-1 switching from a regimen containing tenofovir disoproxil fumarate to an elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide single-tablet regimen: A multicentre, open-label, phase 3b, randomised trial. Lancet HIV 2019, 6, e655–e666. [Google Scholar] [CrossRef] [PubMed]
- Negredo, E.; Domingo, P.; Perez-Alvarez, N.; Gutierrez, M.; Mateo, G.; Puig, J.; Escrig, R.; Echeverria, P.; Bonjoch, A.; Clotet, B. Improvement in bone mineral density after switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: Two-centre randomized pilot study (OsteoTDF study). J. Antimicrob. Chemother. 2014, 69, 3368–3371. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, K.; Glidden, D.V.; Anderson, P.L.; Liu, A.; McMahan, V.; Gonzales, P.; Ramirez-Cardich, M.E.; Namwongprom, S.; Chodacki, P.; de Mendonca, L.M.; et al. Effects of Emtricitabine/Tenofovir on Bone Mineral Density in HIV-Negative Persons in a Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Infect. Dis. 2015, 61, 572–580. [Google Scholar] [CrossRef]
- Havens, P.L.; Stephensen, C.B.; Van Loan, M.D.; Schuster, G.U.; Woodhouse, L.R.; Flynn, P.M.; Gordon, C.M.; Pan, C.G.; Rutledge, B.; Liu, N.; et al. Decline in Bone Mass with Tenofovir Disoproxil Fumarate/Emtricitabine Is Associated with Hormonal Changes in the Absence of Renal Impairment When Used by HIV-Uninfected Adolescent Boys and Young Men for HIV Preexposure Prophylaxis. Clin. Infect. Dis. 2017, 64, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, R.; Sahin, A.; Ozturk, O.; Calhan, T.; Sayar, S.; Kanat, E.; Doganay, L.; Ozdil, K. Effects of Long-Term Tenofovir and Entecavir Treatment on Bone Mineral Density in Patients with Chronic Hepatitis B. Turk. J. Gastroenterol. 2022, 33, 35–43. [Google Scholar] [CrossRef]
- Gill, U.S.; Zissimopoulos, A.; Al-Shamma, S.; Burke, K.; McPhail, M.J.; Barr, D.A.; Kallis, Y.N.; Marley, R.T.; Kooner, P.; Foster, G.R.; et al. Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: Can the fracture risk assessment tool identify those at greatest risk? J. Infect. Dis. 2015, 211, 374–382. [Google Scholar] [CrossRef]
- Da Wang, F.; Zhou, J.; Li, L.Q.; Li, Y.J.; Wang, M.L.; Tao, Y.C.; Zhang, D.M.; Wang, Y.H.; Chen, E.Q. Improved bone and renal safety in younger tenofovir disoproxil fumarate experienced chronic hepatitis B patients after switching to tenofovir alafenamide or entecavir. Ann. Hepatol. 2023, 28, 101119. [Google Scholar] [CrossRef]
- Shiau, S.; Arpadi, S.M.; Yin, M.T. Bone Update: Is It Still an Issue without Tenofovir Disoproxil Fumarate? Curr. HIV/AIDS Rep. 2020, 17, 1–5. [Google Scholar] [CrossRef]
- Carnovali, M.; Banfi, G.; Mora, S.; Mariotti, M. Tenofovir and bone: Age-dependent effects in a zebrafish animal model. Antivir. Ther. 2016, 21, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Conesa-Buendía, F.M.; Llamas-Granda, P.; Larrañaga-Vera, A.; Wilder, T.; Largo, R.; Herrero-Beaumont, G.; Cronstein, B.; Mediero, A. Tenofovir Causes Bone Loss via Decreased Bone Formation and Increased Bone Resorption, Which Can Be Counteracted by Dipyridamole in Mice. J. Bone Miner. Res. 2019, 34, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Conradie, M.M.; van de Vyver, M.; Andrag, E.; Conradie, M.; Ferris, W.F. A Direct Comparison of the Effects of the Antiretroviral Drugs Stavudine, Tenofovir and the Combination Lopinavir/Ritonavir on Bone Metabolism in a Rat Model. Calcif. Tissue Int. 2017, 101, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Nowak, B.; Nikodem, A.; Jedrzejuk, D.; Szkudlarek, D.; Zduniak, K.; Filipiak, J.; Sznadruk-Bender, M.; Tomkalski, T.; Ceremuga, I.; et al. Effects of efavirenz and tenofovir on bone tissue in Wistar rats. Adv. Clin. Exp. Med. 2020, 29, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.B.; Tarantal, A.F.; Watnik, M.R.; Martin, R.B. Tenofovir treatment at 30 mg/kg/day can inhibit cortical bone mineralization in growing rhesus monkeys (Macaca mulatta). J. Orthop. Res. 2002, 20, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Jalal, M.M.K.; Lalloo, D.G.; Hamish, R.W.S.A. The effect of anti-retroviral therapy on fracture healing: An in vivo animal model. Bone Jt. Res. 2022, 11, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, J.; Martins, C.S.W.; Galvao, J.; Furukawa, L.N.; Domingues, W.V.; Oliveira, I.B.; Dos Reis, L.M.; Pereira, R.M.; Nickolas, T.L.; Yin, M.T.; et al. Treatment of Human Immunodeficiency Virus Infection with Tenofovir Disoproxil Fumarate-Containing Antiretrovirals Maintains Low Bone Formation Rate, but Increases Osteoid Volume on Bone Histomorphometry. J. Bone Miner. Res. 2019, 34, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), S131–S139. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. 2011, 6, 121–145. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Katagiri, T.; Takahashi, N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral. Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Okagu, I.U.; Ezeorba, T.P.C.; Aguchem, R.N.; Ohanenye, I.C.; Aham, E.C.; Okafor, S.N.; Bollati, C.; Lammi, C. A Review on the Molecular Mechanisms of Action of Natural Products in Preventing Bone Diseases. Int. J. Mol. Sci. 2022, 23, 8468. [Google Scholar] [CrossRef] [PubMed]
- Devlin, M.J.; Rosen, C.J. The bone-fat interface: Basic and clinical implications of marrow adiposity. Lancet Diabetes Endocrinol. 2015, 3, 141–147. [Google Scholar] [CrossRef]
- Grigsby, I.F.; Pham, L.; Mansky, L.M.; Gopalakrishnan, R.; Carlson, A.E.; Mansky, K.C. Tenofovir treatment of primary osteoblasts alters gene expression profiles: Implications for bone mineral density loss. Biochem. Biophys. Res. Commun. 2010, 394, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Fukai, Y.; Masuda, N.; Miyazaki, T.; Nakajima, M.; Sohda, M.; Manda, R.; Tsukada, K.; Kato, H.; Kuwano, H. High-level expression of the Smad ubiquitin ligase Smurf2 correlates with poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Res. 2002, 62, 7162–7165. [Google Scholar] [PubMed]
- Boyce, B.F.; Yao, Z.; Xing, L. Osteoclasts have multiple roles in bone in addition to bone resorption. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Komatsu, N.; Okamoto, K.; Sawa, S.; Nakashima, T.; Oh-hora, M.; Kodama, T.; Tanaka, S.; Bluestone, J.A.; Takayanagi, H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat. Med. 2014, 20, 62–68. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Kodama, J.; Kaito, T. Osteoclast Multinucleation: Review of Current Literature. Int. J. Mol. Sci. 2020, 21, 5685. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ng, B.N.; Rostam, M.K.I.; Muhammad Fadzil, N.F.D.; Raman, V.; Mohamed Yunus, F.; Syed Hashim, S.A.; Ekeuku, S.O. A Mini Review on Osteoporosis: From Biology to Pharmacological Management of Bone Loss. J. Clin. Med. 2022, 11, 6434. [Google Scholar] [CrossRef] [PubMed]
- Foger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Osterhoff, G.; Morgan, E.F.; Shefelbine, S.J.; Karim, L.; McNamara, L.M.; Augat, P. Bone mechanical properties and changes with osteoporosis. Injury 2016, 47 (Suppl. S2), S11–S20. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. Weaker bones and white skin as adaptions to improve anthropological “fitness” for northern environments. Osteoporos. Int. 2020, 31, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Ozono, K.; Michigami, T.; Minagawa, M.; Okazaki, R.; Sugimoto, T.; Takeuchi, Y.; Matsumoto, T. Pathogenesis and diagnostic criteria for rickets and osteomalacia—Proposal by an expert panel supported by Ministry of Health, Labour and Welfare, Japan, the Japanese Society for Bone and Mineral Research and the Japan Endocrine Society. Endocr. J. 2015, 62, 665–671. [Google Scholar] [CrossRef]
- Zimmerman, L.; McKeon, B. Osteomalacia. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551616/ (accessed on 28 December 2023).
- Motosuneya, T.; Asazuma, T.; Yasuoka, H.; Tsuji, T.; Fujikawa, K. Severe kyphoscoliosis associated with osteomalacia. Spine J. 2006, 6, 587–590. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Ima-Nirwana, S.; Chin, K.Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef]
- Abraham, P.; Ramamoorthy, H.; Isaac, B. Depletion of the cellular antioxidant system contributes to tenofovir disoproxil fumarate—Induced mitochondrial damage and increased oxido-nitrosative stress in the kidney. J. Biomed. Sci. 2013, 20, 61. [Google Scholar] [CrossRef]
- Canale, D.; de Bragança, A.C.; Gonçalves, J.G.; Shimizu, M.H.; Sanches, T.R.; Andrade, L.; Volpini, R.A.; Seguro, A.C. Vitamin D deficiency aggravates nephrotoxicity, hypertension and dyslipidemia caused by tenofovir: Role of oxidative stress and renin-angiotensin system. PLoS ONE 2014, 9, e103055. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in bone metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef]
- Dessordi, R.; Watanabe, L.M.; Guimaraes, M.P.; Romao, E.A.; de Lourdes Candolo Martinelli, A.; de Carvalho Santana, R.; Navarro, A.M. Bone loss in hepatitis B virus-infected patients can be associated with greater osteoclastic activity independently of the retroviral use. Sci. Rep. 2021, 11, 10162. [Google Scholar] [CrossRef]
- Titanji, K.; Vunnava, A.; Foster, A.; Sheth, A.N.; Lennox, J.L.; Knezevic, A.; Shenvi, N.; Easley, K.A.; Ofotokun, I.; Weitzmann, M.N. T-cell receptor activator of nuclear factor-kappaB ligand/osteoprotegerin imbalance is associated with HIV-induced bone loss in patients with higher CD4+ T-cell counts. AIDS 2018, 32, 885–894. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Ott, S.M. Bone strength: More than just bone density. Kidney Int. 2016, 89, 16–19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).