Effects of 1H-1,2,3-Triazole Derivatives of 3-O-Acetyl-11-Keto-Beta-Boswellic Acid from Boswellia sacra Resin on T-Cell Proliferation and Activation
Abstract
1. Introduction
2. Results
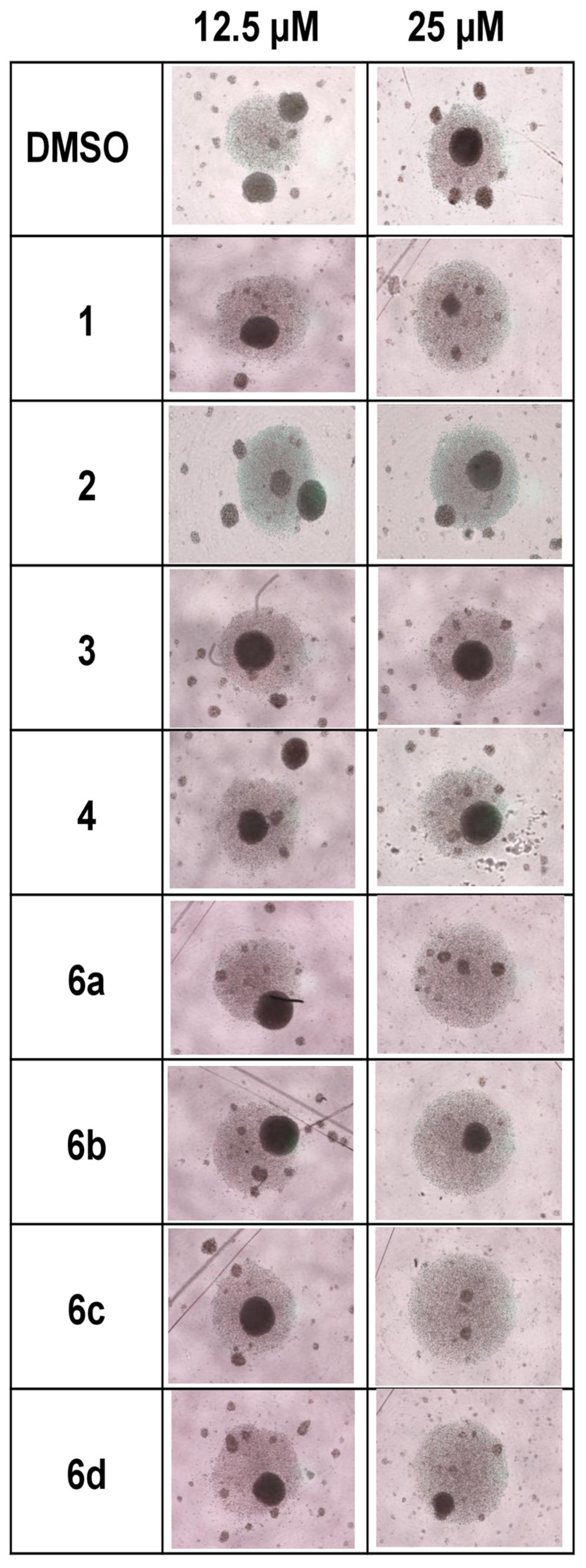
2.1. Effect of 1H-1,2,3-Triazole Derivatives of β-AKBA on T-Cell Expansion
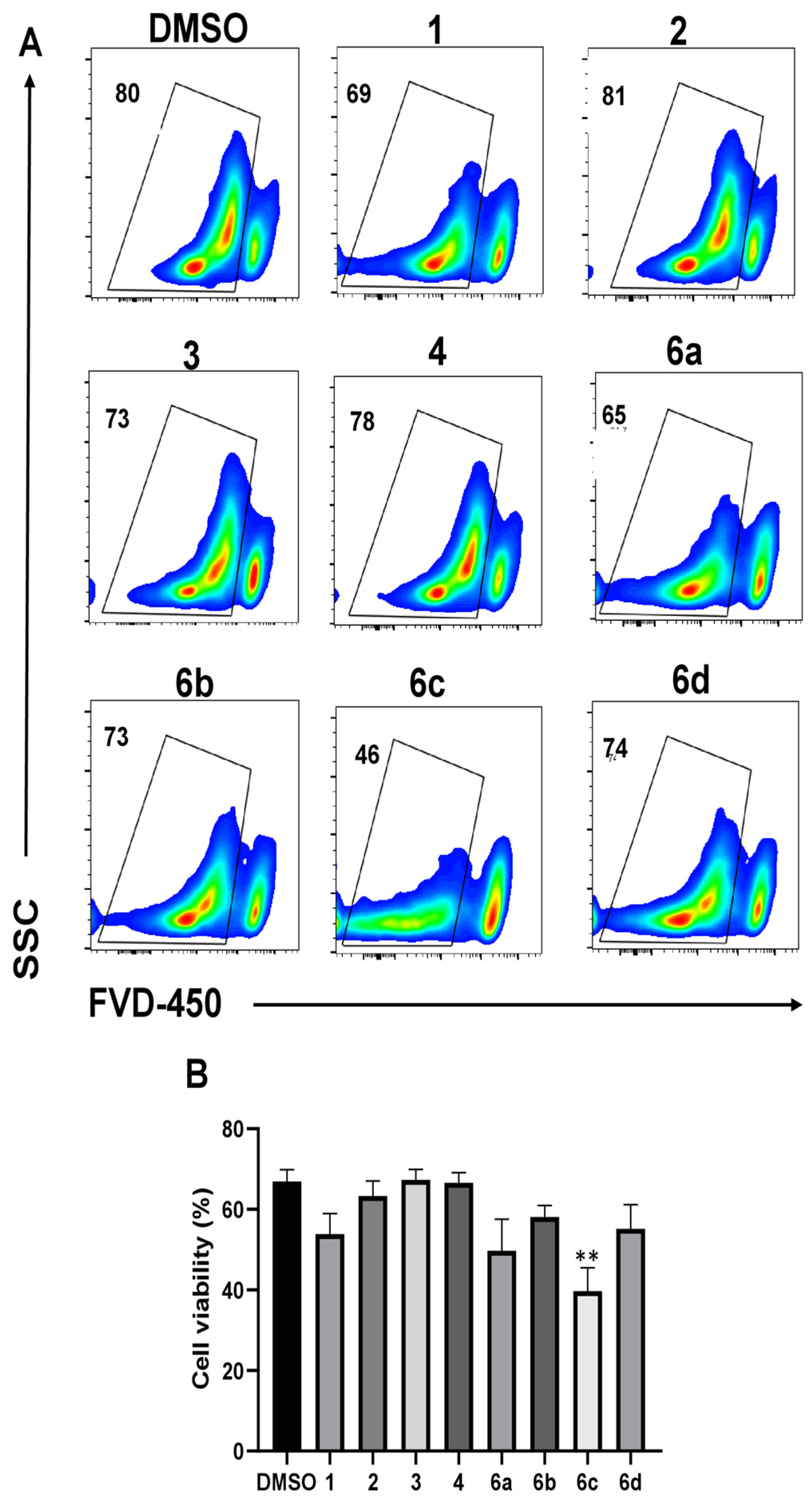
2.2. Effect of 1H-1,2,3-Triazole Derivatives of β-AKBA on T-Cell Viability
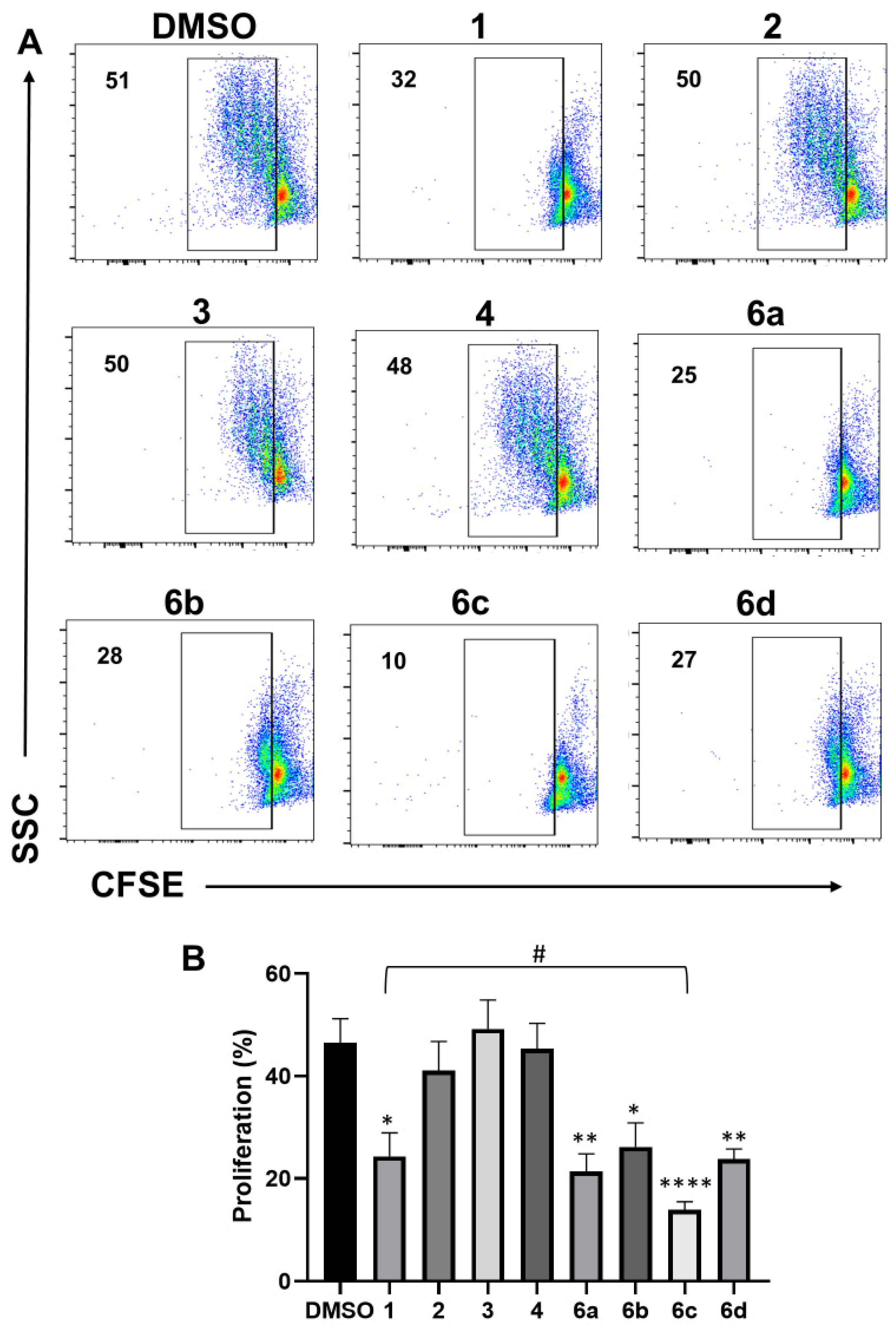
2.3. Effect of 1H-1,2,3-Triazole Derivatives of β-AKBA on T-Cell Proliferation
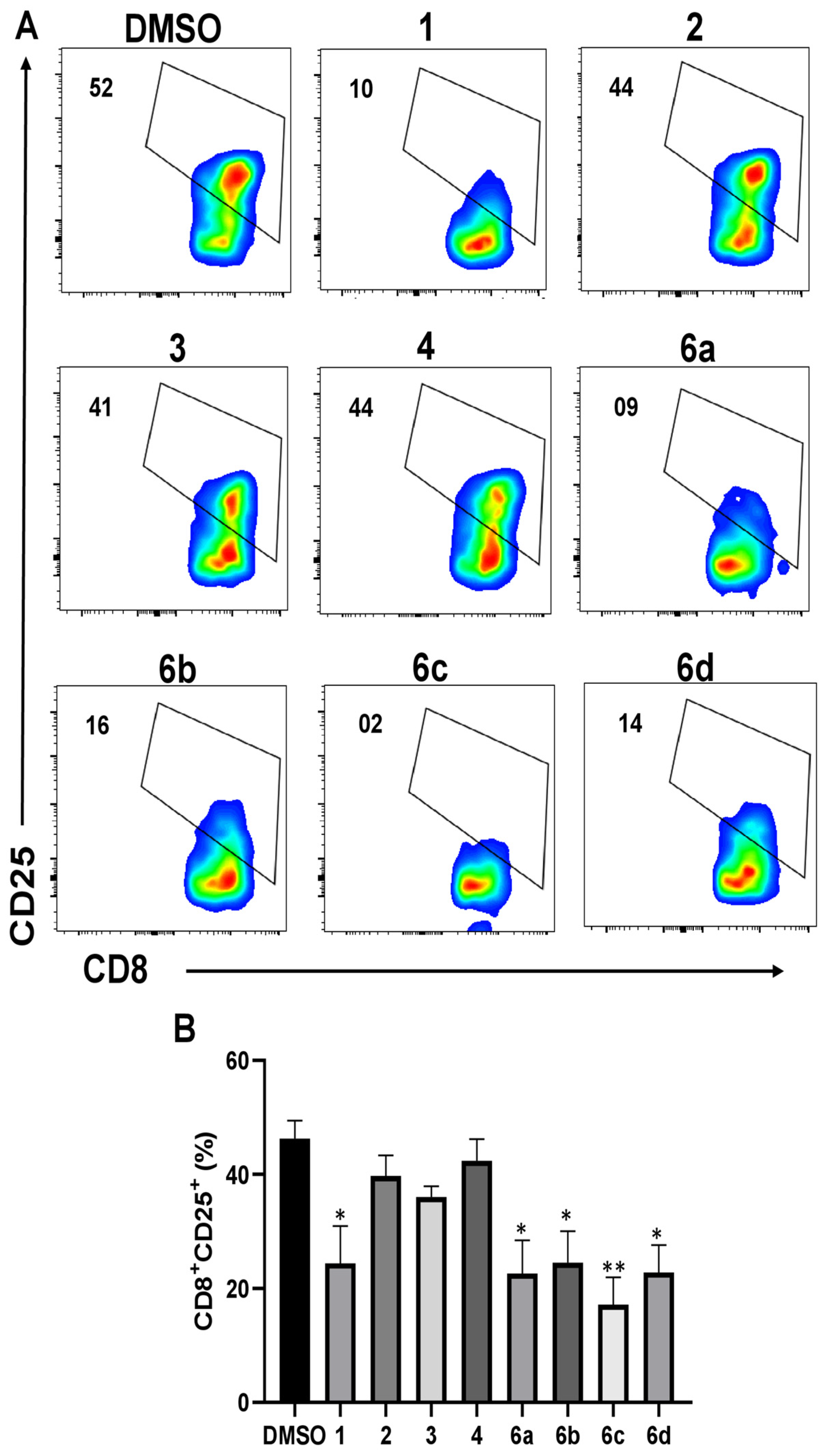
2.4. Effect of 1H-1,2,3-Triazole Derivatives of β-AKBA on CD25 Expression
3. Discussion
4. Materials and Methods
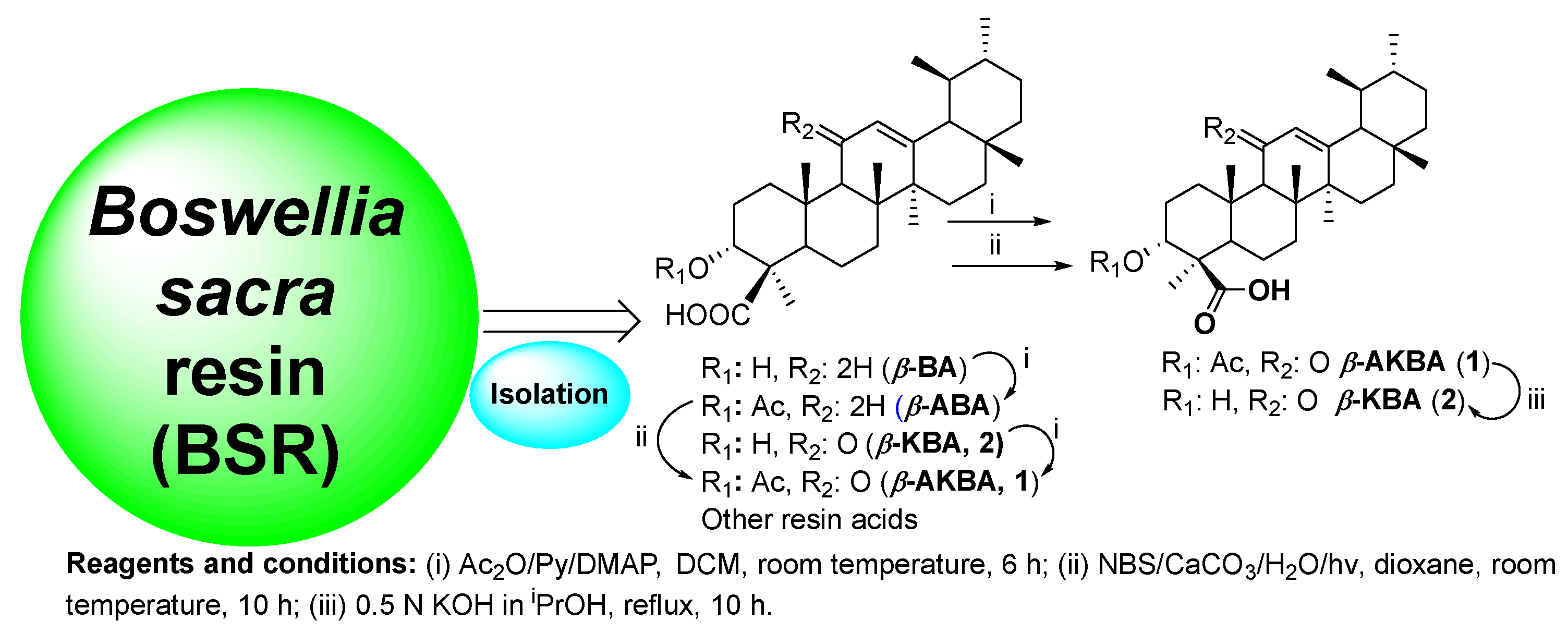
4.1. Preparation of Boswellic Acid (BA) Cluster
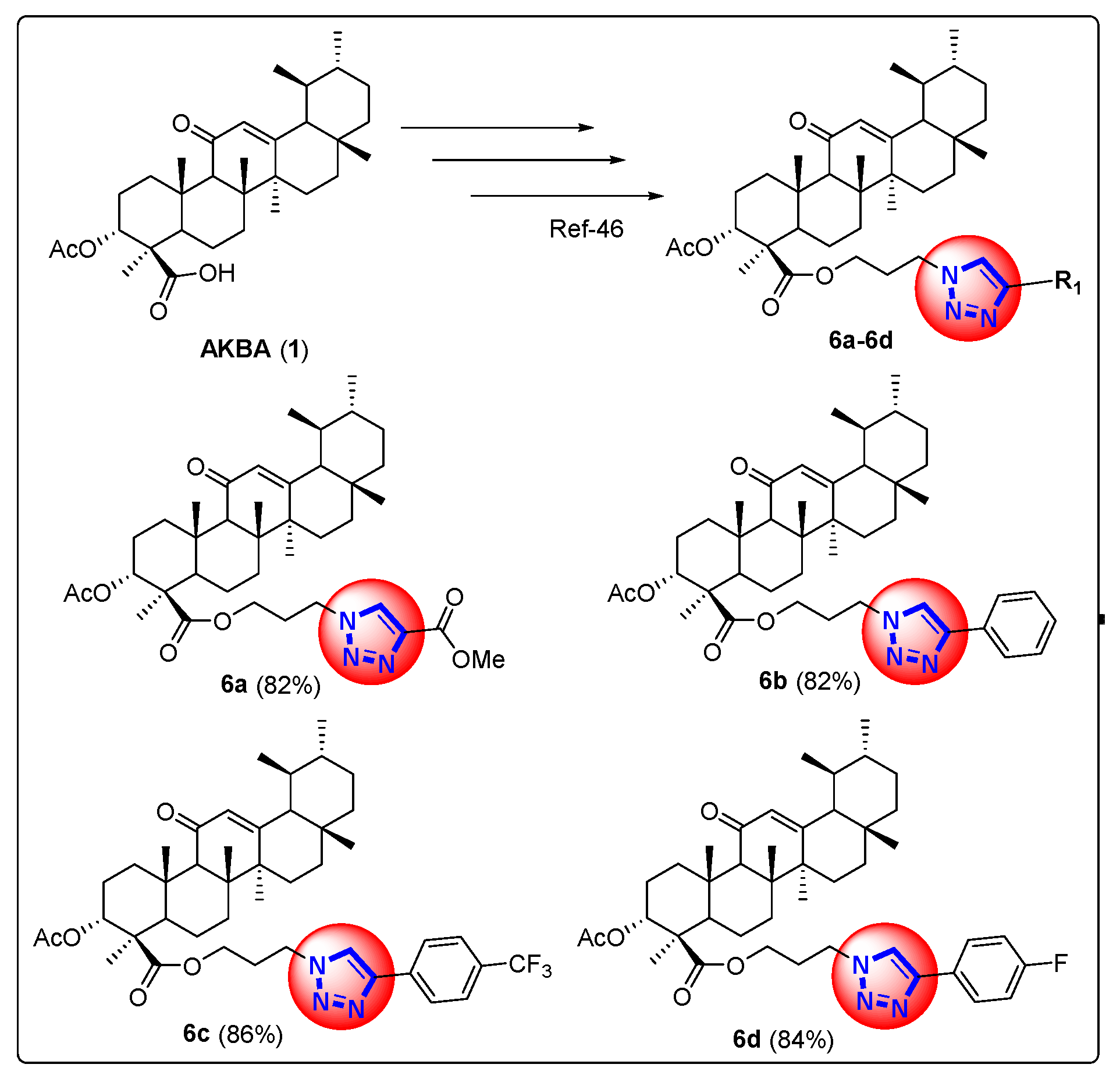
4.2. Synthesis of 1H-1,2,3-Triazole Derivatives of β-AKBA (6a–6d)
4.3. Antibodies and Reagents
4.4. Isolation of Peripheral Blood Mononuclear Cells
4.5. T-Cell Proliferation Assays
4.6. Cell Staining and Flow Cytometry
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Harrasi, A.; Al-Saidi, S. Phytochemical analysis of the essential oil from botanically certified oleogum resin of Boswellia sacra (Omani Luban). Molecules 2008, 13, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Thulin, M.; Warfa, A.M. The frankincense trees (Boswellia spp., Burseraceae) of northern Somalia and southern Arabia. Kew Bull. 1987, 42, 487–500. [Google Scholar] [CrossRef]
- Masyita, A.; Mustika Sari, R.; Dwi Astuti, A.; Yasir, B.; Rahma Rumata, N.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Al-Yasiry, A.R.; Kiczorowska, B. Frankincense--therapeutic properties. Postep. Hig. Med. Dosw. 2016, 70, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Alluri, V.K.; Kundimi, S.; Sengupta, K.; Golakoti, T.; Kilari, E.K. An Anti-Inflammatory Composition of Boswellia serrata Resin Extracts Alleviates Pain and Protects Cartilage in Monoiodoacetate-Induced Osteoarthritis in Rats. Evid. -Based Complement. Altern. Med. Ecam 2020, 2020, 7381625. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, X.; Sang, L.; Liu, H.; Xu, Q.; Liu, Z. Boswellic acid attenuates asthma phenotypes by downregulation of GATA3 via pSTAT6 inhibition in a murine model of asthma. Int. J. Clin. Exp. Pathol. 2015, 8, 236–243. [Google Scholar]
- Zhang, P.; Jiang, H. Acetyl-11-keto-β-boswellic Acid Confers Protection in DSS-Induced Colitis via the JNK-p38 MAPK and NF-κB Signaling Pathways. Adv. Biol. 2023, 7, e2200247. [Google Scholar] [CrossRef] [PubMed]
- Abou Zaid, E.S.; Mansour, S.Z.; El-Sonbaty, S.M.; Moawed, F.S.; Kandil, E.I.; Haroun, R.A. Boswellic acid coated zinc nanoparticles attenuate NF-κB-mediated inflammation in DSS-induced ulcerative colitis in rats. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320221150720. [Google Scholar] [CrossRef]
- Raja, A.F.; Ali, F.; Khan, I.A.; Shawl, A.S.; Arora, D.S.; Shah, B.A.; Taneja, S.C. Antistaphylococcal and biofilm inhibitory activities of acetyl-11-keto-β-boswellic acid from Boswellia serrata. BMC Microbiol. 2011, 11, 54. [Google Scholar] [CrossRef]
- Mohamed, T.M.; Youssef, M.A.M.; Bakry, A.A.; El-Keiy, M.M. Alzheimer’s disease improved through the activity of mitochondrial chain complexes and their gene expression in rats by boswellic acid. Metab. Brain Dis. 2021, 36, 255–264. [Google Scholar] [CrossRef]
- Takahashi, M.; Sung, B.; Shen, Y.; Hur, K.; Link, A.; Boland, C.R.; Aggarwal, B.B.; Goel, A. Boswellic acid exerts antitumor effects in colorectal cancer cells by modulating expression of the let-7 and miR-200 microRNA family. Carcinogenesis 2012, 33, 2441–2449. [Google Scholar] [CrossRef] [PubMed]
- Schmiech, M.; Ulrich, J.; Lang, S.J.; Büchele, B.; Paetz, C.; St-Gelais, A.; Syrovets, T.; Simmet, T. 11-Keto-α-Boswellic Acid, a Novel Triterpenoid from Boswellia spp. with Chemotaxonomic Potential and Antitumor Activity against Triple-Negative Breast Cancer Cells. Molecules 2021, 26, 366. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Shao, S.; Zhang, Q.; Zhuang, X.; Qiao, T. Acetyl-11-Keto-β-Boswellic Acid Exerts the Anti-Cancer Effects via Cell Cycle Arrest, Apoptosis Induction and Autophagy Suppression in Non-Small Cell Lung Cancer Cells. Onco Targets Ther. 2020, 13, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.X.; He, X.P.; Huang, P.Y.; Qi, Q.; Sun, W.H.; Liu, G.S.; Hua, J. Acetyl-11-keto-β-boswellic acid inhibits proliferation and induces apoptosis of gastric cancer cells through the phosphatase and tensin homolog /Akt/ cyclooxygenase-2 signaling pathway. World J. Gastroenterol. 2020, 26, 5822–5835. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P.T. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef]
- Ammon, H.P. Boswellic Acids and Their Role in Chronic Inflammatory Diseases. Adv. Exp. Med. Biol. 2016, 928, 291–327. [Google Scholar] [CrossRef]
- Chevrier, M.R.; Ryan, A.E.; Lee, D.Y.; Zhongze, M.; Wu-Yan, Z.; Via, C.S. Boswellia carterii extract inhibits TH1 cytokines and promotes TH2 cytokines in vitro. Clin. Diagn. Lab. Immunol. 2005, 12, 575–580. [Google Scholar] [CrossRef]
- Stürner, K.H.; Verse, N.; Yousef, S.; Martin, R.; Sospedra, M. Boswellic acids reduce Th17 differentiation via blockade of IL-1β-mediated IRAK1 signaling. Eur. J. Immunol. 2014, 44, 1200–1212. [Google Scholar] [CrossRef]
- Bishnoi, M.; Patil, C.S.; Kumar, A.; Kulkarni, S.K. Co-administration of acetyl-11-keto-beta-boswellic acid, a specific 5-lipoxygenase inhibitor, potentiates the protective effect of COX-2 inhibitors in kainic acid-induced neurotoxicity in mice. Pharmacology 2007, 79, 34–41. [Google Scholar] [CrossRef]
- Lv, M.; Zhuang, X.; Zhang, Q.; Cheng, Y.; Wu, D.; Wang, X.; Qiao, T. Acetyl-11-keto-β-boswellic acid enhances the cisplatin sensitivity of non-small cell lung cancer cells through cell cycle arrest, apoptosis induction, and autophagy suppression via p21-dependent signaling pathway. Cell Biol. Toxicol. 2021, 37, 209–228. [Google Scholar] [CrossRef]
- Wang, X.; Huang, B.; Liu, X.; Zhan, P. Discovery of bioactive molecules from CuAAC click-chemistry-based combinatorial libraries. Drug Discov. Today 2016, 21, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M. 1,2,3-Triazole hybrids as anticancer agents: A review. Arch. Pharm. 2022, 355, e2100158. [Google Scholar] [CrossRef] [PubMed]
- Kishore Kumar, A.; Sunitha, V.; Shankar, B.; Ramesh, M.; Murali Krishna, T.; Jalapathi, P. Synthesis, biological evaluation, and molecular docking studies of novel 1,2,3-triazole derivatives as potent anti-inflammatory agents. Russ. J. Gen. Chem. 2016, 86, 1154–1162. [Google Scholar] [CrossRef]
- Esmaili, S.; Ebadi, A.; Khazaei, A.; Ghorbani, H.; Faramarzi, M.A.; Mojtabavi, S.; Mahdavi, M.; Najafi, Z. Novel Pyrano[3,2-c]quinoline-1,2,3-triazole Hybrids as Potential Anti-Diabetic Agents: In Vitro α-Glucosidase Inhibition, Kinetic, and Molecular Dynamics Simulation. ACS Omega 2023, 8, 23412–23424. [Google Scholar] [CrossRef]
- Poonia, N.; Kumar, A.; Kumar, V.; Yadav, M.; Lal, K. Recent Progress in 1H-1,2,3-triazoles as Potential Antifungal Agents. Curr. Top. Med. Chem. 2021, 21, 2109–2133. [Google Scholar] [CrossRef]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1, 2, 4-Triazoles as Antifungal Agents. Biomed. Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef]
- Agouram, N. 1,2,3-triazole derivatives as antiviral agents. Med. Chem. Res. 2023, 32, 2458–2472. [Google Scholar] [CrossRef]
- Karypidou, K.; Ribone, S.R.; Quevedo, M.A.; Persoons, L.; Pannecouque, C.; Helsen, C.; Claessens, F.; Dehaen, W. Synthesis, biological evaluation and molecular modeling of a novel series of fused 1,2,3-triazoles as potential anti-coronavirus agents. Bioorg Med. Chem. Lett. 2018, 28, 3472–3476. [Google Scholar] [CrossRef]
- Ashok, D.; Gundu, S.; Aamate, V.K.; Devulapally, M.G.; Bathini, R.; Manga, V. Dimers of coumarin-1,2,3-triazole hybrids bearing alkyl spacer: Design, microwave-assisted synthesis, molecular docking and evaluation as antimycobacterial and antimicrobial agents. J. Mol. Struct. 2018, 1157, 312–321. [Google Scholar] [CrossRef]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Todorov, L.; Kostova, I. 1,2,3-Triazoles and their metal chelates with antimicrobial activity. Front. Chem. 2023, 11, 1247805. [Google Scholar] [CrossRef] [PubMed]
- Meyiah, A.; Shawkat, M.Y.; Ur Rehman, N.; Al-Harrasi, A.; Elkord, E. Effect of Boswellic acids on T cell proliferation and activation. Int. Immunopharmacol. 2023, 122, 110668. [Google Scholar] [CrossRef] [PubMed]
- Dixit, D.; Verma, P.K.; Marwaha, R.K. A review on ‘triazoles’: Their chemistry, synthesis and pharmacological potentials. J. Iran. Chem. Soc. 2021, 18, 2535–2565. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.; Lv, Z.; Zhang, G.; Xu, Z. Recent updates on 1,2,3-triazole-containing hybrids with in vivo therapeutic potential against cancers: A mini-review. Eur. J. Med. Chem. 2023, 251, 115254. [Google Scholar] [CrossRef]
- Liu, J.Q.; Yan, Y.Q.; Liu, J.T.; Wang, Y.R.; Wang, X. Curcumin prevents experimental autoimmune encephalomyelitis by inhibiting proliferation and effector CD4+ T cell activation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9108–9116. [Google Scholar] [CrossRef]
- Chyuan, I.T.; Tsai, H.F.; Wu, C.S.; Sung, C.C.; Hsu, P.N. TRAIL-Mediated Suppression of T Cell Receptor Signaling Inhibits T Cell Activation and Inflammation in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Morishima, C.; Shuhart, M.C.; Wang, C.C.; Paschal, D.M.; Apodaca, M.C.; Liu, Y.; Sloan, D.D.; Graf, T.N.; Oberlies, N.H.; Lee, D.Y.; et al. Silymarin inhibits in vitro T-cell proliferation and cytokine production in hepatitis C virus infection. Gastroenterology 2010, 138, 671–681. [Google Scholar] [CrossRef]
- Gayathri, B.; Manjula, N.; Vinaykumar, K.S.; Lakshmi, B.S.; Balakrishnan, A. Pure compound from Boswellia serrata extract exhibits anti-inflammatory property in human PBMCs and mouse macrophages through inhibition of TNFalpha, IL-1beta, NO and MAP kinases. Int. Immunopharmacol. 2007, 7, 473–482. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Makboul, R.M.; Al-Mokhtar, M.A.; Nicola, M.A. Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resistance of diabetic rats through inhibition of GSK3β activity, oxidative stress and pro-inflammatory cytokines. Biomed. Pharmacother. 2019, 109, 281–292. [Google Scholar] [CrossRef]
- Kim, T.W.; Yong, Y.; Shin, S.Y.; Jung, H.; Park, K.H.; Lee, Y.H.; Lim, Y.; Jung, K.Y. Synthesis and biological evaluation of phenyl-1H-1,2,3-triazole derivatives as anti-inflammatory agents. Bioorganic Chem. 2015, 59, 1–11. [Google Scholar] [CrossRef]
- Kumar, A.; Alam, M.S.; Hamid, H.; Chugh, V.; Tikla, T.; Kaul, R.; Dhulap, A.; Sharma, S.K. Design and synthesis of anti–inflammatory 1,2,3–triazolylpyrrolobenzodiazepinone derivatives and impact of molecular structure on COX–2 selective targeting. J. Mol. Struct. 2023, 1272, 134151. [Google Scholar] [CrossRef]
- Jahan, H.; Siddiqui, N.N.; Iqbal, S.; Basha, F.Z.; Shaikh, S.; Pizzi, M.; Choudhary, M.I. Suppression of COX-2/PGE2 levels by carbazole-linked triazoles via modulating methylglyoxal-AGEs and glucose-AGEs—Induced ROS/NF-κB signaling in monocytes. Cell. Signal. 2022, 97, 110372. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Alam, M.S.; Hamid, H.; Shafi, S.; Nargotra, A.; Mahajan, P.; Nazreen, S.; Kalle, A.M.; Kharbanda, C.; Ali, Y.; et al. Synthesis of novel 1,2,3-triazole based benzoxazolinones: Their TNF-α based molecular docking with in-vivo anti-inflammatory, antinociceptive activities and ulcerogenic risk evaluation. Eur. J. Med. Chem. 2013, 70, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.C.; de Paiva, J.C.; da Silva, E.Q.G.; Santos, M.R.; de Almeida Lima, G.D.; Moreira, G.A.; Silva, L.V.G.; de Melo Agripino, J.; de Souza, A.P.M.; de Oliveira Mendes, T.A.; et al. Immunomodulatory activity of trifluoromethyl arylamides derived from the SRPK inhibitor SRPIN340 and their potential use as vaccine adjuvant. Life Sci. 2022, 307, 120849. [Google Scholar] [CrossRef]
- Pattanayak, P.; Nikhitha, S.; Halder, D.; Ghosh, B.; Chatterjee, T. Exploring the impact of trifluoromethyl (-CF(3)) functional group on the anti-cancer activity of isoxazole-based molecules: Design, synthesis, biological evaluation and molecular docking analysis. RSC Adv. 2024, 14, 18856–18870. [Google Scholar] [CrossRef]
- Avula, S.K.; Rehman, N.U.; Khan, M.; Halim, S.A.; Khan, A.; Rafiq, K.; Csuk, R.; Das, B.; Al-Harrasi, A. New synthetic 1H-1,2,3-triazole derivatives of 3-O-acetyl-β-boswellic acid and 3-O-acetyl-11-keto-β-boswellic acid from Boswellia sacra inhibit carbonic anhydrase II in vitro. Med. Chem. Res. 2021, 30, 1185–1198. [Google Scholar] [CrossRef]
- Johann Jauch, J.B. An Efficient Method for the Large-Scale Preparation of 3-O-Acetyl-11-oxo-β-boswellic Acid and Other Boswellic Acids. Eur. JOC Dec. 2003, 2003, 4752–4756. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyiah, A.; Avula, S.K.; Al-Harrasi, A.; Elkord, E. Effects of 1H-1,2,3-Triazole Derivatives of 3-O-Acetyl-11-Keto-Beta-Boswellic Acid from Boswellia sacra Resin on T-Cell Proliferation and Activation. Pharmaceuticals 2024, 17, 1650. https://doi.org/10.3390/ph17121650
Meyiah A, Avula SK, Al-Harrasi A, Elkord E. Effects of 1H-1,2,3-Triazole Derivatives of 3-O-Acetyl-11-Keto-Beta-Boswellic Acid from Boswellia sacra Resin on T-Cell Proliferation and Activation. Pharmaceuticals. 2024; 17(12):1650. https://doi.org/10.3390/ph17121650
Chicago/Turabian StyleMeyiah, Abdo, Satya Kumar Avula, Ahmed Al-Harrasi, and Eyad Elkord. 2024. "Effects of 1H-1,2,3-Triazole Derivatives of 3-O-Acetyl-11-Keto-Beta-Boswellic Acid from Boswellia sacra Resin on T-Cell Proliferation and Activation" Pharmaceuticals 17, no. 12: 1650. https://doi.org/10.3390/ph17121650
APA StyleMeyiah, A., Avula, S. K., Al-Harrasi, A., & Elkord, E. (2024). Effects of 1H-1,2,3-Triazole Derivatives of 3-O-Acetyl-11-Keto-Beta-Boswellic Acid from Boswellia sacra Resin on T-Cell Proliferation and Activation. Pharmaceuticals, 17(12), 1650. https://doi.org/10.3390/ph17121650







