Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Component | Internal Standard | LLOQ [ng/mL] | Analytical Measuring Range [ng/mL] |
|---|---|---|---|
| Δ8-THCV | Δ9-THCV-d5 | 0.5 | 0.5–400 |
| Δ9-THCV | Δ9-THCV-d5 | 0.5 | 0.5–400 |
| 11-OH-Δ8-THCV | 7-OH-CBD-d3 | 0.5 | 0.5–400 |
| Δ8-THCV-COOH | 7-CBD-COOH-d3 | 1.0 | 1.0–400 |
| Δ9-THCV-COOH | Δ9-THC-COOH-d3 | 1.0 | 1.0–400 |
| Δ8-THC | Δ8-THC-d9 | 0.5 | 0.5–400 |
| Δ9-THC | Δ9-THC-d9 | 0.5 | 0.5–400 |
| 9S-Δ10-THC | Δ8-THC-d9 | 0.5 | 0.5–400 |
| 9R-Δ10-THC | Δ8-THC-d9 | 0.5 | 0.5–400 |
| 11-OH-Δ8-THC | 11-OH-Δ9-THC-d3 | 1.0 | 1.0–400 |
| 11-OH-Δ9-THC | 11-OH-Δ9-THC-d3 | 1.0 | 1.0–400 |
| Δ8-THC-COOH | Δ9-THC-COOH-d3 | 1.0 | 1.0–400 |
| Δ9-THC-COOH | Δ9-THC-COOH-d3 | 1.0 | 1.0–400 |
| Intra-Day Summary | Inter-Day Summary | |||||||
|---|---|---|---|---|---|---|---|---|
| Component | Mean Accuracy | Accuracy Range | Mean Precision | Precision Range | Mean Accuracy | Accuracy Range | Mean Precision | Precision Range |
| Δ8-THCV | 103.1 | 95.0–106.4 | 4.66 | 3.19–18.2 | 103.1 | 94.2–106.7 | 4.91 | 4.33–14.7 |
| Δ9-THCV | 97.1 | 80.3–105.2 | 9.96 | 1.68–6.78 | 95.8 | 82.9–100.7 | 7.64 | 4.55–12.3 |
| 11-OH-Δ8-THCV | 100.8 | 96.4–104.1 | 2.90 | 3.91–9.69 | 99.0 | 96.3–102.7 | 2.58 | 5.09–12.4 |
| Δ8-THCV-COOH | 95.3 | 86.7–101.8 | 6.66 | 5.81–14.4 | 97.1 | 90.0–104.3 | 5.74 | 7.51–16.1 |
| Δ9-THCV-COOH | 97.1 | 93.2–100.0 | 3.22 | 5.90–18.3 | 96.0 | 91.6–99.7 | 4.16 | 7.80–17.5 |
| Δ8-THC | 105.1 | 102.7–109.9 | 2.66 | 4.09–18.5 | 105.6 | 101.6–108.3 | 2.34 | 5.65–15.5 |
| Δ9-THC | 101.6 | 98.2–106.6 | 3.05 | 4.38–14.5 | 99.5 | 97.9–101.6 | 1.35 | 5.78–11.3 |
| Δ10-9S-THC | 100.2 | 89.7–109.3 | 8.84 | 2.49–18.6 | 100.9 | 89.2–107.8 | 7.31 | 6.16–29.0 |
| Δ10-9R-THC | 102.5 | 88.9–110.2 | 8.81 | 3.76–43.0 | 103.5 | 91.6–108.8 | 7.08 | 4.78–27.1 |
| 11-OH-Δ8-THC | 95.9 | 83.6–101.0 | 7.34 | 6.70–17.7 | 98.6 | 87.5–103.1 | 6.62 | 7.20–16.5 |
| 11-OH-Δ9-THC | 106.7 | 100.5–119.2 | 6.88 | 4.32–23.3 | 101.8 | 95.7–105.2 | 3.82 | 4.71–25.3 |
| Δ8-THC-COOH | 104.5 | 99.8–114.1 | 5.42 | 3.58–15.6 | 100.4 | 96.9–103.5 | 2.66 | 6.24–15.7 |
| Δ9-THC-COOH | 99.8 | 96.0–102.0 | 2.47 | 3.37–15.5 | 96.1 | 88.8–99.4 | 4.72 | 5.97–12.4 |
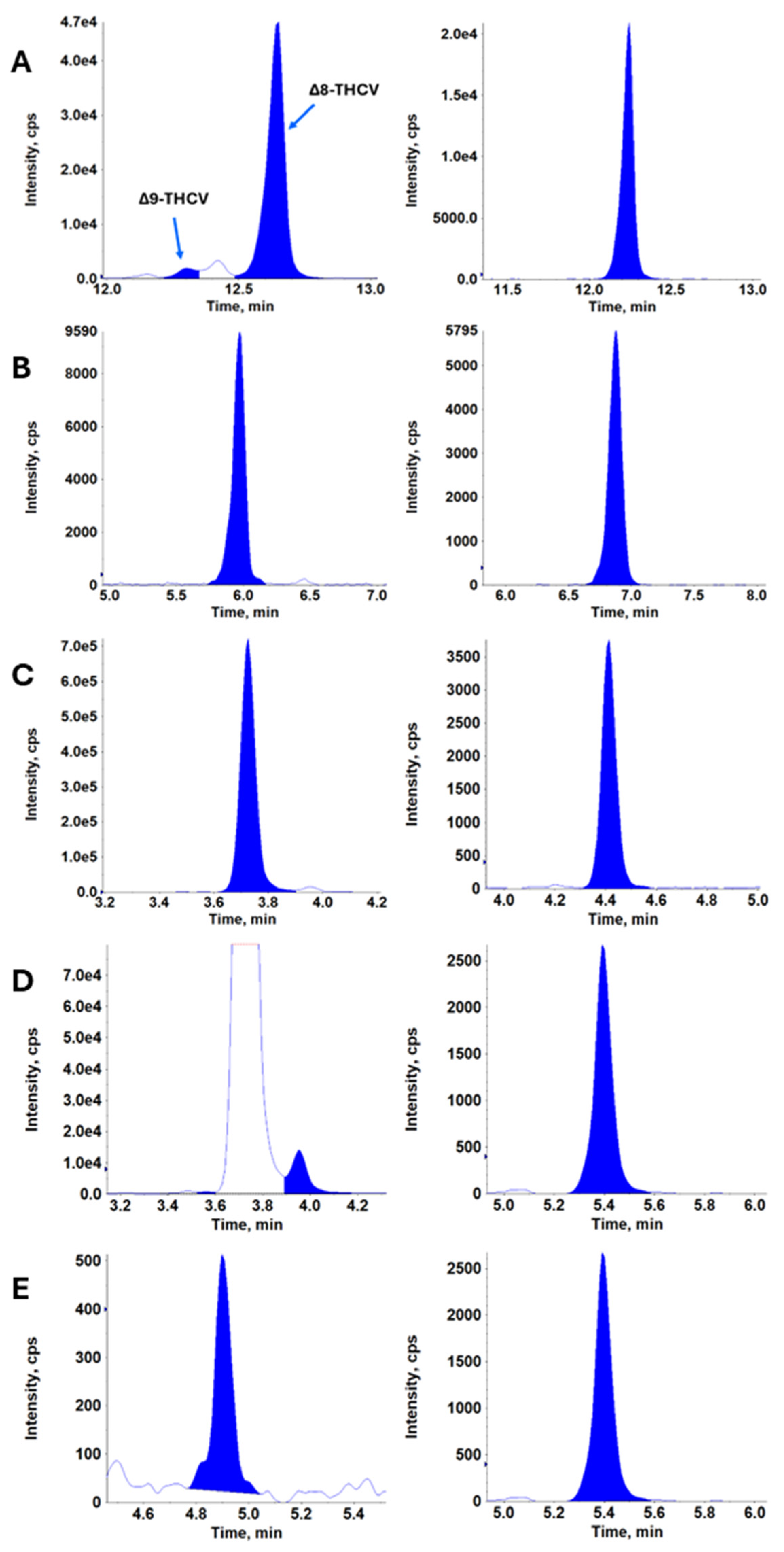
| Timepoint | 12.5 mg | 25 mg | 50 mg | 100 mg | 200 mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BLOQ (n = 15) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 14) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 14) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 14) | Mean (SD, %CV) | Median (IQ Range) | BLOQ (n = 15) | Mean (SD, %CV) | Median (IQ Range) | |
| Δ8-THCV | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 12 | 0.2 (±0.5, 254) | 0 (0–0) | 14 | 0.1 (±0.3, 387) | 0 (0–0) |
| 1 h | 12 | 0.1 (±0.3, 212) | 0 (0–0) | 11 | 0.3 (±0.6, 238) | 0 (0–0) | 10 | 0.5 (±1, 201) | 0 (0–0.5) | 6 | 4.2 (±6.1, 145) | 2.1 (0–4.9) | 6 | 3.6 (±4.5, 124) | 2 (0–5.2) |
| 2 h | 9 | 0.4 (±0.8, 184) | 0 (0–0.7) | 7 | 1.4 (±1.9, 140) | 0.3 (0–2.1) | 8 | 2.6 (±5.3, 204) | 0 (0–1.4) | 4 | 7.1 (±7.9, 113) | 5.5 (0.2–8) | 5 | 21.4 (±21.8, 102) | 14.3 (0–36.6) |
| 4 h | 7 | 0.7 (±1.1, 145) | 0.6 (0–0.9) | 1 | 1.8 (±1.4, 78.9) | 1.5 (1–2.3) | 3 | 4.3 (±4.6, 109) | 2.4 (1.3–6.8) | 1 | 11.4 (±12.4, 109) | 7.3 (4.6–11.6) | 1 | 29.4 (±21.2, 72.2) | 33.3 (12.6–38.6) |
| 6 h | 11 | 0.5 (±0.8, 178) | 0 (0–0.7) | 6 | 0.9 (±1.1, 120) | 0.7 (0–1.4) | 1 | 6.1 (±7.3, 120) | 2.8 (1.7–7.3) | 0 | 12.3 (±13.6, 110) | 7.7 (2.8–18.5) | 0 | 26.6 (±20.3, 76.2) | 21.8 (12.9–40.9) |
| 8 h | 11 | 0.2 (±0.3, 172) | 0 (0–0.3) | 7 | 0.4 (±0.5, 117) | 0.3 (0–0.7) | 1 | 2.2 (±1.6, 71.4) | 2.2 (0.8–3.2) | 0 | 4.1 (±3.1, 74.8) | 3.1 (1.8–5.9) | 0 | 21.2 (±23.2, 109) | 13.4 (5.3–28.3) |
| 11-OH-Δ8-THCV | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 1 h | 14 | 0 (±0.1, 387) | 0 (0–0) | 13 | 0.1 (±0.2, 374) | 0 (0–0) | 12 | 0.1 (±0.4, 274) | 0 (0–0) | 7 | 1.4 (±2.2, 161) | 0.5 (0–1.5) | 8 | 1.2 (±1.5, 126) | 0 (0–1.8) |
| 2 h | 13 | 0.1 (±0.3, 282) | 0 (0–0) | 10 | 0.3 (±0.4, 174) | 0 (0–0.4) | 12 | 0.4 (±1.1, 264) | 0 (0–0) | 4 | 1.8 (±2.3, 132) | 1 (0.1–1.5) | 5 | 4.5 (±5.7, 128) | 1.8 (0–5.9) |
| 4 h | 13 | 0.1 (±0.3, 264) | 0 (0–0) | 11 | 0.2 (±0.4, 203) | 0 (0–0) | 9 | 0.6 (±0.9, 154) | 0 (0–1.1) | 3 | 2.1 (±2.8, 129) | 1.3 (0.9–2.6) | 1 | 5.2 (±5.4, 104) | 3.4 (2.4–5.9) |
| 6 h | 14 | 0.1 (±0.2, 374) | 0 (0–0) | 11 | 0.2 (±0.3, 201) | 0 (0–0) | 5 | 1 (±1, 103) | 0.9 (0–1.5) | 2 | 2.4 (±2.3, 95.6) | 1.5 (1–2.9) | 0 | 4.6 (±2.9, 63.1) | 3.7 (2.2–6) |
| 8 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 5 | 0.6 (±0.5, 87.9) | 0.6 (0–0.9) | 2 | 1.3 (±1, 80.3) | 1.1 (0.7–1.6) | 0 | 3.7 (±2.6, 71.6) | 3.1 (1.7–4.9) |
| Δ8-THCV-COOH | |||||||||||||||
| Predose | 13 | 0.2 (±0.4, 267) | 0 (0–0) | 13 | 0.3 (±1, 374) | 0 (0–0) | 10 | 0.8 (±1.6, 187) | 0 (0–0.9) | 10 | 0.5 (±0.8, 166) | 0 (0–1) | 14 | 0.1 (±0.3, 387) | 0 (0–0) |
| 0.5 h | 12 | 0.4 (±0.9, 237) | 0 (0–0) | 9 | 2.7 (±5.2, 194) | 0 (0–3.2) | 6 | 4.7 (±10.4, 223) | 1.3 (0–2.8) | 5 | 13 (±22.1, 170) | 1.7 (0–17.8) | 10 | 5.6 (±16.2, 289) | 0 (0–1.4) |
| 1 h | 5 | 15 (±22, 147) | 3 (0–19.9) | 2 | 28.5 (±35.7, 125) | 7.1 (2.3–61.6) | 4 | 27.3 (±42.3, 155) | 4.8 (0.6–36.7) | 2 | 202 (±229, 113) | 79.5 (4–426) | 3 | 158 (±231, 146) | 108.5 (4.7–224) |
| 2 h | 2 | 30.1 (±34.2, 114) | 15.5 (2.1–44.4) | 0 | 54.6 (±48.9, 89.6) | 55.6 (5.2–94.4) | 1 | 84.1 (±118, 140) | 26.5 (8.7–101) | 0 | 306 (±371, 121) | 275 (27.5–372) | 1 | 653 (±732, 112) | 318.8 (8.5–1009) |
| 4 h | 0 | 84.6 (±47.9, 56.7) | 73.1 (47.9–116) | 0 | 136 (±83.3, 61.1) | 108 (94.1–143) | 0 | 214 (±157, 73.2) | 177 (93.5–307) | 0 | 556 (±333, 59.9) | 546.6 (439–755) | 0 | 1302 (±838, 64.4) | 1111 (530–1902) |
| 6 h | 1 | 62.2 (±39.8, 63.9) | 53.3 (37.5–66.4) | 0 | 96.3 (±50.9, 52.9) | 82.6 (64.7–132) | 0 | 233 (±173, 74) | 200 (131–276) | 0 | 512 (±259, 50.7) | 496.8 (401–602) | 0 | 1167 (±508, 43.6) | 1023 (841–1310) |
| 8 h | 0 | 46.8 (±23, 49.1) | 38.2 (34.6–51.8) | 0 | 71.6 (±33.8, 47.2) | 78.3 (43.9–86.7) | 0 | 166 (±60.3, 36.4) | 148 (125–208) | 0 | 319 (±118, 37.1) | 303.5 (241–359) | 0 | 917 (±582, 63.4) | 855 (467–1040) |
| Δ9-THCV | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 1 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 14 | 0 (±0.2, 387) | 0 (0–0) |
| 2 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 12 | 0.1 (±0.3, 258) | 0 (0–0) | 9 | 0.6 (±0.8, 134) | 0 (0–1.5) |
| 4 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 10 | 0.3 (±0.6, 183) | 0 (0–0) | 6 | 0.9 (±1, 112) | 0.7 (0–1.5) |
| 6 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 10 | 0.3 (±0.6, 194) | 0 (0–0) | 7 | 0.7 (±0.8, 112) | 0.6 (0–1.5) |
| 8 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 9 | 0.6 (±1.1, 186) | 0 (0–0.7) |
| Δ9-THCV-COOH | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 12 | 0.3 (±0.7, 257) | 0 (0–0) | 14 | 0.1 (±0.6, 387) | 0 (0–0) |
| 1 h | 14 | 0.1 (±0.3, 387) | 0 (0–0) | 9 | 0.7 (±1.1, 144) | 0 (0–1.7) | 11 | 0.6 (±1.3, 204) | 0 (0–0) | 6 | 5.8 (±6.9, 118) | 1.9 (0–13.7) | 7 | 4.9 (±7, 143) | 2.7 (0–7.6) |
| 2 h | 12 | 0.5 (±1.1, 217) | 0 (0–0) | 6 | 1.6 (±1.5, 96.6) | 1.7 (0–2.9) | 8 | 2.3 (±3.9, 170) | 0 (0–2.1) | 4 | 9.2 (±10.2, 111) | 8.7 (0.6–11.6) | 5 | 19.1 (±21.6, 113) | 9.4 (0–30.7) |
| 4 h | 3 | 1.9 (±1.3, 70.7) | 1.8 (1.1–3.1) | 0 | 3.7 (±1.8, 48.1) | 3.6 (2.5–4.9) | 2 | 5.4 (±4.2, 78.3) | 5 (2.4–7.9) | 0 | 16.1 (±11.1, 68.7) | 16 (11.7–19) | 1 | 37.1 (±22.4, 60.4) | 40 (17.6–57.4) |
| 6 h | 6 | 1.5 (±1.6, 107) | 1.3 (0–1.9) | 1 | 3 (±1.6, 53.2) | 2.4 (2.1–4.5) | 0 | 6.7 (±3.9, 58.4) | 5.9 (4.1–8) | 0 | 15.6 (±6.9, 44.2) | 17.5 (11.1–20.1) | 0 | 32.3 (±13.1, 40.4) | 28.5 (24.2–35.6) |
| 8 h | 6 | 1.2 (±1.1, 92.5) | 1.4 (0–2.1) | 2 | 2.3 (±1.4, 61.7) | 2.3 (1.4–3.2) | 0 | 5.4 (±1.9, 36) | 5.2 (4.2–6.5) | 0 | 10.8 (±3.7, 34.7) | 10.5 (7.7–14.1) | 0 | 29.2 (±16.1, 55.3) | 26.3 (17.4–35.5) |
| Δ8-THC-COOH | |||||||||||||||
| Predose | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 0.5 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 1 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 15 | 0 (±0, --) | 0 (0–0) |
| 2 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 11 | 0.4 (±0.7, 175) | 0 (0–0.5) |
| 4 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 10 | 0.4 (±0.7, 167) | 0 (0–0.8) | 6 | 1.3 (±1.2, 90.5) | 1.7 (0–2.2) |
| 6 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 8 | 0.6 (±0.7, 124) | 0 (0–1.2) | 2 | 1.8 (±1, 55.2) | 2 (1.3–2.4) |
| 8 h | 15 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 14 | 0 (±0, --) | 0 (0–0) | 13 | 0.1 (±0.3, 374) | 0 (0–0) | 3 | 1.6 (±1, 62.6) | 1.6 (1.1–2.2) |
References
- Hanus, L.O. Pharmacological and therapeutic secrets of plant and brain (endo)cannabinoids. Med. Res. Rev. 2009, 29, 213–271. [Google Scholar] [CrossRef] [PubMed]
- Potter, D.J. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test. Anal. 2014, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.P. Medical Use of Cannabis in 2019. JAMA 2019, 322, 974–975. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor cannabinoids: Biosynthesis, molecular pharmacology and potential therapeutic uses. Front. Pharmacol. 2021, 12, 777804. [Google Scholar] [CrossRef]
- Zagzoog, A.; Mohamed, K.A.; Kim, H.J.J.; Kim, E.D.; Frank, C.S.; Black, T.; Jadhav, P.D.; Holbrook, L.A.; Laprairie, R.B. In vitro and in vivo pharmacological activity of minor cannabinoids isolated from Cannabis sativa. Sci. Rep. 2020, 10, 20405. [Google Scholar] [CrossRef]
- Peters, E.N.; MacNair, L.; Harrison, A.; Feldner, M.T.; Eglit, G.M.L.; Babalonis, S.; Turcotte, C.; Bonn-Miller, M.O. A two-phase, dose-ranging, placebo-controlled study of the safety and preliminary test of acute effects of oral Δ8-tetrahydrocannabivarin in healthy participants. Cannabis Cannabinoid Res. 2023, 8, S71–S82. [Google Scholar] [CrossRef]
- Mehmedic, Z.; Chandra, S.; Slade, D.; Denham, H.; Foster, S.; Patel, A.S.; Ross, S.A.; Khan, I.A.; ElSohly, M.A. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic. Sci. 2010, 55, 1209–1217. [Google Scholar] [CrossRef]
- Haghdoost, M.; Peters, E.N.; Roberts, M.; Bonn-Miller, M.O. Tetrahydrocannabivarin is not tetrahydrocannabinol. Cannabis Cannabinoid Res. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Haghdoost, M.; Lopez de Los Santos, Y.; Brunstetter, M.; Ferretti, M.L.; Roberts, M.; Bonn-Miller, M.O. Using in silico molecular docking to explain differences in receptor binding behavior of HHC and THCV isomers: Revealing new binding modes. Pharmaceuticals 2024, 17, 637. [Google Scholar] [CrossRef]
- Batkai, S.; Mukhopadhyay, P.; Horvath, B.; Rajesh, M.; Gao, R.Y.; Mahadevan, A.; Amere, M.; Battista, N.; Lichtman, A.H.; Gauson, L.A.; et al. Δ8-Tetrahydrocannabivarin prevents hepatic ischaemia/reperfusion injury by decreasing oxidative stress and inflammatory responses through cannabinoid CB2 receptors. Br. J. Pharmacol. 2012, 165, 2450–2461. [Google Scholar] [CrossRef]
- Abioye, A.; Ayodele, O.; Marinkovic, A.; Patidar, R.; Akinwekomi, A.; Sanyaolu, A. Δ9-Tetrahydrocannabivarin (THCV): A commentary on potential therapeutic benefit for the management of obesity and diabetes. J. Cannabis Res. 2020, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Sempio, C.; Campos-Palomino, J.; Klawitter, J.; Harrison, A.; Peters, E.N.; MacNair, L.; Haghdoost, M.; Bonn-Miller, M.; Babalonis, S.; Huestis, M.A.; et al. LC-MS-MS quantification of Delta8-THC, Delta9-THC, THCV isomers and their main metabolites in human plasma. J. Anal. Toxicol. 2024, 48, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Assanangkornchai, S.; Kalayasiri, R.; Ratta-Apha, W.; Tanaree, A. Effects of cannabis legalization on the use of cannabis and other substances. Curr. Opin. Psychiatry 2023, 36, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, J.; Rosado, T.; Soares, S.; Simao, A.Y.; Caramelo, D.; Luis, A.; Fernandez, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and its secondary metabolites: Their use as therapeutic drugs, toxicological aspects, and analytical determination. Medicines 2019, 6, 31. [Google Scholar] [CrossRef]
- Englund, A.; Atakan, Z.; Kralj, A.; Tunstall, N.; Murray, R.; Morrison, P. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: A placebo-controlled, double-blind, crossover pilot trial. J. Psychopharmacol. 2016, 30, 140–151. [Google Scholar] [CrossRef]
- Jadoon, K.A.; Ratcliffe, S.H.; Barrett, D.A.; Thomas, E.L.; Stott, C.; Bell, J.D.; O’Sullivan, S.E.; Tan, G.D. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care 2016, 39, 1777–1786. [Google Scholar] [CrossRef]
- Newmeyer, M.N.; Swortwood, M.J.; Barnes, A.J.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral cannabis administration in frequent and occasional cannabis users: Identification of recent cannabis intake. Clin. Chem. 2016, 62, 1579–1592. [Google Scholar] [CrossRef]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef]
- Moore, C.F.; Weerts, E.M.; Kulpa, J.; Schwotzer, D.; Dye, W.; Jantzi, J.; McDonald, J.D.; Lefever, T.W.; Bonn-Miller, M.O. Pharmacokinetics of oral minor cannabinoids in blood and brain. Cannabis Cannabinoid Res. 2023, 8, S51–S61. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Feng, S.; Murphy, T.P.; Warrington, A.W.; Ross, S.; Nimrod, A.; Mehmedic, Z.; Fortner, N. Identification and quantitation of 11-nor-delta9-tetrahydrocannabivarin-9-carboxylic acid, a major metabolite of delta9-tetrahydrocannabivarin. J. Anal. Toxicol. 2001, 25, 476–480. [Google Scholar] [CrossRef]
- Rao, Q.; Zhang, T.; Pu, Q.L.; Li, B.; Zhao, Q.; Yan, D.M.; Wu, Z.E.; Li, F. Comparative metabolism of THCA and THCV using UHPLC-Q-Exactive Orbitrap-MS. Xenobiotica 2023, 53, 46–59. [Google Scholar] [CrossRef]
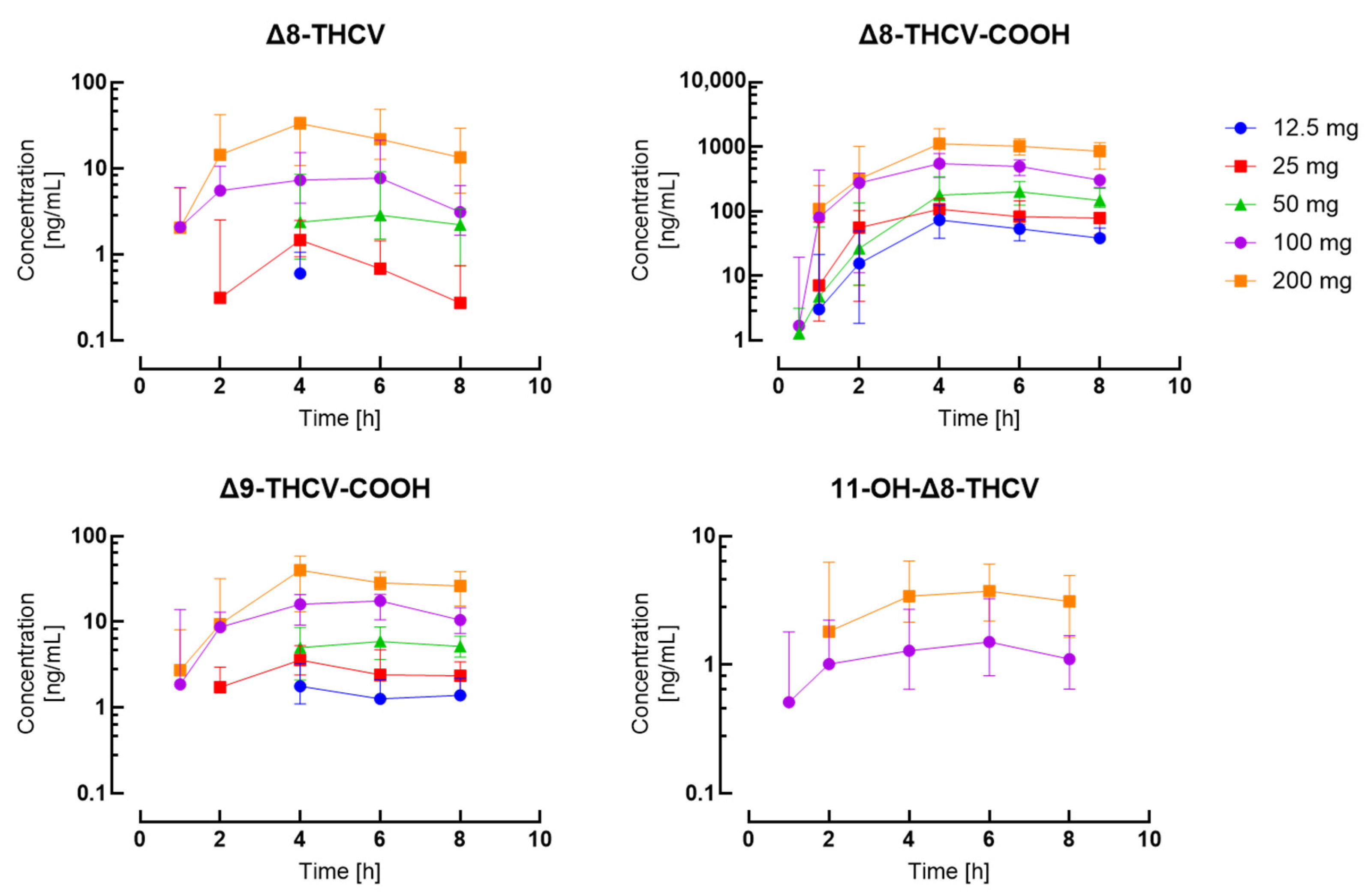
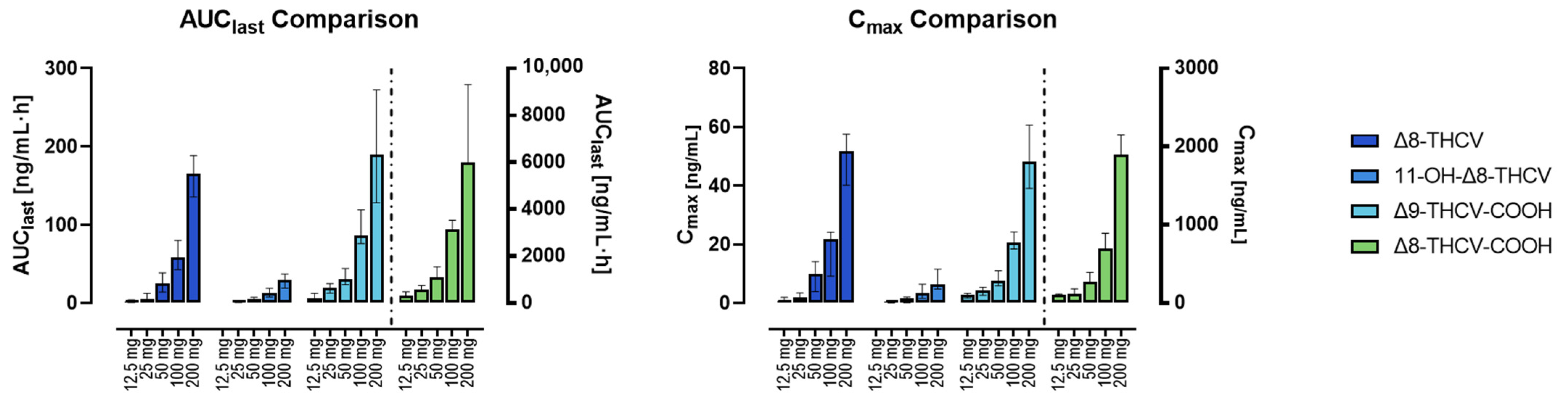
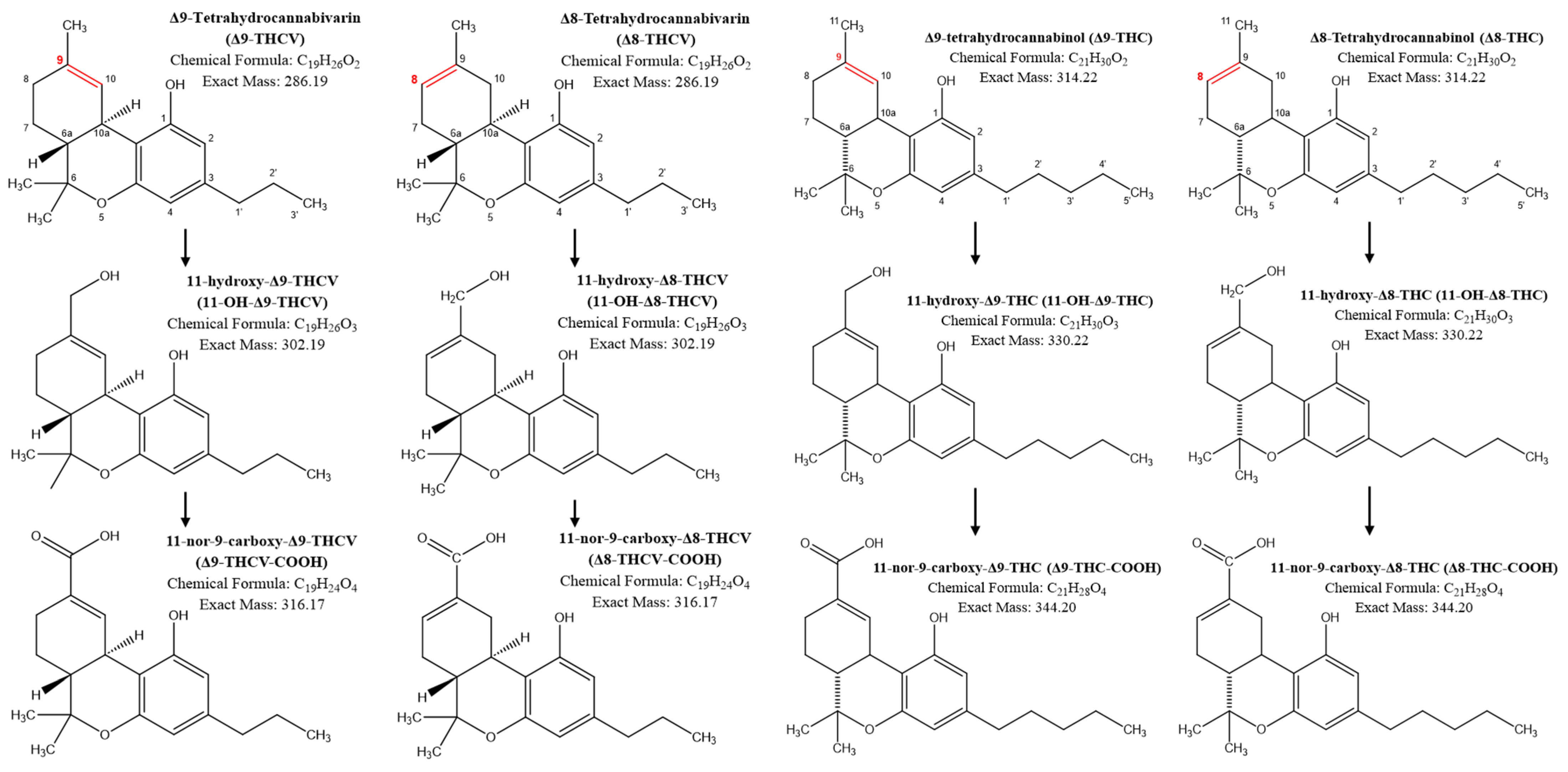
| Timepoint | 12.5 mg | 25 mg | 50 mg | 100 mg | 200 mg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | n | Mean (±SD) | Median (Range) | |
| Δ8-THCV | |||||||||||||||
| tmax | 12 | 3.9 (±2) | 4 (1–8) | 14 | 3.8 (±1.8) | 4 (1–6) | 14 | 5 (±2) | 6 (2–8) | 14 | 4.2 (±2) | 4 (1–8) | 15 | 4.7 (±2.2) | 4 (2–8) |
| Cmax | 12 | 1.6 (±1.1) | 1.1 (0.6–3.3) | 14 | 2.6 (±1.7) | 2.1 (0.9–5.7) | 14 | 10 (±6.8) | 10 (2.4–24.7) | 14 | 21.5 (±13.3) | 22 (5.7–50.4) | 15 | 52.9 (±16.3) | 51.7 (33.3–87.6) |
| tlast | 12 | 5.3 (±2.3) | 5 (2–8) | 14 | 6.3 (±1.9) | 7 (4–8) | 14 | 7.9 (±0.5) | 8 (6–8) | 14 | 8 (±0) | 8 (8–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 12 | 0.9 (±0.3) | 0.7 (0.6–1.5) | 14 | 1.1 (±0.4) | 1 (0.5–1.8) | 14 | 2.3 (±1.5) | 2.2 (0.6–5.5) | 14 | 4.1 (±3.1) | 3.1 (0.8–9.9) | 15 | 21.2 (±23.2) | 13.4 (1.5–87.6) |
| AUClast | 12 | 3.5 (±3.1) | 2.1 (0.3–9.3) | 14 | 7.4 (±5.5) | 5.2 (1.4–16.5) | 14 | 27.3 (±18.5) | 24.9 (2.6–68.6) | 14 | 65.5 (±34.3) | 58.4 (15.7–137) | 15 | 168 (±54.3) | 165 (93.9–299) |
| MRT | 12 | 4 (±1.9) | 4 (1.4–8) | 14 | 4.1 (±1.1) | 4.2 (2.4–5.8) | 14 | 5.2 (±1.4) | 5.4 (2.5–8) | 14 | 4.5 (±1.3) | 4.1 (2.4–7.1) | 15 | 4.7 (±1.3) | 4.7 (2.7–6.5) |
| 11-OH-Δ8-THCV | |||||||||||||||
| tmax | 5 | 3.4 (±1.9) | 4 (1–6) | 7 | 3.7 (±1.8) | 4 (2–6) | 10 | 5 (±1.9) | 6 (2–8) | 14 | 4.2 (±2.4) | 5 (1–8) | 15 | 4.7 (±2) | 4 (2–8) |
| Cmax | 5 | 0.8 (±0.2) | 0.8 (0.6–1.1) | 7 | 0.9 (±0.2) | 0.8 (0.6–1.3) | 10 | 2 (±1) | 1.9 (0.6–3.7) | 14 | 4.3 (±3) | 3.4 (1.3–10.6) | 15 | 8.7 (±5.4) | 6.4 (2.6–22.2) |
| tlast | 5 | 3.6 (±1.7) | 4 (2–6) | 7 | 4.3 (±1.8) | 4 (2–6) | 10 | 7.8 (±0.6) | 8 (6–8) | 14 | 7.6 (±1.2) | 8 (4–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 5 | 0.8 (±0.2) | 0.8 (0.6–1.1) | 7 | 0.8 (±0.1) | 0.8 (0.6–1) | 10 | 0.9 (±0.3) | 0.8 (0.6–1.5) | 14 | 1.4 (±0.9) | 1.1 (0.5–3.8) | 15 | 3.7 (±2.6) | 3.1 (1.2–11.3) |
| AUClast | 5 | 0.7 (±0.1) | 0.8 (0.6–0.8) | 7 | 1.5 (±1.9) | 0.8 (0.4–5.8) | 10 | 6.2 (±4) | 5.3 (0.6–14.7) | 14 | 13.9 (±9.9) | 12.4 (1.5–40.3) | 15 | 30.8 (±18.6) | 29.5 (10.6–87.8) |
| MRT | 5 | 1.5 (±1.7) | 1.4 (0–4) | 7 | 3.6 (±1.4) | 3.6 (2–6) | 10 | 5.7 (±1.5) | 6.1 (3–8) | 14 | 4.6 (±1.6) | 4.3 (2.3–8) | 15 | 4.8 (±1.2) | 4.7 (3.2–6.6) |
| Δ8-THCV-COOH | |||||||||||||||
| tmax | 15 | 4.9 (±1.7) | 4 (2–8) | 14 | 4.6 (±0.9) | 4 (4–6) | 14 | 5.3 (±1.3) | 6 (4–8) | 14 | 4.7 (±1.9) | 4 (2–8) | 15 | 5.3 (±1.8) | 6 (2–8) |
| Cmax | 15 | 99 (±37) | 101 (39.2–178) | 14 | 151 (±76.8) | 116 (91.7–357) | 14 | 324 (±165) | 275 (114–765) | 14 | 756 (±302) | 699 (307–1392) | 15 | 1738 (±579) | 1899 (868–2594) |
| tlast | 15 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 15 | 46.8 (±23) | 38.2 (20.1–102) | 14 | 71.6 (±33.8) | 78.3 (25.5–146) | 14 | 166 (±60.3) | 148 (78.9–264) | 14 | 319 (±118) | 304 (153–590) | 15 | 917 (±582) | 855 (364–2402) |
| AUClast | 15 | 398 (±151) | 331 (216–787) | 14 | 642 (±298) | 574 (264–1311) | 14 | 1209 (±582) | 1093 (364–2194) | 14 | 3071 (±1101) | 3132 (577–4951) | 15 | 6955 (±2671) | 5999 (3345–10759) |
| MRT | 15 | 4.9 (±0.8) | 4.9 (3.7–6.6) | 14 | 4.8 (±0.6) | 4.6 (3.8–5.9) | 14 | 5.3 (±0.9) | 5.3 (4–7.4) | 14 | 4.8 (±1) | 4.6 (3.6–6.7) | 15 | 5.1 (±1) | 5.1 (3.6–6.6) |
| Δ9-THCV | |||||||||||||||
| tmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 4.6 (±1.8) | 5 (1–6) | 13 | 4.9 (±2.3) | 4 (2–8) |
| Cmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.1 (±0.5) | 0.9 (0.5–1.9) | 13 | 2 (±0.9) | 1.7 (0.8–4.1) |
| tlast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 4.8 (±1.5) | 5 (2–6) | 13 | 6.2 (±2.1) | 6 (2–8) |
| Clast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.1 (±0.5) | 0.8 (0.5–1.9) | 13 | 1.5 (±1) | 1.4 (0.5–4.1) |
| AUClast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.4 (±0.8) | 1.2 (0.5–3.1) | 13 | 4.8 (±2.9) | 4.5 (1–10.2) |
| MRT | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 4.4 (±1.7) | 4.4 (1.3–6) | 13 | 4.5 (±1.6) | 4.6 (2–7.2) |
| Δ9-THCV-COOH | |||||||||||||||
| tmax | 15 | 5.1 (±2) | 4 (2–8) | 14 | 4.6 (±1.5) | 4 (2–8) | 14 | 5.1 (±1.9) | 6 (2–8) | 14 | 4.5 (±2) | 4 (1–8) | 15 | 5.3 (±2.1) | 6 (2–8) |
| Cmax | 15 | 2.7 (±1.2) | 2.8 (1.1–5) | 14 | 4.2 (±1.6) | 4.4 (1.9–7.5) | 14 | 8.8 (±3.7) | 7.7 (3.7–16.1) | 14 | 22.5 (±8.3) | 20.6 (11.3–41.6) | 15 | 50.3 (±13.8) | 48.2 (31.4–71.1) |
| tlast | 15 | 6.8 (±1.7) | 8 (4–8) | 14 | 7.6 (±1.2) | 8 (4–8) | 14 | 8 (±0) | 8 (8–8) | 14 | 8 (±0) | 8 (8–8) | 15 | 8 (±0) | 8 (8–8) |
| Clast | 15 | 1.9 (±0.8) | 1.7 (1.1–3.3) | 14 | 2.6 (±1) | 2.4 (1.3–4.4) | 14 | 5.4 (±1.9) | 5.2 (2.4–8.5) | 14 | 10.8 (±3.7) | 10.5 (6.2–16.1) | 15 | 29.2 (±16.1) | 26.3 (10.9–63.8) |
| AUClast | 15 | 7.9 (±5.7) | 5.6 (1.1–20.1) | 14 | 18.3 (±8.7) | 19.6 (2.5–30.6) | 14 | 33.4 (±15.8) | 30.8 (8.7–62.7) | 14 | 92.5 (±35.3) | 86.5 (17.6–149.2) | 15 | 201 (±77.9) | 190 (89.7–336) |
| MRT | 15 | 5.1 (±1.3) | 5.3 (3.2–8) | 14 | 4.8 (±0.7) | 4.6 (3.9–6.1) | 14 | 5.5 (±1) | 5.5 (4–7.4) | 14 | 4.9 (±1) | 4.6 (3.7–7) | 15 | 5.1 (±1) | 5.2 (3.7–6.7) |
| Δ8-THC-COOH | |||||||||||||||
| tmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 5 (±1.1) | 5 (4–6) | 15 | 6.1 (±1.6) | 6 (4–8) |
| Cmax | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.4 (±0.3) | 1.4 (1–1.7) | 15 | 2.4 (±0.7) | 2.5 (1.4–3.8) |
| tlast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 5.8 (±1.3) | 6 (4–8) | 15 | 7.5 (±1.2) | 8 (4–8) |
| Clast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 1.3 (±0.2) | 1.3 (1–1.7) | 15 | 1.9 (±0.6) | 1.8 (1.1–2.8) |
| AUClast | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 2.4 (±1.5) | 1.6 (1–4.7) | 15 | 8.1 (±4.5) | 9.6 (1.4–15) |
| MRT | -- | -- | -- | -- | -- | -- | -- | -- | -- | 8 | 5.2 (±1) | 5.3 (4–6.5) | 15 | 5.7 (±1.3) | 5.8 (3.1–8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sempio, C.; Campos-Palomino, J.; Klawitter, J.; Peters, E.N.; MacNair, L.; Haghdoost, M.; Bonn-Miller, M.O.; Harrison, A.; Babalonis, S.; Christians, U.; et al. Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants. Pharmaceuticals 2024, 17, 1603. https://doi.org/10.3390/ph17121603
Sempio C, Campos-Palomino J, Klawitter J, Peters EN, MacNair L, Haghdoost M, Bonn-Miller MO, Harrison A, Babalonis S, Christians U, et al. Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants. Pharmaceuticals. 2024; 17(12):1603. https://doi.org/10.3390/ph17121603
Chicago/Turabian StyleSempio, Cristina, Jorge Campos-Palomino, Jelena Klawitter, Erica N. Peters, Laura MacNair, Mehdi Haghdoost, Marcel O. Bonn-Miller, Amy Harrison, Shanna Babalonis, Uwe Christians, and et al. 2024. "Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants" Pharmaceuticals 17, no. 12: 1603. https://doi.org/10.3390/ph17121603
APA StyleSempio, C., Campos-Palomino, J., Klawitter, J., Peters, E. N., MacNair, L., Haghdoost, M., Bonn-Miller, M. O., Harrison, A., Babalonis, S., Christians, U., & Klawitter, J. (2024). Pharmacokinetics of Oral Cannabinoid Δ8-Tetrahydrocannabivarin and Its Main Metabolites in Healthy Participants. Pharmaceuticals, 17(12), 1603. https://doi.org/10.3390/ph17121603







