Atopic Dermatitis-Related Problems in Daily Life, Goals of Therapy and Deciding Factors for Systemic Therapy: A Review
Abstract
1. Introduction
2. The Impact of Atopic Dermatitis on Emotional and Psychological States
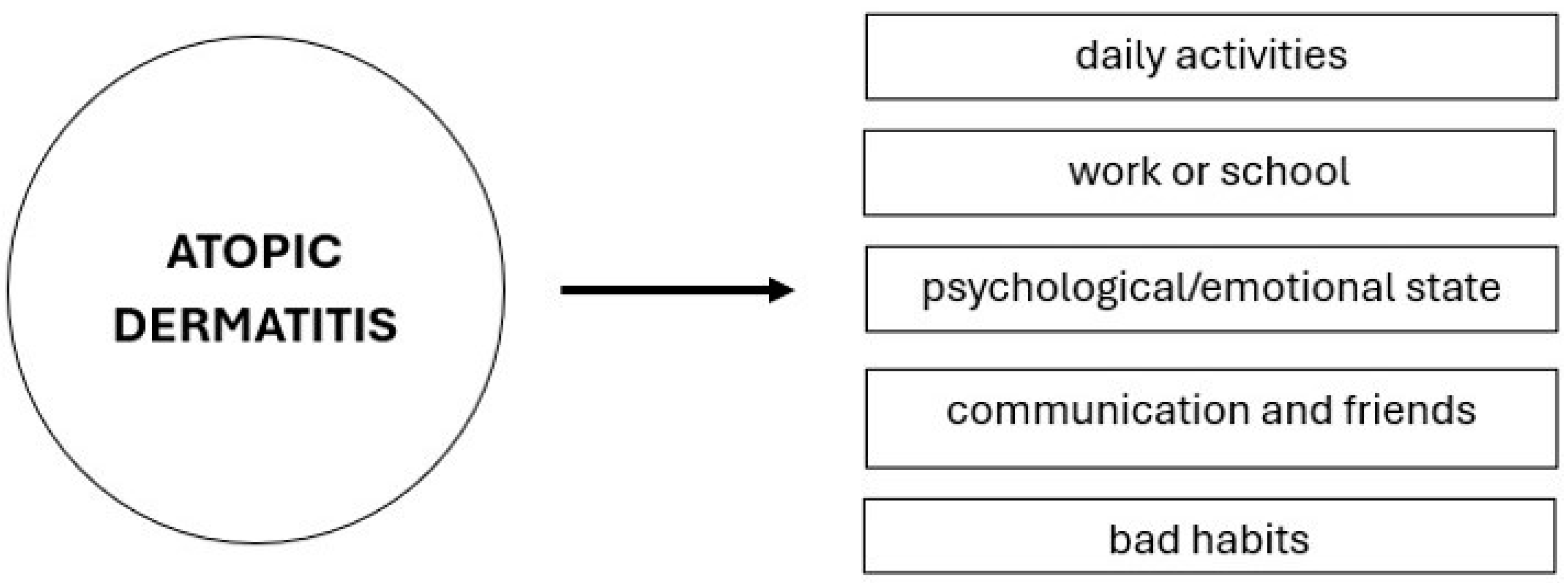
3. The Impact of Atopic Dermatitis on Daily Activities, Work Productivity, and Quality of Life
4. Economic Costs of Atopic Dermatitis
5. Standard Therapy and Advanced Treatment Possibilities
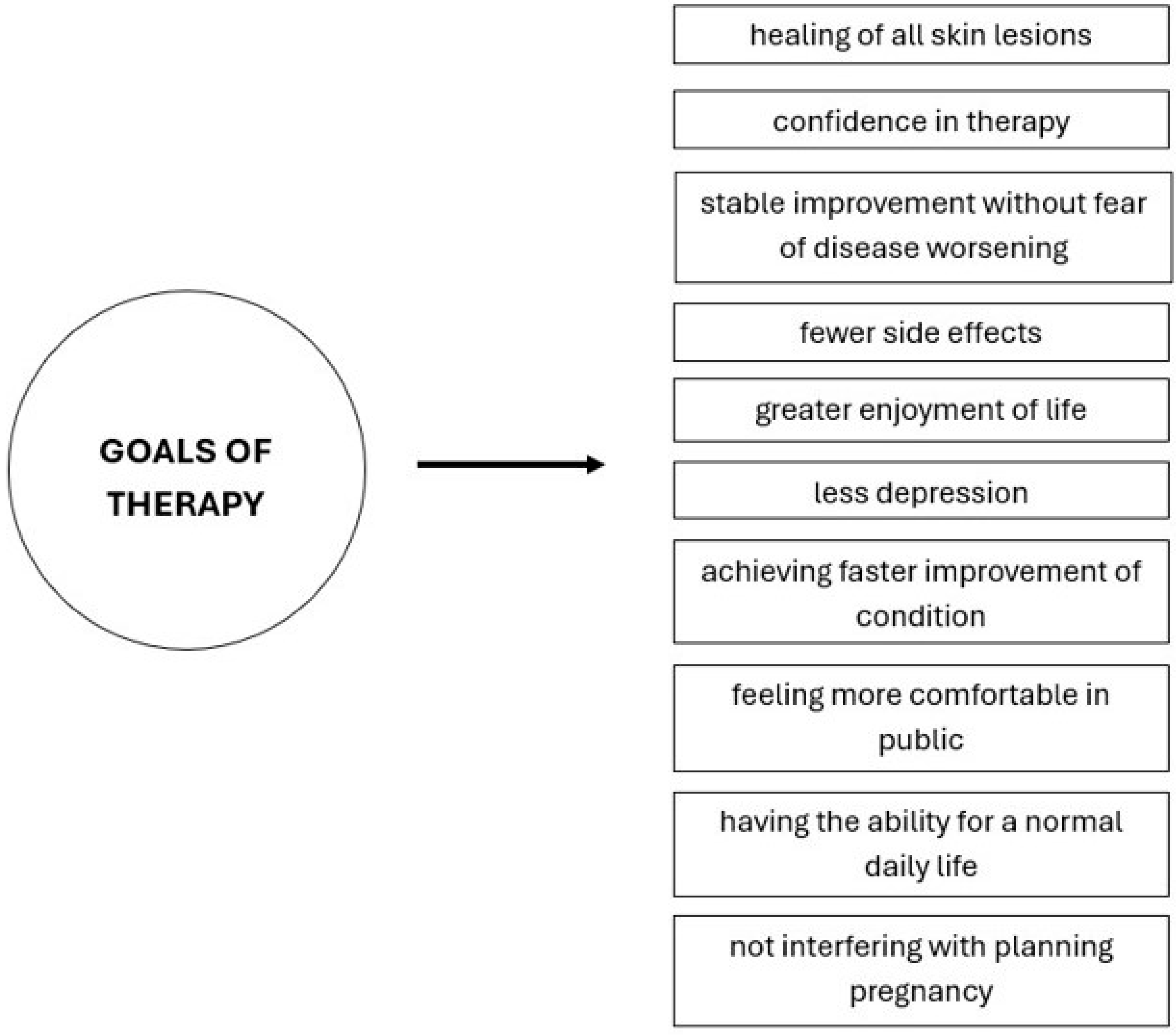
6. Achieving Therapeutic Goals
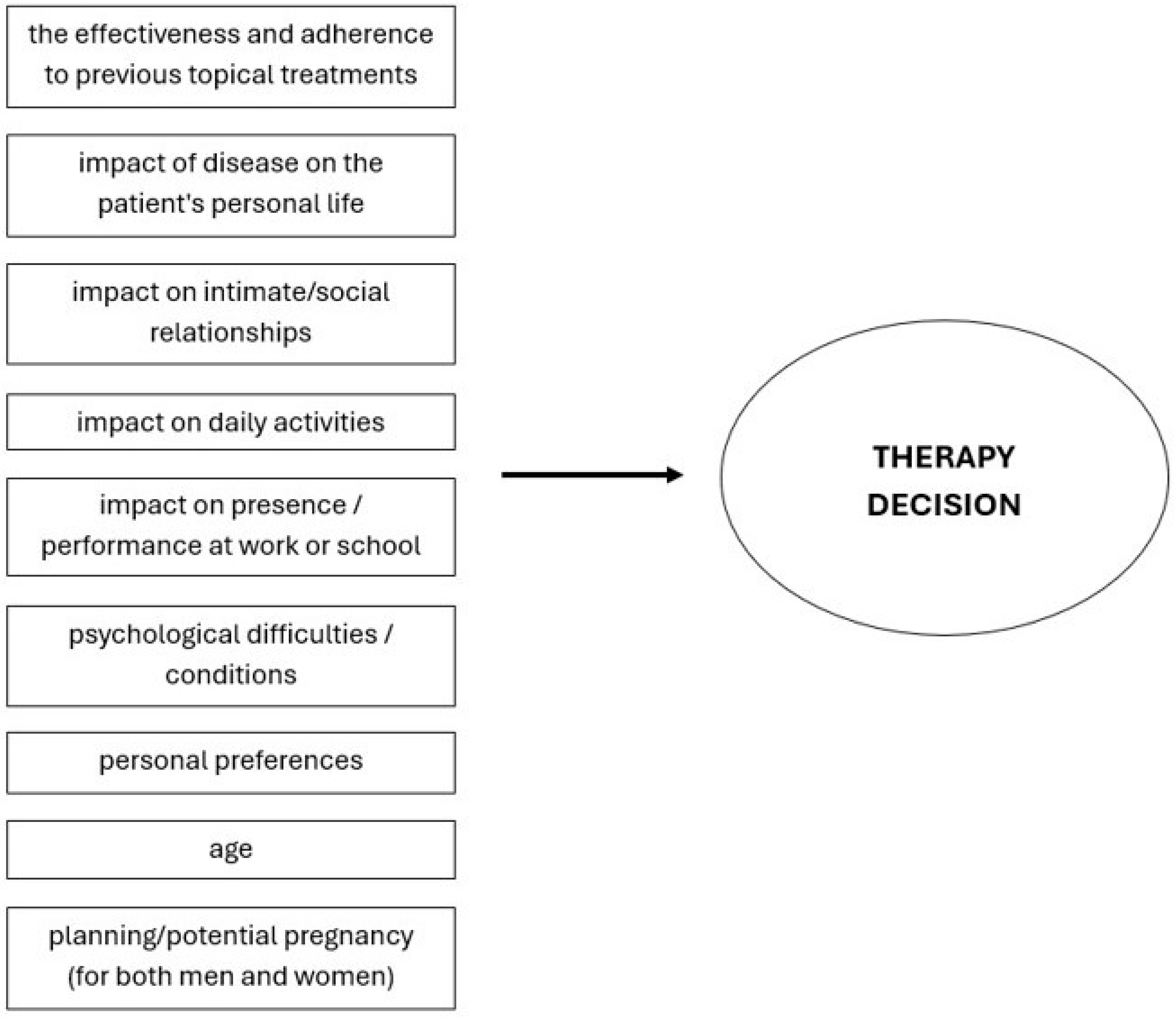
7. Decision to Initiate Systemic Therapy
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Russo, F.; Santi, F.; Cioppa, V.; Orsini, C.; Lazzeri, L.; Cartocci, A.; Rubegni, P. Meeting the needs of patients with atopic dermatitis: A multidisciplinary approach. Dermatitis 2022, 33, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Potočnjak, I.; Cindrić, T.; Ilić, I.; Lovrić, I.; Skalicki, L.; Bešlić, I.; Pondeljak, N. Atopic dermatitis: Disease features, therapeutic options, and a multidisciplinary approach. Life 2023, 13, 1419. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Rabe, A.; Blanthorn-Hazell, S.; Millward, R. Prevalence and clinical profile of atopic dermatitis (AD) in England—A population-based cohort study using the Clinical Practice Research Datalink (CPRD) and Hospital Episode Statistics (HES). Value Health 2020, 23, 745. [Google Scholar] [CrossRef]
- Augustin, M.; Langenbruch, A.; Blome, C.; Gutknecht, M.; Werfel, T.; Ständer, S.; Steinke, S.; Kirsten, N.; Silva, N.; Sommer, R. Characterizing treatment-related patient needs in atopic eczema: Insights for personalized goal-oriented care. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 142–152. [Google Scholar] [CrossRef]
- Chee, A.; Branca, L.; Jeker, F.; Vogt, D.R.; Schwegler, S.; Navarini, A.; Itin, P.; Mueller, S.M. When life is itchy: What harms, helps, and heals from a patient’s perspective? Differences and similarities in skin diseases. Dermatol. Ther. 2020, 33, e13606. [Google Scholar] [CrossRef]
- Falissard, B.; Simpson, E.L.; Guttman-Yassky, E.; Papp, K.A.; Barbarot, S.; Gadkari, A.; Saba, G.; Gautier, L.; Abbe, A.; Eckert, L. Qualitative assessment of adult patients’ perceptions of atopic dermatitis using natural language processing analysis in a cross-sectional study. Dermatol. Ther. 2020, 10, 297–305. [Google Scholar] [CrossRef]
- Xie, Q.W.; Dai, X.; Tang, X.; Chan, C.H.Y.; Chan, C.L.W. Risk of mental disorders in children and adolescents with atopic dermatitis: A systematic review and meta-analysis. Front. Psychol. 2019, 10, 1773. [Google Scholar] [CrossRef]
- El Hachem, M.; Di Mauro, G.; Rotunno, R.; Giancristoforo, S.; De Ranieri, C.; Carlevaris, C.M.; Verga, M.C.; Dello Iacono, I. Pruritus in pediatric patients with atopic dermatitis: A multidisciplinary approach-summary document of the Italian expert group. Ital. J. Pediatr. 2020, 46, 11. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis: An expanded therapeutic framework for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef]
- Williams, H.; Stewart, A.; von Mutius, E.; Cookson, W.; Anderson, H.R. Is eczema really on the increase worldwide? J. Allergy Clin. Immunol. 2008, 121, 947–954.e15. [Google Scholar] [CrossRef]
- Ring, J.; Zink, A.; Arents, B.W.M.; Seitz, I.A.; Mensing, U.; Schielein, M.C.; Wettemann, N.; de Carlo, G.; Fink-Wagner, A. Atopic eczema: Disease burden and individual suffering—Results from a large EU study in adults. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Laughter, M.R.; Maymone, M.B.C.; Mashayekhi, S.; Arents, B.W.M.; Karimkhani, C.; Langan, S.M.; Dellavalle, R.P.; Flohr, C. The global burden of atopic dermatitis: Lessons from the Global Burden of Disease Study 1990–2017. Br. J. Dermatol. 2021, 184, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Fasseeh, A.N.; Elezbawy, B.; Korra, N.; Tannira, M.; Dalle, H.; Aderian, S.; Abaza, S.; Kaló, Z. The burden of atopic dermatitis in adults and adolescents: A systematic review of the literature. Dermatol. Ther. 2022, 12, 2653–2668. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.; Dreher, M.; Weess, H.G.; Staubach, P. Sleep disorders in patients with urticaria and atopic dermatitis: An underestimated burden. Acta Derm. Venereol. 2020, 100, 5678. [Google Scholar] [CrossRef]
- Pedersen, C.J.; Uddin, M.J.; Saha, S.K.; Darmstadt, G.L. Prevalence and psychosocial impact of atopic dermatitis in Bangladeshi children and families. PLoS ONE 2021, 16, e0249824. [Google Scholar] [CrossRef]
- Marron, S.E.; Cebrian-Rodriguez, J.; Alcalde-Herrero, V.M.; Garcia-Latasa de Aranibar, F.J.; Tomas-Aragones, L. Psychosocial impact of atopic dermatitis in adults: A qualitative study. Actas Dermosfiliogr. 2020, 111, 513–517. [Google Scholar] [CrossRef]
- Devleesschauwer, B.; Maertens de Noordhout, C.; Smit, G.S.; Duchateau, L.; Dorny, P.; Stein, C.; Van Oyen, H.; Speybroeck, N. Quantifying the burden of disease to support public health policy in Belgium: Opportunities and limitations. BMC Public Health 2014, 14, 1196. [Google Scholar] [CrossRef]
- Jaworek, A.K.; Jaworek, M.; Szafraniec, K.; Wojas-Pelc, A.; Szepietowski, J.C. Melatonin and sleep disorders in patients with severe atopic dermatitis. Postepy Dermatol. Alergol. 2021, 38, 746–751. [Google Scholar] [CrossRef]
- Bešlić, I.; Lugović-Mihić, L.; Vrtarić, A.; Bešlić, A.; Škrinjar, I.; Hanžek, M.; Crnković, D.; Artuković, M. Melatonin in dermatologic allergic diseases and other skin conditions: Current trends and reports. Int. J. Mol. Sci. 2023, 24, 4039. [Google Scholar] [CrossRef]
- Yu, S.H.; Attarian, H.; Zee, P.; Silverberg, J.I. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis 2016, 27, 50–58. [Google Scholar] [CrossRef]
- Meštrović-Štefekov, J.; Novak-Bilić, G.; Kuna, M.; Pap, N.; Lugović-Mihić, L. Psychological stress in patients with atopic dermatitis. Acta Dermatovenerol. Croat. 2018, 26, 297–303. [Google Scholar] [PubMed]
- Lee, S.H.; Lee, S.H.; Lee, S.Y.; Lee, B.; Lee, S.H.; Park, Y.L. Psychological health status and health-related quality of life in adults with atopic dermatitis: A national cross-sectional study in South Korea. Acta Derm. Venereol. 2018, 98, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, A.; Fujita, H.; Arima, K.; Inoue, T.; Dorey, J.; Fukushima, A.; Taguchi, Y. Health-care resource use and current treatment of adult atopic dermatitis patients in Japan: A retrospective claims database analysis. J. Dermatol. 2019, 46, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Lei, D.; Yousaf, M.; Janmohamed, S.R.; Vakharia, P.P.; Chopra, R.; Chavda, R.; Gabriel, S.; Patel, K.R.; Singam, V.; et al. Association of atopic dermatitis severity with cognitive function in adults. J. Am. Acad. Dermatol. 2020, 83, 1349–1359. [Google Scholar] [CrossRef]
- Arima, K.; Gupta, S.; Gadkari, A.; Hiragun, T.; Kono, T.; Katayama, I.; Demiya, S.; Eckert, L. The burden of atopic dermatitis among Japanese adults: An analysis of data from the 2013 National Health and Wellness Survey. J. Dermatol. 2018, 45, 390–396. [Google Scholar] [CrossRef]
- Eckert, L.; Gupta, S.; Gadkari, A.; Mahajan, P.; Gelfand, J.M. Disease burden in adults with atopic dermatitis: Analysis of survey data from France, Germany, Italy, Spain, and the United Kingdom. J. Am. Acad. Dermatol. 2019, 81, 187–195. [Google Scholar] [CrossRef]
- Vinh, N.M.; Trang, V.T.T.; Dac Thuy, L.N.; Tam, H.T.X.; Hang, L.T.T.; Bac, P.V. The anxiety and depression disorder in adults with atopic dermatitis: Experience of a dermatology hospital. Dermatol. Rep. 2022, 15, 9524. [Google Scholar]
- Kwon, J.A.; Park, E.-C.; Lee, M.; Yoo, K.-B.; Park, S. Does stress increase the risk of atopic dermatitis in adolescents? Results of the Korean Youth Risk Behavior Web-Based Survey (KYRBWS-VI). PLoS ONE 2013, 8, e67890. [Google Scholar]
- Schut, C.; Weik, U.; Tews, N.; Gieler, U.; Deinzer, R.; Kupfer, J. Psychophysiological effects of stress management in patients with atopic dermatitis: A randomized controlled trial. Acta Derm. Venereol. 2013, 93, 57–61. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Cvitanović, H.; Bulat, V.; Duvančić, T.; Pondeljak, N.; Tolušić-Levak, M.; Lazić-Mosler, E.; Novak-Bilić, G. The COVID-19 pandemic and recent earthquake in Zagreb together significantly increased the disease severity of patients with atopic dermatitis. Dermatology 2023, 239, 91–98. [Google Scholar] [CrossRef]
- Choi, C. Factors affecting life satisfaction among adolescents with atopic dermatitis. Stud. Korean Youth 2015, 26, 111–144. [Google Scholar]
- Sandhu, J.K.; Wu, K.K.; Bui, T.-L.; Armstrong, A.W. Association between atopic dermatitis and suicidality: A systematic review and meta-analysis. JAMA Dermatol. 2019, 155, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Pondeljak, N.; Lugović-Mihić, L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin. Ther. 2020, 42, 757–770. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Meštrović-Štefekov, J.; Ferček, I.; Pondeljak, N.; Lazić-Mosler, E.; Gašić, A. Atopic dermatitis severity, patient perception of the disease, and personality characteristics: How are they related to quality of life? Life 2021, 11, 1434. [Google Scholar] [CrossRef]
- McFadden, J.; Thyssen, J.; Basketter, D.; Puangpet, P.; Kimber, I. T-helper 2 immune skewing in pregnancy/early life: Chemical exposures and development of atopic disease and allergy. Br. J. Dermatol. 2015, 172, 584–591. [Google Scholar] [CrossRef]
- Nakashima, C.; Ishida, Y.; Kitoh, A.; Otsuka, A.; Kabashima, K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp. Dermatol. 2019, 28, 1405–1411. [Google Scholar] [CrossRef]
- Zhu, Y.-B.; Xu, L.; Wang, Y.; Zhang, R.; Wang, Y.C.; Li, J.B.; Mu, D. The posterior thalamic nucleus mediates histaminergic itch of the face. Neuroscience 2020, 444, 54–63. [Google Scholar] [CrossRef]
- Kong, S.; Koo, J.; Lim, S.K. Association between stress and physical activity in Korean adolescents with atopic dermatitis based on the Korea Youth Risk Behavior Survey 2018–2019. Int. J. Environ. Res. Public Health 2020, 17, 8175. [Google Scholar] [CrossRef]
- Mücke, M.; Ludyga, S.; Colledge, F.; Gerber, M. The impact of regular physical activity and fitness on stress reactivity measured using the Trier Social Stress Test protocol: A systematic review. Sports Med. 2018, 48, 2607–2622. [Google Scholar] [CrossRef]
- Siddiqui, A.F. Self-Perceived social support of patients with chronic skin diseases in Saudi Arabia: A cross-sectional survey. J. Clin. Med. 2023, 12, 5406. [Google Scholar] [CrossRef]
- Koszorú, K.; Borza, J.; Gulácsi, L.; Sárdy, M. Quality of life of patients with atopic dermatitis. Cutis 2019, 104, 174–177. [Google Scholar] [PubMed]
- Japundžić, I.; Bembić, M.; Špiljak, B.; Parać, E.; Macan, J.; Lugović-Mihić, L. Work-Related Hand Eczema in Healthcare Workers: Etiopathogenic Factors, Clinical Features, and Skin Care. Cosmetics 2023, 10, 134. [Google Scholar] [CrossRef]
- Lugović-Mihić, L.; Ferček, I.; Duvančić, T.; Bulat, V.; Ježovita, J.; Novak-Bilić, G.; Šitum, M. Occupational contact dermatitis amongst dentists and dental technicians. Acta Clin. Croat. 2016, 55, 293–300. [Google Scholar] [CrossRef][Green Version]
- Wei, W.; Ghorayeb, E.; Andria, M.; Walker, V.; Schnitzer, J.; Kennedy, M.; Chen, Z.; Belland, A.; White, J.; Silverberg, J.I. Real-world study evaluating the adequacy of existing systemic treatments for patients with moderate-to-severe atopic dermatitis (QUEST-AD): Baseline treatment patterns and unmet needs assessment. Ann. Allergy Asthma Immunol. 2019, 123, 381–388.e2. [Google Scholar] [CrossRef]
- Eckert, L.; Gupta, S.; Amand, C.; Gadkari, A.; Mahajan, P.; Gelfand, J.M. The burden of atopic dermatitis in adults in the United States: Data on healthcare resource utilization from the 2013 National Health and Wellness Survey. J. Am. Acad. Dermatol. 2018, 78, 54–61. [Google Scholar] [CrossRef]
- Ariëns, L.F.M.; Van Nimwegen, K.J.M.; Shams, M.; De Bruin, D.T.; Van der Schaft, J.; Van Os-Medendorp, H.; De Bruin-Weller, M. Economic burden of adult patients with moderate-to-severe atopic dermatitis indicated for systemic treatment. Acta Derm. Venereol. 2019, 99, 762–768. [Google Scholar] [CrossRef]
- Murota, H.; Inoue, S.; Yoshida, K.; Ishimoto, A. Cost-of-illness study for adult atopic dermatitis in Japan: A cross-sectional web-based survey. J. Dermatol. 2020, 47, 689–698. [Google Scholar] [CrossRef]
- Le, P.H.; Vo, T.Q. Economic burden and productivity loss associated with eczema: A prevalence-based follow-up study in Vietnam. J. Pak. Med. Assoc. 2019, 69, S57–S63. [Google Scholar]
- Lin, Y.; Chu, C.; Cho, Y.; Lee, C.; Tsai, C.; Tang, C. PSY11 Work productivity and activity impairment among patients with atopic dermatitis in Taiwan. Value Health 2019, 22, S376. [Google Scholar] [CrossRef]
- Ezzedine, K.; Shourick, J.; Merhand, S.; Sampogna, F.; Taïeb, C. Impact of atopic dermatitis in adolescents and their parents: A French study. Acta Derm. Venereol. 2020, 100, 00294. [Google Scholar] [CrossRef]
- Andersen, L.; Nyeland, M.E.; Nyberg, F. Increasing severity of atopic dermatitis is associated with a negative impact on work productivity among adults with atopic dermatitis in France, Germany, the UK, and the USA. Br. J. Dermatol. 2020, 182, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Shalom, G.; Babaev, M.; Kridin, K.; Schonmann, Y.; Horev, A.; Dreiher, J.; Cohen, A.D. Health service utilization by 116,816 patients with atopic dermatitis in Israel. Acta Derm. Venereol. 2019, 99, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Eckert, L.; Gupta, S.; Amand, C.; Gadkari, A.; Mahajan, P.; Gelfand, J.M. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: Analysis using the National Health and Wellness Survey. J. Am. Acad. Dermatol. 2017, 77, 274–279.e3. [Google Scholar] [CrossRef] [PubMed]
- Sicras-Mainar, A.; Navarro-Artieda, R.; Carrillo, J.C. Economic impact of atopic dermatitis in adults: A population-based study (IDEA study). Actas Dermosifiliogr. 2018, 109, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Dieris-Hirche, J.; Gieler, U.; Petrak, F.; Milch, W.; Te Wildt, B.; Dieris, B.; Herpertz, S. Suicidal ideation in adult patients with atopic dermatitis: A German cross-sectional study. Acta Derm. Venereol. 2017, 97, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Girolomoni, G.; Luger, T.; Nosbaum, A.; Gruben, D.; Romero, W.; Llamado, L.J.; DiBonaventura, M. Economic and psychosocial burden of comorbidities in adults with moderate to severe atopic dermatitis in Europe: A cross-sectional survey analysis. Dermatol. Ther. 2020, 11, 117–130. [Google Scholar] [CrossRef]
- Drucker, A.M.; Qureshi, A.A.; Amand, C.; Villeneuve, S.; Gadkari, A.; Chao, J.; Kuznik, A.; Bégo-Le-Bagousse, G.; Eckert, L. Healthcare resource utilization and costs among adults with atopic dermatitis in the United States: A claims-based analysis. J. Allergy Clin. Immunol. Pract. 2018, 6, 1342–1348. [Google Scholar] [CrossRef]
- Launois, R.; Ezzedine, K.; Cabout, E.; Reguai, Z.; Merrhand, S.; Heas, S.; Seneschal, J.; Misery, L.; Taieb, C. The importance of out-of-pocket costs for adult patients with atopic dermatitis in France. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1921–1927. [Google Scholar] [CrossRef]
- Väkevä, L.; Niemelä, S.; Lauha, M.; Pasternack, R.; Hannuksela-Svahn, A.; Hjerppe, A.; Joensuu, A.; Soronen, M.; Ylianttila, L.; Pastila, R.; et al. Narrowband ultraviolet B phototherapy improves quality of life in patients with psoriasis and atopic dermatitis up to 3 months: Results of an observational multicenter study. Photodermatol. Photoimmunol. Photomed. 2019, 35, 332–338. [Google Scholar] [CrossRef]
- Schild, M.; Weber, V.; Galetzka, W.; Enders, D.; Zügel, F.S.; Gothe, H. Health resource utilization and associated costs among patients with atopic dermatitis—A retrospective cohort study based on German health claims data. Value Health 2020, 23, S745. [Google Scholar] [CrossRef]
- Reed, B.; Blaiss, M.S. The burden of atopic dermatitis. Allergy Asthma Proc. 2018, 39, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Buethe, M.G.; Kellogg, C.; Seo, Y.J.; Vuong, C.; Eichenfield, L.F. Topical therapy for atopic dermatitis: What is new and the new paradigm. Dermatol. Clin. 2024, 42, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Braschi, É.; Young, J.; Allan, G.M. Topical corticosteroids for atopic dermatitis. Can. Fam. Physician 2024, 70, 558. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Howe, W. Atopic Dermatitis (Eczema): Pathogenesis, Clinical Manifestations, and Diagnosis. Available online: https://www.uptodate.com/contents/atopic-dermatitis-eczema-pathogenesis-clinical-manifestations-and-diagnosis (accessed on 18 September 2024).
- Shergill, M.; Bajwa, B.; Yilmaz, O.; Tailor, K.; Bouadi, N.; Mukovozov, I. Biologic and small molecule therapy in atopic dermatitis. Biomedicines 2024, 12, 1841. [Google Scholar] [CrossRef]
- Flohr, C.; Rosala-Hallas, A.; Jones, A.P.; Beattie, P.; Baron, S.; Browne, F.; Brown, S.J.; Gach, J.E.; Greenblatt, D.; Hearn, R.; et al. Efficacy and safety of ciclosporin versus methotrexate in the treatment of severe atopic dermatitis in children and young people (TREAT): A multicentre parallel group assessor-blinded clinical trial. Br. J. Dermatol. 2024, 190, e13. [Google Scholar] [CrossRef]
- Drljevic-Nielsen, A.; Heilskov, S.; Deleuran, M.S.; Vestergaard, C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: An appraisal of the literature. Dermatol. Venerol. 2024, 159, 23–33. [Google Scholar] [CrossRef]
- Kojanova, M.; Tanczosova, M.; Strosova, D.; Cetkovska, P.; Fialova, J.; Dolezal, T.; Machovcova, A.; Gkalpakiotis, S. BIOREP Study Group. Dupilumab for the treatment of atopic dermatitis: Real-world data from the Czech Republic BIOREP registry. J. Dermatol. Treat. 2022, 33, 2578–2586. [Google Scholar] [CrossRef]
- Berger, T.G. Evaluation and Management of Severe Refractory Atopic Dermatitis (eczema) in Adults. Available online: https://www.uptodate.com/contents/evaluation-and-management-of-severe-refractory-atopic-dermatitis-eczema-in-adults#H1361784051 (accessed on 18 September 2024).
- Huang, L.; Zhao, D.; Lin, H.; Zheng, H.; Li, X.; Chen, L.; Tang, P. Efficacy and safety of upadacitinib in the treatment of moderate-to-severe atopic dermatitis in adolescents: A systematic review and meta-analysis of randomized controlled trials. Medicine 2024, 103, e39826. [Google Scholar] [CrossRef]
- Lauffer, F.; Biedermann, T. Einschätzungen zur Therapie der moderaten bis schweren atopischen dermatitis mit januskinaseinhibitoren [Janus kinase inhibitors for the treatment of atopic dermatitis-evaluation of current data and practical experience]. Dermatologie 2022, 73, 520–528. [Google Scholar] [CrossRef]
- Sharma, D.; Gart, S.; Kitrell, B.; Lonowski, S.; Arthur, M.; Wei, E.X. Analysis of patient experiences regarding JAK inhibitors for atopic dermatitis, psoriasis, alopecia areata, and vitiligo. Arch. Dermatol. Res. 2024, 316, 630. [Google Scholar] [CrossRef] [PubMed]
- Haag, C.; Alexis, A.; Aoki, V.; Bissonnette, R.; Blauvelt, A.; Chovatiya, R.; Cork, M.J.; Danby, S.G.; Eichenfield, L.; Eyerich, K.; et al. A Practical guide to using oral JAK Inhibitors for atopic dermatitis from the International Eczema Council. Br. J. Dermatol. 2024, 342, ljae342. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Gooderham, M.; Katoh, N.; Aoki, V.; Pink, A.E.; Binamer, Y.; Rademaker, M.; Fomina, D.; Gutermuth, J.; Ahn, J.; et al. Combining treat-to-target principles and shared decision-making: International expert consensus-based recommendations with a novel concept for minimal disease activity criteria in atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N.B.; Silverberg, J.I. Atopic Dermatitis: The era of excellence in care. Dermatol. Clin. 2024, 42, 13–14. [Google Scholar] [CrossRef]
- Simpson, E.L.; Bruin-Weller, M.; Flohr, C.; Ardern-Jones, M.R.; Barbarot, S.; Deleuran, M.; Bieber, T.; Vestergaard, C.; Brown, S.J.; Cork, M.J.; et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J. Am. Acad. Dermatol. 2017, 77, 623–633. [Google Scholar] [CrossRef]
- Feldman, S.R.; Guerin, A.; Gauthier-Loiselle, M.; Claxton, A.J.; Hazra, N.C.; Meng, Y.; Gallant, K.; Balu, S. Patient preferences for treatment attributes in moderate-to-severe atopic dermatitis: A discrete choice experiment. J. Dermatol. Treat. 2024, 35, 2345739. [Google Scholar] [CrossRef]
- Chiei-Gallo, A.; Barei, F.; Calzari, P.; Pisapia, A.; Marzano, A.V.; Ferrucci, S.M. Long-term efficacy and safety of dupilumab in adolescents with severe atopic dermatitis: A 3-year real-life study. Int. J. Dermatol. 2024; in press. [Google Scholar] [CrossRef]
- Nevid, M.; Boguniewicz, M. Current and emerging biologics for atopic dermatitis. Immunol. Allergy Clin. N. Am. 2024, 44, 577–594. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Werfel, T.; Barbarot, S.; Hunter, H.J.A.; Pierce, E.; Sun, L.; Cirri, L.; Buchanan, A.S.; Lu, N.; Wollenberg, A. Maintained improvement in physician- and patient-reported outcomes with baricitinib in adults with moderate-to-severe atopic dermatitis who were treated for up to 104 weeks in a randomized trial. J. Dermatol. Treat. 2023, 34, 2190430. [Google Scholar] [CrossRef]
- Pols, D.H.; Wartna, J.B.; Moed, H.; van Alphen, E.I.; Bohnen, A.M.; Bindels, P.J. Atopic dermatitis, asthma and allergic rhinitis in general practice and the open population: A systematic review. Scan J. Prim. Health Care 2016, 34, 143–150. [Google Scholar] [CrossRef]
- Kelava, N.; Lugović-Mihić, L.; Duvančić, T.; Romić, R.; Šitum, M. Oral allergy syndrome—The need of a multidisciplinary approach. Acta Clin. Croat. 2014, 53, 210–219. [Google Scholar] [PubMed]
- Myers, K.; Silverberg, J.I.; Parasuraman, S.; Pierce, A.; Eichenfield, L.F.; Poulos, C. Treatment preferences among patients with mild-to-moderate atopic dermatitis. J. Dermatol. Treat. 2023, 34, 2215356. [Google Scholar] [CrossRef] [PubMed]
- Boeri, M.; Sutphin, J.; Hauber, B.; Cappelleri, J.C.; Romero, W.; Di Bonaventura, M. Quantifying patient preferences for systemic atopic dermatitis treatments using a discrete-choice experiment. J. Dermatol. Treat. 2022, 33, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Brownstone, N.D.; Farberg, A.S.; Litchman, G.H.; Quick, A.P.; Siegel, J.J.; Hurton, L.V.; Goldberg, M.S.; Lio, P.A. Improving systemic therapy selection for inflammatory skin diseases: A clinical need survey. JAAD Int. 2024, 16, 49–56. [Google Scholar] [CrossRef]
- Schaarschmidt, M.L.; Kromer, D.; Wellmann, P.; Peitsch, W.K.; Kromer, C. Patients’ preferences for systemic treatment of atopic dermatitis: Safety and efficacy count the most. J. Dermatol. Treat. 2024, 35, 2308682. [Google Scholar] [CrossRef]
- Thomas, C.; Raibouaa, A.; Wollenberg, A.; Capron, J.P.; Krucien, N.; Karn, H.; Tervonen, T. Patient preferences for atopic dermatitis medications in the UK, France and Spain: A discrete choice experiment. BMJ Open 2022, 12, 058799. [Google Scholar] [CrossRef]
- Kwatra, S.G.; Lio, P.; Weidinger, S.; Calimlim, B.; Ladizinski, B.; Vigna, N.; Botha, W.; Mansfield, C. Patient preferences for atopic dermatitis treatments: A discrete choice experiment. J. Dermatol. Treat. 2023, 34, 2222201. [Google Scholar] [CrossRef]
- Feldman, S.R.; Thyssen, J.P.; Boeri, M.; Gerber, R.; Neary, M.P.; Cha, A.; Hauber, B.; Cappelleri, J.C.; Xenakis, J.; Leach, C.; et al. Adult, adolescent, and caregiver preferences for attributes of topical treatments for mild-to-moderate atopic dermatitis: A discrete-choice experiment. J. Dermatol. Treat. 2024, 35, 2304020. [Google Scholar] [CrossRef]
- Ameen, M.; Alhusayen, R.; Brandi, H.; Bøgelund, M.; Jensen, H.H.; Reitzel, S.B.; Thyssen, J.P. Patient preferences in the treatment of moderate-to-severe atopic dermatitis. Acta Derm. Venereol. 2024, 104, adv24339. [Google Scholar] [CrossRef]
- Okubo, Y.; Ho, K.A.; Fifer, S.; Fujita, H.; Oki, Y.; Taguchi, Y. Patient and physician preferences for atopic dermatitis injection treatments in Japan. J. Dermatol. Treat. 2020, 31, 821–830. [Google Scholar] [CrossRef]
| Author, Year | Analysed Factors | Methods and Examinees/Patients | Results | Conclusions |
|---|---|---|---|---|
| Myers K, et al., 2023 [84] | AD patients’ treatment preferences | Cross-sectional, web-based DCE survey administered to 300 US adults with mild to moderate AD | Most valued treatment outcome: achieving clear skin within 3–4 months. Topical creams applied 2× daily preferred over systemic treatments Respondents with lower self-assessed AD burden were more open to topicals and less concerned about side effects. | → Findings support shared decision-making in managing mild to moderate AD. |
| Boeri M, et al., 2022 [85] | Identification of key treatment attributes and preferences for systemic AD treatment | Qualitative interviews with 21 adults with moderate to severe AD; online DCE survey with 320 participants (74% F; mean age 35 yrs) | Top treatment concerns: annual risk of malignancy, mode of administration, probability of clear skin, and time to itch relief. Daily oral treatment preferred over injectable treatment. Higher AE risks accepted for more effective treatments. | → Findings support joint patient–physician decision-making in managing moderate to severe AD. |
| Brownstone ND, et al., 2024 [86] | Clinicians’ choices of systemic therapies for AD and PSO without molecular testing, and the frequency of treatment switching | Twenty-question survey assessing treatment strategies for AD and PSO, completed by 265 dermatology conference attendees in 2022 | “Reported efficacy” was the top treatment factor, however, 62% of clinicians reported needing ≥2 medications to reach it. A total of 90% found molecular testing useful to improve treatment selection. | → Molecular tests may help determine the most efficacious drug for individual patients. |
| Feldman SR, et al., 2024 [90] | Assessment of treatment preferences for those with moderate to severe AD | Online DCE with 300 US adults (70% F) who reported moderate to severe AD or who had tried systemic therapy after finding topical treatments ineffective (June 2023) RI calculated | RI of treatment attributes: itch control (38%), risk of cancer (23%), respiratory infection risk (18%), heart problem risk (11%), sustained improvement in skin appearance (5%), blood test frequency (3%), and frequency and mode of administration (2%). AE attributes accounted for more than half of RI. | → Treatment efficacy and safety preferred over mode of administration. |
| Schaarschmidt ML, et al., 2024 [87] | Patient preferences for systemic AD treatments | Online DCE of 182 AD patients in Germany (75.3% F) analysing treatment outcome and process preferences | AEs most important (RIS 31.2), followed by (almost) clear skin (RIS 24.2) and probability of itch improvement (RIS 16.0). Less relevant: application method (RIS 14.4), itch relief onset (RIS 7.4), and lab test frequency (RIS 6.8). Preferences significantly influenced by sex, age, psychiatric comorbidity, current therapy, and HRQOL. | → Participants prioritize safety and symptom control. |
| Augustin M, et al., 2020 [4] | Therapeutic needs of patients with AD in routine care | Nationwide cross-sectional study involving 1678 patients (60.5% F) from 91 dermatology practices and outpatient clinics in Germany | High AD burden (mean SCORAD 42.26 ± 18.63, mean DLQI of 8.49 ± 6.45, and mean EQ VAS of 63.62 ± 21.98). ‘Quite important’/‘very important’ patient needs: ‘to be free of itching’ (96.0%), ‘to get better skin quickly’ (87.7%), and ‘to be healed of all lesions’ (85.7%). Treatment needs rated more important by older people, women, and those diagnosed with AD for ≤1 year. Key factors for higher needs: skin-related QoL, greater AD severity, and age. | → AD patients exhibit diverse therapeutic needs based on individual burdens; identifying these can enhance personalized care and shared decision-making. |
| Thomas C, et al., 2022 [88] | Patient preferences for AD treatment attributes | Online DCE survey completed by 404 AD patients (65% F) who used AD treatments during the past 2 yrs | Priorities: achieving significant itch reduction and minimizing infection risk. Patients willing to accept lower efficacy for treatments with rapid onset, oral administration, and less frequent check-ups. | → Understanding patients’ preferences can enhance patient–physician decision-making |
| Kwatra SG, et al., 2023 [89] | AD patients’ willingness to balance the risks and benefits of systemic treatments | Online DCE survey involving 200 patients with moderate to severe AD, who assessed treatment attribute preferences | Patients prioritized itch reduction, speed of itch relief, and clearing skin and were willing to accept some risk of serious infections and acne for better treatment outcomes. | → Patients with moderate to severe AD may accept associated risks of systemic treatment. Attention to preferences can enhance patient–physician decision-making |
| Feldman SR, et al., 2024 [90] | CRI of topical treatments attributes for mild to moderate AD | DCE survey administered to 300 adults and 331 adolescents with AD and 330 caregivers of children with AD in the US | Adults prioritized avoiding skin colour changes (CRI 29.0) and time until itch improvement (26.6). Adolescents less concerned about skin colour changes. Caregivers less concerned about time until clear skin in patients. | → Physicians should consider age-related differences in treatment preferences. |
| Ameen M, et al., 2024 [91] | Treatment preferences and priorities of moderate to severe AD patients | Online DCE survey of 713 adults from Denmark, France, the UK, and Canada | Patients prioritized avoiding severe AEs. Daily oral pills preferred over biweekly injections. Less important factors: time to full effect and monitoring. | → Safety is the highest priority for moderate to severe AD patients, followed by ease of administration. |
| Okubo Y, et al., 2024 [92] | Patient and physician preferences for new biologic AD treatments | Online DCE survey in Japan involving 323 AD patients and 121 physicians | A total of 46.24% of patients and 76.67% of physicians chose new treatments. Physicians prioritized rash treatment efficacy and cost. Patients favoured add-on therapies and clinic-administered injections. | → Findings support shared decision-making in clinical practice. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugović-Mihić, L.; Barac, E.; Tomašević, R.; Parać, E.; Zanze, L.; Ljevar, A.; Dolački, L.; Štrajtenberger, M. Atopic Dermatitis-Related Problems in Daily Life, Goals of Therapy and Deciding Factors for Systemic Therapy: A Review. Pharmaceuticals 2024, 17, 1455. https://doi.org/10.3390/ph17111455
Lugović-Mihić L, Barac E, Tomašević R, Parać E, Zanze L, Ljevar A, Dolački L, Štrajtenberger M. Atopic Dermatitis-Related Problems in Daily Life, Goals of Therapy and Deciding Factors for Systemic Therapy: A Review. Pharmaceuticals. 2024; 17(11):1455. https://doi.org/10.3390/ph17111455
Chicago/Turabian StyleLugović-Mihić, Liborija, Ema Barac, Renata Tomašević, Ena Parać, Lucija Zanze, Ana Ljevar, Lorena Dolački, and Maja Štrajtenberger. 2024. "Atopic Dermatitis-Related Problems in Daily Life, Goals of Therapy and Deciding Factors for Systemic Therapy: A Review" Pharmaceuticals 17, no. 11: 1455. https://doi.org/10.3390/ph17111455
APA StyleLugović-Mihić, L., Barac, E., Tomašević, R., Parać, E., Zanze, L., Ljevar, A., Dolački, L., & Štrajtenberger, M. (2024). Atopic Dermatitis-Related Problems in Daily Life, Goals of Therapy and Deciding Factors for Systemic Therapy: A Review. Pharmaceuticals, 17(11), 1455. https://doi.org/10.3390/ph17111455






