Efficacy of Quercetin and Quercetin Loaded Chitosan Nanoparticles Against Cisplatin-Induced Renal and Testicular Toxicity via Attenuation of Oxidative Stress, Inflammation, and Apoptosis
Abstract
1. Introduction
2. Results
2.1. Characterization of Quercetin-Loaded Chitosan Nanoparticles (QUE.NPs)
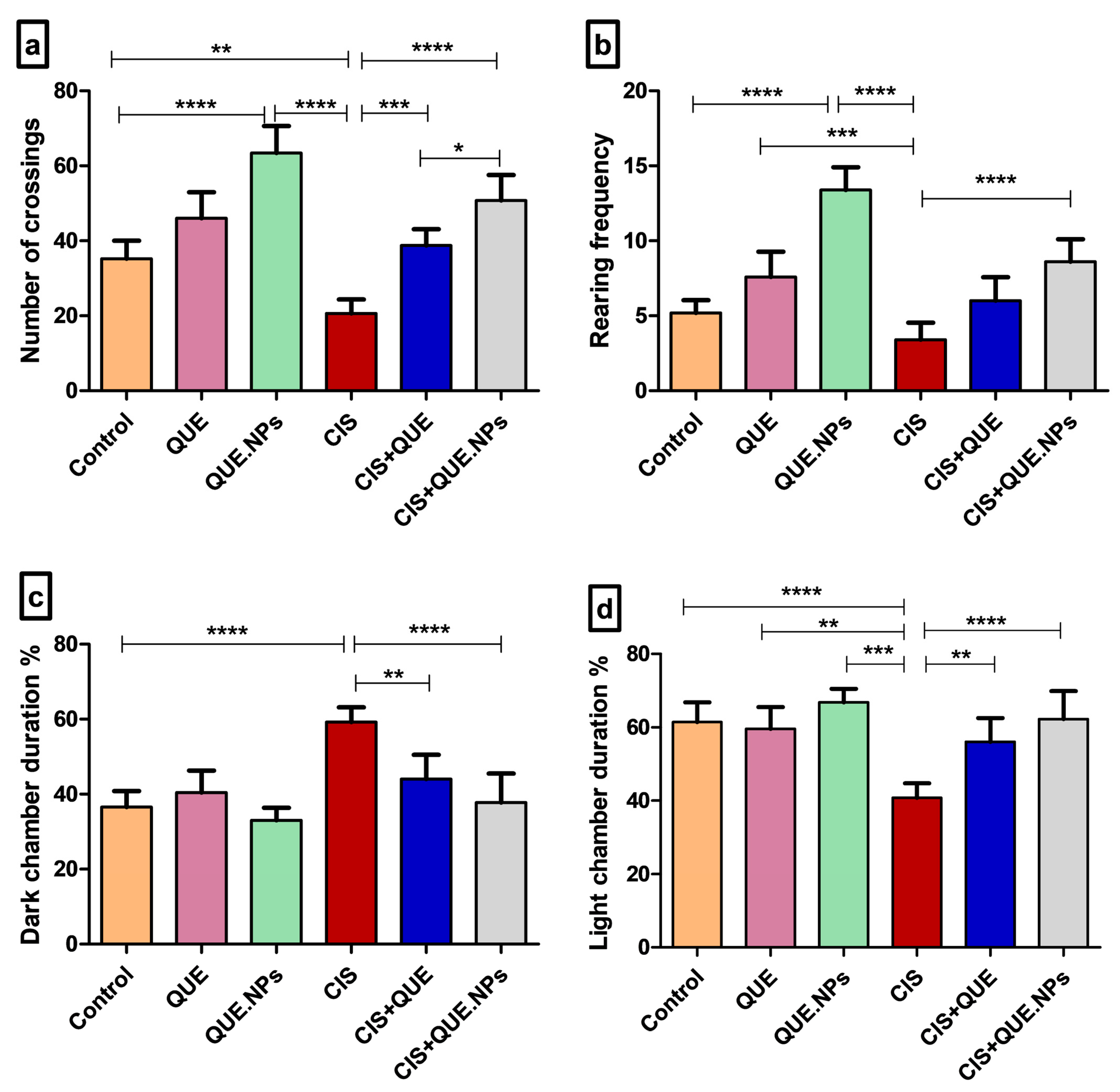
2.2. QUE and QUE.NPs Inhibit Anxiety and Emotional Disturbance Induced by Cisplatin
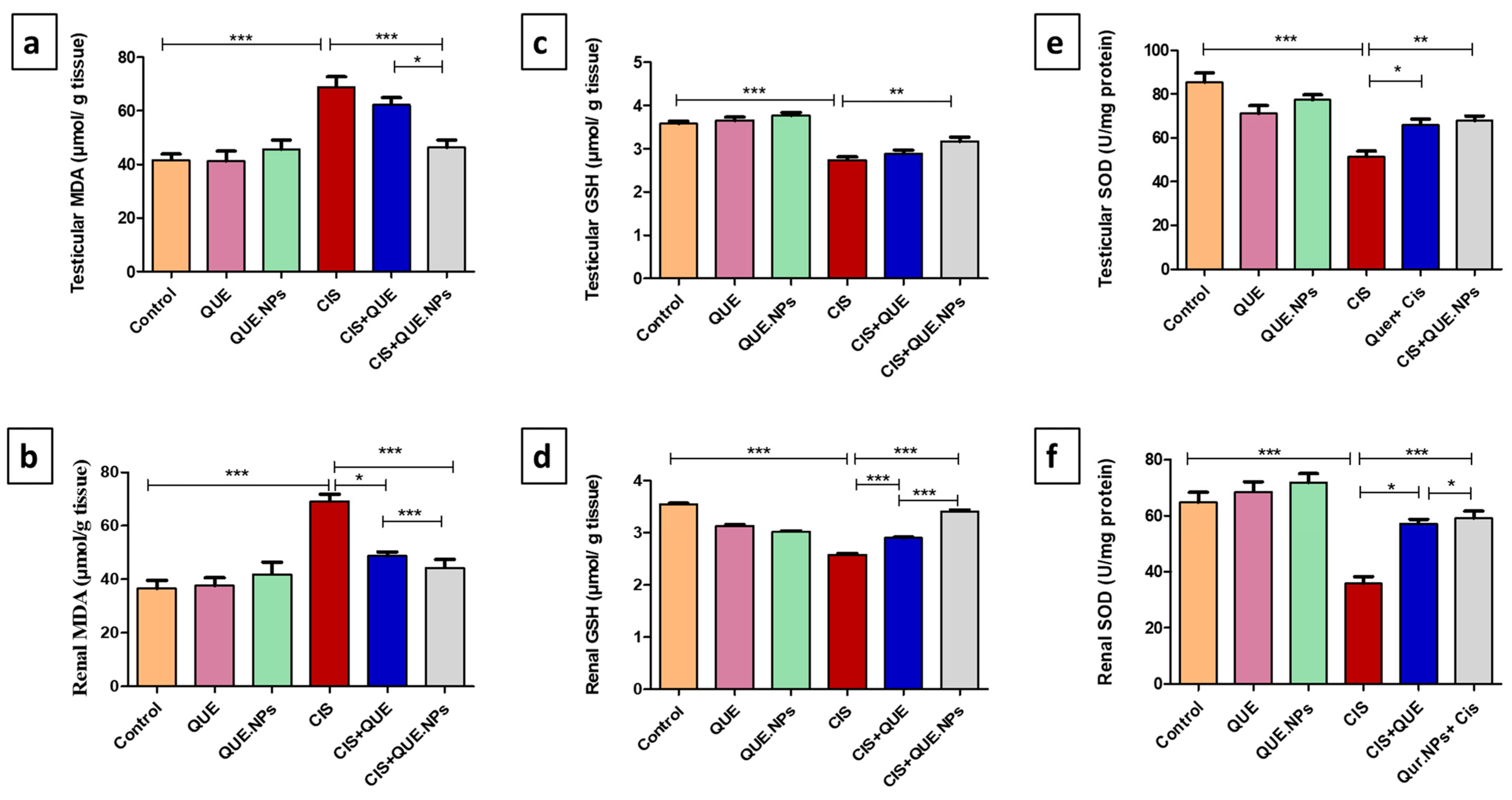
2.3. QUE and QUE.NPs Counteract CIS-Induced Testicular and Renal Oxidative Stress
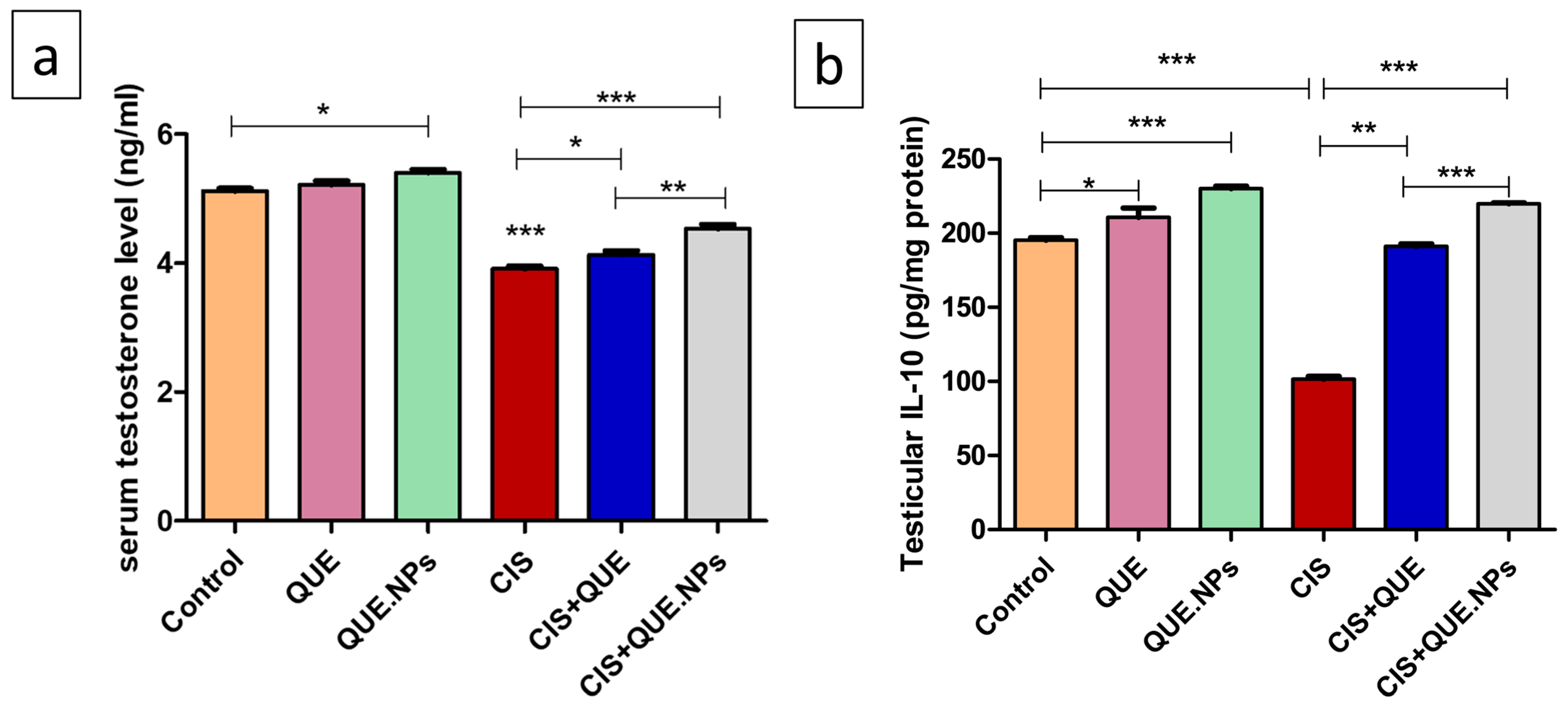
2.4. QUE and Its Nanoform Mitigate the Reduction in Both Serum Testosterone and Testicular IL-10
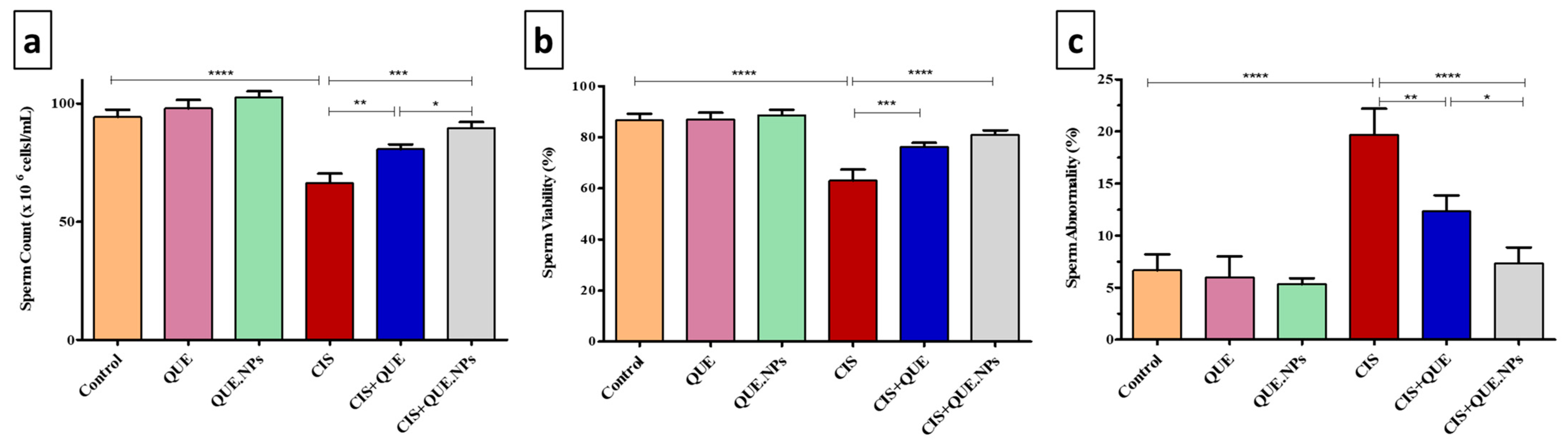
2.5. Sperm Concentration, Viability, and Morphology
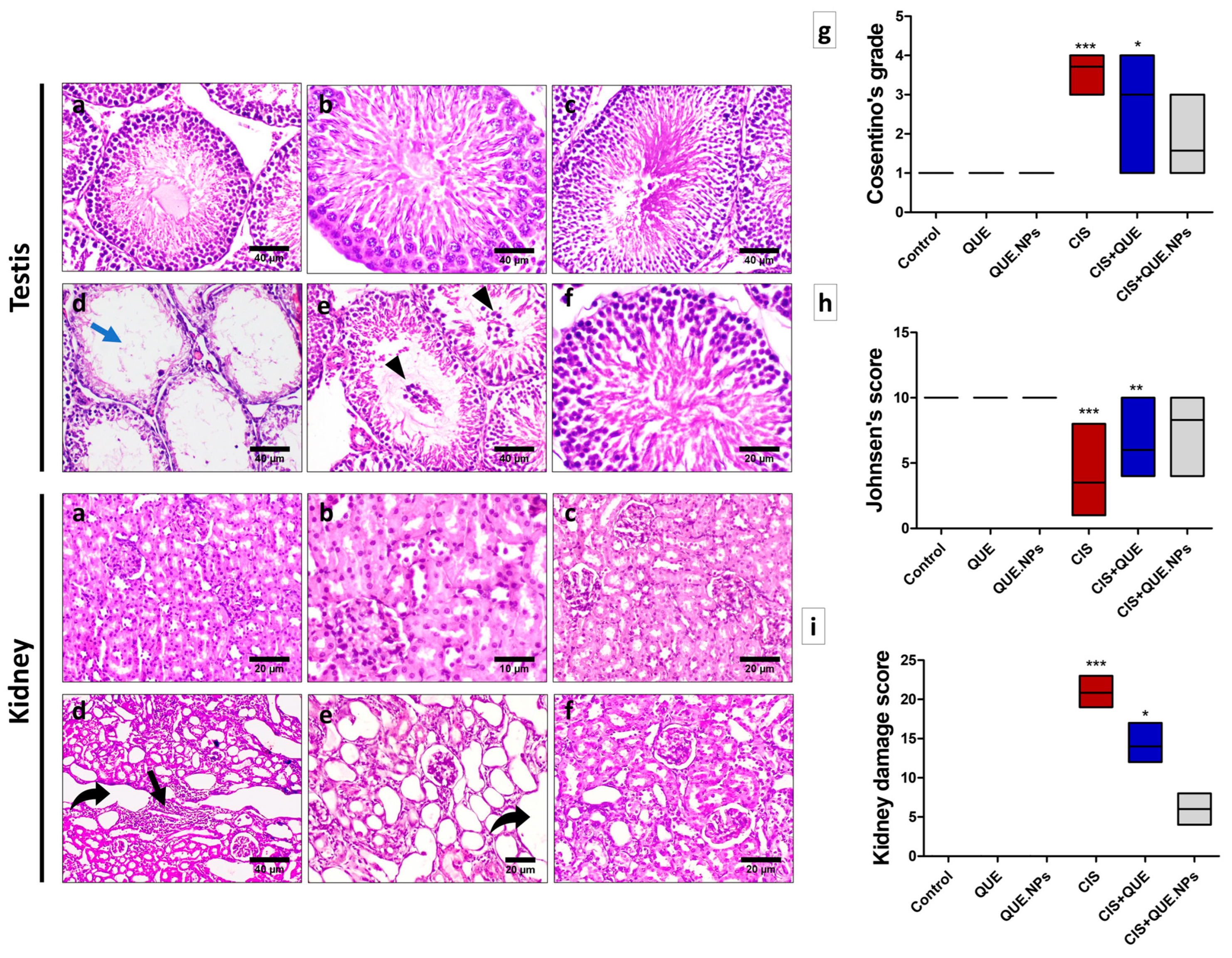
2.6. QUE and QUE.NPs Attenuate CIS-Induced Kidney Damage
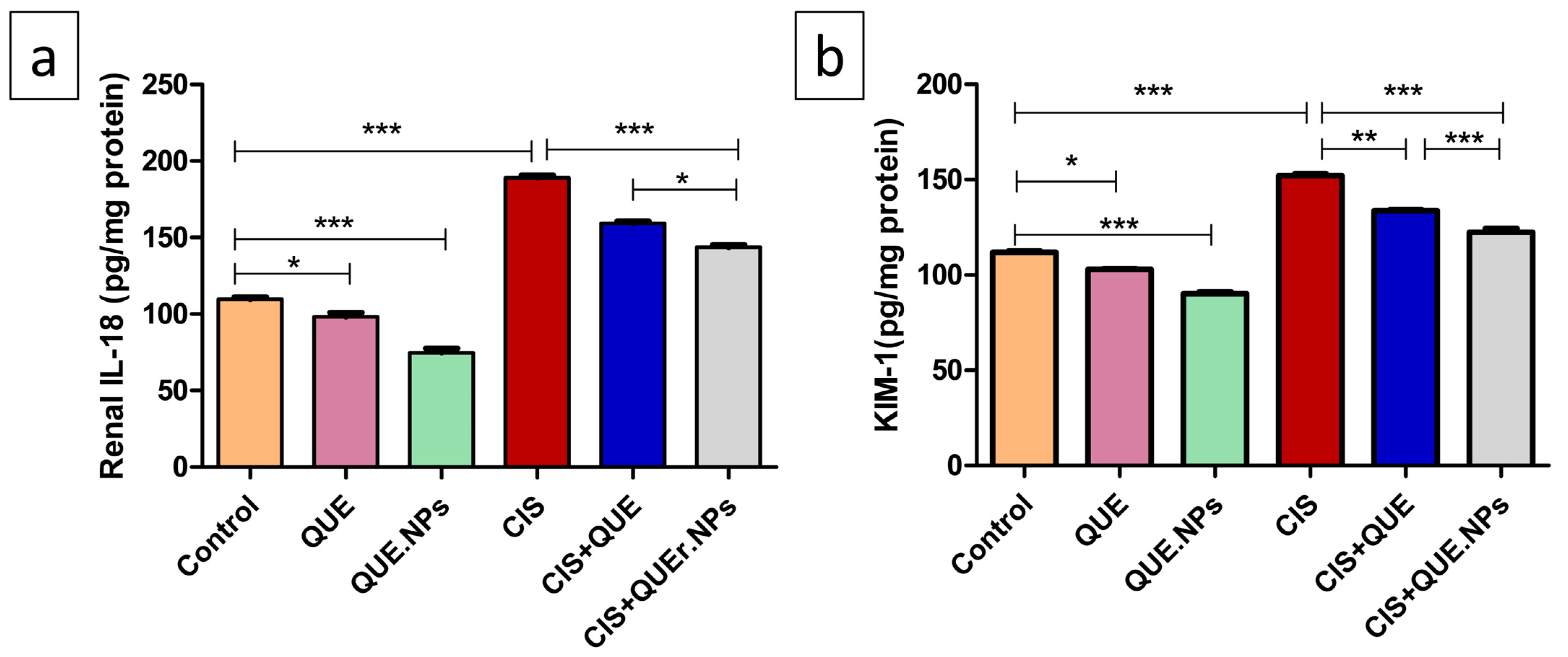
2.7. QUE and QUE.NPs Adjust Interleukin-18 (IL-18) and Kidney Injury Molecule-1 (KIM-1) Levels in Renal Tissue
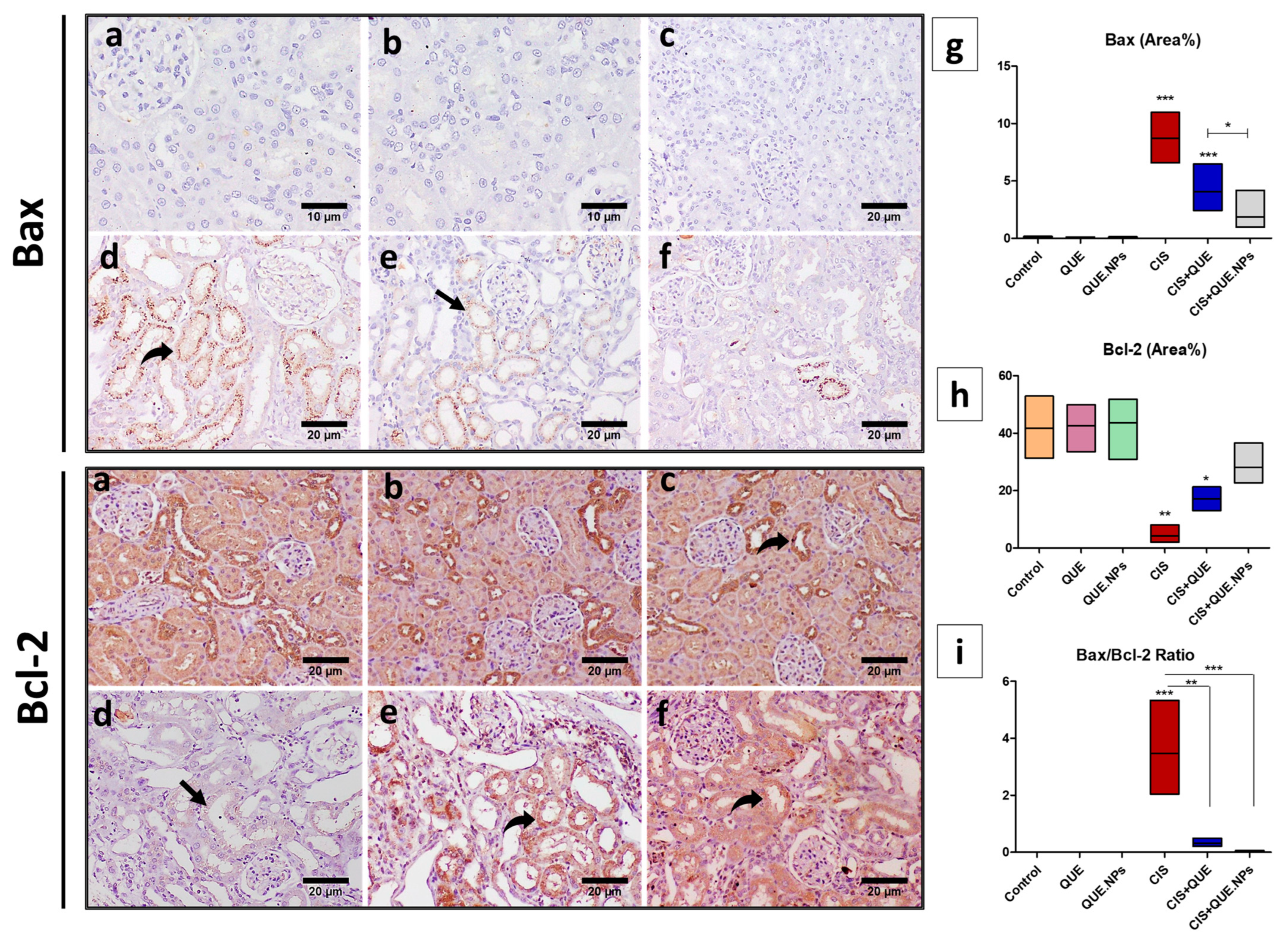
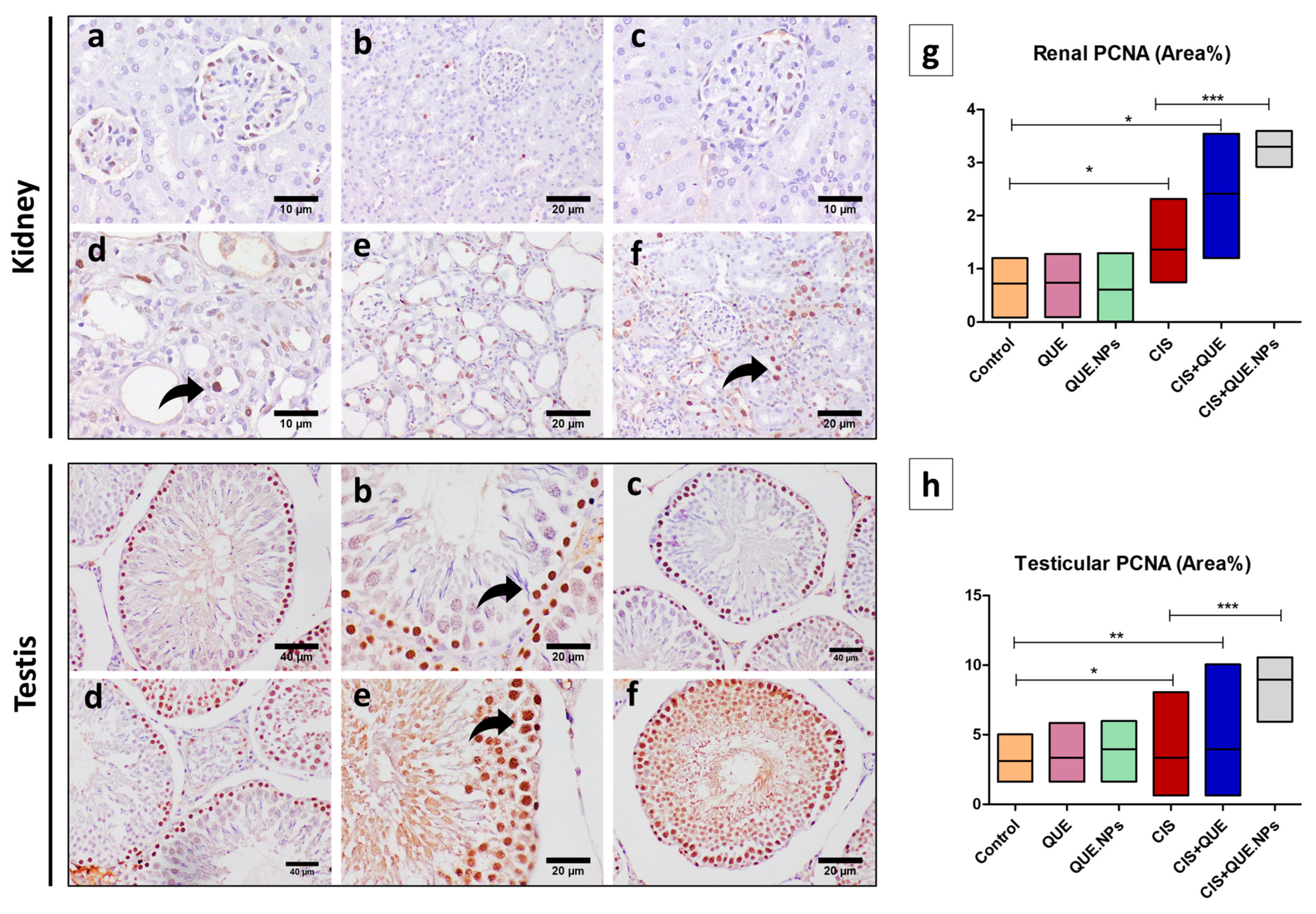
2.8. QUE and QUE.NPs Protect the Histological Structure of the Testis and Kidney
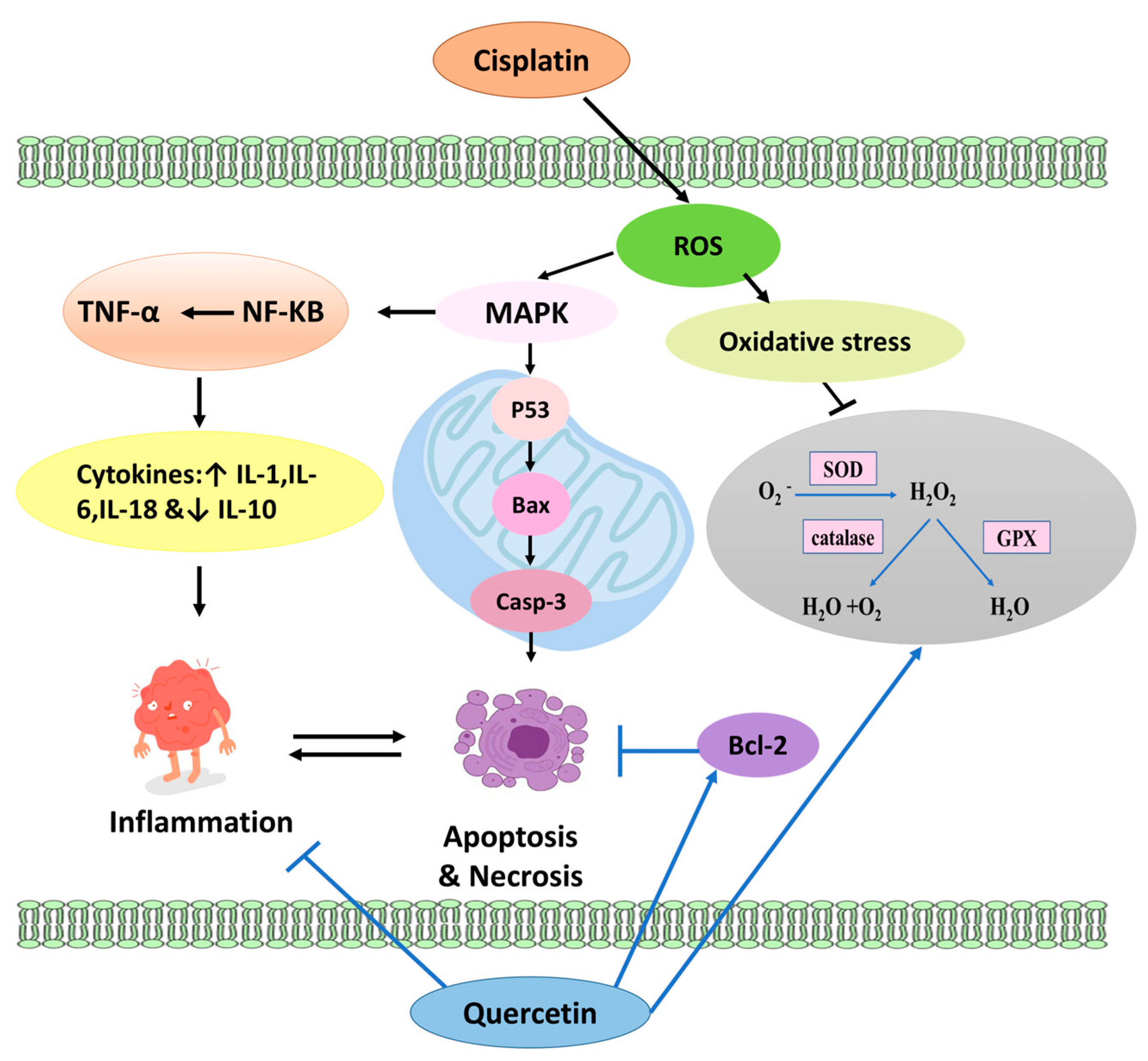
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Spectral Analysis
4.3. Isolation of Quercetin
4.4. Quercetin-Loaded Chitosan Nanoparticles Synthesis
4.5. Quercetin-Loaded Chitosan Nanoparticles Characterization
4.6. Experimental Designs
4.7. Behavioral Analysis
4.8. Biochemical Analysis
4.8.1. Assessment of Oxidative Stress in Both Testicular and Renal Tissue Homogenates Using HPLC
4.8.2. Determination of Serum Total Testosterone Testicular IL-10 Content Using an Enzyme-Linked Immunosorbent Assay (ELISA)
4.8.3. Sperm Concentration, Viability, and Morphology
4.8.4. Assessment of Serum Kidney Functions
4.8.5. Assessment of Renal Interleukin-18 and Kidney Injury Molecule-1 Using ELISA
4.9. Histopathological Analysis of Renal and Testicular Tissue
4.10. Immunohistochemical Staining of Bcl-2, Bax, and PCNA of Renal and Testicular Tissue
4.11. Statistical Analysis
5. Conclusions
6. Limitations of Our Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Poorvu, P.D.; Frazier, A.L.; Feraco, A.M.; Manley, P.E.; Ginsburg, E.S.; Laufer, M.R.; LaCasce, A.S.; Diller, L.R.; Partridge, A.H. Cancer Treatment-Related Infertility: A Critical Review of the Evidence. JNCI Cancer Spectr. 2019, 3, pkz008. [Google Scholar] [CrossRef]
- Linkermann, A.; Himmerkus, N.; Rölver, L.; Keyser, K.A.; Steen, P.; Bräsen, J.-H.; Bleich, M.; Kunzendorf, U.; Krautwald, S. Renal tubular Fas ligand mediates fratricide in cisplatin-induced acute kidney failure. Kidney Int. 2011, 79, 169–178. [Google Scholar] [CrossRef]
- Pearce, A.; Haas, M.; Viney, R.; Pearson, S.-A.; Haywood, P.; Brown, C.; Ward, R. Incidence and severity of self-reported chemotherapy side effects in routine care: A prospective cohort study. PLoS ONE 2017, 12, e0184360. [Google Scholar] [CrossRef]
- Le, X.; Hanna, E.Y. Optimal regimen of cisplatin in squamous cell carcinoma of head and neck yet to be determined. Ann. Transl. Med. 2018, 6, 229. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Li, M.; Yu, Z.; Qi, R.; Ding, J.; Zhang, Z.; Chen, X. Self-Stabilized Hyaluronate Nanogel for Intracellular Codelivery of Doxorubicin and Cisplatin to Osteosarcoma. Adv. Sci. 2018, 5, 1800811. [Google Scholar] [CrossRef]
- Fennell, D.; Summers, Y.; Cadranel, J.; Benepal, T.; Christoph, D.; Lal, R.; Das, M.; Maxwell, F.; Visseren-Grul, C.; Ferry, D. Cisplatin in the modern era: The backbone of first-line chemotherapy for non-small cell lung cancer. Cancer Treat. Rev. 2016, 44, 42–50. [Google Scholar] [CrossRef]
- Byrski, T.; Huzarski, T.; Dent, R.; Marczyk, E.; Jasiowka, M.; Gronwald, J.; Jakubowicz, J.; Cybulski, C.; Wisniowski, R.; Godlewski, D.; et al. Pathologic complete response to neoadjuvant cisplatin in BRCA1-positive breast cancer patients. Breast Cancer Res. Treat. 2014, 147, 401–405. [Google Scholar] [CrossRef]
- Yu, L.; Gu, C.; Zhong, D.; Shi, L.; Kong, Y.; Zhou, Z.; Liu, S. Induction of autophagy counteracts the anticancer effect of cisplatin in human esophageal cancer cells with acquired drug resistance. Cancer Lett. 2014, 355, 34–45. [Google Scholar] [CrossRef]
- Kim, E.-A.; Kim, T.G.; Sung, E.-G.; Song, I.-H.; Kim, J.-Y.; Doh, K.-O.; Lee, T.-J. miR-148a increases the sensitivity to cisplatin by targeting Rab14 in renal cancer cells. Int. J. Oncol. 2017, 50, 984–992. [Google Scholar] [CrossRef]
- de Vries, G.; Rosas-Plaza, X.; van Vugt, M.A.; Gietema, J.A.; de Jong, S. Testicular cancer: Determinants of cisplatin sensitivity and novel therapeutic opportunities. Cancer Treat. Rev. 2020, 88, 102054. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of cisplatin-induced acute kidney injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Yucel, C.; Arslan, F.D.; Ekmekci, S.; Ulker, V.; Kisa, E.; Yucel, E.E.; Ucar, M.; Ilbey, Y.O.; Celik, O.; Basok, B.I.; et al. Protective Effect of All-Trans Retinoic Acid in Cisplatin-Induced Testicular Damage in Rats. World J. Men’s Health 2019, 37, 249–256. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from Shallots (Allium cepa L. var.aggregatum) Is More Bioavailable Than Its Glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D. Effects of the Dietary Flavonoid Quercetin Upon Performance and Health. Curr. Sports Med. Rep. 2009, 8, 206–213. [Google Scholar] [CrossRef]
- Maalik, A.; Khan, F.; Mumtaz, A.; Mehmood, A.; Azhar, S.; Atif, M.; Karim, S.; Altaf, Y.; Tariq, I. Pharmacological Applications of Quercetin and its Derivatives: A Short Review. Trop. J. Pharm. Res. 2014, 13, 1561. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The Pharmacological Activity, Biochemical Properties, and Pharmacokinetics of the Major Natural Polyphenolic Flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Agbor, G.A. Anti-viral and anti-inflammatory effects of kaempferol and quercetin and COVID-2019: A scoping review. Asian Pac. J. Trop. Biomed. 2021, 11, 327. [Google Scholar] [CrossRef]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of quercetin glucuronides and effect on biological activity. Free. Radic. Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Lin, Y.-L.; Li, C.-C.; Chuang, C.-H. Comparing the metabolism of quercetin in rats, mice and gerbils. Eur. J. Nutr. 2016, 55, 413–422. [Google Scholar] [CrossRef]
- Ferri, P.; Angelino, D.; Gennari, L.; Benedetti, S.; Ambrogini, P.; Del Grande, P.; Ninfali, P. Enhancement of flavonoid ability to cross the blood–brain barrier of rats by co-administration with α-tocopherol. Food Funct. 2015, 6, 394–400. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.M.; Braidy, N.; Setzer, W.N.; Ahmed, T.; Nabavi, S.F. Quercetin and the mitochondria: A mechanistic view. Biotechnol. Adv. 2016, 34, 532–549. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Kim, S. Competitive Biological Activities of Chitosan and Its Derivatives: Antimicrobial, Antioxidant, Anticancer, and Anti-Inflammatory Activities. Int. J. Polym. Sci. 2018, 2018, 1708172. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, A.; Sinha, M.; Saha, S. Biological Efficacy of Medicinal Plant Extracts in Preventing Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 2018, 7904349. [Google Scholar] [CrossRef]
- Oh, G.-S.; Kim, H.-J.; Shen, A.; Bin Lee, S.; Khadka, D.; Pandit, A.; So, H.-S. Cisplatin-induced Kidney Dysfunction and Perspectives on Improving Treatment Strategies. Electrolytes Blood Press. 2014, 12, 55–65. [Google Scholar] [CrossRef]
- Mohammadnejad, D.; Abedelahi, A.; Soleimani-Rad, J.; Mohammadi-Roshandeh, A.; Rashtbar, M.; Azami, A. Degenerative effect of Cisplatin on testicular germinal epithelium. Adv. Pharm. Bull. 2012, 2, 173–177. [Google Scholar] [CrossRef]
- Nematbakhsh, M.; Ashrafi, F.; Pezeshki, Z.; Fatahi, Z.; Kianpoor, F.; Sanei, M.-H.; Talebi, A. A histopathological study of nephrotoxicity, hepatoxicity or testicular toxicity: Which one is the first observation as side effect of Cisplatin-induced toxicity in animal model? J. Nephropathol. 2012, 1, 190. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. Aaps Pharmscitech 2019, 20, 69. [Google Scholar] [CrossRef]
- Shu, X.Z.; Zhu, K.J. The influence of multivalent phosphate structure on the properties of ionically cross-linked chitosan films for controlled drug release. Eur. J. Pharm. Biopharm. 2002, 54, 235–243. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef]
- Schiller, J.; Naji, L.; Trampel, R.; Ngwa, W.; Knauss, R.; Arnold, K. Pulsed-Field Gradient-Nuclear Magnetic Resonance (PFG NMR) to Measure the Diffusion of Ions and Polymers in Cartilage. In Cartilage and Osteoarthritis; Springer: Berlin/Heidelberg, Germany, 2004; pp. 287–302. [Google Scholar]
- Oyarzun-Ampuero, F.; Brea, J.; Loza, M.; Torres, D.; Alonso, M. Chitosan–hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef]
- Jingou, J.; Shilei, H.; Weiqi, L.; Danjun, W.; Tengfei, W.; Yi, X. Preparation, characterization of hydrophilic and hydrophobic drug in combine loaded chitosan/cyclodextrin nanoparticles and in vitro release study. Colloids Surf. B Biointerfaces 2011, 83, 103–107. [Google Scholar] [CrossRef]
- Abdollahzadeh, M.; Panahpour, H.; Ghaheri, S.; Saadati, H. Calcitriol supplementation attenuates cisplatin-induced behavioral and cognitive impairments through up-regulation of BDNF in male rats. Brain Res. Bull. 2022, 181, 21–29. [Google Scholar] [CrossRef]
- Kosari-Nasab, M.; Shokouhi, G.; Ghorbanihaghjo, A.; Mesgari-Abbasi, M.; Salari, A.-A. Quercetin mitigates anxiety-like behavior and normalizes hypothalamus–pituitary–adrenal axis function in a mouse model of mild traumatic brain injury. Behav. Pharmacol. 2019, 30, 282–289. [Google Scholar] [CrossRef]
- Rifaai, R.A.; Mokhemer, S.A.; Saber, E.A.; El-Aleem, S.A.A.; El-Tahawy, N.F.G. Neuroprotective effect of quercetin nanoparticles: A possible prophylactic and therapeutic role in alzheimer’s disease. J. Chem. Neuroanat. 2020, 107, 101795. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Khan, M.R.; Almajwal, A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer 2017, 17, 883. [Google Scholar] [CrossRef]
- Morigi, M.; Perico, L.; Rota, C.; Longaretti, L.; Conti, S.; Rottoli, D.; Novelli, R.; Remuzzi, G.; Benigni, A. Sirtuin 3–dependent mitochondrial dynamic improvements protect against acute kidney injury. J. Clin. Investig. 2015, 125, 715–726. [Google Scholar] [CrossRef]
- Holditch, S.J.; Brown, C.N.; Lombardi, A.M.; Nguyen, K.N.; Edelstein, C.L. Recent Advances in Models, Mechanisms, Biomarkers, and Interventions in Cisplatin-Induced Acute Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3011. [Google Scholar] [CrossRef]
- Moradi, M.; Goodarzi, N.; Faramarzi, A.; Cheraghi, H.; Hashemian, A.H.; Jalili, C. Melatonin protects rats testes against bleomycin, etoposide, and cisplatin-induced toxicity via mitigating nitro-oxidative stress and apoptosis. Biomed. Pharmacother. 2021, 138, 111481. [Google Scholar] [CrossRef]
- Aly, H.A.; Eid, B.G. Cisplatin induced testicular damage through mitochondria mediated apoptosis, inflammation and oxidative stress in rats: Impact of resveratrol. Endocr. J. 2020, 67, 969–980. [Google Scholar] [CrossRef]
- Attia, S.M. The impact of quercetin on cisplatin-induced clastogenesis and apoptosis in murine marrow cells. Mutagenesis 2010, 25, 281–288. [Google Scholar] [CrossRef]
- Aldemir, M.; Okulu, E.; Kösemehmetoğlu, K.; Ener, K.; Topal, F.; Evirgen, O.; Gürleyik, E.; Avcı, A. Evaluation of the protective effect of quercetin against cisplatin-induced renal and testis tissue damage and sperm parameters in rats. Andrologia 2014, 46, 1089–1097. [Google Scholar] [CrossRef]
- Almaghrabi, O.A. Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J. Biol. Sci. 2015, 22, 227–231. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Akimoto, Y.; Sakurai, M.; Matsunaga, I.; Nishimuro, H.; Ippoushi, K.; Oike, H.; Ohnishi-Kameyama, M. Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. J. Funct. Foods 2015, 15, 551–560. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. -Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59, ii91–ii97. [Google Scholar] [CrossRef]
- Stoiber, W.; Obermayer, A.; Steinbacher, P.; Krautgartner, W.-D. The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans. Biomolecules 2015, 5, 702–723. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Gao, Y.; Li, L.; Tang, C.; Wen, G.; Zhou, Y.; Zhou, M.; Mao, L.; Fan, Y. Protective Effects of Quercetin on Mitochondrial Biogenesis in Experimental Traumatic Brain Injury via the Nrf2 Signaling Pathway. PLoS ONE 2016, 11, e0164237. [Google Scholar] [CrossRef]
- Shati, A.A. Resveratrol improves sperm parameter and testicular apoptosis in cisplatin-treated rats: Effects on ERK1/2, JNK, and Akt pathways. Syst. Biol. Reprod. Med. 2019, 65, 236–249. [Google Scholar] [CrossRef]
- Nna, V.U.; Ujah, G.A.; Suleiman, J.B.; Mohamed, M.; Nwokocha, C.; Akpan, T.J.; Ekuma, H.C.; Fubara, V.V.; Kekung-Asu, C.B.; Osim, E.E. Tert-butylhydroquinone preserve testicular steroidogenesis and spermatogenesis in cisplatin-intoxicated rats by targeting oxidative stress, inflammation and apoptosis. Toxicology 2020, 441, 152528. [Google Scholar] [CrossRef]
- Boroja, T.; Katanić, J.; Rosić, G.; Selaković, D.; Joksimović, J.; Mišić, D.; Stanković, V.; Jovičić, N.; Mihailović, V. Summer savory (Satureja hortensis L.) extract: Phytochemical profile and modulation of cisplatin-induced liver, renal and testicular toxicity. Food Chem. Toxicol. 2018, 118, 252–263. [Google Scholar] [CrossRef]
- Yoshida, T. Determination of reduced and oxidized glutathione in erythrocytes by high-performance liquid chromatography with ultraviolet absorbance detection. J. Chromatogr. B Biomed. Sci. Appl. 1996, 678, 157–164. [Google Scholar] [CrossRef]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and male infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef]
- Pérez, C.V.; Theas, M.S.; Jacobo, P.V.; Jarazo-Dietrich, S.; Guazzone, V.A.; Lustig, L. Dual role of immune cells in the testis: Protective or pathogenic for germ cells? Spermatogenesis 2013, 3, e23870. [Google Scholar] [CrossRef]
- Okkay, U.; Okkay, I.F.; Aydin, I.C.; Bayram, C.; Ertugrul, M.S.; Gezer, A.; Hacimuftuoglu, A. Effects of Achillea millefolium on cisplatin induced ocular toxicity: An experimental study. Cutan. Ocul. Toxicol. 2021, 40, 214–220. [Google Scholar] [CrossRef]
- Fouad, A.A.; Refaie, M.M.M.; Abdelghany, M.I. Naringenin palliates cisplatin and doxorubicin gonadal toxicity in male rats. Toxicol. Mech. Methods 2019, 29, 67–73. [Google Scholar] [CrossRef]
- Almeer, R.S.; Abdel Moneim, A.E. Evaluation of the Protective Effect of Olive Leaf Extract on Cisplatin-Induced Testicular Damage in Rats. Oxidative Med. Cell. Longev. 2018, 2018, 8487248. [Google Scholar] [CrossRef]
- Eid, A.H.; Abdelkader, N.F.; El-Raouf, O.M.A.; Fawzy, H.M.; El-Denshary, E.-E.S. Carvedilol alleviates testicular and spermatological damage induced by cisplatin in rats via modulation of oxidative stress and inflammation. Arch. Pharmacal Res. 2016, 39, 1693–1702. [Google Scholar] [CrossRef]
- Wang, L.; He, Y.; Li, Y.; Pei, C.; Olatunji, O.J.; Tang, J.; Famurewa, A.C.; Wang, H.; Yan, B. Protective Effects of Nucleosides-Rich Extract from Cordyceps cicadae against Cisplatin Induced Testicular Damage. Chem. Biodivers. 2020, 17, e2000671. [Google Scholar] [CrossRef]
- Spermon, J.; Ramos, L.; Wetzels, A.; Sweep, C.; Braat, D.; Kiemeney, L.; Witjes, J. Sperm integrity pre- and post-chemotherapy in men with testicular germ cell cancer. Hum. Reprod. 2006, 21, 1781–1786. [Google Scholar] [CrossRef]
- Lacey, M.; Bohday, J.; Fonseka, S.M.; Ullah, A.I.; Whitehead, S.A. Dose–response effects of phytoestrogens on the activity and expression of 3β-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. J. Steroid Biochem. Mol. Biol. 2005, 96, 279–286. [Google Scholar] [CrossRef]
- El-Diasty, H.H.; El-Sayyad, H.; Refat, S.; El-Ghaweet, H.A. Efficacy of Quercetin-Sensitized Cisplatin against N-Nitroso-N-Methylurea Induced Testicular Carcinogenesis in Wistar Rats. Asian Pac. J. Cancer Prev. 2021, 22, 75. [Google Scholar] [CrossRef]
- Chen, X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn. Mag. 2010, 6, 135–141. [Google Scholar] [CrossRef]
- Schanz, M.; Schricker, S.; Pfister, F.; Alscher, M.D.; Kimmel, M. Renal complications of cancer therapies. Drugs Today 2018, 54, 561–575. [Google Scholar] [CrossRef]
- Qian, W.; Nishikawa, M.; Haque, A.M.; Hirose, M.; Mashimo, M.; Sato, E.; Inoue, M. Mitochondrial density determines the cellular sensitivity to cisplatin-induced cell death. Am. J. Physiol. Cell Physiol. 2005, 289, C1466–C1475. [Google Scholar] [CrossRef]
- Siew, E.D.; Ikizler, T.A.; Gebretsadik, T.; Shintani, A.; Wickersham, N.; Bossert, F.; Peterson, J.F.; Parikh, C.R.; May, A.K.; Ware, L.B. Elevated Urinary IL-18 Levels at the Time of ICU Admission Predict Adverse Clinical Outcomes. Clin. J. Am. Soc. Nephrol. 2010, 5, 1497–1505. [Google Scholar] [CrossRef]
- Ho, E.; Fard, A.; Maisel, A. Evolving use of biomarkers for kidney injury in acute care settings. Curr. Opin. Crit. Care 2010, 16, 399–407. [Google Scholar] [CrossRef]
- Bonventre, J.V. Diagnosis of acute kidney injury: From classic parameters to new biomarkers. Acute Kidney Inj. 2007, 156, 213–219. [Google Scholar]
- Kim, S.G.; Kim, J.-R.; Choi, H.C. Quercetin-Induced AMP-Activated Protein Kinase Activation Attenuates Vasoconstriction Through LKB1-AMPK Signaling Pathway. J. Med. Food 2018, 21, 146–153. [Google Scholar] [CrossRef]
- Marunaka, Y.; Niisato, N.; Miyazaki, H.; Nakajima, K.; Taruno, A.; Sun, H.; Marunaka, R.; Okui, M.; Yamamoto, T.; Kanamura, N.; et al. Quercetin is a Useful Medicinal Compound Showing Various Actions Including Control of Blood Pressure, Neurite Elongation and Epithelial Ion Transport. Curr. Med. Chem. 2018, 25, 4876–4887. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Souza, M.T.d.S.; Duarte, A.B.S.; Sousa, D.P.d. Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury. Molecules 2020, 25, 5772. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Han, X.; Sun, Y.; Zhang, L.; Liu, W.; Liu, X.; Li, W.; Liu, Y. Kidney protection effect of ginsenoside re and its underlying mechanisms on cisplatin-induced kidney injury. Cell. Physiol. Biochem. 2018, 48, 2219–2229. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Ren, S.; Yan, X.-T.; Li, H.-P.; Li, W.; Zheng, B.; Wang, Z.; Liu, Y.-Y. Improvement of Cisplatin-induced renal dysfunction by Schisandra chinensis stems via anti-inflammation and anti-apoptosis effects. J. Ethnopharmacol. 2018, 217, 228–237. [Google Scholar] [CrossRef]
- Zhang, W.; Hou, J.; Yan, X.; Leng, J.; Li, R.; Zhang, J.; Xing, J.; Chen, C.; Wang, Z.; Li, W. Platycodon grandiflorum saponins ameliorate cisplatin-induced acute nephrotoxicity through the NF-κB-mediated inflammation and PI3K/Akt/apoptosis signaling pathways. Nutrients 2018, 10, 1328. [Google Scholar] [CrossRef]
- Tillhon, M.; Cazzalini, O.; Dutto, I.; Stivala, L.A.; Prosperi, E. p21CDKN1A and DNA repair systems: Recent findings and future perspectives. In DNA Repair—New Research Directions; InTech: Rijeka, Croatia, 2013; pp. 249–279. [Google Scholar]
- Baltaci, B.B.; Uygur, R.; Caglar, V.; Aktas, C.; Aydin, M.; Ozen, O.A. Protective effects of quercetin against arsenic-induced testicular damage in rats. Andrologia 2016, 48, 1202–1213. [Google Scholar] [CrossRef]
- Potočnjak, I.; Domitrović, R. Carvacrol attenuates acute kidney injury induced by cisplatin through suppression of ERK and PI3K/Akt activation. Food Chem. Toxicol. 2016, 98, 251–261. [Google Scholar] [CrossRef]
- Cazzalini, O.; Donà, F.; Savio, M.; Tillhon, M.; Maccario, C.; Perucca, P.; Stivala, L.A.; Scovassi, A.I.; Prosperi, E. p21CDKN1A participates in base excision repair by regulating the activity of poly(ADP-ribose) polymerase-1. DNA Repair 2010, 9, 627–635. [Google Scholar] [CrossRef]
- Jiang, M.; Wei, Q.; Dong, G.; Komatsu, M.; Su, Y.; Dong, Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012, 82, 1271–1283. [Google Scholar] [CrossRef]
- Beniston, R.G.; Campo, M.S. Quercetin elevates p27Kip1 and arrests both primary and HPV16 E6/E7 transformed human keratinocytes in G1. Oncogene 2003, 22, 5504–5514. [Google Scholar] [CrossRef]
- Hirpara, K.V.; Aggarwal, P.; Mukherjee, A.J.; Joshi, N.; Burman, A.C. Quercetin and Its Derivatives: Synthesis, Pharmacological Uses with Special Emphasis on Anti-Tumor Properties and Prodrug with Enhanced Bio-Availability. Anti-Cancer Agents Med. Chem. 2009, 9, 138–161. [Google Scholar] [CrossRef]
- Wang, Y.; Quan, F.; Cao, Q.; Lin, Y.; Yue, C.; Bi, R.; Cui, X.; Yang, H.; Yang, Y.; Birnbaumer, L.; et al. Quercetin alleviates acute kidney injury by inhibiting ferroptosis. J. Adv. Res. 2021, 28, 231–243. [Google Scholar] [CrossRef]
- Najafi, M.; Tavakol, S.; Zarrabi, A.; Ashrafizadeh, M. Dual role of quercetin in enhancing the efficacy of cisplatin in chemotherapy and protection against its side effects: A review. Arch. Physiol. Biochem. 2020, 128, 1438–1452. [Google Scholar] [CrossRef]
- Harwood, M.; Danielewska-Nikiel, B.; Borzelleca, J.F.; Flamm, G.W.; Williams, G.M.; Lines, T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007, 45, 2179–2205. [Google Scholar] [CrossRef]
- Ren, G.; Hou, J.; Fang, Q.; Sun, H.; Liu, X.; Zhang, L.; Wang, P.G. Synthesis of flavonol 3-O-glycoside by UGT78D1. Glycoconj. J. 2012, 29, 425–432. [Google Scholar] [CrossRef]
- Popova, E.; Zorin, I.; Domnina, N.; Novikova, I.; Krasnobaeva, I. Chitosan–tripolyphosphate nanoparticles: Synthesis by the Ionic gelation method, properties, and biological activity. Russ. J. Gen. Chem. 2020, 90, 1304–1311. [Google Scholar] [CrossRef]
- Khalil, S.K.; El-Feky, G.S.; El-Banna, S.T.; Khalil, W.A. Preparation and evaluation of warfarin-beta-cyclodextrin loaded chitosan nanoparticles for transdermal delivery. Carbohydr Polym. 2012, 90, 1244–1253. [Google Scholar] [CrossRef]
- Rashedi, J.; Haghjo, A.G.; Abbasi, M.M.; Tabrizi, A.D.; Yaqoubi, S.; Sanajou, D.; Jigheh, Z.A.; Namvaran, A.; Mohammadi, A.; Khoshraj, J.M.; et al. Anti-tumor Effect of Quercetin Loaded Chitosan Nanoparticles on Induced Colon Cancer in Wistar Rats. Adv. Pharm. Bull. 2019, 9, 409–415. [Google Scholar] [CrossRef]
- Iranmanesh, P.; Ehsani, A.; Khademi, A.; Asefnejad, A.; Shahriari, S.; Soleimani, M.; Nejad, M.G.; Saber-Samandari, S.; Khandan, A. Application of 3D Bioprinters for Dental Pulp Regeneration and Tissue Engineering (Porous architecture). Transp. Porous Media 2022, 142, 265–293. [Google Scholar] [CrossRef]
- Ali, K.A.; El-Naa, M.M.; Bakr, A.F.; Mahmoud, M.Y.; Abdelgawad, E.M.; Matoock, M.Y. The dual gastro- and neuroprotective effects of curcumin loaded chitosan nanoparticles against cold restraint stress in rats. Biomed. Pharmacother. 2022, 148, 112778. [Google Scholar] [CrossRef]
- Vindya, N.S.; Mohamad, A.; Razdan, R. Allantoin attenuates deficits of behavioural and motor nerve conduction in an animal model of cisplatin-induced neurotoxicity in rats. Anim. Model. Exp. Med. 2019, 2, 114–120. [Google Scholar] [CrossRef]
- Anjaneyulu, M.; Chopra, K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 244–248. [Google Scholar] [CrossRef]
- Khalil, H.M.; Eliwa, H.A.; El-Shiekh, R.A.; Al-Mokaddem, A.K.; Hassan, M.; Tawfek, A.M.; El-Maadawy, W.H. Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J. Ethnopharmacol. 2021, 277, 114141. [Google Scholar] [CrossRef]
- Zaki, S.M.; Hussein, G.H.; Khalil, H.M.; Algaleel, W.A.A. Febuxostat ameliorates methotrexate-induced lung damage. Folia Morphol. 2021, 80, 392–402. [Google Scholar] [CrossRef]
- Jayatilleke, E.; Shaw, S. A High-Performance Liquid Chromatographic Assay for Reduced and Oxidized Glutathione in Biological Samples. Anal. Biochem. 1993, 214, 452–457. [Google Scholar] [CrossRef]
- Lazzarino, G.; Di Pierro, D.; Tavazzi, B.; Cerroni, L.; Giardina, B. Simultaneous separation of malondialdehyde, ascorbic acid, and adenine nucleotide derivatives from biological samples by ion-pairing high-performance liquid chromatography. Anal. Biochem. 1991, 197, 191–196. [Google Scholar] [CrossRef]
- Rarani, F.Z.; Golshan-Iranpour, F.; Dashti, G.R. Correlation between sperm motility and sperm chromatin/DNA damage before and after cryopreservation and the effect of folic acid and nicotinic acid on post-thaw sperm quality in normozoospermic men. Cell Tissue Bank. 2019, 20, 367–378. [Google Scholar] [CrossRef]
- Akorede, G.J.; Ambali, S.F.; Hudu, M.G.; Olatunji, A.O.; Shittu, M.; Aremu, A.; Basiru, A.; Biobaku, K.T.; Ahmed, A.O.; Ameen, S.A. Protective effect of vitamin C on chronic carbamazepine-induced reproductive toxicity in male wistar rats. Toxicol. Rep. 2020, 7, 269–276. [Google Scholar] [CrossRef]
- Bancroft, J.D. Histochemical Techniques; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Baky, M.H.; Badawy, M.T.; Bakr, A.F.; Hegazi, N.M.; Abdellatif, A.; Farag, M.A. Metabolome-based profiling of African baobab fruit (Adansonia digitata L.) using a multiplex approach of MS and NMR techniques in relation to its biological activity. RSC Adv. 2021, 11, 39680–39695. [Google Scholar] [CrossRef]
- Cosentino, M.J.; Nishida, M.; Rabinowitz, R.; Cockett, A.T. Histological Changes Occurring in the Contralateral Testes of Prepubertal Rats Subjected to Various Durations of Unilateral Spermatic Cord Torsion. J. Urol. 1985, 133, 906–911. [Google Scholar] [CrossRef]
- Johnsen, S.G. Testicular Biopsy Score Count–A Method for Registration of Spermatogenesis in Human Testes: Normal Values and Results in 335 Hypogonadal Males. Horm. Res. Paediatr. 1970, 1, 2–25. [Google Scholar] [CrossRef]


| Groups | Parameters | |||
|---|---|---|---|---|
| Creatinine (mg/dL) | Urea (mg/dL) | Potassium-K+ (mEq/L) | Sodium-Na+ (mEq/L) | |
| Control | 0.81 ± 0.052 | 44.29 ± 1.11 | 3.45 ± 0.041 | 149.0 ± 3.85 |
| QUE | 0.81 ± 0.035 | 42.85 ± 0.96 | 3.26 ± 0.029 | 154.4 ± 4.63 |
| QUE.NPs | 0.83 ± 0.054 | 42.87 ± 1.26 | 3.43 ± 0.064 | 151.1 ± 4.99 |
| CIS | 1.31 ± 0.042 * (p < 0.0001) | 57.52 ± 1.27 * (p < 0.001) | 2.50 ± 0.039 * (p < 0.001) | 210.0 ± 5.90 * (p < 0.001) |
| CIS + QUE | 1.10 ± 0.027 ** (p < 0.05) | 54.39 ± 1.04 ** (p < 0.05) | 2.42 ± 0.034 ** | 201.1 ± 5.92 |
| CIS + QUE.NPs | 0.94 ± 0.059 *** (p < 0.05) | 49.30 ± 0.87 *** (p < 0.01) | 2.90 ± 0.051 *** (p < 0.001) | 180.0 ± 3.77 *** (p < 0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakr, A.F.; El-Shiekh, R.A.; Mahmoud, M.Y.; Khalil, H.M.A.; Alyami, M.H.; Alyami, H.S.; Galal, O.; Mansour, D.F. Efficacy of Quercetin and Quercetin Loaded Chitosan Nanoparticles Against Cisplatin-Induced Renal and Testicular Toxicity via Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Pharmaceuticals 2024, 17, 1384. https://doi.org/10.3390/ph17101384
Bakr AF, El-Shiekh RA, Mahmoud MY, Khalil HMA, Alyami MH, Alyami HS, Galal O, Mansour DF. Efficacy of Quercetin and Quercetin Loaded Chitosan Nanoparticles Against Cisplatin-Induced Renal and Testicular Toxicity via Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Pharmaceuticals. 2024; 17(10):1384. https://doi.org/10.3390/ph17101384
Chicago/Turabian StyleBakr, Alaa F., Riham A. El-Shiekh, Mohamed Y. Mahmoud, Heba M. A. Khalil, Mohammad H. Alyami, Hamad S. Alyami, Omneya Galal, and Dina F. Mansour. 2024. "Efficacy of Quercetin and Quercetin Loaded Chitosan Nanoparticles Against Cisplatin-Induced Renal and Testicular Toxicity via Attenuation of Oxidative Stress, Inflammation, and Apoptosis" Pharmaceuticals 17, no. 10: 1384. https://doi.org/10.3390/ph17101384
APA StyleBakr, A. F., El-Shiekh, R. A., Mahmoud, M. Y., Khalil, H. M. A., Alyami, M. H., Alyami, H. S., Galal, O., & Mansour, D. F. (2024). Efficacy of Quercetin and Quercetin Loaded Chitosan Nanoparticles Against Cisplatin-Induced Renal and Testicular Toxicity via Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Pharmaceuticals, 17(10), 1384. https://doi.org/10.3390/ph17101384








