Transgenic Drosophila Expressing Active Human LH Receptor in the Gonads Exhibit a Decreased Fecundity: Towards a Platform to Identify New Orally Active Modulators of Gonadotropin Receptor Activity
Abstract
1. Introduction
2. Results
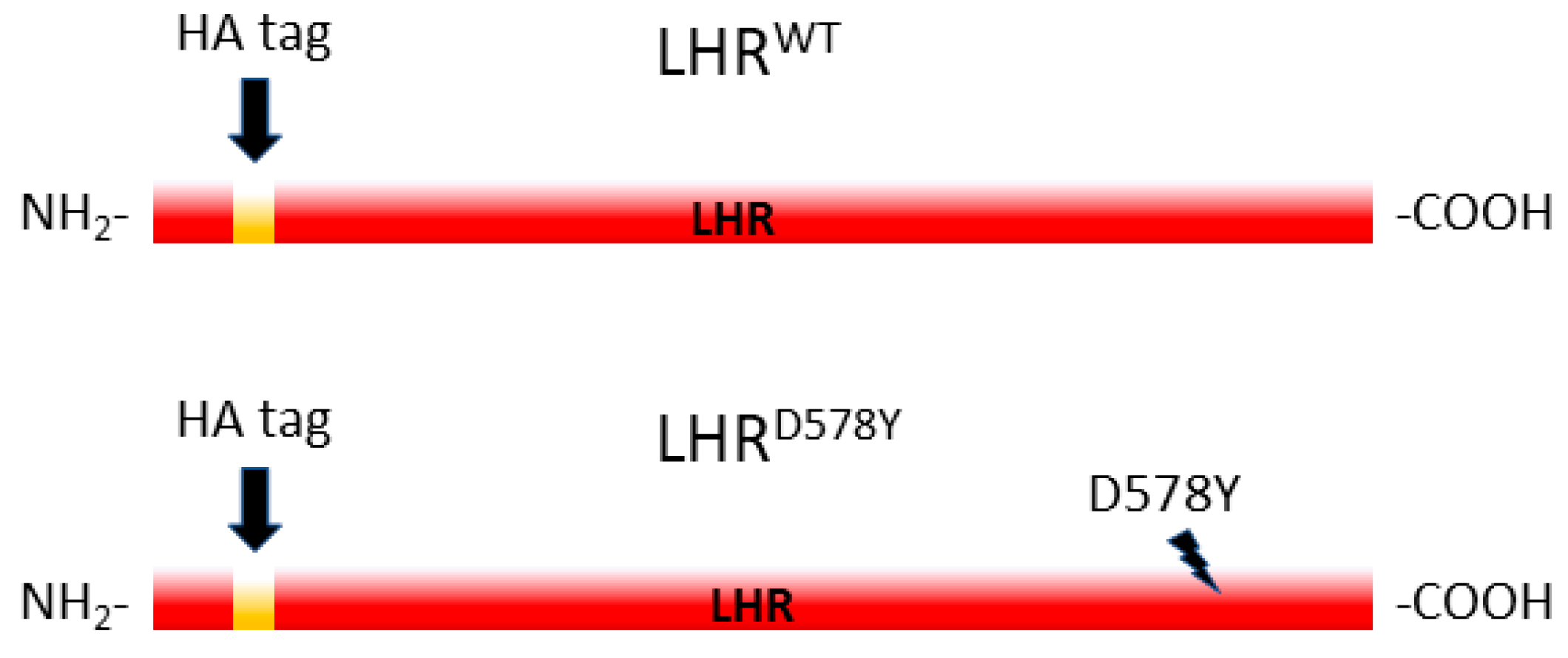
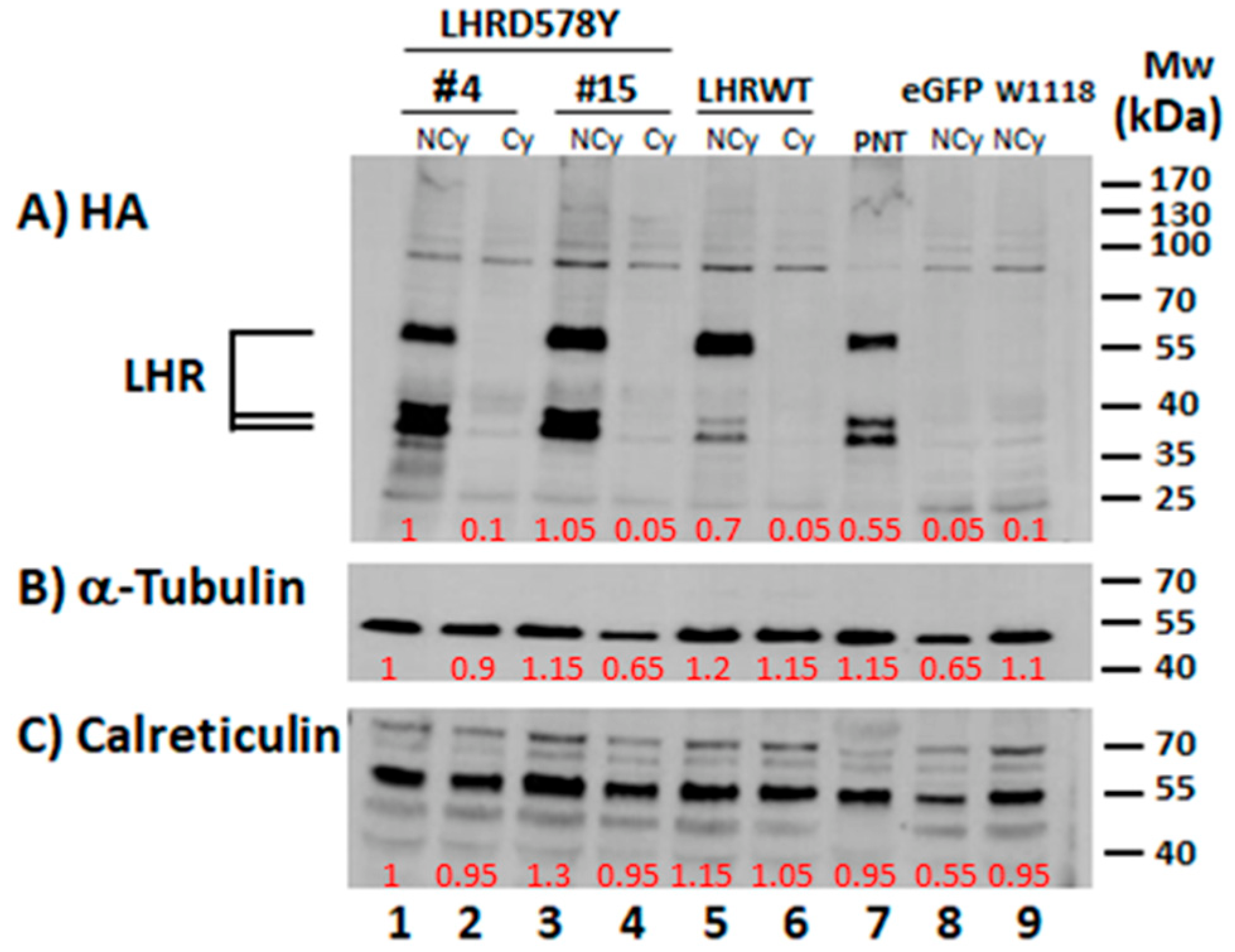
2.1. Expression of the Human LHR in the Fly Ovary
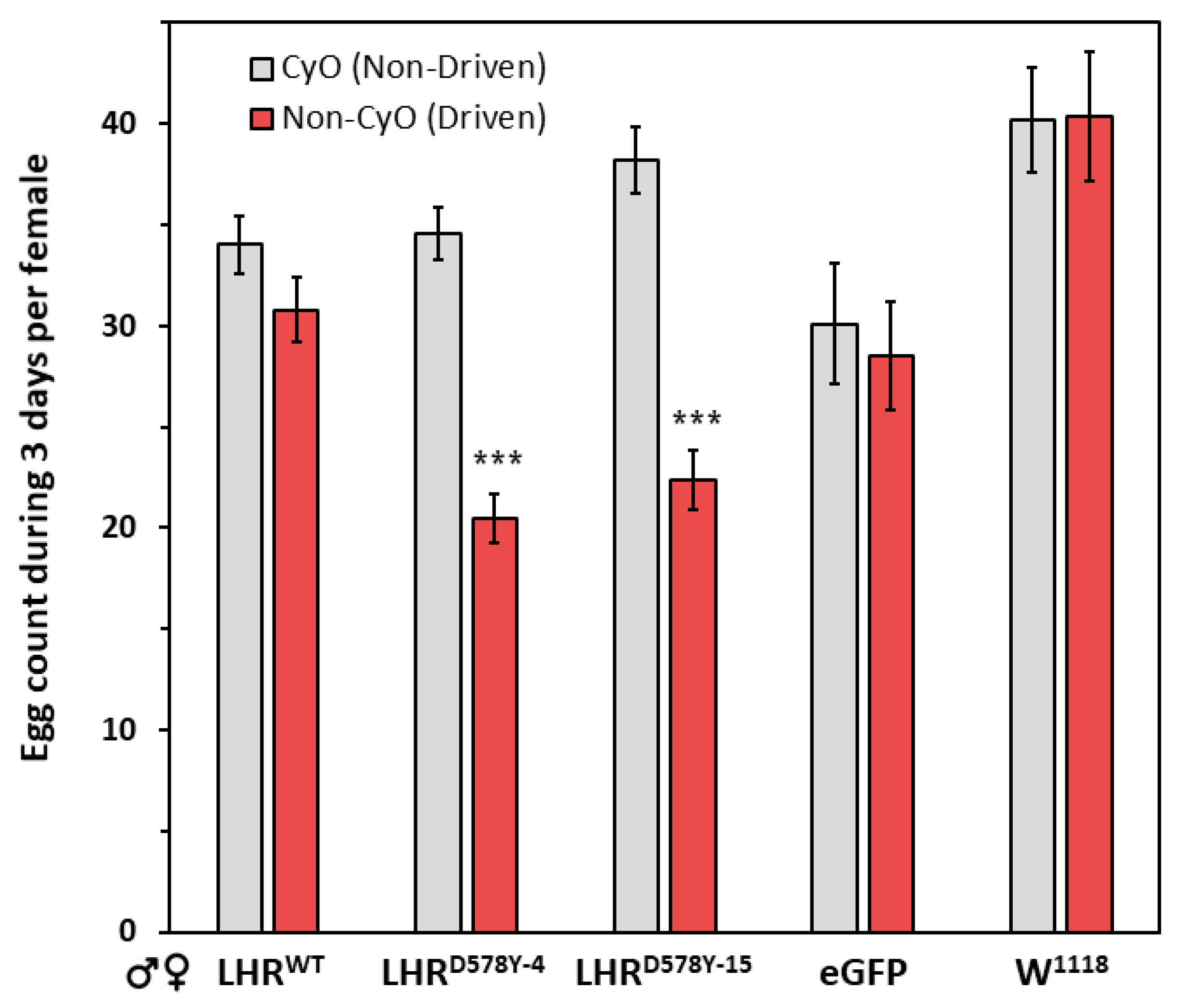
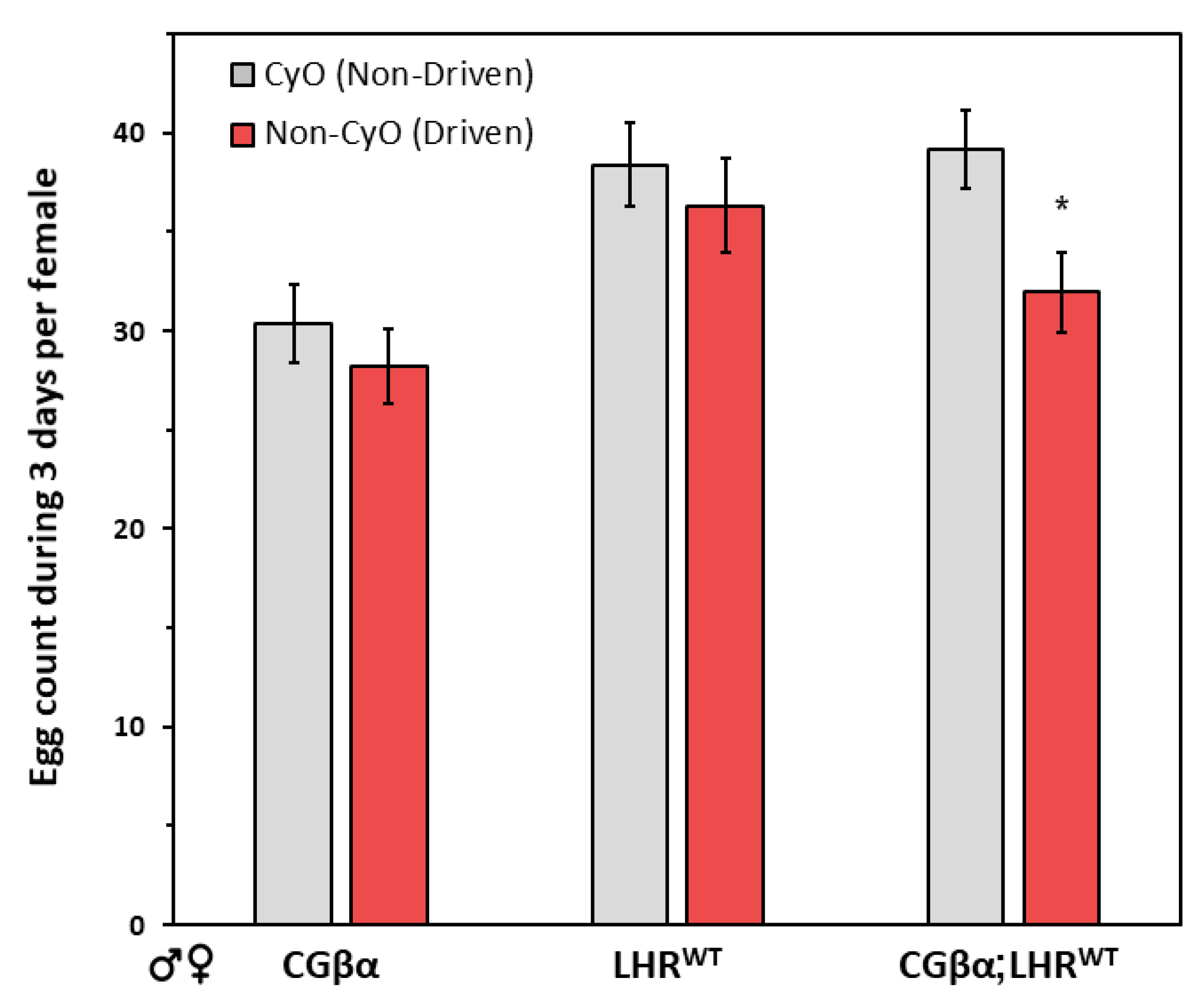
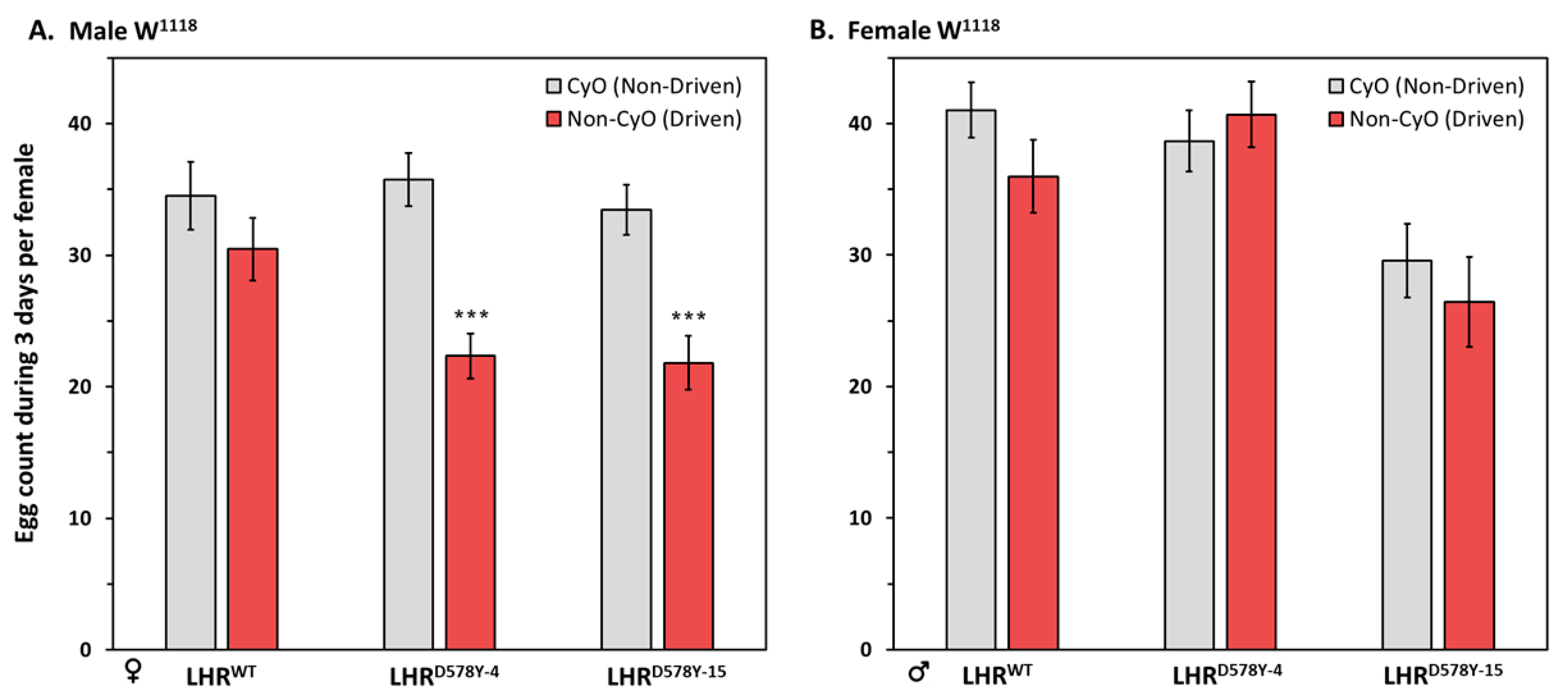
2.2. Fecundity of LHR Flies
2.3. Gender Analysis of the Gonad Phenotype
3. Discussion
4. Materials and Methods
4.1. Fly Strains and Crosses
4.2. Western Blot Analysis of Receptor Expression in the Ovaries and Pupae
4.3. Fecundity Test
4.4. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belchetz, P.E.; Plant, T.M.; Nakai, Y.; Keogh, E.J.; Knobil, E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 1978, 202, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Themmen, A.P.N.; Huhtaniemi, I.T. Mutations of gonadotropins and gonadotropin receptors: Elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr. Rev. 2000, 21, 551–583. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.; McGee, E.A.; Hayashi, M.; Hsu, S.Y. Hormonal regulation of early follicle development in the rat ovary. Mol. Cell. Endocrinol. 2000, 163, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Hunzicker-Dunn, M.; Maizels, E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell Signal. 2006, 18, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.C.; Steele, G.L. Intracellular signaling in the gonads. Endocr. Rev. 1992, 13, 476–498. [Google Scholar] [PubMed]
- Richards, J.S. New signaling pathways for hormones and cyclic adenosine 3′,5′-monophosphate action in endocrine cells. Mol. Endocrinol. 2001, 15, 209–218. [Google Scholar] [PubMed]
- Ascoli, M.; Fanelli, F.; Segaloff, D.L. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 2002, 23, 141–174. [Google Scholar] [CrossRef]
- Casarini, L.; Santi, D.; Brigante, G.; Simoni, M. Two Hormones for One Receptor: Evolution, Biochemistry, Actions, and Pathophysiology of LH and hCG. Endocr. Rev. 2018, 39, 549–592. [Google Scholar] [CrossRef]
- Pierce, J.G.; Parsons, T.F. Glycoprotein hormones: Structure and function. Annu. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef]
- Murphy, B.D.; Martinuk, S.D. Equine Chorionic Gonadotropin. Endocr. Rev. 1991, 12, 27–44. [Google Scholar] [CrossRef]
- Simoni, M.; Gromoll, J.; Nieschlag, E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [PubMed]
- Kakar, S.S.; Musgrove, L.C.; Devor, D.C.; Sellers, J.C.; Neill, J.D. Cloning, sequencing, and expression of human gonadotropin releasing hormone (GnRH) receptor. Biochem. Biophys. Res. Commun. 1992, 189, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C. Drosophila melanogaster: A model and a tool to investigate malignancy and identify new therapeutics. Nat. Rev. Cancer 2013, 13, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.; Cagan, R. Drosophila as a Model for Human Disease. In Vogel and Motulsky’s Human Genetics; Speicher, M.R., Motulsky, A.G., Antonarakis, S.E., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Moulton, M.J.; Letsou, A. Modeling congenital disease and inborn errors of development in Drosophila melanogaster. Dis. Model. Mech. 2016, 9, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Read, R.D.; Goodfellow, P.J.; Mardis, E.R.; Novak, N.; Armstrong, J.R.; Cagan, R.L. A Drosophila model of multiple endocrine neoplasia type 2. Genetics 2005, 171, 1057–1081. [Google Scholar] [CrossRef]
- Vidal, M.; Wells, S.; Ryan, A.; Cagan, R. ZD6474 suppresses oncogenic RET isoforms in a Drosophila model for type 2 multiple endocrine neoplasia syndromes and papillary thyroid carcinoma. Cancer Res. 2005, 65, 3538–3541. [Google Scholar] [CrossRef]
- Adashi, E.Y.; Hsueh, A.J.; Yen, S.S. Insulin enhancement of luteinizing hormone and follicle-stimulating hormone release by cultured pituitary cells. Endocrinology 1981, 108, 1441–1449. [Google Scholar] [CrossRef]
- Conti, M.; Hsieh, M.; Park, J.Y.; Su, Y.Q. Role of the epidermal growth factor network in ovarian follicles. Mol. Endocrinol. 2006, 20, 715–723. [Google Scholar] [CrossRef]
- Eimerl, S.; Orly, J. Regulation of steroidogenic genes by insulin-like growth factor-1 and follicle-stimulating hormone: Differential responses of cytochrome P450 side-chain cleavage, steroidogenic acute regulatory protein, and 3beta-hydroxysteroid dehydrogenase/isomerase in rat granulosa cells. Biol. Reprod. 2002, 67, 900–910. [Google Scholar] [CrossRef]
- Zhou, J.; Kumar, T.R.; Matzuk, M.M.; Bondy, C. Insulin-like growth factor I regulates gonadotropin responsiveness in the murine ovary. Mol. Endocrinol. 1997, 11, 1924–1933. [Google Scholar] [CrossRef]
- Ben-Menahem, D. GnRH-Related Neurohormones in the Fruit Fly Drosophila melanogaster. Int. J. Mol. Sci. 2021, 22, 5035. [Google Scholar] [CrossRef] [PubMed]
- Juengel, J.L.; McNatty, K.P. The role of proteins of the transforming growth factor-beta superfamily in the intraovarian regulation of follicular development. Hum. Reprod. Update 2005, 11, 143–160. [Google Scholar] [CrossRef]
- Ben-Ami, I.; Armon, L.; Freimann, S.; Strassburger, D.; Ron-El, R.; Amsterdam, A. EGF-like growth factors as LH mediators in the human corpus luteum. Hum. Reprod. 2009, 24, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Burns, K.H.; Viveiros, M.M.; Eppig, J.J. Intercellular communication in the mammalian ovary: Oocytes carry the conversation. Science 2002, 296, 2178–2180. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Su, Y.Q.; Ariga, M.; Law, E.; Jin, S.L.; Conti, M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004, 303, 682–684. [Google Scholar] [CrossRef]
- Vale, W.; Wiater, E.; Gray, P.; Harrison, C.; Bilezikjian, L.; Choe, S. Activins and inhibins and their signaling. Ann. N. Y. Acad. Sci. 2004, 1038, 142–147. [Google Scholar] [CrossRef]
- Hauser, F.; Nothacker, H.P.; Grimmelikhuijzen, C.J. Molecular cloning, genomic organization, and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to members of the thyroid-stimulating hormone, follicle-stimulating hormone, luteinizing hormone/choriogonadotropin receptor family from mammals. J. Biol. Chem. 1997, 272, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Hauser, F.; Sondergaard, L.; Grimmelikhuijzen, C.J. Molecular cloning, genomic organization and developmental regulation of a novel receptor from Drosophila melanogaster structurally related to gonadotropin-releasing hormone receptors for vertebrates. Biochem. Biophys. Res. Commun. 1998, 249, 822–828. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Nakabayashi, K.; Bhalla, A. Evolution of glycoprotein hormone subunit genes in bilateral metazoa: Identification of two novel human glycoprotein hormone subunit family genes, GPA2 and GPB5. Mol. Endocrinol. 2002, 16, 1538–1551. [Google Scholar] [CrossRef]
- Mendive, F.M.; Van Loy, T.; Claeysen, S.; Poels, J.; Williamson, M.; Hauser, F.; Grimmelikhuijzen, C.J.; Vassart, G.; Vanden Broeck, J. Drosophila molting neurohormone bursicon is a heterodimer and the natural agonist of the orphan receptor DLGR2. FEBS Lett. 2005, 579, 2171–2176. [Google Scholar] [CrossRef]
- Sudo, S.; Kuwabara, Y.; Park, J.I.; Hsu, S.Y.; Hsueh, A.J. Heterodimeric fly glycoprotein hormone-alpha2 (GPA2) and glycoprotein hormone-beta5 (GPB5) activate fly leucine-rich repeat-containing G protein-coupled receptor-1 (DLGR1) and stimulation of human thyrotropin receptors by chimeric fly GPA2 and human GPB5. Endocrinology 2005, 146, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.D.; Celniker, S.E.; Holt, R.A.; Evans, C.A.; Gocayne, J.D.; Amanatides, P.G.; Scherer, S.E.; Li, P.W.; Hoskins, R.A.; Galle, R.F.; et al. The genome sequence of Drosophila melanogaster. Science 2000, 287, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- De Loof, A.; Baggerman, G.; Breuer, M.; Claeys, I.; Cerstiaens, A.; Clynen, E.; Janssen, T.; Schoofs, L.; Vanden Broeck, J. Gonadotropins in insects: An overview. Arch. Insect Biochem. Physiol. 2001, 47, 129–138. [Google Scholar] [CrossRef]
- Kuczer, M.; Rosinski, G.; Konopinska, D. Insect gonadotropic peptide hormones: Some recent developments. J. Pept. Sci. 2007, 13, 16–26. [Google Scholar] [CrossRef]
- Graves, J.; Markman, S.; Alegranti, Y.; Gechtler, J.; Johnson, R.I.; Cagan, R.; Ben-Menahem, D. The LH/CG receptor activates canonical signaling pathway when expressed in Drosophila. Mol. Cell. Endocrinol. 2015, 413, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef]
- Li, M.A.; Alls, J.D.; Avancini, R.M.; Koo, K.; Godt, D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat. Cell Biol. 2003, 5, 994–1000. [Google Scholar] [CrossRef]
- Olivieri, D.; Sykora, M.M.; Sachidanandam, R.; Mechtler, K.; Brennecke, J. An in vivo RNAi assay identifies major genetic and cellular requirements for primary piRNA biogenesis in Drosophila. EMBO J. 2010, 29, 3301–3317. [Google Scholar] [CrossRef]
- Latronico, A.C.; Segaloff, D.L. Naturally occurring mutations of the luteinizing-hormone receptor: Lessons learned about reproductive physiology and G protein-coupled receptors. Am. J. Hum. Genet. 1999, 65, 949–958. [Google Scholar] [CrossRef]
- Laue, L.; Chan, W.Y.; Hsueh, A.J.; Kudo, M.; Hsu, S.Y.; Wu, S.M.; Blomberg, L.; Cutler, G.B., Jr. Genetic heterogeneity of constitutively activating mutations of the human luteinizing hormone receptor in familial male-limited precocious puberty. Proc. Natl. Acad. Sci. USA 1995, 92, 1906–1910. [Google Scholar] [CrossRef]
- Liu, G.; Duranteau, L.; Carel, J.C.; Monroe, J.; Doyle, D.A.; Shenker, A. Leydig-cell tumors caused by an activating mutation of the gene encoding the luteinizing hormone receptor. N. Engl. J. Med. 1999, 341, 1731–1736. [Google Scholar] [CrossRef] [PubMed]
- Hai, L.; McGee, S.R.; Rabideau, A.C.; Paquet, M.; Narayan, P. Infertility in Female Mice with a Gain-of-Function Mutation in the Luteinizing Hormone Receptor Is Due to Irregular Estrous Cyclicity, Anovulation, Hormonal Alterations, and Polycystic Ovaries. Biol. Reprod. 2015, 93, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McGee, S.R.; Narayan, P. Precocious puberty and Leydig cell hyperplasia in male mice with a gain of function mutation in the LH receptor gene. Endocrinology 2013, 154, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Risma, K.A.; Clay, C.M.; Nett, T.M.; Wagner, T.; Yun, J.; Nilson, J.H. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc. Natl. Acad. Sci. USA 1995, 92, 1322–1326. [Google Scholar] [CrossRef]
- Baker, V.L.; Brown, M.B.; Luke, B.; Smith, G.W.; Ireland, J.J. Gonadotropin dose is negatively correlated with live birth rate: Analysis of more than 650,000 assisted reproductive technology cycles. Fertil. Steril. 2015, 104, 1145–1152. [Google Scholar] [CrossRef]
- Dekel, N.; Ayalon, D.; Lewysohn, O.; Nevo, N.; Kaplan-Kraicer, R.; Shalgi, R. Experimental extension of the time interval between oocyte maturation and ovulation: Effect on fertilization and first cleavage. Fertil. Steril. 1995, 64, 1023–1028. [Google Scholar] [CrossRef]
- Ireland, J.J.; Ward, F.; Jimenez-Krassel, F.; Ireland, J.L.; Smith, G.W.; Lonergan, P.; Evans, A.C. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum. Reprod. 2007, 22, 1687–1695. [Google Scholar] [CrossRef]
- Maman, E.; Yung, Y.; Kedem, A.; Yerushalmi, G.M.; Konopnicki, S.; Cohen, B.; Dor, J.; Hourvitz, A. High expression of luteinizing hormone receptors messenger RNA by human cumulus granulosa cells is in correlation with decreased fertilization. Fertil. Steril. 2012, 97, 592–598. [Google Scholar] [CrossRef]
- Orisaka, M.; Hattori, K.; Fukuda, S.; Mizutani, T.; Miyamoto, K.; Sato, T.; Tsang, B.K.; Kotsuji, F.; Yoshida, Y. Dysregulation of ovarian follicular development in female rat: LH decreases FSH sensitivity during preantral-early antral transition. Endocrinology 2013, 154, 2870–2880. [Google Scholar] [CrossRef]
- Bindhani, B.; Maity, S.; Chakrabarti, I.; Saha, S.K. Roles of matrix metalloproteinases in development, immunology, and ovulation in fruit Fly (Drosophila). Arch. Insect Biochem. Physiol. 2022, 109, e21849. [Google Scholar] [CrossRef]
- Castrillon, D.H.; Wasserman, S.A. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development 1994, 120, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Deady, L.D.; Shen, W.; Mosure, S.A.; Spradling, A.C.; Sun, J. Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila. PLoS Genet. 2015, 11, e1004989. [Google Scholar] [CrossRef] [PubMed]
- Fortini, M.E.; Skupski, M.P.; Boguski, M.S.; Hariharan, I.K. A survey of human disease gene counterparts in the Drosophila genome. J. Cell Biol. 2000, 150, F23-30. [Google Scholar] [CrossRef] [PubMed]
- Shore, T.; Levi, T.; Kalifa, R.; Dreifuss, A.; Rekler, D.; Weinberg-Shukron, A.; Nevo, Y.; Bialistoky, T.; Moyal, V.; Gold, M.Y.; et al. Nucleoporin107 mediates female sexual differentiation via Dsx. Elife 2022, 11, e72632. [Google Scholar] [CrossRef] [PubMed]
- Tsafriri, A. Ovulation as a tissue remodelling process. Proteolysis and cumulus expansion. Adv. Exp. Med. Biol. 1995, 377, 121–140. [Google Scholar] [CrossRef]
- Weinberg-Shukron, A.; Renbaum, P.; Kalifa, R.; Zeligson, S.; Ben-Neriah, Z.; Dreifuss, A.; Abu-Rayyan, A.; Maatuk, N.; Fardian, N.; Rekler, D.; et al. A mutation in the nucleoporin-107 gene causes XX gonadal dysgenesis. J. Clin. Invest. 2015, 125, 4295–4304. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, J.; Huang, Y.; Wang, Y.; Xiao, S.; Hadden, M.K.; Woodruff, T.K.; Sun, J. A platform utilizing Drosophila ovulation for nonhormonal contraceptive screening. Proc. Natl. Acad. Sci. USA 2021, 118, e2026403118. [Google Scholar] [CrossRef]
- Crawford, E.D.; Schally, A.V. The role of FSH and LH in prostate cancer and cardiometabolic comorbidities. Can. J. Urol. 2020, 27, 10167–10173. [Google Scholar]
- Davies, S.; Bax, C.M.; Chatzaki, E.; Chard, T.; Iles, R.K. Regulation of endometrial cancer cell growth by luteinizing hormone (LH) and follicle stimulating hormone (FSH). Br. J. Cancer 2000, 83, 1730–1734. [Google Scholar] [CrossRef][Green Version]
- Mey, M.; Bhatta, S.; Casadesus, G. Luteinizing hormone and the aging brain. Vitam. Horm. 2021, 115, 89–104. [Google Scholar] [CrossRef]
- Nerattini, M.; Rubino, F.; Jett, S.; Andy, C.; Boneu, C.; Zarate, C.; Carlton, C.; Loeb-Zeitlin, S.; Havryliuk, Y.; Pahlajani, S.; et al. Elevated gonadotropin levels are associated with increased biomarker risk of Alzheimer’s disease in midlife women. Front. Dement. 2023, 2, 1303256. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Yu, H.; Hao, X.; Dong, W.; Yin, X.; Lin, M.; Zheng, J.; Zhou, B.O. Luteinizing hormone signaling restricts hematopoietic stem cell expansion during puberty. EMBO J. 2018, 37, e98984. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V. Involvement of Luteinizing Hormone in Alzheimer Disease Development in Elderly Women. Reprod. Sci. 2017, 24, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Short, R.A.; Bowen, R.L.; O’Brien, P.C.; Graff-Radford, N.R. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin. Proc. 2001, 76, 906–909. [Google Scholar] [CrossRef] [PubMed]
- Soni, S.; Menon, M.C.; Bhaskaran, M.; Jhaveri, K.D.; Molmenti, E.; Muoio, V. Elevated human chorionic gonadotropin levels in patients with chronic kidney disease: Case series and review of literature. Indian. J. Nephrol. 2013, 23, 424–427. [Google Scholar] [CrossRef]
- Stroomberg, H.V.; Jorgensen, A.; Brasso, K.; Nielsen, J.E.; Juul, A.; Frederiksen, H.; Blomberg Jensen, M.; Roder, M.A. Novel functions of the luteinizing hormone/chorionic gonadotropin receptor in prostate cancer cells and patients. PLoS ONE 2020, 15, e0238814. [Google Scholar] [CrossRef]
- Webber, K.M.; Perry, G.; Smith, M.A.; Casadesus, G. The contribution of luteinizing hormone to Alzheimer disease pathogenesis. Clin. Med. Res. 2007, 5, 177–183. [Google Scholar] [CrossRef]
- Narayan, P.; Wu, C.; Puett, D. Functional expression of yoked human chorionic gonadotropin in baculovirus-infected insect cells. Mol. Endocrinol. 1995, 9, 1720–1726. [Google Scholar] [CrossRef][Green Version]
- Sugahara, T.; Pixley, M.R.; Minami, S.; Perlas, E.; Ben-Menahem, D.; Hsueh, A.J.; Boime, I. Biosynthesis of a biologically active single peptide chain containing the human common alpha and chorionic gonadotropin beta subunits in tandem. Proc. Natl. Acad. Sci. USA 1995, 92, 2041–2045. [Google Scholar] [CrossRef]
- Nurwakagari, P.; Breit, A.; Hess, C.; Salman-Livny, H.; Ben-Menahem, D.; Gudermann, T. A conformational contribution of the luteinizing hormone-receptor ectodomain to receptor activation. J. Mol. Endocrinol. 2007, 38, 259–275. [Google Scholar] [CrossRef][Green Version]
- Sangkuhl, K.; Schulz, A.; Schultz, G.; Schoneberg, T. Structural requirements for mutational lutropin/choriogonadotropin receptor activation. J. Biol. Chem. 2002, 277, 47748–47755. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahamid, A.; Ben-Menahem, D. Transgenic Drosophila Expressing Active Human LH Receptor in the Gonads Exhibit a Decreased Fecundity: Towards a Platform to Identify New Orally Active Modulators of Gonadotropin Receptor Activity. Pharmaceuticals 2024, 17, 1267. https://doi.org/10.3390/ph17101267
Mahamid A, Ben-Menahem D. Transgenic Drosophila Expressing Active Human LH Receptor in the Gonads Exhibit a Decreased Fecundity: Towards a Platform to Identify New Orally Active Modulators of Gonadotropin Receptor Activity. Pharmaceuticals. 2024; 17(10):1267. https://doi.org/10.3390/ph17101267
Chicago/Turabian StyleMahamid, Amir, and David Ben-Menahem. 2024. "Transgenic Drosophila Expressing Active Human LH Receptor in the Gonads Exhibit a Decreased Fecundity: Towards a Platform to Identify New Orally Active Modulators of Gonadotropin Receptor Activity" Pharmaceuticals 17, no. 10: 1267. https://doi.org/10.3390/ph17101267
APA StyleMahamid, A., & Ben-Menahem, D. (2024). Transgenic Drosophila Expressing Active Human LH Receptor in the Gonads Exhibit a Decreased Fecundity: Towards a Platform to Identify New Orally Active Modulators of Gonadotropin Receptor Activity. Pharmaceuticals, 17(10), 1267. https://doi.org/10.3390/ph17101267




