Liuwei Dihuang Pills Enhance Osteogenic Differentiation in MC3T3-E1 Cells through the Activation of the Wnt/β-Catenin Signaling Pathway
Abstract
1. Introduction
2. Results
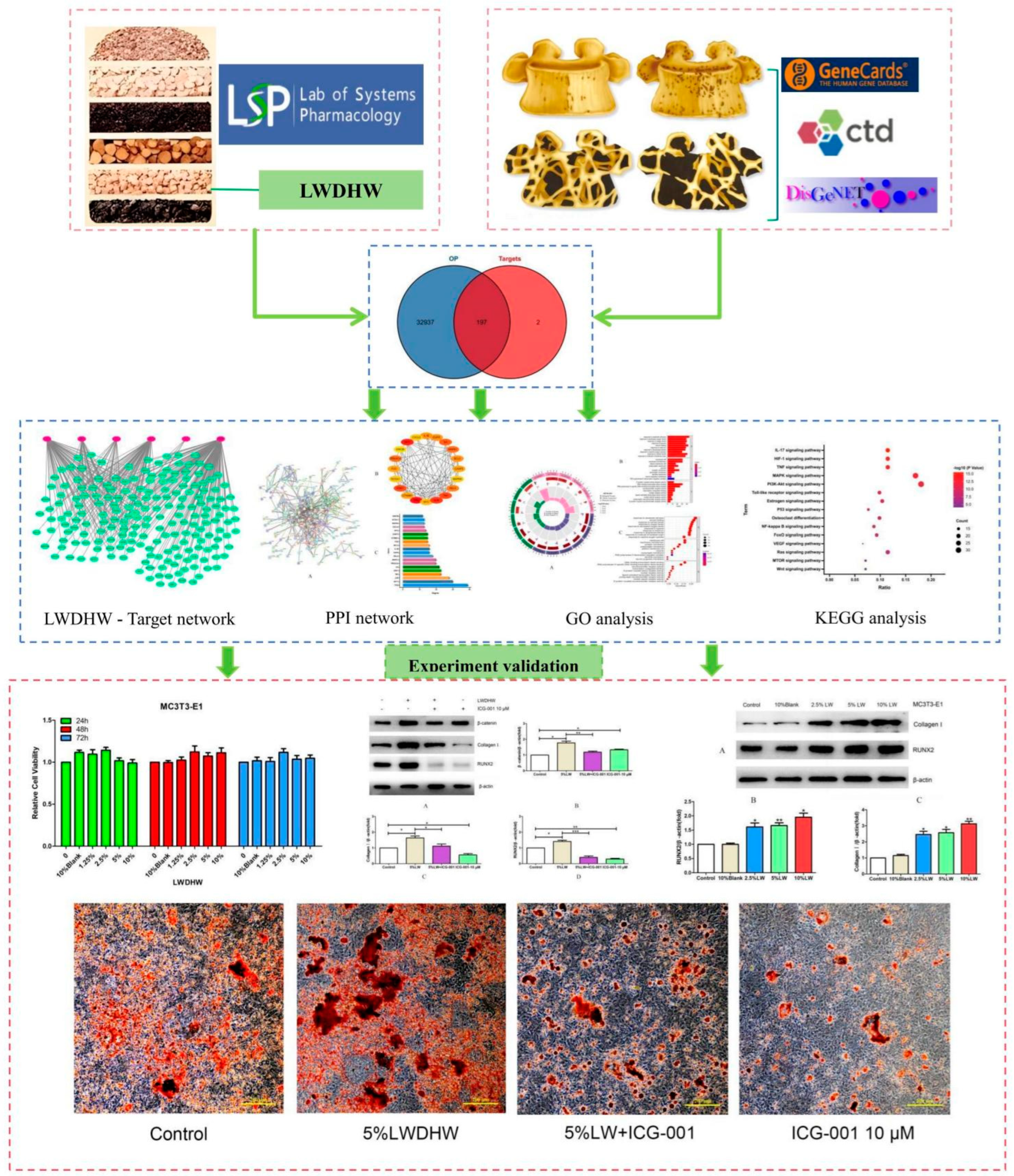
2.1. Network Pharmacology Results
2.1.1. Active Ingredients and Targets of LWDH Pills
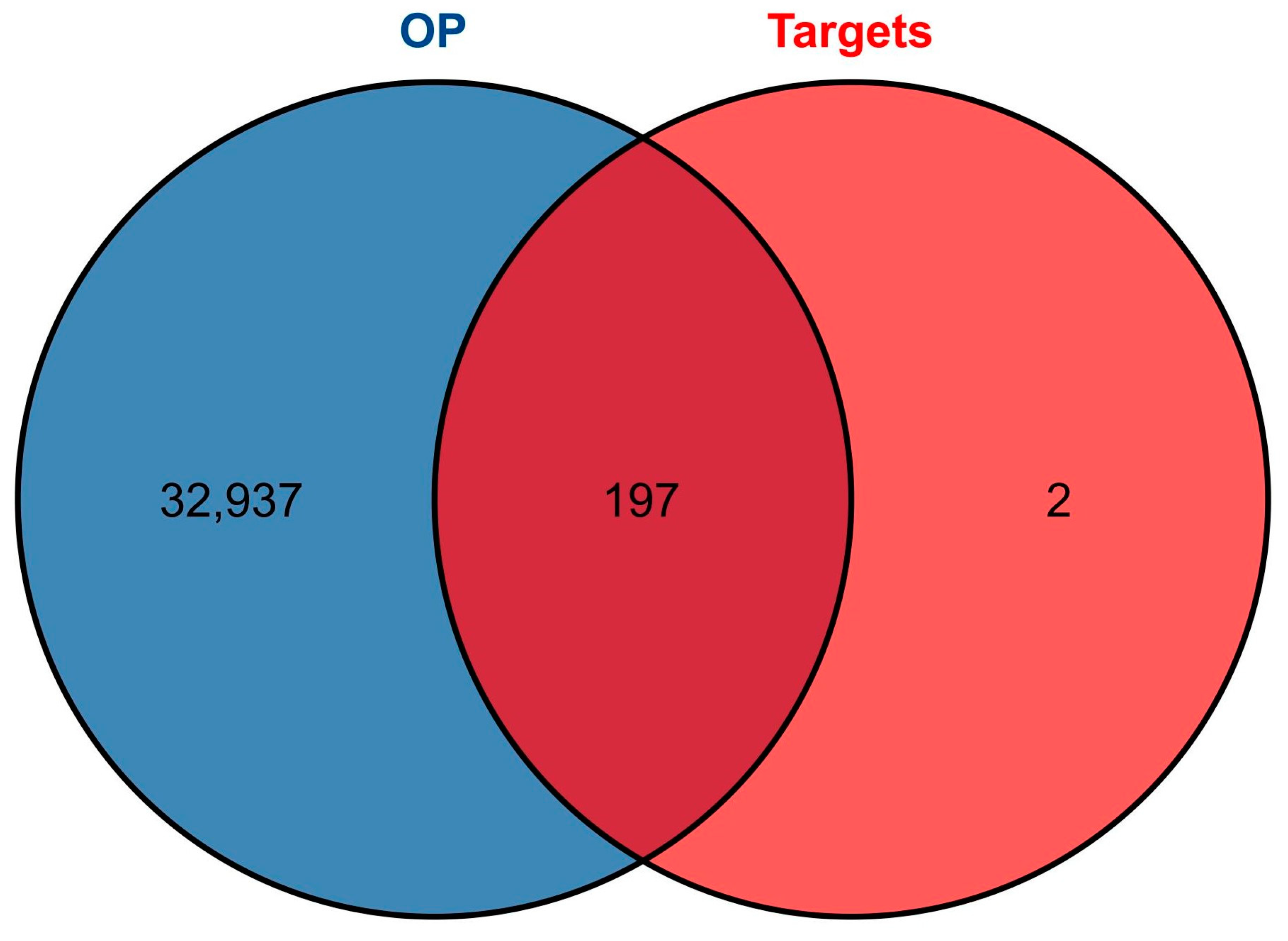
2.1.2. Intersecting Targets between OP and LWDH Pills
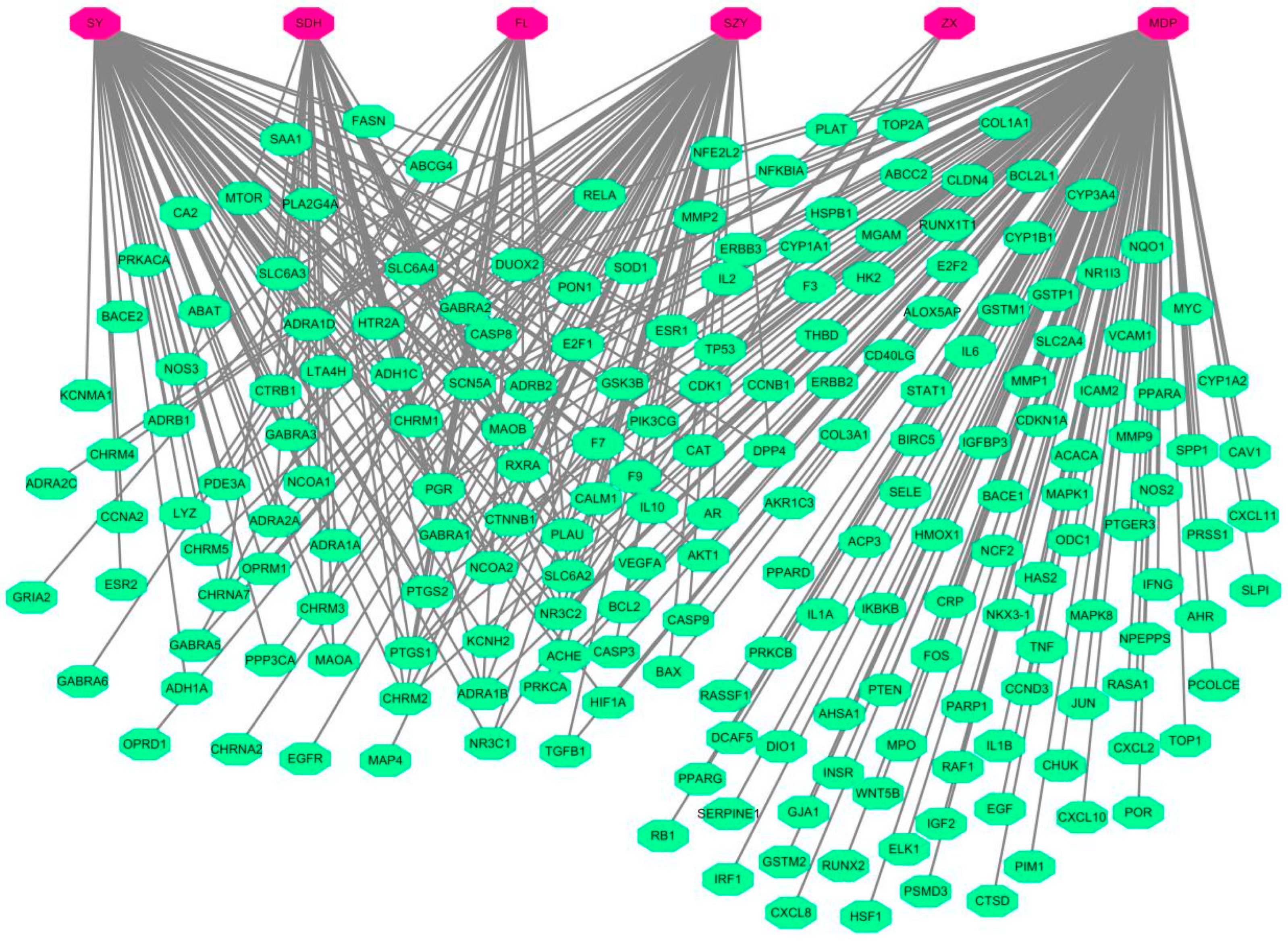
2.1.3. Construction of TCM-Target Network
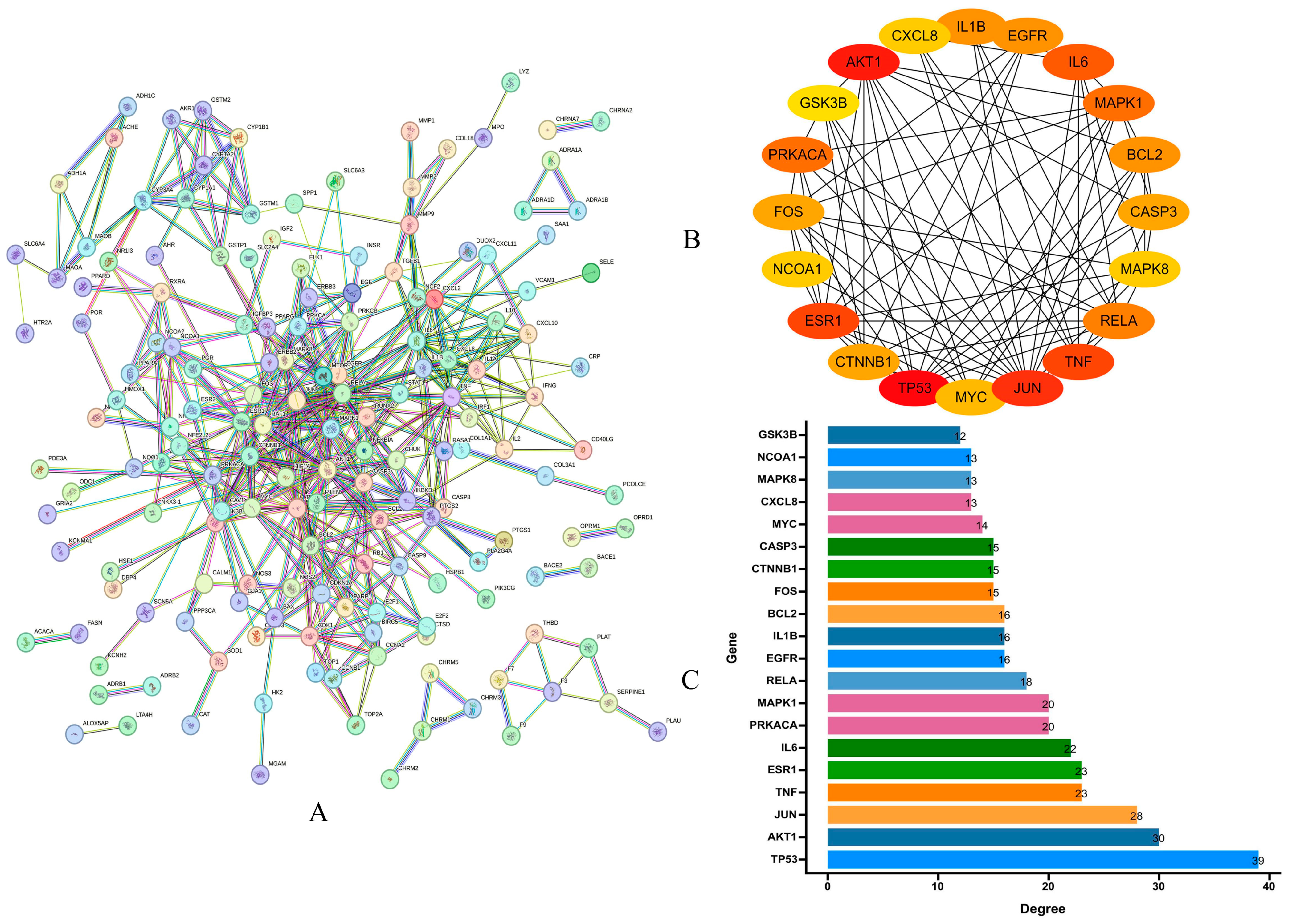
2.1.4. PPI Analysis and Core Targets
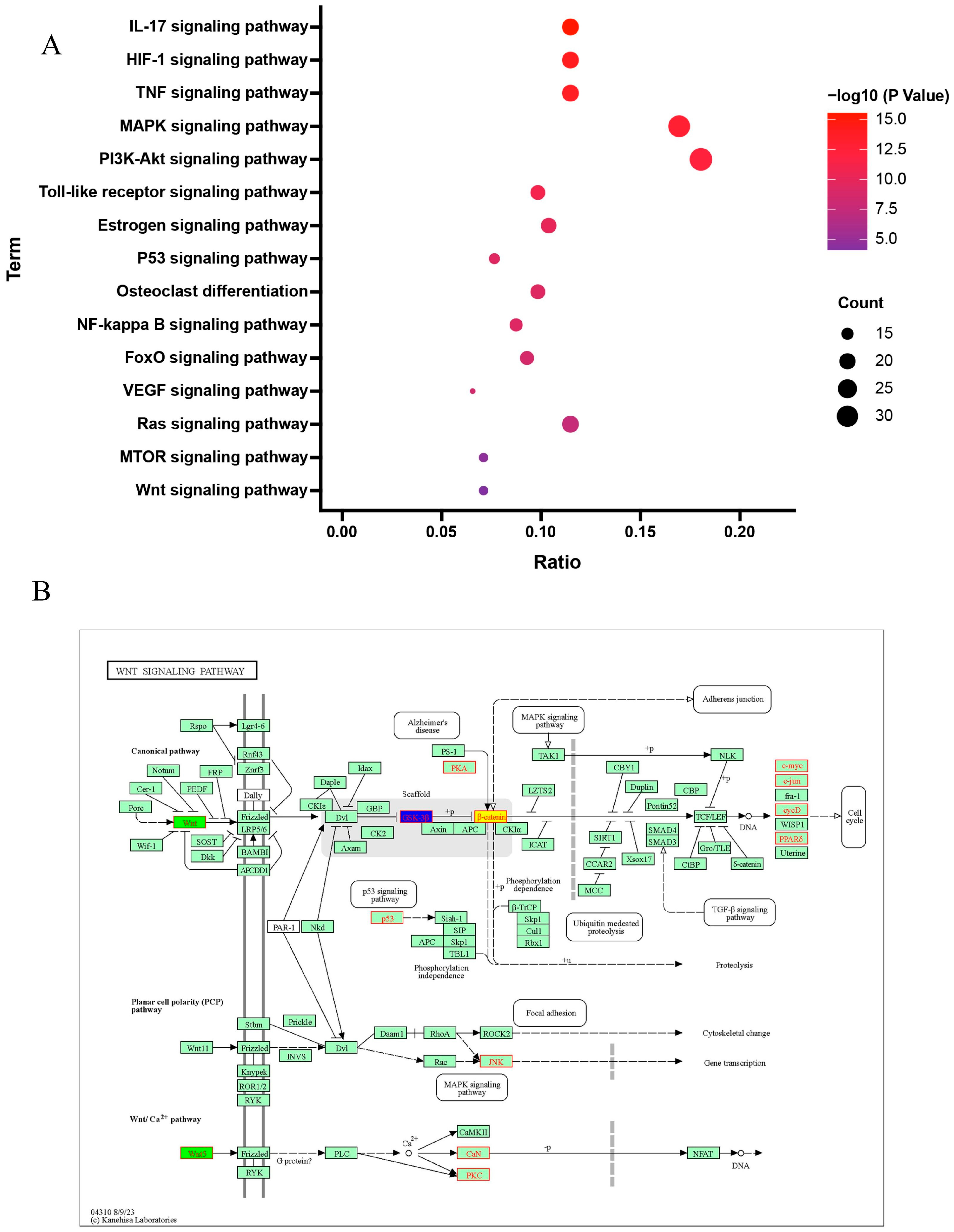
2.1.5. Results of GO and KEGG Signaling Pathway Enrichment
2.2. In Vitro Experimental Validation
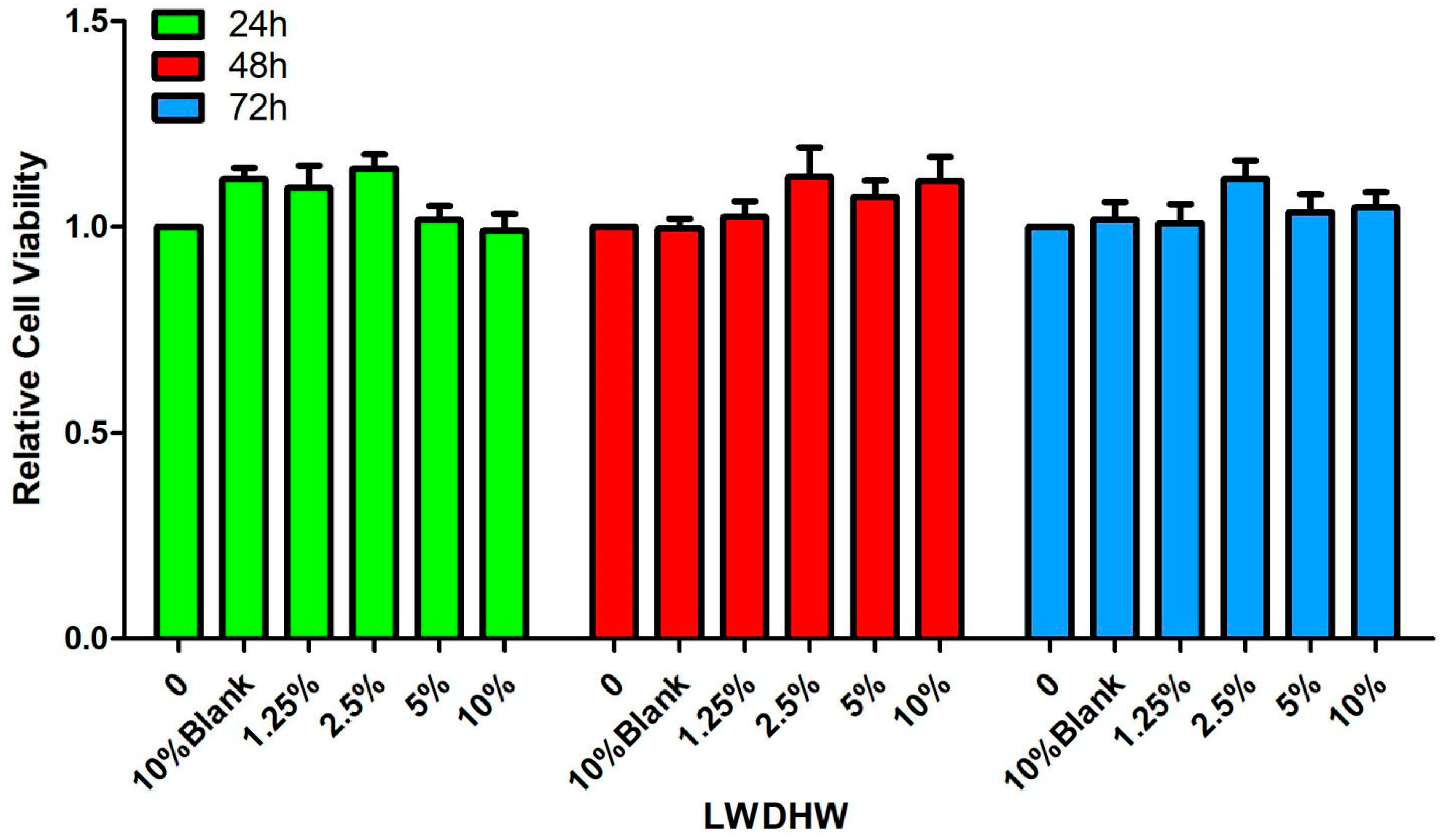
2.2.1. Effect of LWDH Pill-Medicated Serum on the Viability of MC3T3-E1 Cells
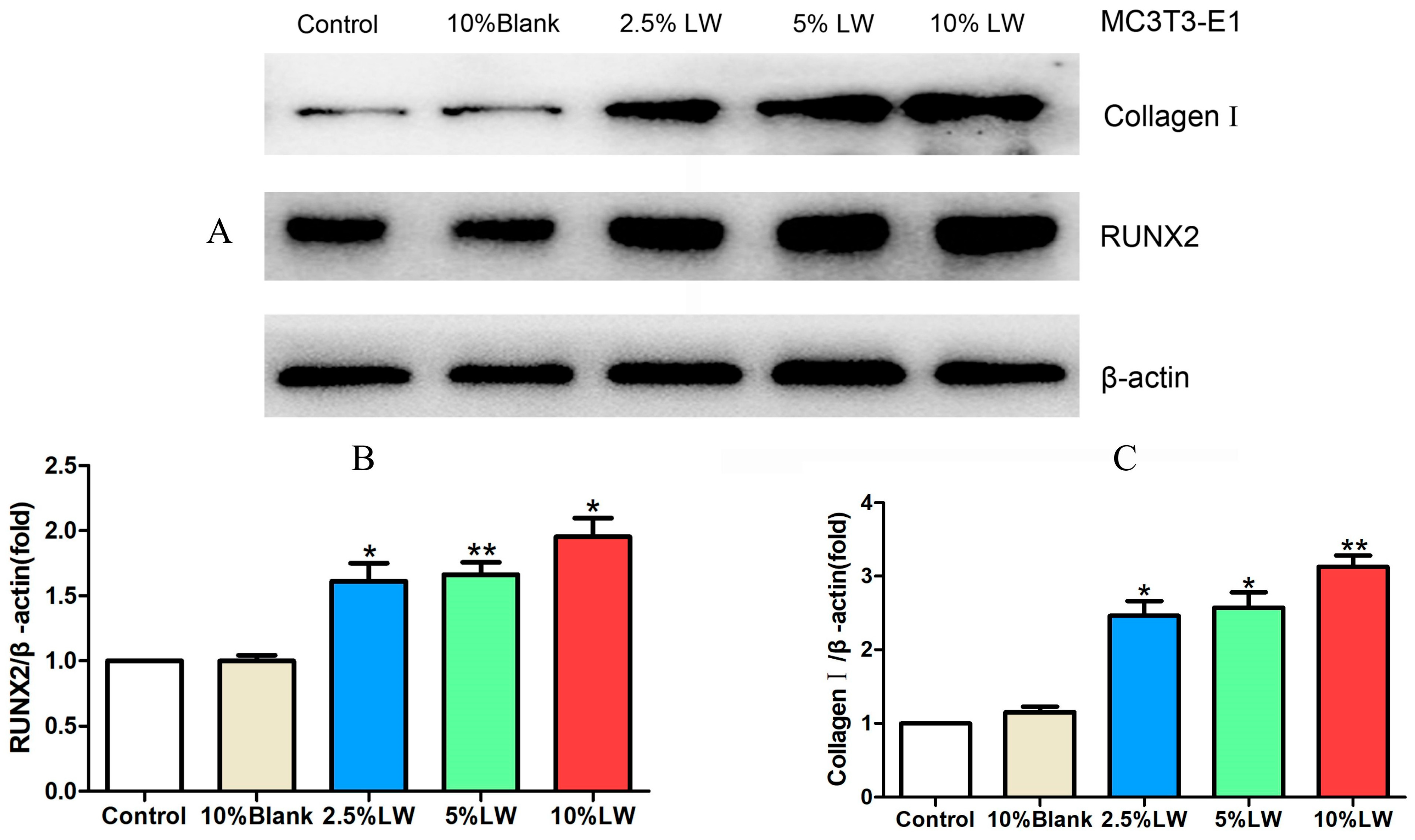
2.2.2. Impact of LWDH Pill-Medicated Serum on the Levels of Osteogenic Differentiation-Related Proteins in MC3T3-E1 Cells
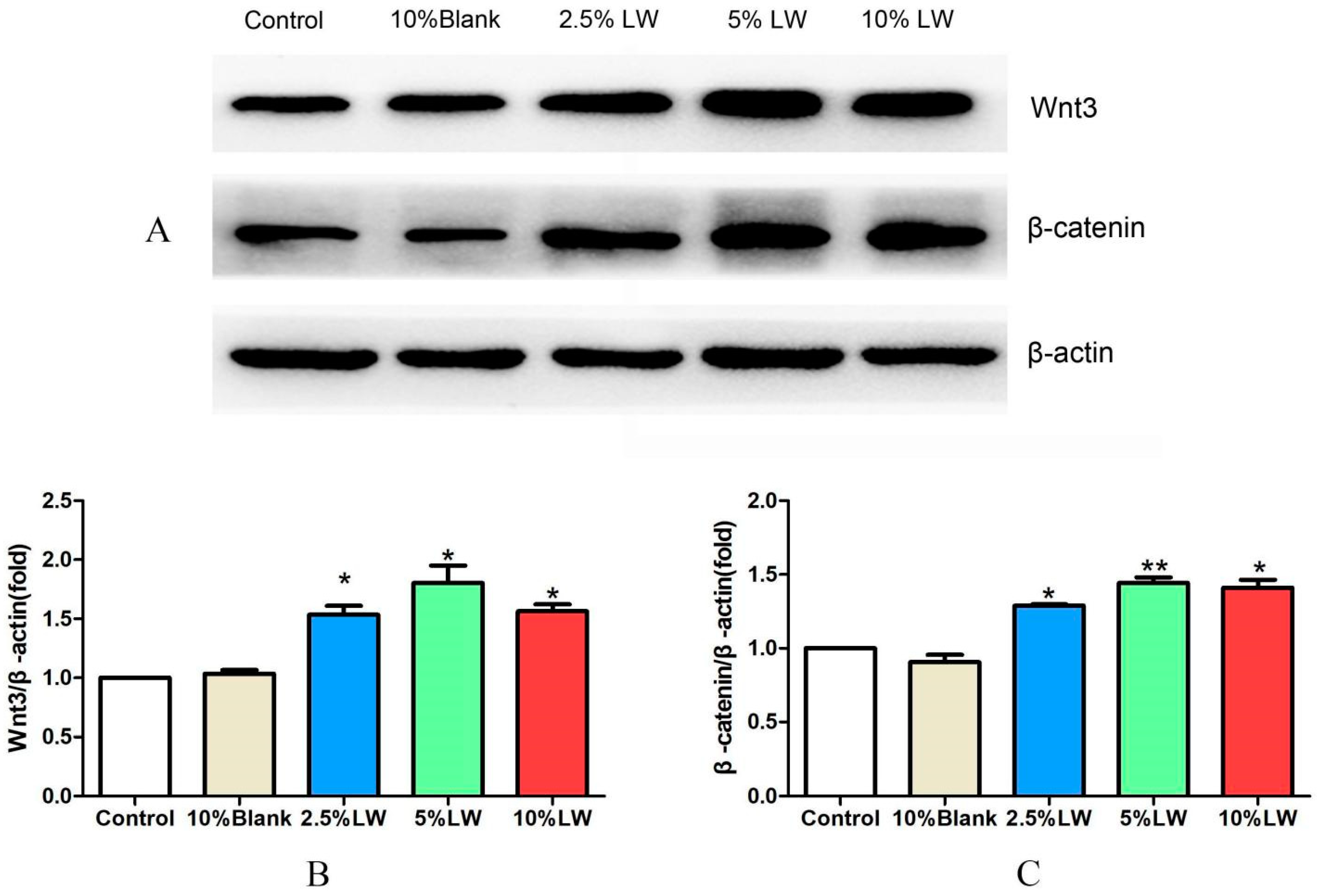
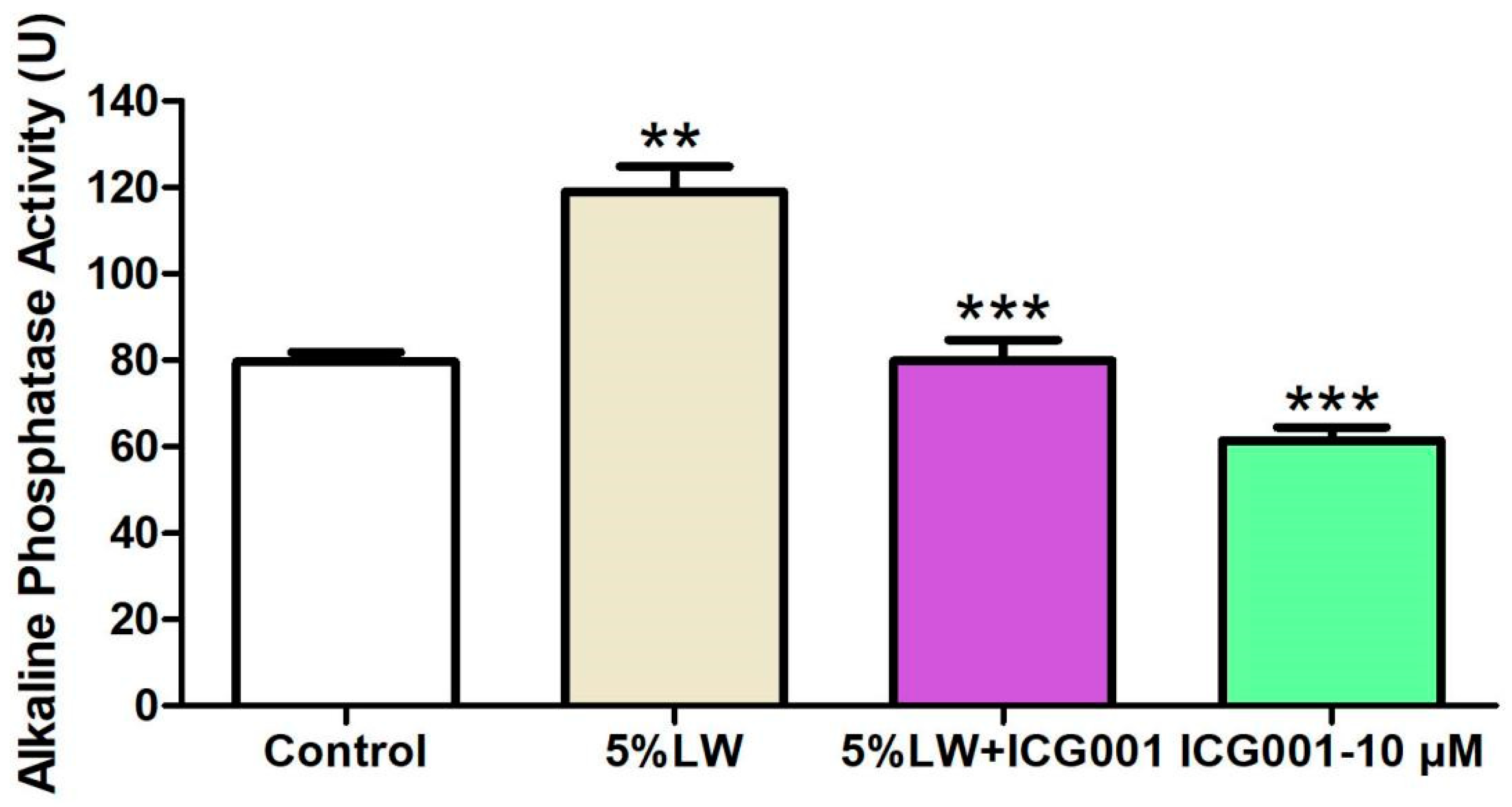
2.2.3. Effect of LWDH Pill-Medicated Serum on the Levels of Wnt/β-catenin Signaling Pathway-Related Proteins in MC3T3-E1 Cells
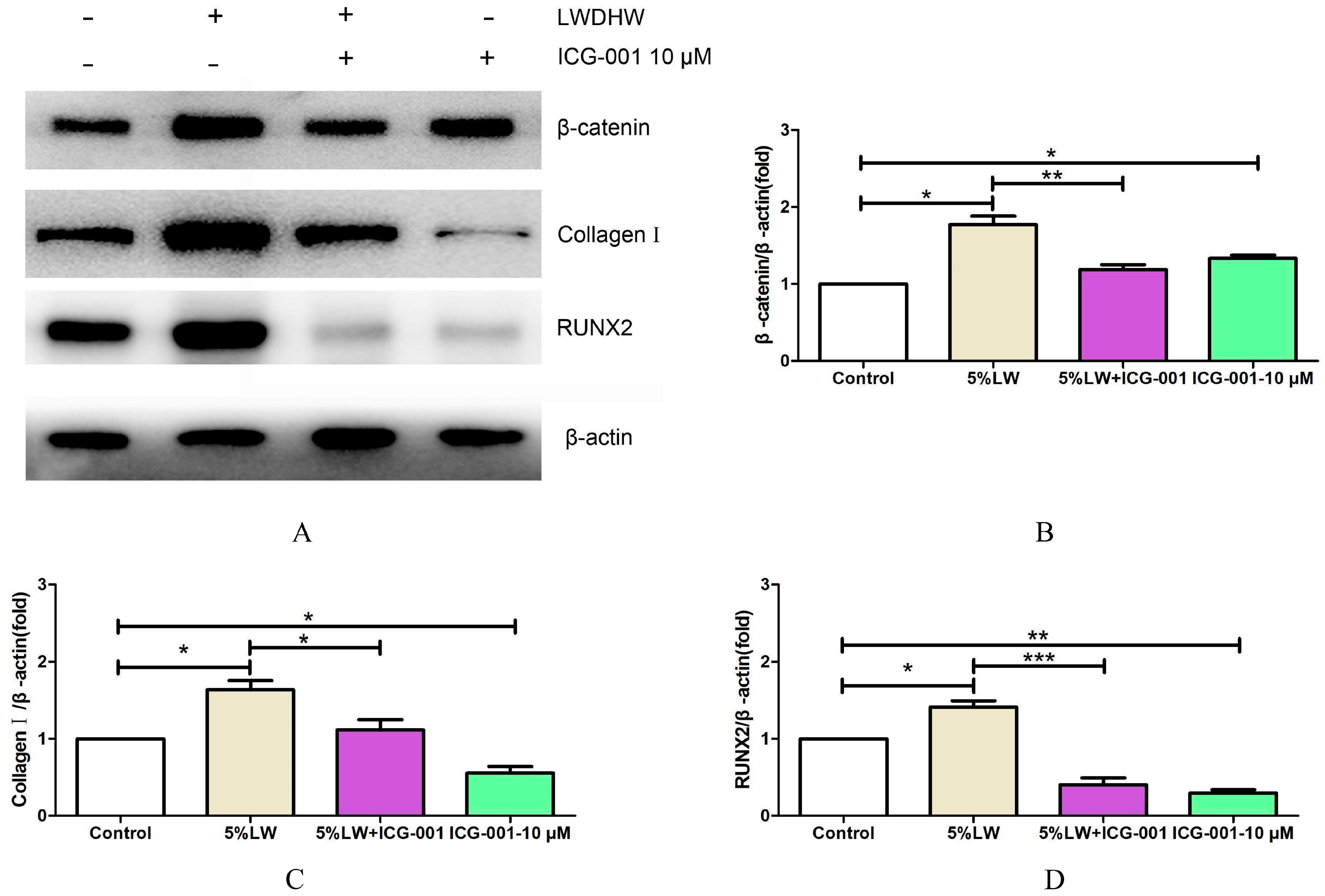
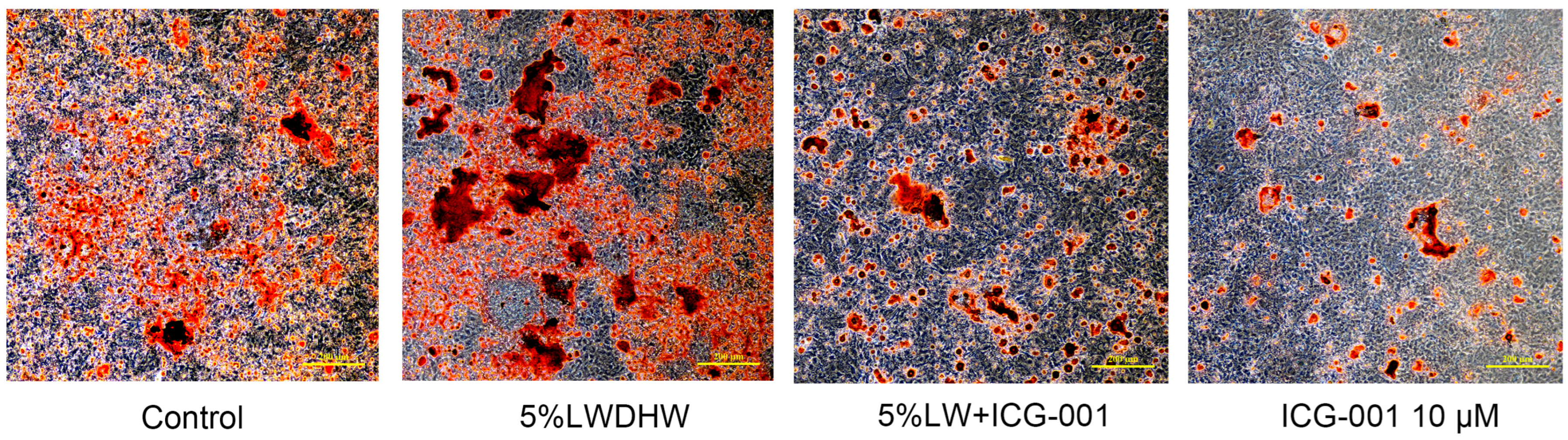
2.2.4. Impact of the Wnt/β-catenin Signaling Pathway on Augmenting the Osteogenic Differentiation of MC3T3-E1 Cells
3. Discussion
4. Methods
4.1. Network Pharmacology and Bioinformatics Analysis
4.1.1. Active Ingredients and Targets of LWDH Pills
4.1.2. Disease Targets for OP
4.1.3. Intersecting Targets between LWDH Pills and OP
4.1.4. Visualization Network of LWDH Pills-Target Proteins
4.1.5. Protein-Protein Interaction (PPI) Analysis
4.1.6. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analyses
4.2. Experimental Validation
4.2.1. Experimental Animals
4.2.2. Drugs and Reagents
4.2.3. Preparation of LWDH Pill-Medicated Serum
4.2.4. Cell Counting Kit-8 (CCK-8) Methods
4.2.5. Western Blot (WB) Method
4.2.6. Osteogenic Differentiation and Mineralization Assessment
4.2.7. Alkaline Phosphatase (ALP) Activity Assay
4.2.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Foessl, I.; Dimai, H.P.; Obermayer-Pietsch, B. Long-term and sequential treatment for osteoporosis. Nat. Rev. Endocrinol. 2023, 19, 520–533. [Google Scholar] [CrossRef]
- Chen, J.; Liao, X.; Gan, J. Review on the protective activity of osthole against the pathogenesis of osteoporosis. Front. Pharmacol. 2023, 14, 1236893. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, B.; Annweiler, C.; Legrand, E. Osteoporosis in older adults. Jt. Bone Spine 2021, 88, 105135. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.R.; Macey, R.; Lewis, J.; Stokes, J.; Gill, J.R.; Cook, J.A.; Eardley, W.G.; Parker, M.J.; Griffin, X.L. Surgical interventions for treating extracapsular hip fractures in older adults: A network meta-analysis. Cochrane Database Syst. Rev. 2022, 2022, CD013405. [Google Scholar] [CrossRef]
- Salari, N.; Ghasemi, H.; Mohammadi, L.; Behzadi, M.H.; Rabieenia, E.; Shohaimi, S.; Mohammadi, M. The global prevalence of osteoporosis in the world: A comprehensive systematic review and meta-analysis. J. Orthop. Surg. Res. 2021, 16, 609. [Google Scholar] [CrossRef]
- Xiao, P.-L.; Cui, A.-Y.; Hsu, C.-J.; Peng, R.; Jiang, N.; Xu, X.-H.; Ma, Y.-G.; Liu, D.; Lu, H.-D. Global, regional prevalence, and risk factors of osteoporosis according to the World Health Organization diagnostic criteria: A systematic review and meta-analysis. Osteoporos. Int. 2022, 33, 2137–2153. [Google Scholar] [CrossRef]
- Niznik, J.D.; Gilliam, M.A.; Colón-Emeric, C.; Thorpe, C.T.; Lund, J.L.; Berry, S.D.; Hanson, L.C. Controversies in osteoporosis treatment of nursing home residents. J. Am. Med. Dir. Assoc. 2022, 23, 1928–1934. [Google Scholar] [CrossRef]
- Xie, Y.; Tian, Y.; Zhang, Y.; Zhang, Z.; Chen, R.; Li, M.; Tang, J.; Bian, J.; Li, Z.; Xu, X. Overview of the development of selective androgen receptor modulators (sarms) as pharmacological treatment for osteoporosis (1998–2021). Eur. J. Med. Chem. 2022, 230, 114119. [Google Scholar] [CrossRef]
- Appelman-Dijkstra, N.M.; Oei, H.D.; Vlug, A.G.; Winter, E.M. The effect of osteoporosis treatment on bone mass. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101623. [Google Scholar] [CrossRef]
- Zhu, F.; Li, W.; Wang, L.; Dai, B.; Liu, Z.; Wu, H.; Deng, T. Study on the treatment of postmenopausal osteoporosis with quercetin in liuwei dihuang Pill based on network pharmacology. J. Orthop. Surg. Res. 2023, 18, 21. [Google Scholar] [CrossRef]
- Ge, J.-R.; Xie, L.-H.; Chen, J.; Li, S.-Q.; Xu, H.-J.; Lai, Y.-L.; Qiu, L.-L.; Ni, C.-B. Liuwei dihuang pill treats postmenopausal osteoporosis with shen (kidney) yin deficiency via janus kinase/signal transducer and activator of transcription signal pathway by up-regulating cardiotrophin-like cytokine factor 1 expression. Chin. J. Integr. Med. 2018, 24, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, P.; Shi, X.; Li, H.; Zhang, X.; Zeng, S.; Xu, Z.; Lai, D.; Zhang, M. Liuwei Dihuang Decoction for primary osteoporosis: A protocol for a systematic review and meta-analysis. Medicine 2019, 98, e15282. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, H.; Song, X.; Zhao, J.; Xu, D.; Liu, M. The wnt/β-catenin signaling pathway inhibits osteoporosis by regulating the expression of TERT: An in vivo and in vitro study. Aging 2023, 15, 11471–11488. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Lin, W.-Y.; Wu, F.-J.; Luo, C.-W. Unveiling the transcriptomic landscape and the potential antagonist feedback mechanisms of TGF-β superfamily signaling module in bone and osteoporosis. Cell Commun. Signal. 2022, 20, 190. [Google Scholar] [CrossRef]
- Huang, K.; Cai, S.; Fu, T.; Zhu, Q.; Liu, L.; Yao, Z.; Rao, P.; Lan, X.; Li, Q.; Xiao, J. Wnt10b regulates osteogenesis of adipose-derived stem cells through Wnt/β-catenin signalling pathway in osteoporosis. Cell Prolif. 2023, 57, e13522. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Tang, Z.; Yu, Y.; Chen, H.; Yu, Q.; Zhang, D.; Yan, C. Curculigo orchioides polysaccharide COP70-1 stimulates osteogenic differentiation of MC3T3-E1 cells by activating the BMP and Wnt signaling pathways. Int. J. Biol. Macromol. 2023, 248, 125879. [Google Scholar] [CrossRef]
- Shen, Y.; Huang, X.; Wu, J.; Lin, X.; Zhou, X.; Zhu, Z.; Pan, X.; Xu, J.; Qiao, J.; Zhang, T.; et al. The global burden of osteoporosis, low bone mass, and its related fracture in 204 countries and territories, 1990–2019. Front. Endocrinol. 2022, 13, 882241. [Google Scholar] [CrossRef]
- Ohta, H.; Uemura, Y.; Sone, T.; Tanaka, S.; Soen, S.; Mori, S.; Hagino, H.; Fukunaga, M.; Nakamura, T.; Orimo, H.; et al. Effect of bone resorption inhibitors on serum cholesterol level and fracture risk in osteoporosis: Randomized comparative study between minodronic acid and raloxifene. Calcif. Tissue Int. 2023, 112, 430–439. [Google Scholar] [CrossRef]
- Ahire, J.J.; Kumar, V.; Rohilla, A. Understanding osteoporosis: Human bone density, genetic mechanisms, gut microbiota, and future prospects. Probiotics Antimicrob. Proteins 2023. online ahead of print. [Google Scholar] [CrossRef]
- Catalano, A.; Loddo, S.; Bellone, F.; Pecora, C.; Lasco, A.; Morabito, N. Pulsed electromagnetic fields modulate bone metabolism via rankl/opg and wnt/β-catenin pathways in women with postmenopausal osteoporosis: A pilot study. Bone 2018, 116, 42–46. [Google Scholar] [CrossRef]
- Ma, Q.; Yu, J.; Zhang, X.; Wu, X.; Deng, G. Wnt/β-catenin signaling pathway-a versatile player in apoptosis and autophagy. Biochimie 2023, 211, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Shen, H.; Chen, B.; Hu, H.; Liu, C.; Chen, Y.; Cong, W. The recent progress of peptide regulators for the Wnt/β-catenin signaling pathway. Front. Med. 2023, 10, 1164656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Y.; Xie, Z.; Li, M.; Liu, Y.; Tu, X. Activating Wnt/β-catenin signaling in osteocytes promotes osteogenic differentiation of BMSCs through BMP-7. Int. J. Mol. Sci. 2022, 23, 16045. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, M.; Wu, X.; Chen, D.; Xie, M.; Pan, H. TGM2 accelerates migration and differentiation of BMSCs by activating Wnt/β-catenin signaling. J. Orthop. Surg. Res. 2023, 18, 168. [Google Scholar] [CrossRef]
- Bodine, P.V.N. Wnt signaling control of bone cell apoptosis. Cell Res. 2008, 18, 248–253. [Google Scholar] [CrossRef]
- Li, B.; Zhang, H.; Zeng, M.; He, W.; Li, M.; Huang, X.; Deng, D.Y.B.; Wu, J. Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting the Wnt/β-catenin pathway. Cell Biol. Int. 2015, 39, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Arafa, E.-S.A.; Elgendy, N.O.; Elhemely, M.A.; Abdelaleem, E.A.; Mohamed, W.R. Diosmin mitigates dexamethasone-induced osteoporosis in vivo: Role of Runx2, RANKL/OPG, and oxidative stress. Biomed. Pharmacother. 2023, 161, 114461. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, H.; Hong, J.; Kim, S.H.L.; Kwon, E.; Park, T.H.; Hwang, N.S. Bone-targeted delivery of cell-penetrating-RUNX2 fusion protein in osteoporosis model. Adv. Sci. 2023, 10, e2301570. [Google Scholar] [CrossRef]
- Lu, X.-M.; Zhao, H.; Wang, E.-H. A high-fat diet induces obesity and impairs bone acquisition in young male mice. Mol. Med. Rep. 2013, 7, 1203–1208. [Google Scholar] [CrossRef]
- Zheng, H.-L.; Xu, W.-N.; Zhou, W.-S.; Yang, R.-Z.; Chen, P.-B.; Liu, T.; Jiang, L.-S.; Jiang, S.-D. Beraprost ameliorates postmenopausal osteoporosis by regulating Nedd4-induced Runx2 ubiquitination. Cell Death Dis. 2021, 12, 497. [Google Scholar] [CrossRef]
- Takebe, H.; Shalehin, N.; Hosoya, A.; Shimo, T.; Irie, K. Sonic hedgehog regulates bone fracture healing. Int. J. Mol. Sci. 2020, 21, 677. [Google Scholar] [CrossRef] [PubMed]
- Raiyan, S.; Rahman, A.; Al Mamun, A.; Asim, M.H.; Makki, A.; Hajjar, D.; Alelwani, W.; Tangpong, J.; Mathew, B. Natural compounds from Leea macrophylla enhance phagocytosis and promote osteoblasts differentiation by alkaline phosphatase, type 1 collagen, and osteocalcin gene expression. J. Biomed. Mater. Res. 2021, 109, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Xu, B.; Sun, Y.; Xiao, L.; Pan, J.; Jin, H.; Tong, P. The effects of Liuwei Dihuang on canonical Wnt/β-catenin signaling pathway in osteoporosis. J. Ethnopharmacol. 2014, 153, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]


| Molecule Name | Source | Structure | Molecular Formula | Molecular Weight (g/mol) | OB (%) | DL | Degree |
|---|---|---|---|---|---|---|---|
| quercetin | MDP | 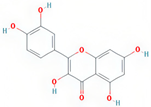 | C15H10O7 | 302.23 | 46.43 | 0.28 | 154 |
| stigmasterol | SDH/SY/SZY | 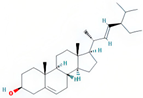 | C29H48O | 412.7 | 43.83 | 0.76 | 31 |
| kaempferol | MDP | 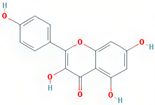 | C15H10O6 | 286.25 | 41.88 | 0.24 | 63 |
| beta-sitosterol | SZY | 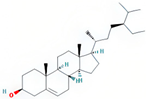 | C29H50O | 414.79 | 36.91 | 0.75 | 38 |
| tetrahydroalstonine | SZY | 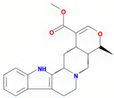 | C21H24N2O3 | 352.47 | 32.42 | 0.81 | 28 |
| kadsurenone | SY |  | C21H24O5 | 356.45 | 54.72 | 0.38 | 27 |
| hederagenin | FL |  | C30H48O4 | 414.79 | 36.91 | 0.75 | 24 |
| hancinone C | SY |  | C23H28O6 | 400.51 | 59.05 | 0.39 | 22 |
| diosgenin | SY |  | C27H42O3 | 414.69 | 80.88 | 0.81 | 16 |
| AIDS180907 | SY |  | C23H22O6 | 394.45 | 45.33 | 0.77 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Liang, G.; Yang, J.; Huang, H.; Dou, Y.; Gu, Z.; Liu, J.; Zeng, L.; Yang, W. Liuwei Dihuang Pills Enhance Osteogenic Differentiation in MC3T3-E1 Cells through the Activation of the Wnt/β-Catenin Signaling Pathway. Pharmaceuticals 2024, 17, 99. https://doi.org/10.3390/ph17010099
Zhao J, Liang G, Yang J, Huang H, Dou Y, Gu Z, Liu J, Zeng L, Yang W. Liuwei Dihuang Pills Enhance Osteogenic Differentiation in MC3T3-E1 Cells through the Activation of the Wnt/β-Catenin Signaling Pathway. Pharmaceuticals. 2024; 17(1):99. https://doi.org/10.3390/ph17010099
Chicago/Turabian StyleZhao, Jinlong, Guihong Liang, Junzheng Yang, Hetao Huang, Yaoxing Dou, Zhuoxu Gu, Jun Liu, Lingfeng Zeng, and Weiyi Yang. 2024. "Liuwei Dihuang Pills Enhance Osteogenic Differentiation in MC3T3-E1 Cells through the Activation of the Wnt/β-Catenin Signaling Pathway" Pharmaceuticals 17, no. 1: 99. https://doi.org/10.3390/ph17010099
APA StyleZhao, J., Liang, G., Yang, J., Huang, H., Dou, Y., Gu, Z., Liu, J., Zeng, L., & Yang, W. (2024). Liuwei Dihuang Pills Enhance Osteogenic Differentiation in MC3T3-E1 Cells through the Activation of the Wnt/β-Catenin Signaling Pathway. Pharmaceuticals, 17(1), 99. https://doi.org/10.3390/ph17010099







