Risk of Melanoma and Non-Melanoma Skin Cancer in Patients with Psoriasis and Psoriatic Arthritis Treated with Targeted Therapies: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
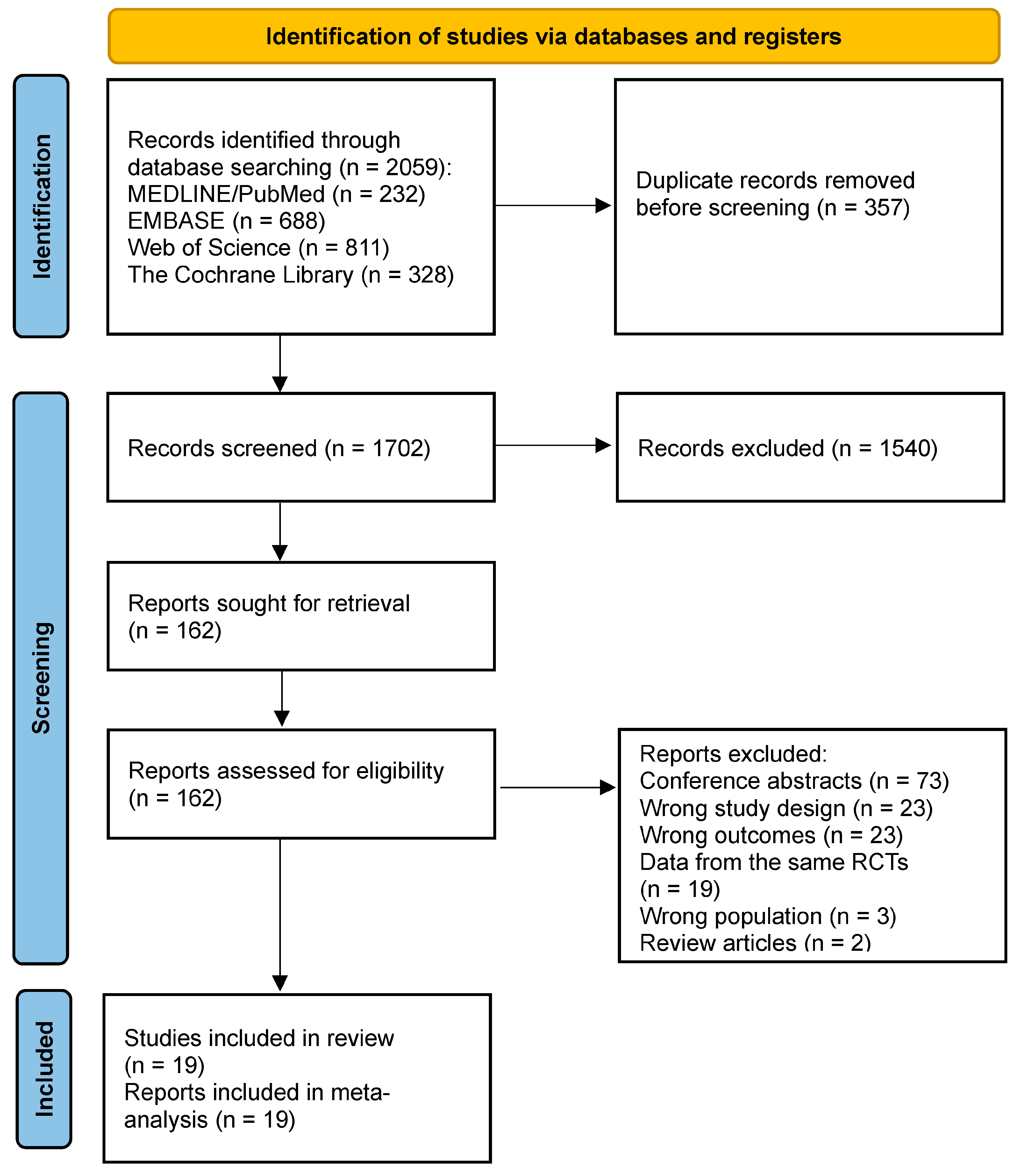
2. Methods
2.1. Search Strategy
2.2. Eligibility Assessment
2.3. Data Extraction and Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Included Studies
3.2. Melanoma Risk
3.3. NMSC Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michalek, I.M.; Loring, B.; John, S.M. A Systematic Review of Worldwide Epidemiology of Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, F.; Calov, M.; Kristensen, L.E.; Gladman, D.D.; Coates, L.C.; Jullien, D.; Gottlieb, A.B.; Gisondi, P.; Wu, J.J.; Thyssen, J.P.; et al. Prevalence of Psoriatic Arthritis in Patients with Psoriasis: A Systematic Review and Meta-Analysis of Observational and Clinical Studies. J. Am. Acad. Dermatol. 2019, 80, 251–265.e19. [Google Scholar] [CrossRef] [PubMed]
- Vaengebjerg, S.; Skov, L.; Egeberg, A.; Loft, N.D. Prevalence, Incidence, and Risk of Cancer in Patients with Psoriasis and Psoriatic Arthritis. JAMA Dermatol. 2020, 156, 421. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.S. The Risk of Melanoma in Association with Long-Term Exposure to PUVA. J. Am. Acad. Dermatol. 2001, 44, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Polesie, S.; Gillstedt, M.; Sönnergren, H.H.; Osmancevic, A.; Paoli, J. Methotrexate Treatment and Risk for Cutaneous Malignant Melanoma: A Retrospective Comparative Registry-Based Cohort Study. Br. J. Dermatol. 2017, 176, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.; Ho, V.; Lebwohl, M.G.; Leite, L.; Hopkins, L.; Galindo, C.; Goyal, K.; Langholff, W.; Fakharzadeh, S.; Srivastava, B.; et al. Risk of Malignancy with Systemic Psoriasis Treatment in the Psoriasis Longitudinal Assessment Registry. J. Am. Acad. Dermatol. 2017, 77, 845–854.e5. [Google Scholar] [CrossRef] [PubMed]
- Esse, S.; Mason, K.J.; Green, A.C.; Warren, R.B. Melanoma Risk in Patients Treated With Biologic Therapy for Common Inflammatory Diseases. JAMA Dermatol. 2020, 156, 787. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wan, Q.; Zhao, R.; Xiao, H.; Cen, Y.; Xu, X. Risk of Non-Melanoma Skin Cancer with Biological Therapy in Common Inflammatory Diseases: A Systemic Review and Meta-Analysis. Cancer Cell Int. 2021, 21, 614. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital: Ottawa, ON, Canada, 2000. [Google Scholar]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the Metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- Spittal, M.J.; Pirkis, J.; Gurrin, L.C. Meta-Analysis of Incidence Rate Data in the Presence of Zero Events. BMC Med. Res. Methodol. 2015, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Gossec, L.; Theander, E.; Chakravarty, S.D.; Bergmans, P.; Lavie, F.; Noël, W.; Sharaf, M.; Siebert, S.; Smolen, J.S. Response to Treatment in Psoriatic Arthritis, the Effect of Age: Analysis of Patients Receiving Ustekinumab in the PsABio Real-World Study. Arthritis Res. Ther. 2023, 25, 100. [Google Scholar] [CrossRef]
- Blauvelt, A.; Leonardi, C.L.; Gooderham, M.; Papp, K.A.; Philipp, S.; Wu, J.J.; Igarashi, A.; Flack, M.; Geng, Z.; Wu, T.; et al. Efficacy and Safety of Continuous Risankizumab Therapy vs Treatment Withdrawal in Patients with Moderate to Severe Plaque Psoriasis. JAMA Dermatol. 2020, 156, 649. [Google Scholar] [CrossRef]
- Blauvelt, A.; Lebwohl, M.; Langley, R.G.; Rowland, K.; Yang, Y.-W.; Chan, D.; Miller, M.; You, Y.; Yu, J.; Thaҫi, D.; et al. Malignancy Rates through 5 Years of Follow-up in Patients with Moderate-to-Severe Psoriasis Treated with Guselkumab: Pooled Results from the VOYAGE 1 and VOYAGE 2 Trials. J. Am. Acad. Dermatol. 2023, 89, 274–282. [Google Scholar] [CrossRef]
- Burmester, G.R.; Curtis, J.R.; Yun, H.; FitzGerald, O.; Winthrop, K.L.; Azevedo, V.F.; Rigby, W.F.C.; Kanik, K.S.; Wang, C.; Biswas, P.; et al. An Integrated Analysis of the Safety of Tofacitinib in Psoriatic Arthritis across Phase III and Long-Term Extension Studies with Comparison to Real-World Observational Data. Drug. Saf. 2020, 43, 379–392. [Google Scholar] [CrossRef]
- Burmester, G.R.; Winthrop, K.; Blanco, R.; Nash, P.; Goupille, P.; Azevedo, V.F.; Salvarani, C.; Rubbert-Roth, A.; Lesser, E.; Lippe, R.; et al. Safety Profile of Upadacitinib up to 3 Years in Psoriatic Arthritis: An Integrated Analysis of Two Pivotal Phase 3 Trials. Rheumatol. Ther. 2022, 9, 521–539. [Google Scholar] [CrossRef]
- Coates, L.C.; Gossec, L.; Theander, E.; Bergmans, P.; Neuhold, M.; Karyekar, C.S.; Shawi, M.; Noël, W.; Schett, G.; McInnes, I.B. Efficacy and Safety of Guselkumab in Patients with Active Psoriatic Arthritis Who Are Inadequate Responders to Tumour Necrosis Factor Inhibitors: Results through One Year of a Phase IIIb, Randomised, Controlled Study (COSMOS). Ann. Rheum. Dis. 2022, 81, 359–369. [Google Scholar] [CrossRef]
- Coates, L.C.; McInnes, I.B.; Merola, J.F.; Warren, R.B.; Kavanaugh, A.; Gottlieb, A.B.; Gossec, L.; Assudani, D.; Bajracharya, R.; Coarse, J.; et al. Safety and Efficacy of Bimekizumab in Patients with Active Psoriatic Arthritis: Three-Year Results from a Phase IIb Randomized Controlled Trial and Its Open-Label Extension Study. Arthritis Rheumatol. 2022, 74, 1959–1970. [Google Scholar] [CrossRef]
- Combe, B.; Rahman, P.; Kameda, H.; Cañete, J.D.; Gallo, G.; Agada, N.; Xu, W.; Genovese, M.C. Safety Results of Ixekizumab with 1822.2 Patient-Years of Exposure: An Integrated Analysis of 3 Clinical Trials in Adult Patients with Psoriatic Arthritis. Arthritis Res. Ther. 2020, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- Kivitz, A.J.; Nash, P.; Tahir, H.; Everding, A.; Mann, H.; Kaszuba, A.; Pellet, P.; Widmer, A.; Pricop, L.; Abrams, K. Efficacy and Safety of Subcutaneous Secukinumab 150 Mg with or Without Loading Regimen in Psoriatic Arthritis: Results from the FUTURE 4 Study. Rheumatol. Ther. 2019, 6, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.E.; Keiserman, M.; Papp, K.; McCasland, L.; White, D.; Lu, W.; Soliman, A.M.; Eldred, A.; Barcomb, L.; Behrens, F. Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 52-Week Results from the KEEPsAKE 1 Study. Rheumatology 2023, 62, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, M.G.; Blauvelt, A.; Menter, A.; Papp, K.A.; Guenthner, S.; Pillai, R.; Israel, R.J.; Jacobson, A. Efficacy, Safety, and Patient-Reported Outcomes in Patients with Moderate-to-Severe Plaque Psoriasis Treated with Brodalumab for 5 Years in a Long-Term, Open-Label, Phase II Study. Am. J. Clin. Dermatol. 2019, 20, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, C.; Reich, K.; Foley, P.; Torii, H.; Gerdes, S.; Guenther, L.; Gooderham, M.; Ferris, L.K.; Griffiths, C.E.M.; ElMaraghy, H.; et al. Efficacy and Safety of Ixekizumab Through 5 Years in Moderate-to-Severe Psoriasis: Long-Term Results from the UNCOVER-1 and UNCOVER-2 Phase-3 Randomized Controlled Trials. Dermatol. Ther. 2020, 10, 431–447. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Mease, P.J.; Ritchlin, C.T.; Rahman, P.; Gottlieb, A.B.; Kirkham, B.; Kajekar, R.; Delicha, E.-M.; Pricop, L.; Mpofu, S. Secukinumab Sustains Improvement in Signs and Symptoms of Psoriatic Arthritis: 2 Year Results from the Phase 3 FUTURE 2 Study. Rheumatology 2017, 56, 1993–2003. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.J.; Smolen, J.S.; Behrens, F.; Nash, P.; Liu Leage, S.; Li, L.; Tahir, H.; Gooderham, M.; Krishnan, E.; Liu-Seifert, H.; et al. A Head-to-Head Comparison of the Efficacy and Safety of Ixekizumab and Adalimumab in Biological-Naïve Patients with Active Psoriatic Arthritis: 24-Week Results of a Randomised, Open-Label, Blinded-Assessor Trial. Ann. Rheum. Dis. 2020, 79, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Odnopozova, L.; Edin, A.; Sukharev, A.; Wu, T.; Aydin, K.; Kelly, M.; Khotko, A. Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis in the Russian Federation. Dermatol. Ther. 2022, 12, 2063–2075. [Google Scholar] [CrossRef]
- Östör, A.; Van den Bosch, F.; Papp, K.; Asnal, C.; Blanco, R.; Aelion, J.; Lu, W.; Wang, Z.; Soliman, A.M.; Eldred, A.; et al. Efficacy and Safety of Risankizumab for Active Psoriatic Arthritis: 52-Week Results from the KEEPsAKE 2 Study. Rheumatology 2023, 62, 2122–2129. [Google Scholar] [CrossRef]
- Papp, K.A.; Krueger, J.G.; Feldman, S.R.; Langley, R.G.; Thaci, D.; Torii, H.; Tyring, S.; Wolk, R.; Gardner, A.; Mebus, C.; et al. Tofacitinib, an Oral Janus Kinase Inhibitor, for the Treatment of Chronic Plaque Psoriasis: Long-Term Efficacy and Safety Results from 2 Randomized Phase-III Studies and 1 Open-Label Long-Term Extension Study. J. Am. Acad. Dermatol. 2016, 74, 841–850. [Google Scholar] [CrossRef]
- Papp, K.A.; Lebwohl, M.G.; Puig, L.; Ohtsuki, M.; Beissert, S.; Zeng, J.; Rubant, S.; Sinvhal, R.; Zhao, Y.; Soliman, A.M.; et al. Long-term Efficacy and Safety of Risankizumab for the Treatment of Moderate-to-severe Plaque Psoriasis: Interim Analysis of the LIMMitless Open-label Extension Trial beyond 3 Years of Follow-up. Br. J. Dermatol. 2021, 185, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Thaci, D.; Piaserico, S.; Warren, R.B.; Gupta, A.K.; Cantrell, W.; Draelos, Z.; Foley, P.; Igarashi, A.; Langley, R.G.; Asahina, A.; et al. Five-year Efficacy and Safety of Tildrakizumab in Patients with Moderate-to-severe Psoriasis Who Respond at Week 28: Pooled Analyses of Two Randomized Phase III Clinical Trials (ReSURFACE 1 and ReSURFACE 2). Br. J. Dermatol. 2021, 185, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Bertino, L.; Fontana, A.; Calapai, F.; Ingrasciotta, Y.; Berretta, M.; Trifirò, G.; Guarneri, C. Incidence of Skin Cancer in Patients with Chronic Inflammatory Cutaneous Diseases on Targeted Therapies: A Systematic Review and Meta-Analysis of Observational Studies. Front. Oncol. 2021, 11, 687432. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. United States Cancer Statistics: Incidence of Malignant Melanoma of the Skin—United States, 2009–2018. In USCS Data Brief, No. 28; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2018. [Google Scholar]
- Hu, W.; Fang, L.; Ni, R.; Zhang, H.; Pan, G. Changing Trends in the Disease Burden of Non-Melanoma Skin Cancer Globally from 1990 to 2019 and Its Predicted Level in 25 Years. BMC Cancer 2022, 22, 836. [Google Scholar] [CrossRef] [PubMed]
- Noonan, F.P.; Zaidi, M.R.; Wolnicka-Glubisz, A.; Anver, M.R.; Bahn, J.; Wielgus, A.; Cadet, J.; Douki, T.; Mouret, S.; Tucker, M.A.; et al. Melanoma Induction by Ultraviolet A but Not Ultraviolet B Radiation Requires Melanin Pigment. Nat. Commun. 2012, 3, 884. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Płonka, P.M.; Raman, C.; Brożyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Slominski, R.M.; Raman, C.; Chen, J.Y.; Athar, M.; Elmets, C. Neuroendocrine Signaling in the Skin with a Special Focus on the Epidermal Neuropeptides. Am. J. Physiol. Cell Physiol. 2022, 323, C1757–C1776. [Google Scholar] [CrossRef]
- Zattra, E.; Fortina, A.B.; Bordignon, M.; Piaserico, S.; Alaibac, M. Immunosuppression and Melanocyte Proliferation. Melanoma Res. 2009, 19, 63–68. [Google Scholar] [CrossRef]
- Weaver, C.T.; Elson, C.O.; Fouser, L.A.; Kolls, J.K. The Th17 Pathway and Inflammatory Diseases of the Intestines, Lungs, and Skin. Annu. Rev. Pathol. Mech. Dis. 2013, 8, 477–512. [Google Scholar] [CrossRef]
- Loft, N.D.; Vaengebjerg, S.; Halling, A.-S.; Skov, L.; Egeberg, A. Adverse Events with IL-17 and IL-23 Inhibitors for Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-analysis of Phase III Studies. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1151–1160. [Google Scholar] [CrossRef]
- Bezzio, C.; Vernero, M.; Ribaldone, D.G.; Alimenti, E.; Manes, G.; Saibeni, S. Cancer Risk in Patients Treated with the JAK Inhibitor Tofacitinib: Systematic Review and Meta-Analysis. Cancers 2023, 15, 2197. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.D.; Stovin, C.; Alveyn, E.; Adeyemi, O.; Chan, C.K.D.; Patel, V.; Adas, M.A.; Atzeni, F.; Ng, K.K.H.; Rutherford, A.I.; et al. JAK Inhibitors and the Risk of Malignancy: A Meta-Analysis across Disease Indications. Annu. Rheum. Dis. 2023, 82, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Olivera, P.A.; Lasa, J.S.; Bonovas, S.; Danese, S.; Peyrin-Biroulet, L. Safety of Janus Kinase Inhibitors in Patients with Inflammatory Bowel Diseases or Other Immune-Mediated Diseases: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1554–1573.e12. [Google Scholar] [CrossRef] [PubMed]
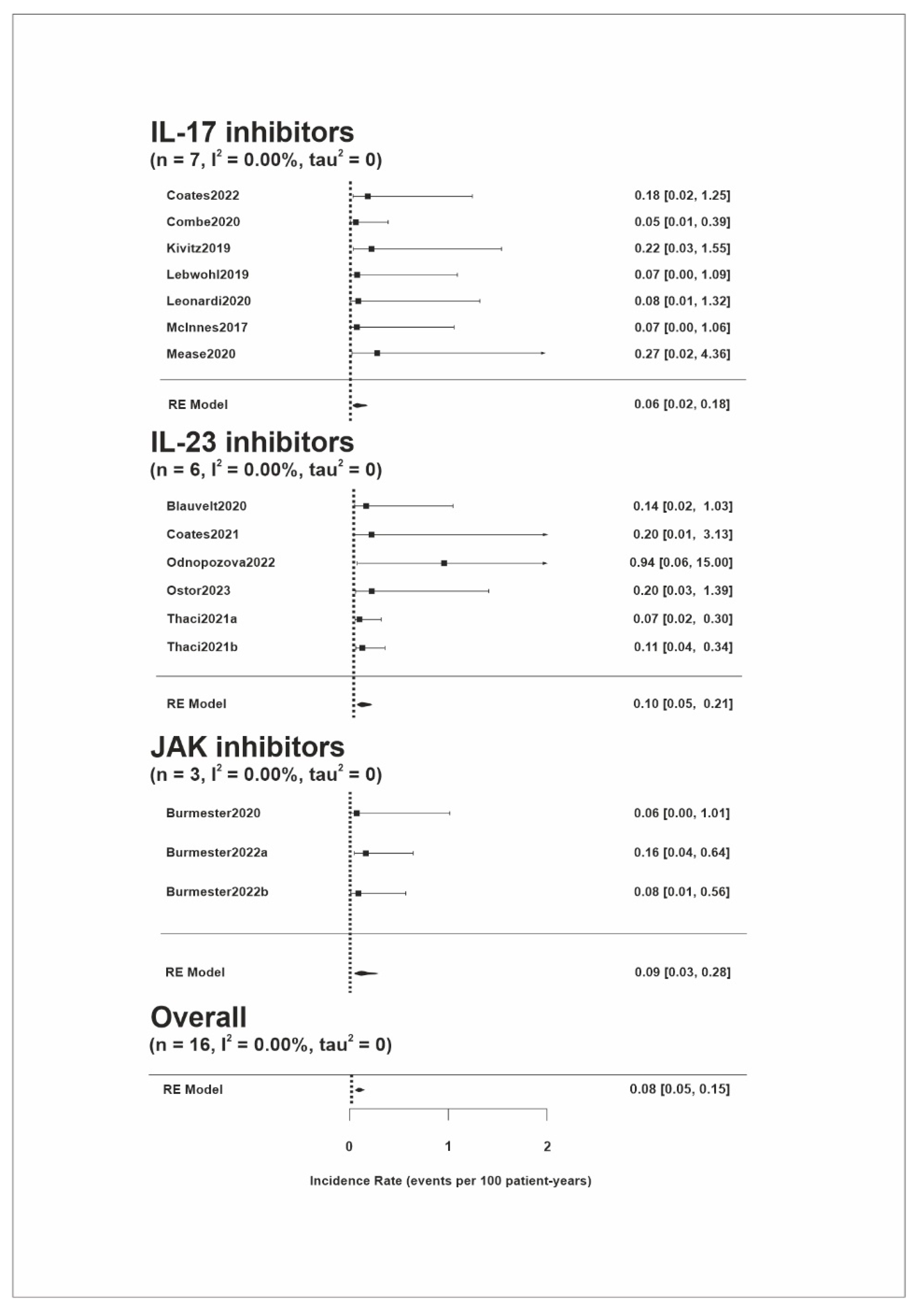

| Source | Identifier | Study Design | Disease | Study Drug | Drug Dose | No. of Participants | Total Patient Years of Exposure | Age, Mean (SD) | Male, n (%) | Melanoma Incidence Rates per 100 Patient Years | NMSC Incidence Rates per 100 Patient Years |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational studies | |||||||||||
| Gossec, 2023 [15] | PsaBIO study (NCT02627768) | Multinational, prospective, real-world, observational study | PSA | Ustekinumab (IL-12/23 inhibitor) | 45 mg/90 mg | 494 | 991.3 | NA | NA | NA | 0.1 |
| Randomized clinical trials | |||||||||||
| Blauvelt, 2020 [16] | IMMhance (NCT02672852) | Phase 3, multinational, double-blind placebo-controlled trial | PSO | Risankizumab (IL-23 inhibitor) | 150 mg | 500 | 690.1 | NA | NA | 0.14 | 0.72 |
| Blauvelt, 2023 [17] | VOYAGE 1 (NCT02207231), VOYAGE 2 (NCT02207244) | Randomized, double-blind, phase 3 studies with OLE | PSO | Guselkumab (IL-23 inhibitor) | 100 mg | 1221 | >5200 | NA | NA | NA | 0.31 (0.17–0.5) |
| Burmester, 2020 [18] | OPAL Broaden (NCT01877668), OPAL Beyond (NCT01882439), OPAL Balance (NCT01976364) | Double-blind, placebo-controlled, parallel-group studies with LTE | PSA | Tofacitinib (JAK inhibitor) | 5 mg/10 mg | 783 | 789 | 48.7 (12.0) | 355 (45) | 0 | 0.5 (0.1–1.3) |
| Burmester, 2022a [19] | SELECT-PsA 1 (NCT03104400), SELECT-PsA 2 (NCT03104374) | Randomized, placebo-controlled phase 3 trials | PSA | Upadacitinib (JAK inhibitor) | 15 mg | 907 | 1247.2 | 51.5 (12.1) | 429 (47) | 0.16 | 0.8 (0.4–1.5) |
| Burmester, 2022b [19] | 30 mg | 921 | 1257.4 | 51.4 (12.3) | 417 (45) | 0.08 | 0.8 (0.4–1.5) | ||||
| Coates, 2021 [20] | COSMOS (NCT03796858) | Phase IIIb, randomized, double-blind study | PSA | Guselkumab (IL-23 inhibitor) | 100 mg | 279 | 255.4 | NA | NA | 0 | 0 |
| Coates, 2022 [21] | BE ACTIVE (NCT02969525) | Randomized, double-blind, placebo-controlled study with OLE | PSA | Bimekizumab (IL-17 inhibitor) | 160 mg/320 mg | 206 | 570.1 | 49.3 (12.4) | 105 (51) | 0.2 | 0 |
| Combe, 2020 [22] | SPIRIT-P1 (NCT01695239), SPIRIT-P2 (NCT02349295), SPIRIT-P3 (NCT02584855) | Phase 3 randomized, double-blind, placebo-controlled, parallel-group studies with LTE; phase 3 study with an open-label period followed by a randomized double-blind withdrawal period | PSA | Ixekizumab (IL-17 inhibitor) | 160 mg → 80 mg | 1118 | 1822.2 | 49.5 (11.9) | 517 (46.2) | 0.05 | 0.4 (0.1–3.0) |
| Kivitz, 2019 [23] | FUTURE 4 (NCT02294227) | Randomized, Double-blind, Placebo-controlled Multicenter Study | PSA | Secukinumab (IL-17 inhibitor) | 150 mg | 334 | 458.4 | NA | NA | 0.22 | 0.22 |
| Kristensen, 2023 [24] | KEEPsAKE 1 (NCT03675308) | Phase 3, double-blind, placebo-controlled study with OLE | PSA | Risankizumab (IL-23 inhibitor) | 150 mg | 946 | 958.1 | NA | NA | NA | 0.6 |
| Lebwohl, 2019 [25] | NCT00975637, NCT01101100 | Phase II, double-blind, placebo-controlled, dose-ranging clinical trial with OLE | PSO | Brodalumab (IL-17 inhibitor) | 210 mg | 181 | 731.7 | 42.7 (12.2) | 117 (65) | 0 | 0 |
| Leonardi, 2020 [26] | UNCOVER-1 (NCT01474512), UNCOVER-2 (NCT01597245) | Multicenter, randomized, double-blinded, placebo-controlled, phase-3 clinical trials with LTE | PSO | Ixekizumab (IL-17 inhibitor) | 160 mg → 80 mg | 206 | 604.3 | Median (range) 43.0 (18–77) | 140 (68) | 0 | 0.17 |
| McInnes, 2017 [27] | FUTURE 2 (NCT01752634) | Multicenter, randomized, double-blind, parallel-group, placebo-controlled study | PSA | Secukinumab (IL-17 inhibitor) | 75 mg/150 mg/300 mg | 387 | 751.3 | NA | NA | 0 | 1.06 |
| Mease, 2020 [28] | SPIRIT-H2H (NCT03151551) | Phase IIIb/IV, multicenter, randomized, open-label, blinded-assessor, parallel-group study | PSA | Ixekizumab (IL-17 inhibitor) | 160 mg→80 mg | 283 | 183.5 | 47.5 (12.0) | 162 (57) | 0 | 0 |
| Odnopozova, 2022 [29] | IMMPress (NCT03518047) | Phase 3, randomized, double-blind, placebo-controlled study | PSO | Risankizumab (IL-23 inhibitor) | 150 mg | 50 | 53.3 | 44.6 (12.9) | 27 (54) | 0 | 0 |
| Östor, 2023 [30] | KEEPsAKE 2 (NCT03671148) | Double-blind, placebo-controlled study with OLE | PSA | Risankizumab (IL-23 inhibitor) | 150 mg | 419 | 509.7 | NA | NA | 0.2 | 1.76 |
| Papp, 2016 [31] | OPT Pivotal 1 (NCT01276639), OPT Pivotal 2 (NCT01309737), NCT01163253 | Phase III, multisite, randomized, double-blind clinical studies with LTE | PSO | Tofacitinib (JAK inhibitor) | 5 mg/10 mg | 1807 | 2704.8 | NA | NA | NA | 0.71 (0.43–1.1) |
| Papp, 2021 [32] | LIMMitless (NCT03047395) | Phase III OLE study | PSO | Risankizumab (IL-23 inhibitor) | 150 mg | 897 | 3106.2 | 46.9 (22.4) | 633 (71) | NA | 0 |
| Thaci, 2021a [33] | reSURFACE 1 (NCT01722331), reSURFACE 2 (NCT01729754) | Randomized, double-blind, placebo-controlled, parallel-group phase III trials with LTE | PSO | Tildrakizumab (IL-23 inhibitor) | 100 mg | 872 | 2688.4 | NA | NA | 0.1 (0.0–0.3) | 0.4 (0.2–0.8) |
| Thaci, 2021b [33] | 200 mg | 928 | 2753.5 | NA | NA | 0.1 (0.0–0.3) | 0.4 (0.2–0.7) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzysztofik, M.; Brzewski, P.; Cuber, P.; Kacprzyk, A.; Kulbat, A.; Richter, K.; Wojewoda, T.; Wysocki, W.M. Risk of Melanoma and Non-Melanoma Skin Cancer in Patients with Psoriasis and Psoriatic Arthritis Treated with Targeted Therapies: A Systematic Review and Meta-Analysis. Pharmaceuticals 2024, 17, 14. https://doi.org/10.3390/ph17010014
Krzysztofik M, Brzewski P, Cuber P, Kacprzyk A, Kulbat A, Richter K, Wojewoda T, Wysocki WM. Risk of Melanoma and Non-Melanoma Skin Cancer in Patients with Psoriasis and Psoriatic Arthritis Treated with Targeted Therapies: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2024; 17(1):14. https://doi.org/10.3390/ph17010014
Chicago/Turabian StyleKrzysztofik, Marta, Paweł Brzewski, Przemysław Cuber, Artur Kacprzyk, Aleksandra Kulbat, Karolina Richter, Tomasz Wojewoda, and Wojciech M. Wysocki. 2024. "Risk of Melanoma and Non-Melanoma Skin Cancer in Patients with Psoriasis and Psoriatic Arthritis Treated with Targeted Therapies: A Systematic Review and Meta-Analysis" Pharmaceuticals 17, no. 1: 14. https://doi.org/10.3390/ph17010014
APA StyleKrzysztofik, M., Brzewski, P., Cuber, P., Kacprzyk, A., Kulbat, A., Richter, K., Wojewoda, T., & Wysocki, W. M. (2024). Risk of Melanoma and Non-Melanoma Skin Cancer in Patients with Psoriasis and Psoriatic Arthritis Treated with Targeted Therapies: A Systematic Review and Meta-Analysis. Pharmaceuticals, 17(1), 14. https://doi.org/10.3390/ph17010014






