Pharmacology of Veratrum californicum Alkaloids as Hedgehog Pathway Antagonists
Abstract
1. Introduction
1.1. Veratrum Californicum Alkaloids
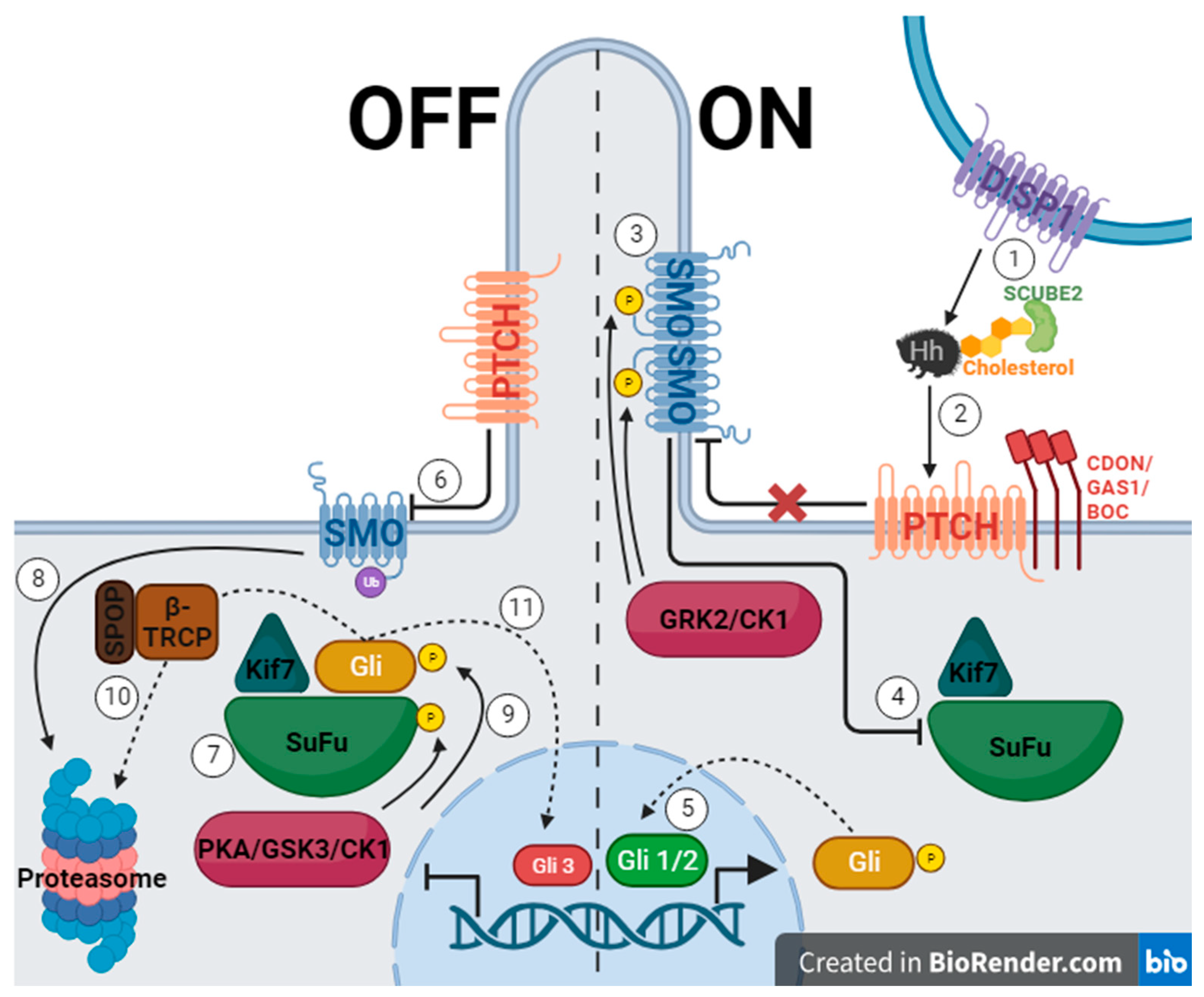
1.2. Hedgehog Signaling Pathway
2. Results and Discussion
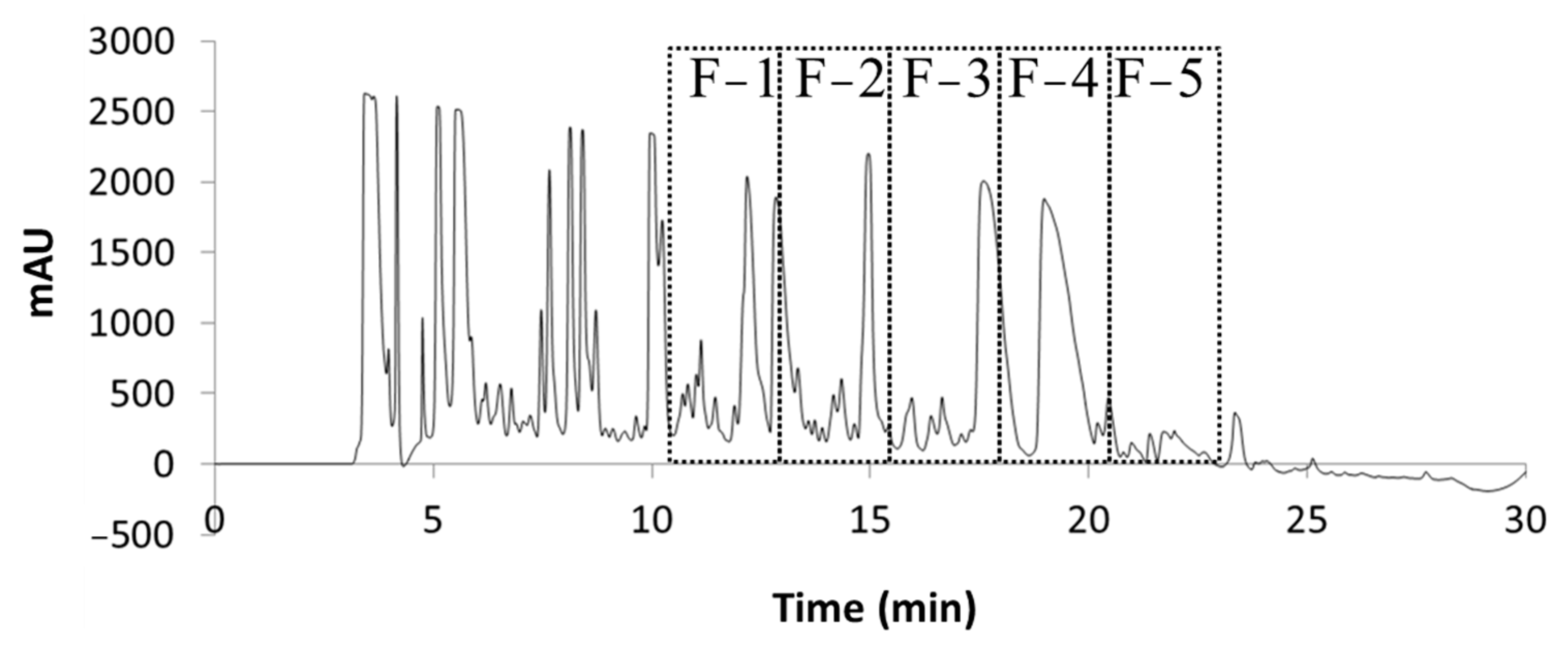
2.1. Extraction and Fraction Collection
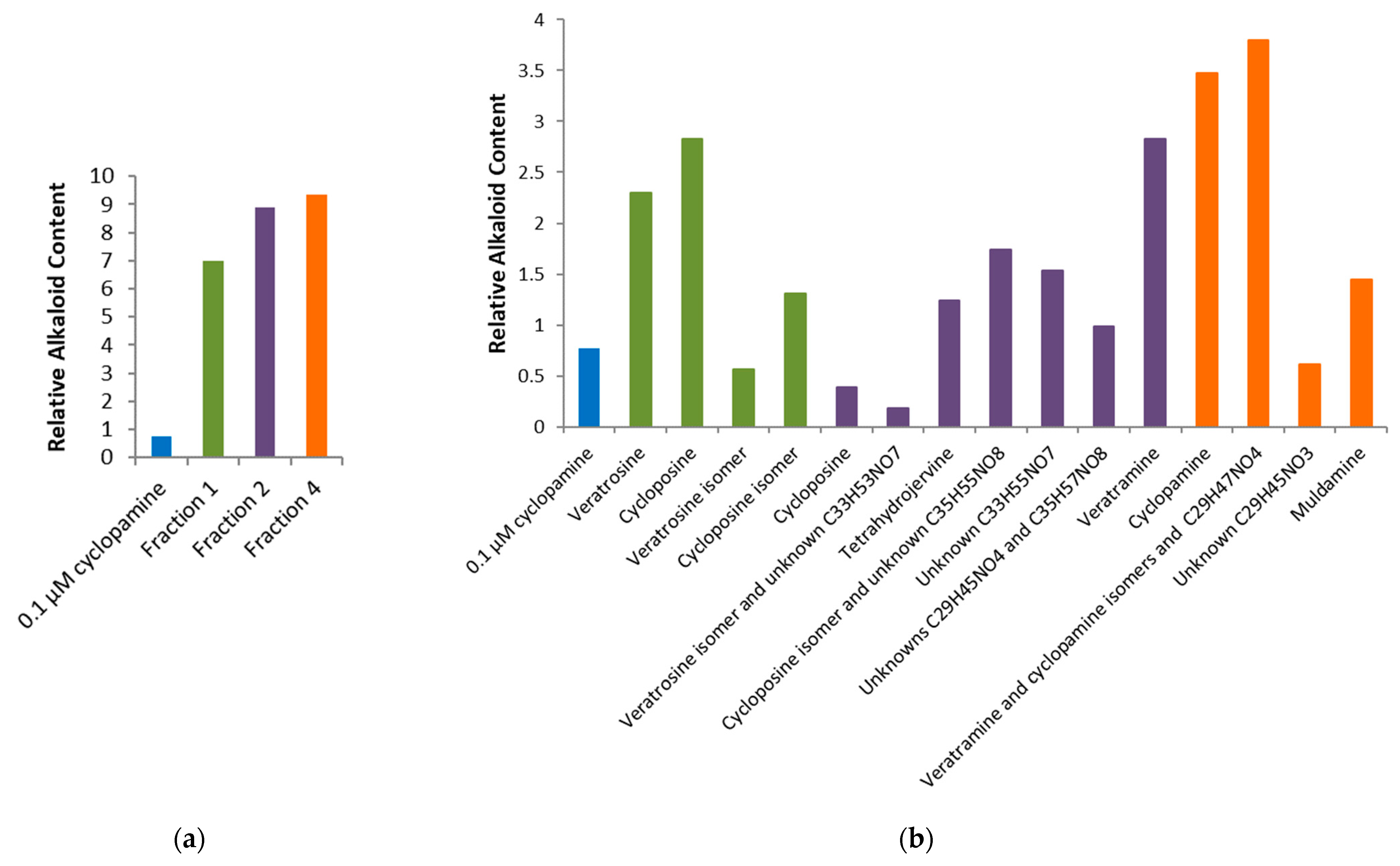
2.2. Bioactivity
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
3.3. Separation
3.4. Identification
3.5. Chemicals and Solvents
3.6. Cell Culture
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauressergues, E.; Heusler, P.; Lestienne, F.; Troulier, D.; Rauly-Lestienne, I.; Lie Tourette, A.; Ailhaud, M.-C.; Cathala, C.; Phanie Tardif, S.; Denais-Laliè Ve, D.; et al. Pharmacological Evaluation of a Series of Smoothened Antagonists in Signaling Pathways and after Topical Application in a Depilated Mouse Model. Pharmacol. Res. Perspect. 2016, 4, 214. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Song, R.; Xie, J. Sonidegib: Mechanism of Action, Pharmacology, and Clinical Utility for Advanced Basal Cell Carcinomas. OncoTargets Ther. 2017, 10, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.M.; McDougal, O.M. Medicinal History of North American Veratrum. Phytochem. Rev. 2013, 13, 671–694. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.W.; Rossi, M.; Campfield, V.; French, J.; Hunt, E.; Wade, E.; McDougal, O.M. Steroidal Alkaloid Variation in Veratrum Californicum as Determined by Modern Methods of Analytical Analysis. Fitoterapia 2019, 137, 104281. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Welch, K.D.; Panter, K.E.; Gardner, D.R.; Garrossian, M.; Chang, C.W.T. Cyclopamine: From Cyclops Lambs to Cancer Treatment. J. Agric. Food Chem. 2014, 62, 7355–7362. [Google Scholar] [CrossRef]
- Aditya, S.; Rattan, A. Vismodegib: A Smoothened Inhibitor for the Treatment of Advanced Basal Cell Carcinoma. Indian. Dermatol. Online J. 2013, 4, 365. [Google Scholar] [CrossRef]
- Tremblay, M.R.; Lescarbeau, A.; Grogan, M.J.; Tan, E.; Lin, G.; Austad, B.C.; Yu, L.C.; Behnke, M.L.; Nair, S.J.; Hagel, M.; et al. Discovery of a Potent and Orally Active Hedgehog Pathway Antagonist (IPI-926). J. Med. Chem. 2009, 52, 4400–4418. [Google Scholar] [CrossRef] [PubMed]
- Keeler, R.F.; Binns, W. Chemical Compounds of Veratrum Californicum Related to Congenital Ovine Cyclopian Malformations: Extraction of Active Material. Proc. Soc. Exp. Biol. Med. 1964, 116, 123–127. [Google Scholar] [CrossRef]
- Keeler, R.F.; Binns, W. Teratogenic Compounds of Veratrum Californicum (Durand). I. Preparation and Characterization of Fractions and Alkaloids for Biologic Testing. Can. J. Biochem. 1966, 44, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Keeler, R.F. Teratogenic Compounds of Veratrum Californicum (Durand)—IV. Phytochemistry 1968, 7, 303–306. [Google Scholar] [CrossRef]
- Keeler, R.F. Teratogenic Compounds of Veratrum Californicum (Durand)—VI. The Structure of Cyclopamine. Phytochemistry 1969, 8, 223–225. [Google Scholar] [CrossRef]
- Keeler, R.F. Teratogenic Compounds of Veratrum Californicum (Durand) VII. The Structure of the Glycosidic Alkaloid Cycloposine. Steroids 1969, 13, 579–588. [Google Scholar] [CrossRef]
- Binns, W.; James, L.F.; Shupe, J.L.; Thacker, E.J. Cyclopian-Type Malformation in Lambs. Am. Med. Assoc. 1962, 5, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Oatis, J.E.; Brunsfeld, P.; Rushing, J.W.; Moeller, P.D.; Bearden, D.W.; Gallien, T.N.; Cooper IV, G. Isolation, Purification, and Full NMR Assignments of Cyclopamine from Veratrum Californicum. Chem. Cent. J. 2008, 2, 12. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chandler, C.M.; Habig, J.W.; Fisher, A.A.; Ambrose, K.V.; Jiménez, S.T.; Mcdougal, O.M. Improved Extraction and Complete Mass Spectral Characterization of Steroidal Alkaloids from Veratrum Californicum. Nat. Prod. Commun. 2013, 8, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.W.; Cruz, R.; Mattos, J.; Baughman, N.; Elwell, J.; Fothergill, J.; Nielsen, A.; Brookhouse, J.; Bartlett, A.; Malek, P.; et al. Cyclopamine Bioactivity by Extraction Method from Veratrum Californicum. Bioorganic Med. Chem. 2016, 24, 3752–3757. [Google Scholar] [CrossRef]
- Turner, M.W.; Cruz, R.; Elwell, J.; French, J.; Mattos, J.; McDougal, O.M. Native V. Californicum Alkaloid Combinations Induce Differential Inhibition of Sonic Hedgehog Signaling. Molecules 2018, 23, 2222. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Takebe, N.; LoRusso, P. Targeting the Hedgehog Pathway in Cancer. Ther. Adv. Med. Oncol. 2010, 2, 237. [Google Scholar] [CrossRef]
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; de Sampaio e Spohr, T.C.L. A Highlight on Sonic Hedgehog Pathway. Cell Commun. Signal. 2018, 16, 11. [Google Scholar] [CrossRef]
- Cooper, M.K.; Porter, J.A.; Young, K.E.; Beachy, P.A. Teratogen-Mediated Inhibition of Target Tissue Response to Shh Signaling. Science 1998, 280, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Taipale, J. Hedgehog: Functions and Mechanisms. Genes Dev. 2008, 22, 2454–2472. [Google Scholar] [CrossRef] [PubMed]
- Echelard, Y.; Epstein, D.J.; St-Jacques, B.; Shen, L.; Mohler, J.; McMahon, J.A.; McMahon, A.P. Sonic Hedgehog, a Member of a Family of Putative Signaling Molecules, Is Implicated in the Regulation of CNS Polarity. Cell 1993, 75, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Concordet, J.P.; Ingham, P.W. A Functionally Conserved Homolog of the Drosophila Segment Polarity Gene Hh Is Expressed in Tissues with Polarizing Activity in Zebrafish Embryos. Cell 1993, 75, 1431–1444. [Google Scholar] [CrossRef] [PubMed]
- Riddle, R.D.; Johnson, R.L.; Laufer, E.; Tabin, C. Sonic Hedgehog Mediates the Polarizing Activity of the ZPA. Cell 1993, 75, 1401–1416. [Google Scholar] [CrossRef] [PubMed]
- Nüsslein-Volhard, C.; Wieschaus, E. Mutations Affecting Segment Number and Polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef]
- Cell Signaling Technology. Hedgehog Signaling. Available online: https://www.cellsignal.com/pathways/hedgehog-signaling-pathway (accessed on 26 July 2023).
- Tukachinsky, H.; Kuzmickas, R.P.; Jao, C.Y.; Liu, J.; Salic, A. Dispatched and Scube Mediate the Efficient Secretion of the Cholesterol-Modified Hedgehog Ligand. Cell Rep. 2012, 2, 308–320. [Google Scholar] [CrossRef]
- Abcam. Hedgehog Signaling Pathway: An Overview. Available online: https://www.abcam.com/pathways/hedgehog-signaling-pathway (accessed on 26 July 2023).
- Bhateja, P.; Cherian, M.; Majumder, S.; Ramaswamy, B. The Hedgehog Signaling Pathway: A Viable Target in Breast Cancer? Cancers 2019, 11, 1126. [Google Scholar] [CrossRef]
- Yang, C.; Qi, Y.; Sun, Z. The Role of Sonic Hedgehog Pathway in the Development of the Central Nervous System and Aging-Related Neurodegenerative Diseases. Front. Mol. Biosci. 2021, 8, 711710. [Google Scholar] [CrossRef]
- Jeng, K.S.; Jeng, C.J.; Jeng, W.J.; Sheen, I.S.; Li, S.Y.; Leu, C.M.; Tsay, Y.G.; Chang, C.F. Sonic Hedgehog Signaling Pathway as a Potential Target to Inhibit the Progression of Hepatocellular Carcinoma (Review). Oncol. Lett. 2019, 18, 4377–4384. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Jia, J. Mechanisms of Smoothened Regulation in Hedgehog Signaling. Cells 2021, 10, 2138. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, C.; Martinelli, G.; Papayannidis, C.; Cortes, J.E. Hedgehog Pathway Inhibitors: A New Therapeutic Class for the Treatment of Acute Myeloid Leukemia. Blood Cancer Discov. 2020, 1, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Varnat, F.; Duquet, A.; Malerba, M.; Zbinden, M.; Mas, C.; Gervaz, P.; Ruiz I Altaba, A. Human Colon Cancer Epithelial Cells Harbour Active HEDGEHOG-GLI Signalling That Is Essential for Tumour Growth, Recurrence, Metastasis and Stem Cell Survival and Expansion. EMBO Mol. Med. 2009, 1, 338–351. [Google Scholar] [CrossRef]
- Clement, V.; Sanchez, P.; de Tribolet, N.; Radovanovic, I.; Ruiz i Altaba, A. HEDGEHOG-GLI1 Signaling Regulates Human Glioma Growth, Cancer Stem Cell Self-Renewal, and Tumorigenicity. Curr. Biol. 2007, 17, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Mas, C.; Clement, V.; Zbinden, M.; Correa, R.; Piguet, V.; Beermann, F.; Ruiz Altaba, A. Melanomas Require HEDGEHOG-GLI Signaling Regulated by Interactions between GLI1 and the RAS-MEK/AKT Pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 5895–5900. [Google Scholar] [CrossRef]
- Berman, D.M.; Karhadkar, S.S.; Maitra, A.; Montes de Oca, R.; Gerstenblith, M.R.; Briggs, K.; Parker, A.R.; Shimada, Y.; Eshleman, J.R.; Watkins, D.N.; et al. Widespread Requirement for Hedgehog Ligand Stimulation in Growth of Digestive Tract Tumours. Nature 2003, 425, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Thayer, S.P.; Pasca di Magliano, M.; Heiser, P.W.; Nielsen, C.M.; Roberts, D.J.; Lauwers, G.Y.; Qi, Y.P.; Gysin, S.; Fernandez-del Castillo, C.; Yajnik, V.; et al. Hedgehog Is an Early and Late of Pancreatic Cancer. Nature 2003, 425, 851–856. [Google Scholar] [CrossRef]
- Feldmann, G.; Dhara, S.; Fendrich, V.; Bedja, D.; Beaty, R.; Mullendore, M.; Karikari, C.; Alvarez, H.; Iacobuzio-Donahue, C.; Jimeno, A.; et al. Blockade of Hedgehog Signaling Inhibits Pancreatic Cancer Invasion and Metastases: A New Paradigm for Combination Therapy in Solid Cancers. Cancer Res. 2007, 67, 2187–2196. [Google Scholar] [CrossRef] [PubMed]
- Karhadkar, S.S.; Bova, G.S.; Abdallah, N.; Dhara, S.; Gardner, D.; Maitra, A.; Isaacs, J.T.; Berman, D.M.; Beachy, P.A. Hedgehog Signalling in Prostate, Neoplasia And. Nature 2004, 431, 707–7112. [Google Scholar] [CrossRef]
- Watkins, D.N.; Berman, D.M.; Burkholder, S.G.; Wang, B.; Beachy, P.A.; Baylin, S.B. Hedgehog Signalling within Airway Epithelial Progenitors and in Small-Cell Lung Cancer. Nature 2003, 422, 313–317. [Google Scholar] [CrossRef]
- Berman, D.M.; Karhadkar, S.S.; Hallahan, A.R.; Pritchard, J.I.; Eberhart, C.G.; Watkins, D.N.; Chen, J.K.; Cooper, M.K.; Taipale, J.; Olson, J.M.; et al. Medulloblastoma Growth Inhibition by Hedgehog Pathway Blockade. Science (1979) 2002, 297, 1559–1561. [Google Scholar] [CrossRef]
- Sanchez, P.; Ruiz I Altaba, A. In Vivo Inhibition of Endogenous Brain Tumors through Systemic Interference of Hedgehog Signaling in Mice. Mech. Dev. 2005, 122, 223–230. [Google Scholar] [CrossRef]
- Chen, J.K. I Only Have Eye for Ewe: The Discovery of Cyclopamine and Development of Hedgehog Pathway-Targeting Drugs. Nat. Prod. Rep. 2016, 33, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Kong, H.; Gadeau, A.-P.; Sharma, K.; Sasai, N.; Jp, N.N.; Toriyama, M.; Kondo, T. Hedgehog Signal and Genetic Disorders. Front. Genet. 2019, 10, 1103. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Study of Patidegib Topical Gel, 2%, for the Reduction of Disease Burden of Persistently Developing Basal Cell Carcinomas (BCCs) in Subjects with Basal Cell Nevus Syndrome (Gorlin Syndrome)—No Study Results Posted. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03703310 (accessed on 20 September 2022).
- FDA. 2012 Notifications. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/2012-notifications (accessed on 24 July 2021).
- FDA. Novel Drug Approvals for 2015. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2015 (accessed on 24 July 2021).
- Dummer, R.; Guminski, A.; Gutzmer, R.; Dirix, L.; Lewis, K.D.; Combemale, P.; Herd, R.M.; Kaatz, M.; Loquai, C.; Stratigos, A.J.; et al. The 12-Month Analysis from Basal Cell Carcinoma Outcomes with LDE225 Treatment (BOLT): A Phase II, Randomized, Double-Blind Study of Sonidegib in Patients with Advanced Basal Cell Carcinoma. J. Am. Acad. Dermatol. 2016, 75, 113–125.e5. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Morese, R.; Campana, I.; Coco, V.; di Pietro, F.R.; Ricci, F.; Pallotta, S.; Candi, E.; Abeni, D.; et al. Basal Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2020, 8, 449. [Google Scholar] [CrossRef] [PubMed]
- Wolska-Washer, A.; Robak, T. Glasdegib in the Treatment of Acute Myeloid Leukemia. Future Oncol. 2019, 15, 3219–3232. [Google Scholar] [CrossRef]
- Turner, M. Comprehensive Investigation of Bioactive Steroidal Alkaloids in Veratrum Californicum; Boise State University: Boise, ID, USA, 2019. [Google Scholar]
- Promega Corporation. Dual-Luciferase® Reporter Assay System Technical Manual; Promega Corporation: Madison, WI, USA, 2015; pp. 1–25. [Google Scholar]
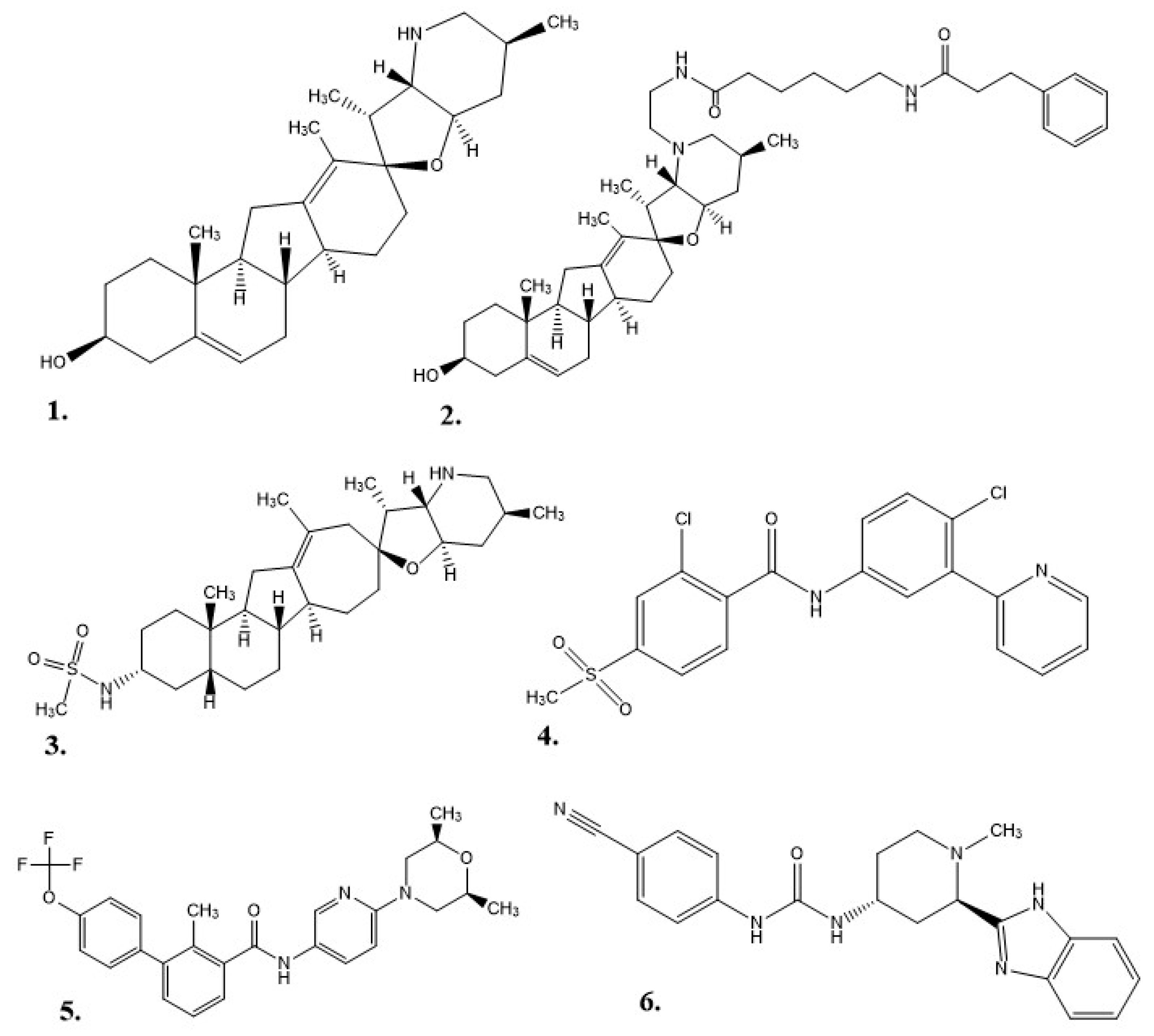

| Rt | m/z | Molecular Formula | Identity | Fraction |
|---|---|---|---|---|
| 15.3 | 572.3 | C33H49NO7 | Veratrosine | 1 |
| 15.7 | 574.3 | C33H51NO7 | Cycloposine | 1, 2 |
| 16 | 572.3 | C33H49NO7 | Isomer of Veratrosine * | 1, 2 |
| 16.1 | 576.4 | C33H53NO7 | ? | 2 |
| 16.2 | 430.3 | C27H43NO3 | Tetrahydrojervine * | 2 |
| 16.5 | 574.3 | C33H51NO7 | Isomer of Cycloposine * | 1, 2 |
| 16.6 | 618.4 | C35H55NO8 | ? | 2 |
| 16.9 | 578.4 | C33H55NO7 | ? | 2 |
| 17.1 | 428.3 | C27H41NO3 | Dihydrojervine * ** | 2 |
| 17.2 | 472.3 | C29H45NO4 | ? | 2 |
| 17.3 | 620.4 | C35H57NO8 | ? | 2 |
| 17.4 | 414.3 | C27H43NO2 | Etioline * ** | 2 |
| 17.5 | 428.3 | C27H41NO3 | Isomer of Dihydrojervine * ** | 2 |
| 17.6 | 410.3 | C27H39NO2 | Veratramine | 2, 3 |
| 18.4/18.5 | 412.3 | C27H41NO2 | Cyclopamine | 3, 4, 5 |
| 18.8 | 410.3 | C27H39NO2 | Isomer of Veratramine * | 4 |
| 18.9 | 412.3 | C27H41NO2 | Isomer of Cyclopamine * | 3, 4 |
| 19.2 | 474.3 | C29H47NO4 | ? | 4, 5 |
| 19.4 | 456.3 | C29H45NO3 | ? | 4, 5 |
| 19.6 | 416.3 | C27H45NO2 | 22-keto-26-aminocholesterol * | 5 |
| 19.5 | 414.3 | C27H43NO2 | Isorubijervine ** | 4 |
| 19.8 | 458.3 | C29H47NO3 | Muldamine | 4, 5 |
| 20 | 416.3 | C27H45NO2 | Isomer of 22-keto-26-aminocholesterol * | 5 |
| 20.3 | 398.3 | C27H43NO | Verazine * | 5 |
| 20.5 | 458.3 | C29H47NO3 | Isomer of Muldamine * | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dirks, M.L.; McDougal, O.M. Pharmacology of Veratrum californicum Alkaloids as Hedgehog Pathway Antagonists. Pharmaceuticals 2024, 17, 123. https://doi.org/10.3390/ph17010123
Dirks ML, McDougal OM. Pharmacology of Veratrum californicum Alkaloids as Hedgehog Pathway Antagonists. Pharmaceuticals. 2024; 17(1):123. https://doi.org/10.3390/ph17010123
Chicago/Turabian StyleDirks, Madison L., and Owen M. McDougal. 2024. "Pharmacology of Veratrum californicum Alkaloids as Hedgehog Pathway Antagonists" Pharmaceuticals 17, no. 1: 123. https://doi.org/10.3390/ph17010123
APA StyleDirks, M. L., & McDougal, O. M. (2024). Pharmacology of Veratrum californicum Alkaloids as Hedgehog Pathway Antagonists. Pharmaceuticals, 17(1), 123. https://doi.org/10.3390/ph17010123







