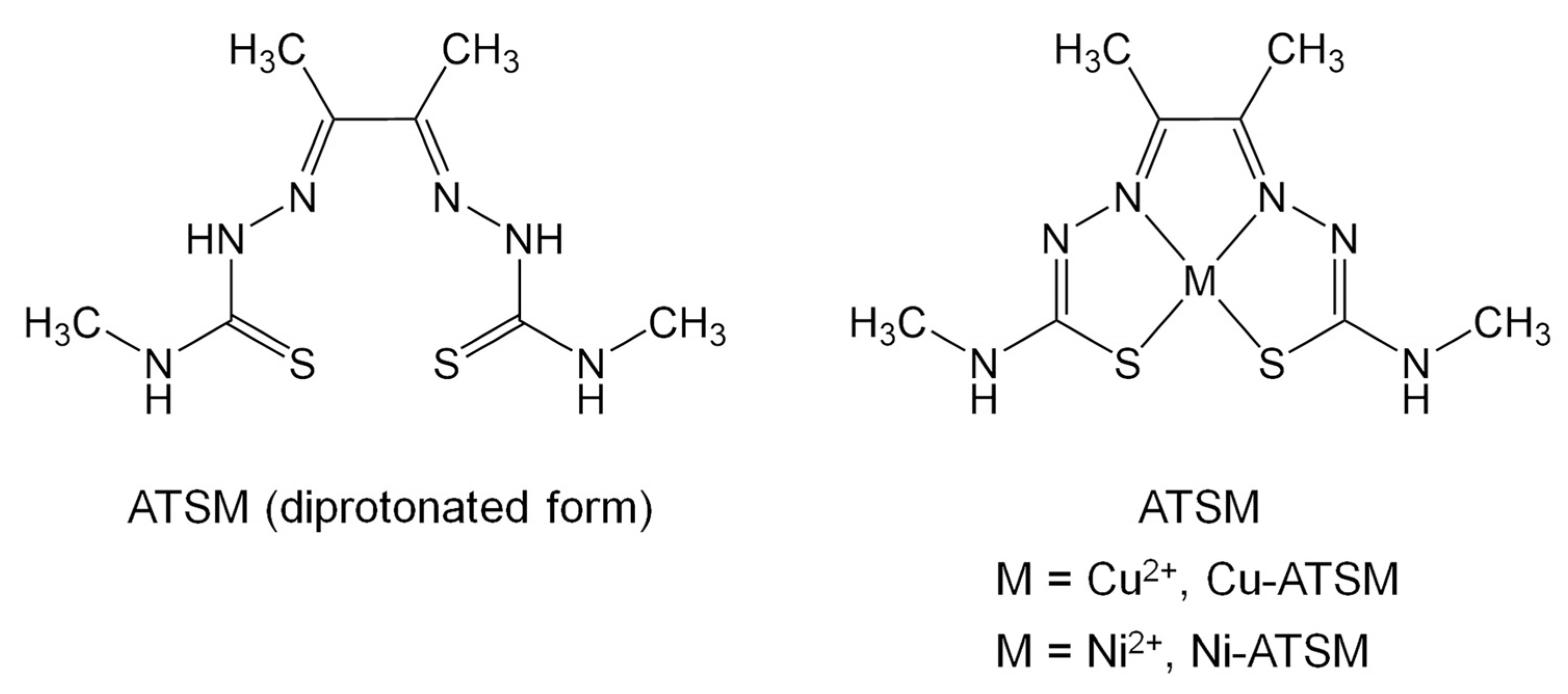
Trace Metal Impurities Effects on the Formation of [64Cu]Cu-diacetyl-bis(N4-methylthiosemicarbazone) ([64Cu]Cu-ATSM)
Abstract
:1. Introduction
2. Results
2.1. Experiment 1: Effect of Trace Metal Impurities on the Formation of [64Cu]Cu-ATSM Complex
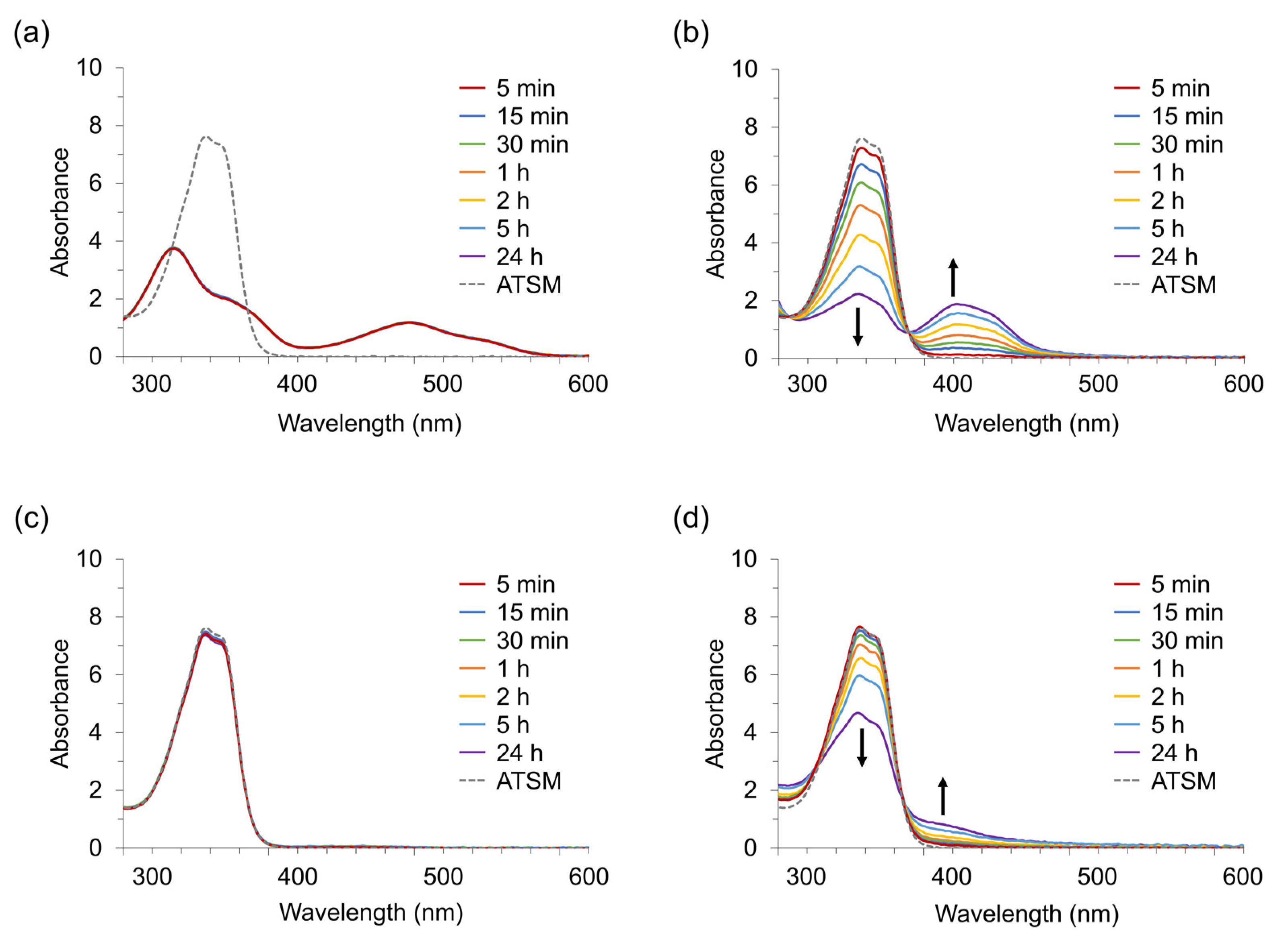
2.2. Experiment 2: Chelate Formation of ATSM with Metal Ions
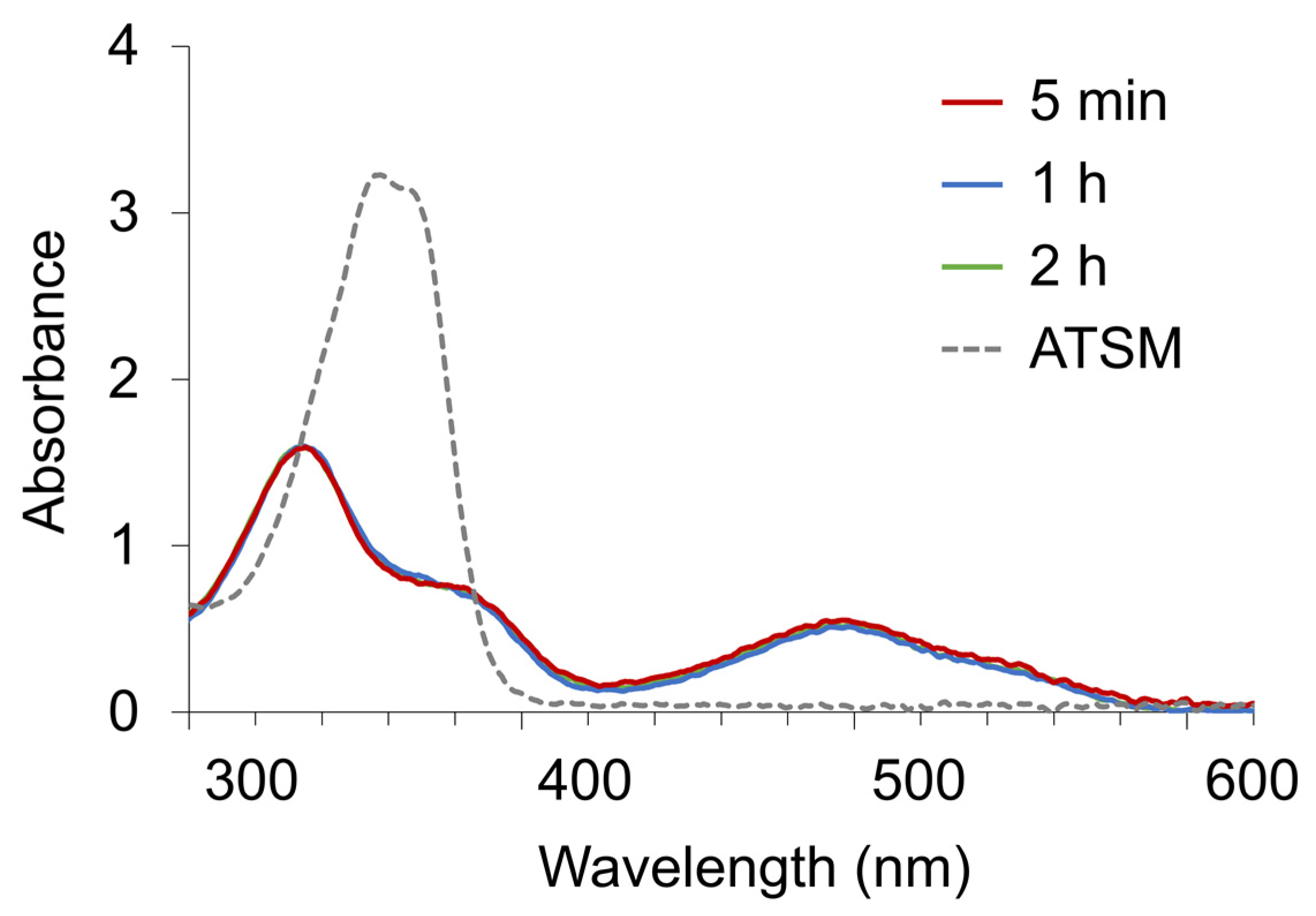
2.3. Experiment 3: Chelate Formation of Zn-ATSM in the Presence of Brønsted Base
2.4. Experiment 4: Differences in the Formation Constants of Cu-ATSM and Ni-ATSM
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Determination of Radiochemical Yield
4.3. Experiment 1: Effect of Trace Metal Impurities on the Formation of [64Cu]Cu-ATSM Complex
4.4. Experiment 2: Chelate Formation of ATSM with Metal Ions
4.5. Experiment 3: Chelate Formation of Zn-ATSM in the Presence of Brønsted Base
4.6. Experiment 4: Differences in the Formation Constants of M-ATSM in the Reaction of ATSM with Cu2+ and Ni2+ Mixtures
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, C.H.; Shyu, W.C.; Chiang, C.Y.; Kuo, J.W.; Shen, W.C.; Liu, R.S. NADPH oxidase subunit 4-mediated reactive oxygen species contribute to cycling hypoxia-promoted tumor progression in glioblastoma multiforme. PLoS ONE 2011, 6, e23945. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Durden, D.L.; Van Meir, E.G.; Brat, D.J. “Pseudopalisading” necrosis in glioblastoma: A familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Benini, A.; Mirandola, P.; Gessi, S.; Varani, K.; Leung, E.; Maclennan, S.; Borea, P.A. Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem. Pharmacol. 2006, 72, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Hypoxia-inducible factor 1 as a possible target for cancer chemoprevention. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Chen, W.W.; Zhang, X. Glioblastoma multiforme: Effect of hypoxia and hypoxia inducible factors on therapeutic approaches. Oncol. Lett. 2016, 12, 2283–2288. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Fujibayashi, Y.; Yonekura, Y.; Welch, M.J.; Waki, A.; Tsuchida, T.; Sadato, N.; Sugimoto, K.; Itoh, H. Evaluation of 62Cu labeled diacetyl-bis(N4-methylthiosemicarbazone) as a hypoxic tissue tracer in patients with lung cancer. Ann. Nucl. Med. 2000, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Laforest, R.; Dehdashti, F.; Grigsby, P.W.; Welch, M.J.; Siegel, B.A. An imaging comparison of 64Cu-ATSM and 60Cu-ATSM in cancer of the uterine cervix. J. Nucl. Med. 2008, 49, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Floberg, J.M.; Wang, L.; Bandara, N.; Rashmi, R.; Mpoy, C.; Garbow, J.R.; Rogers, B.E.; Patti, G.J.; Schwarz, J.K. Alteration of Cellular Reduction Potential Will Change 64Cu-ATSM Signal with or without Hypoxia. J. Nucl. Med. 2020, 61, 427–432. [Google Scholar] [CrossRef]
- Green, M.A.; Mathias, C.J.; Smith, N.J.; Cheng, M.; Hutchins, G.D. In Vivo Quantitative Whole-Body Perfusion Imaging Using Radiolabeled Copper(II) Bis(Thiosemicarbazone) Complexes and Positron Emission Tomography (PET). Methods Mol. Biol. 2022, 2393, 751–771. [Google Scholar] [CrossRef]
- Liu, T.; Karlsen, M.; Karlberg, A.M.; Redalen, K.R. Hypoxia imaging and theranostic potential of [64Cu][Cu(ATSM)] and ionic Cu(II) salts: A review of current evidence and discussion of the retention mechanisms. EJNMMI Res. 2020, 10, 33. [Google Scholar] [CrossRef]
- Maitz, C.A.; Tate, D.; Bechtel, S.; Lunceford, J.; Henry, C.; Flesner, B.; Collins, A.; Varterasian, M.; Tung, D.; Zhang, L.; et al. Paired 18F-Fluorodeoxyglucose (18F-FDG), and 64Cu-Copper(II)-diacetyl-bis(N(4)-methylthiosemicarbazone) (64Cu-ATSM) PET Scans in Dogs with Spontaneous Tumors and Evaluation for Hypoxia-Directed Therapy. Radiat. Res. 2022, 197, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, H.; Ikawa, M.; Tsujikawa, T.; Mori, T.; Makino, A.; Kiyono, Y.; Nakamoto, Y.; Kosaka, H.; Yoneda, M. Cerebral Oxidative Stress in Early Alzheimer’s Disease Evaluated by 64Cu-ATSM PET/MRI: A Preliminary Study. Antioxidants 2022, 11, 1022. [Google Scholar] [CrossRef]
- Peres, E.A.; Toutain, J.; Paty, L.P.; Divoux, D.; Ibazizene, M.; Guillouet, S.; Barre, L.; Vidal, A.; Cherel, M.; Bourgeois, M.; et al. 64Cu-ATSM/64Cu-Cl2 and their relationship to hypoxia in glioblastoma: A preclinical study. EJNMMI Res. 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, J.; Yoneyama, T.; Ohtake, M.; Tateishi, K.; Bae, H.; Kishino, M.; Tateishi, U. Redox reaction and clinical outcome of primary diffuse large B-cell lymphoma of the central nervous system: Prognostic role of metabolic and textural parameters of 62Cu-diacetyl-bis (N4-methylthiosemicarbazone) PET/computed tomography in a small patient cohort. Nucl. Med. Commun. 2020, 41, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.; Packard, A.B. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl. Med. Biol. 2010, 37, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Obata, A.; Yoshimi, E.; Waki, A.; Lewis, J.S.; Oyama, N.; Welch, M.J.; Saji, H.; Yonekura, Y.; Fujibayashi, Y. Retention mechanism of hypoxia selective nuclear imaging/radiotherapeutic agent cu-diacetyl-bis(N4-methylthiosemicarbazone) (Cu-ATSM) in tumor cells. Ann. Nucl. Med. 2001, 15, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Giansiracusa, J.H.; Bell, S.G.; Wong, L.L.; Dilworth, J.R. In vitro kinetic studies on the mechanism of oxygen-dependent cellular uptake of copper radiopharmaceuticals. Phys. Med. Biol. 2009, 54, 2103–2119. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; McCarthy, D.W.; McCarthy, T.J.; Fujibayashi, Y.; Welch, M.J. Evaluation of 64Cu-ATSM in vitro and in vivo in a hypoxic tumor model. J. Nucl. Med. 1999, 40, 177–183. [Google Scholar]
- Maurer, R.I.; Blower, P.J.; Dilworth, J.R.; Reynolds, C.A.; Zheng, Y.; Mullen, G.E. Studies on the mechanism of hypoxic selectivity in copper bis(thiosemicarbazone) radiopharmaceuticals. J. Med. Chem. 2002, 45, 1420–1431. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 radiopharmaceuticals for PET imaging of cancer: Advances in preclinical and clinical research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef]
- McMillan, D.D.; Maeda, J.; Bell, J.J.; Genet, M.D.; Phoonswadi, G.; Mann, K.A.; Kraft, S.L.; Kitamura, H.; Fujimori, A.; Yoshii, Y.; et al. Validation of 64Cu-ATSM damaging DNA via high-LET Auger electron emission. J. Radiat. Res. 2015, 56, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, Y.; Matsumoto, H.; Yoshimoto, M.; Zhang, M.R.; Oe, Y.; Kurihara, H.; Narita, Y.; Jin, Z.H.; Tsuji, A.B.; Yoshinaga, K.; et al. Multiple Administrations of 64Cu-ATSM as a Novel Therapeutic Option for Glioblastoma: A Translational Study Using Mice with Xenografts. Transl. Oncol. 2018, 11, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Igarashi, C.; Kaneko, E.; Hashimoto, H.; Suzuki, H.; Kawamura, K.; Zhang, M.R.; Higashi, T.; Yoshii, Y. Process development of [64Cu]Cu-ATSM: Efficient stabilization and sterilization for therapeutic applications. J. Radioanal. Nucl. Chem. 2019, 322, 467–475. [Google Scholar] [CrossRef]
- Igarashi, C.; Matsumoto, H.; Takahashi, M.; Hihara, F.; Tachibana, T.; Zhang, M.R.; Kurihara, H.; Higashi, T.; Yoshii, Y. Identification and quantitative structure-activity relationship assessment of trace chemical impurities contained in the therapeutic formulation of [64Cu]Cu-ATSM. Nucl. Med. Biol. 2022, 108–109, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Alliot, C.; Michel, N.; Bonraisin, A.-C.; Bossé, V.; Laizé, J.; Bourdeau, C.; Mokili, B.M.; Haddad, F. One step purification process for no-carrier-added 64Cu produced using enriched nickel target. Radiochim. Acta 2011, 99, 627–630. [Google Scholar] [CrossRef]
- Avila-Rodriguez, M.A.; Nye, J.A.; Nickles, R.J. Simultaneous production of high specific activity 64Cu and 61Co with 11.4 MeV protons on enriched 64Ni nuclei. Appl. Radiat. Isot. 2007, 65, 1115–1120. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient production of high specific activity 64Cu using a biomedical cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- Obata, A.; Kasamatsu, S.; McCarthy, D.W.; Welch, M.J.; Saji, H.; Yonekura, Y.; Fujibayashi, Y. Production of therapeutic quantities of 64Cu using a 12 MeV cyclotron. Nucl. Med. Biol. 2003, 30, 535–539. [Google Scholar] [CrossRef]
- Ferreira, C.L.; Yapp, D.T.; Lamsa, E.; Gleave, M.; Bensimon, C.; Jurek, P.; Kiefer, G.E. Evaluation of novel bifunctional chelates for the development of Cu-64-based radiopharmaceuticals. Nucl. Med. Biol. 2008, 35, 875–882. [Google Scholar] [CrossRef]
- Boswell, C.A.; McQuade, P.; Weisman, G.R.; Wong, E.H.; Anderson, C.J. Optimization of labeling and metabolite analysis of copper-64-labeled azamacrocyclic chelators by radio-LC-MS. Nucl. Med. Biol. 2005, 32, 29–38. [Google Scholar] [CrossRef]
- Zeng, D.; Anderson, C.J. Rapid and sensitive LC-MS approach to quantify non-radioactive transition metal impurities in metal radionuclides. Chem. Commun. 2013, 49, 2697–2699. [Google Scholar] [CrossRef] [PubMed]
- Ohya, T.; Nagatsu, K.; Suzuki, H.; Fukada, M.; Minegishi, K.; Hanyu, M.; Fukumura, T.; Zhang, M.R. Efficient preparation of high-quality 64Cu for routine use. Nucl. Med. Biol. 2016, 43, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.P.; Barnard, P.J.; Collison, D.; Dilworth, J.R.; Edge, R.; Green, J.C.; McInnes, E.J. Spectroelectrochemical and computational studies on the mechanism of hypoxia selectivity of copper radiopharmaceuticals. Chemistry 2008, 14, 5890–5907. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.J.; McCleverty, J.A. Complexes of transition metals with Schiff bases and the factors influencing their redox properties. Part I. Nickel and copper complexes of some diketone bisthiosemicarbazones. J. Chem. Soc. A Inorg. Phys. Theor. 1970, 2829–2836. [Google Scholar] [CrossRef]
- Thisgaard, H.; Jensen, M.; Elema, D.R. Medium to large scale radioisotope production for targeted radiotherapy using a small PET cyclotron. Appl. Radiat. Isot. 2011, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tochon-Danguy, H.; Poniger, S.; Sachinidis, J.; Alt, K.; Scott, A.; Hagemeyer, C. Reducing Metal Contamination in Cu-64 Production; Helmholtz-Zentrum Dresden: Rossendorf, Germany, 2014. [Google Scholar]
- Szajek, L.P.; Meyer, W.; Plascjak, P.; Eckelman, W.C. Semi-remote production of [64Cu]CuCl2 and preparation of high specific activity [64Cu]Cu-ATSM for PET studies. Radiochim. Acta 2005, 93, 239–244. [Google Scholar] [CrossRef]
- Thieme, S.; Walther, M.; Pietzsch, H.J.; Henniger, J.; Preusche, S.; Mading, P.; Steinbach, J. Module-assisted preparation of 64Cu with high specific activity. Appl. Radiat. Isot. 2012, 70, 602–608. [Google Scholar] [CrossRef]
- Shigenobu, F.; Yasuhiro, I. Solvation Structures and Solvent Exchange Reactions of Metal Ions in Various Coordinating Solvents. Bull. Chem. Soc. Jpn. 2002, 75, 1901–1925. [Google Scholar] [CrossRef]
- Walczak, R.; Gawęda, W.; Dudek, J.; Choiński, J.; Bilewicz, A. Influence of metal ions on the 44Sc-labeling of DOTATATE. J. Radioanal. Nucl. Chem. 2019, 322, 249–254. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants: First Supplement; Plenum Press: New York, NY, USA, 1974; pp. 132–200. [Google Scholar]
- Irving, H.; Williams, R.J.P. 637. The stability of transition-metal complexes. J. Chem. Soc. (Resumed) 1953, 5, 3192–3210. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Douglas, X.W.; Janeice, S.I.; Gordon, A.B.; Anthony, E.L.; Jesús, V.-M.; Klaus, H.E.; Simón, H.-O. Copper(II) and nickel(II) complexes of 2,3-butanedione bis(N(3)-substituted thiosemicarbazones). Polyhedron 1997, 16, 1895–1905. [Google Scholar] [CrossRef]
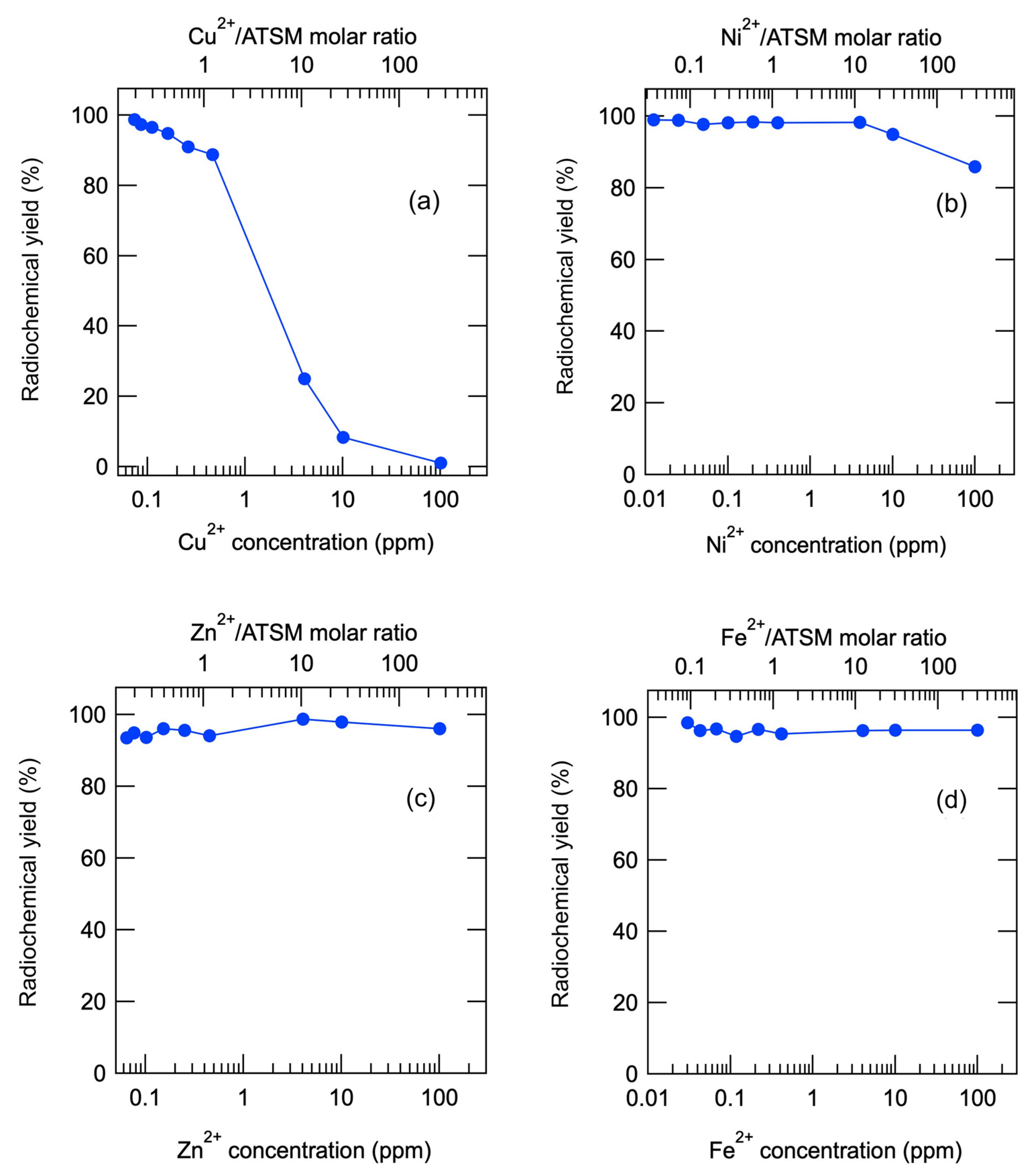


| Cu2+ | Ni2+ | ||||
|---|---|---|---|---|---|
| Metal Ion in ppm (mol) | Radiochemical Yield (%) | Ratio (1) | Metal Ion in ppm (mol) | Radiochemical Yield (%) | Ratio |
| 0.073 (1.9 × 10−8) | 98.8 | 0.19 | 0.0125 (3.4 × 10−9) | 99.0 | 0.036 |
| 0.086 (2.2 × 10−8) | 97.4 | 0.22 | 0.025 (6.9 × 10−9) | 98.8 | 0.072 |
| 0.11 (2.9 × 10−8) | 96.6 | 0.28 | 0.05 (1.4 × 10−8) | 97.7 | 0.14 |
| 0.16 (4.1 × 10−8) | 94.8 | 0.41 | 0.1 (2.8 × 10−8) | 98.2 | 0.29 |
| 0.26 (6.7 × 10−8) | 91.0 | 0.66 | 0.2 (5.5 × 10−8) | 98.4 | 0.58 |
| 0.46 (1.2 × 10−7) | 88.8 | 1.2 | 0.4 (1.1 × 10−7) | 98.2 | 1.2 |
| 4.1 (1.0 × 10−6) | 25.0 | 10 | 4 (1.1 × 10−6) | 98.3 | 12 |
| 10 (2.6 × 10−6) | 8.3 | 26 | 10 (2.8 × 10−6) | 94.9 | 29 |
| 100 (2.6 × 10−5) | 1.0 | 255 | 100 (2.8 × 10−5) | 85.9 | 288 |
| Zn2+ | Fe2+ | ||||
|---|---|---|---|---|---|
| Metal Ion in ppm (mol) | Radiochemical Yield (%) | Ratio | Metal Ion in ppm (mol) | Radiochemical Yield (%) | Ratio |
| 0.064 (1.6 × 10−8) | 93.5 | 0.16 | 0.030 (8.8 × 10−9) | 98.5 | 0.088 |
| 0.077 (1.9 × 10−8) | 94.9 | 0.19 | 0.043 (1.2 × 10−8) | 96.4 | 0.12 |
| 0.10 (2.5 × 10−8) | 93.7 | 0.25 | 0.068 (2.0 × 10−8) | 96.7 | 0.20 |
| 0.15 (3.8 × 10−8) | 96.1 | 0.38 | 0.12 (3.4 × 10−8) | 94.6 | 0.34 |
| 0.25 (6.3 × 10−8) | 95.7 | 0.62 | 0.22 (6.3 × 10−8) | 96.6 | 0.63 |
| 0.45 (1.1 × 10−7) | 94.1 | 1.1 | 0.42 (1.2 × 10−7) | 95.4 | 1.2 |
| 4.1 (1.0 × 10−6) | 98.7 | 10 | 4.0 (1.2 × 10−6) | 96.4 | 12 |
| 10 (2.5 × 10−6) | 98.0 | 25 | 10 (2.9 × 10−6) | 96.4 | 29 |
| 100 (2.5 × 10−5) | 96.1 | 248 | 100 (2.9 × 10−5) | 96.4 | 290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shinada, M.; Suzuki, H.; Hanyu, M.; Igarashi, C.; Matsumoto, H.; Takahashi, M.; Hihara, F.; Tachibana, T.; Sogawa, C.; Zhang, M.-R.; et al. Trace Metal Impurities Effects on the Formation of [64Cu]Cu-diacetyl-bis(N4-methylthiosemicarbazone) ([64Cu]Cu-ATSM). Pharmaceuticals 2024, 17, 10. https://doi.org/10.3390/ph17010010
Shinada M, Suzuki H, Hanyu M, Igarashi C, Matsumoto H, Takahashi M, Hihara F, Tachibana T, Sogawa C, Zhang M-R, et al. Trace Metal Impurities Effects on the Formation of [64Cu]Cu-diacetyl-bis(N4-methylthiosemicarbazone) ([64Cu]Cu-ATSM). Pharmaceuticals. 2024; 17(1):10. https://doi.org/10.3390/ph17010010
Chicago/Turabian StyleShinada, Mitsuhiro, Hisashi Suzuki, Masayuki Hanyu, Chika Igarashi, Hiroki Matsumoto, Masashi Takahashi, Fukiko Hihara, Tomoko Tachibana, Chizuru Sogawa, Ming-Rong Zhang, and et al. 2024. "Trace Metal Impurities Effects on the Formation of [64Cu]Cu-diacetyl-bis(N4-methylthiosemicarbazone) ([64Cu]Cu-ATSM)" Pharmaceuticals 17, no. 1: 10. https://doi.org/10.3390/ph17010010
APA StyleShinada, M., Suzuki, H., Hanyu, M., Igarashi, C., Matsumoto, H., Takahashi, M., Hihara, F., Tachibana, T., Sogawa, C., Zhang, M.-R., Higashi, T., Sato, H., Kurihara, H., Yoshii, Y., & Doi, Y. (2024). Trace Metal Impurities Effects on the Formation of [64Cu]Cu-diacetyl-bis(N4-methylthiosemicarbazone) ([64Cu]Cu-ATSM). Pharmaceuticals, 17(1), 10. https://doi.org/10.3390/ph17010010







