Development of a Bilayer Tablet by Fused Deposition Modeling as a Sustained-Release Drug Delivery System
Abstract
:1. Introduction
2. Results and Discussions
2.1. Formulation and Design Strategies
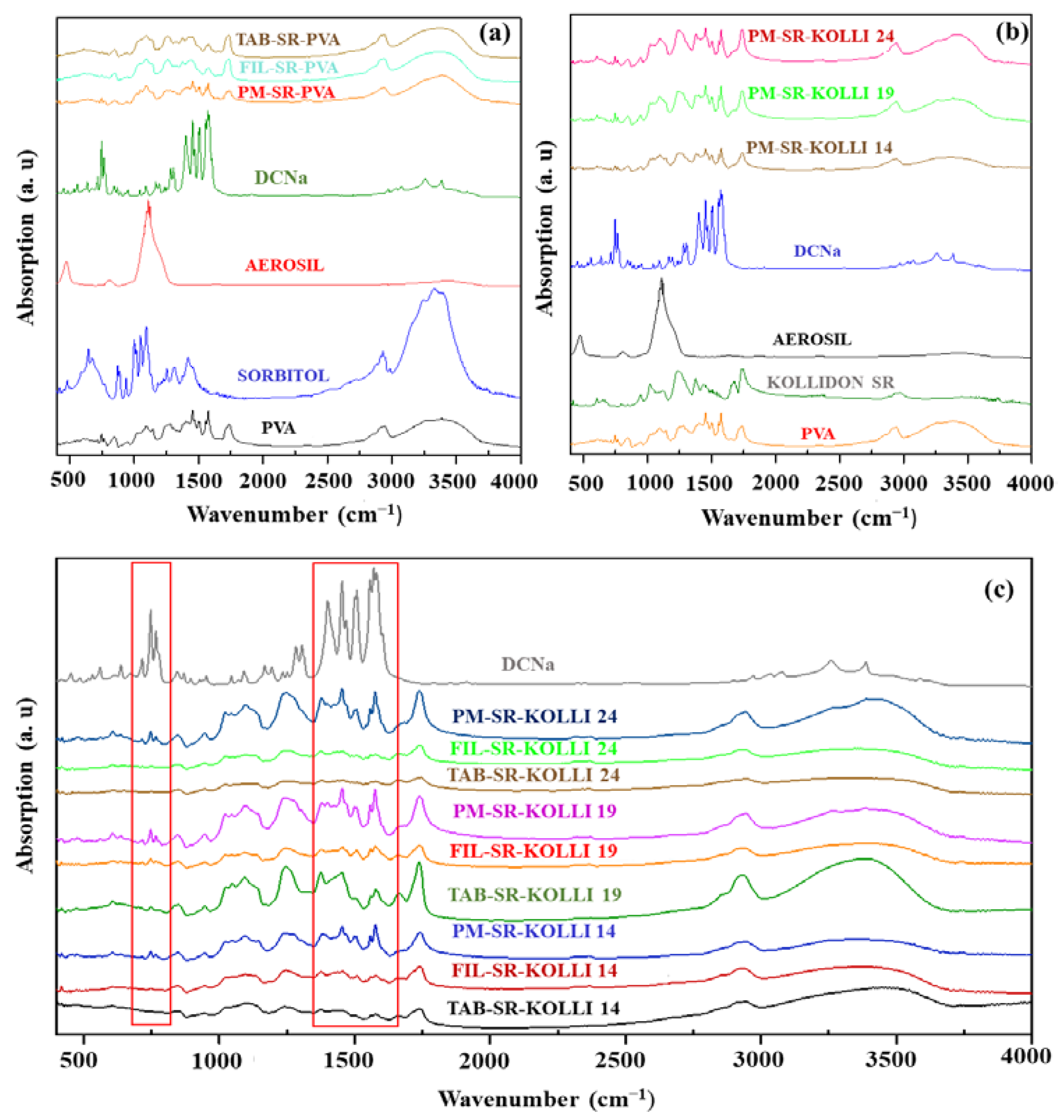
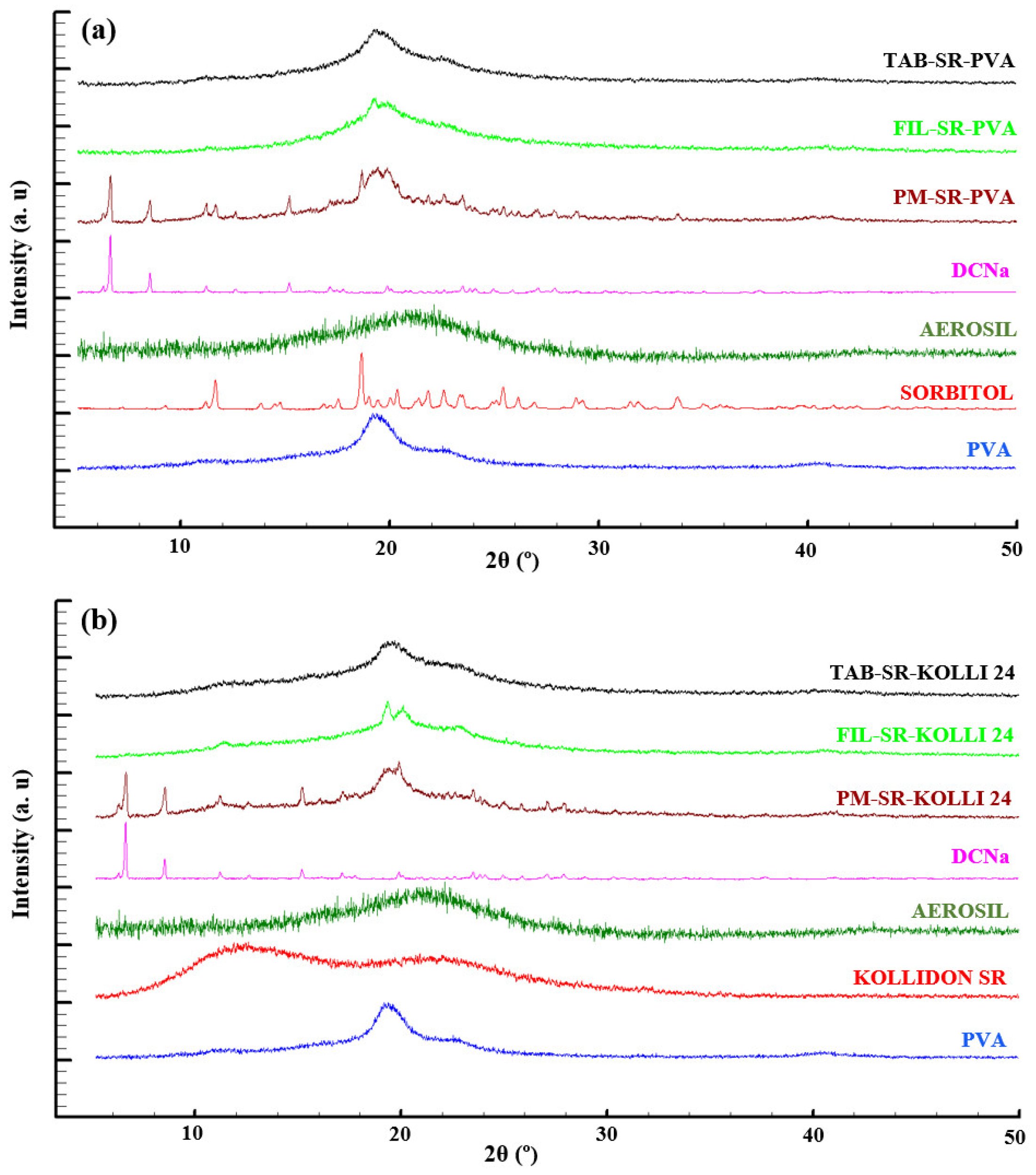
2.2. Solid State Evaluation
2.3. Mechanical Characterization of the Filaments
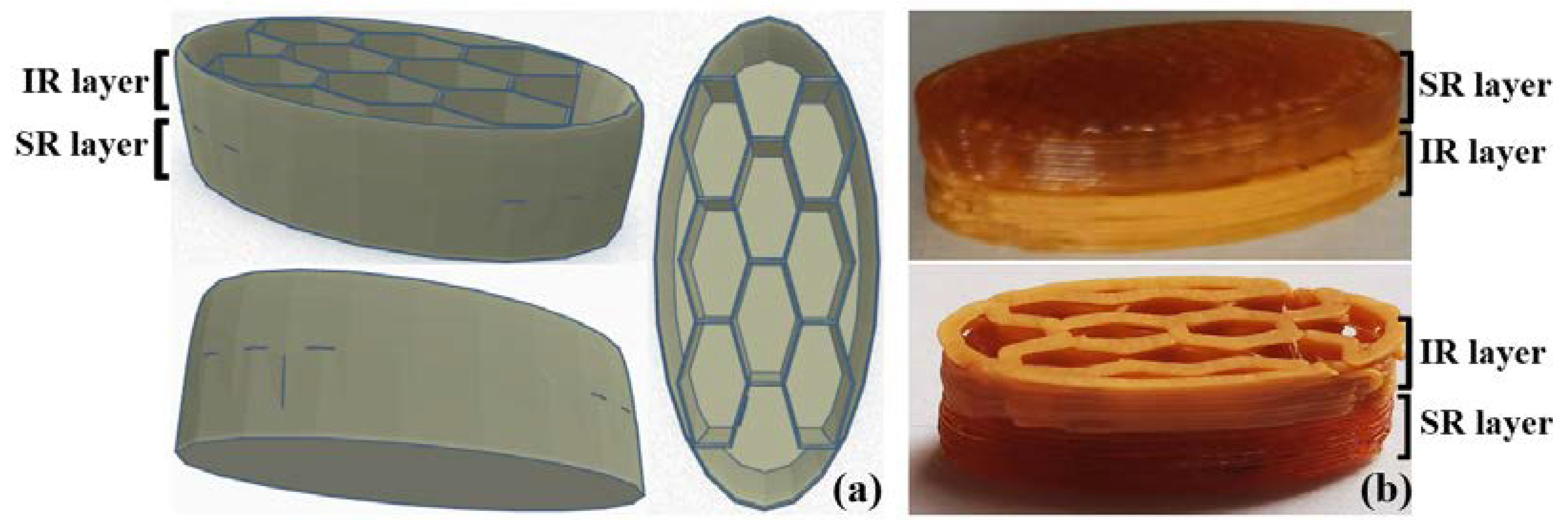
2.4. Physical Characteristics of the Tablets
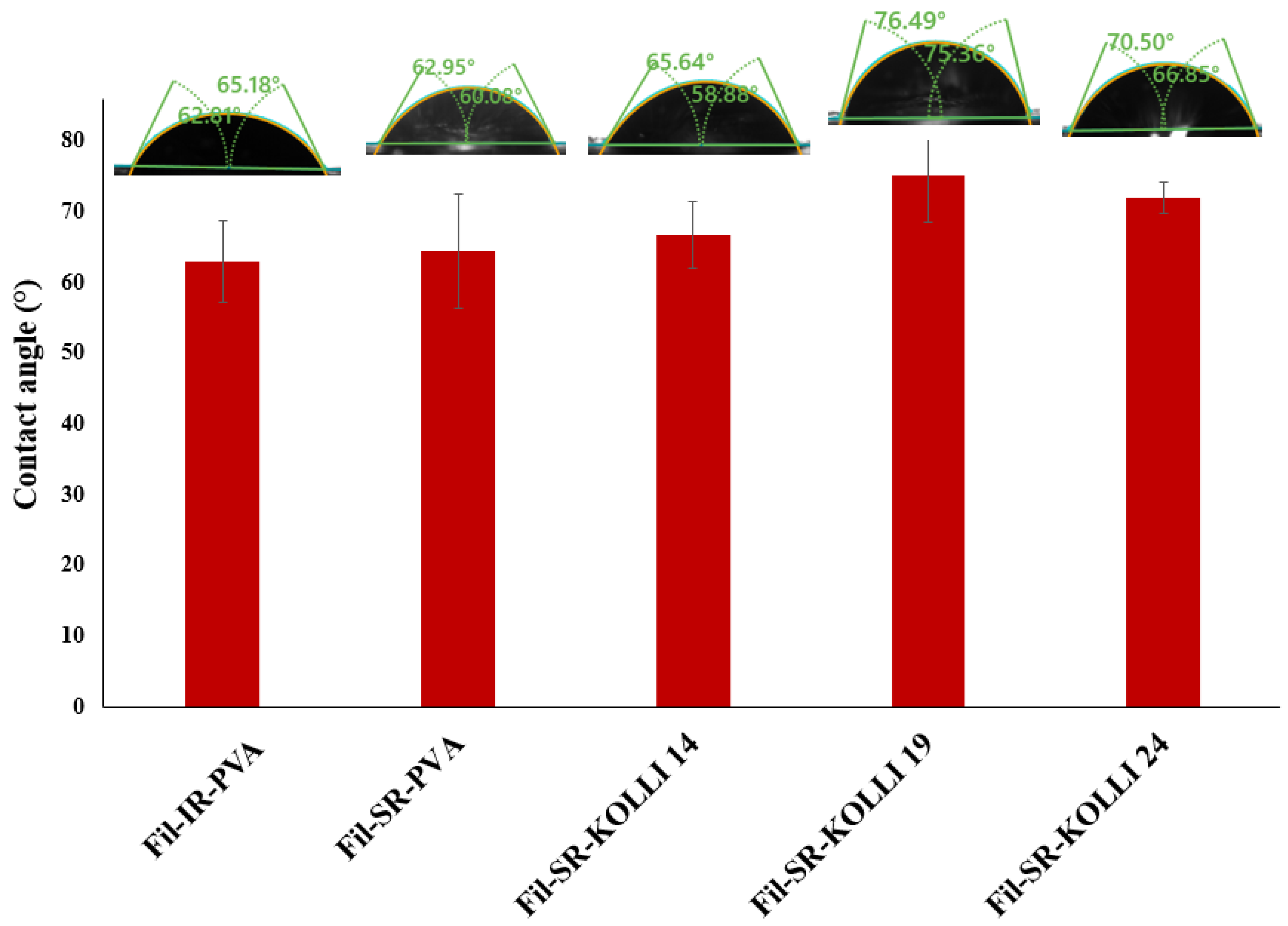
2.5. Contact Angle Measurement
2.6. Drug Content
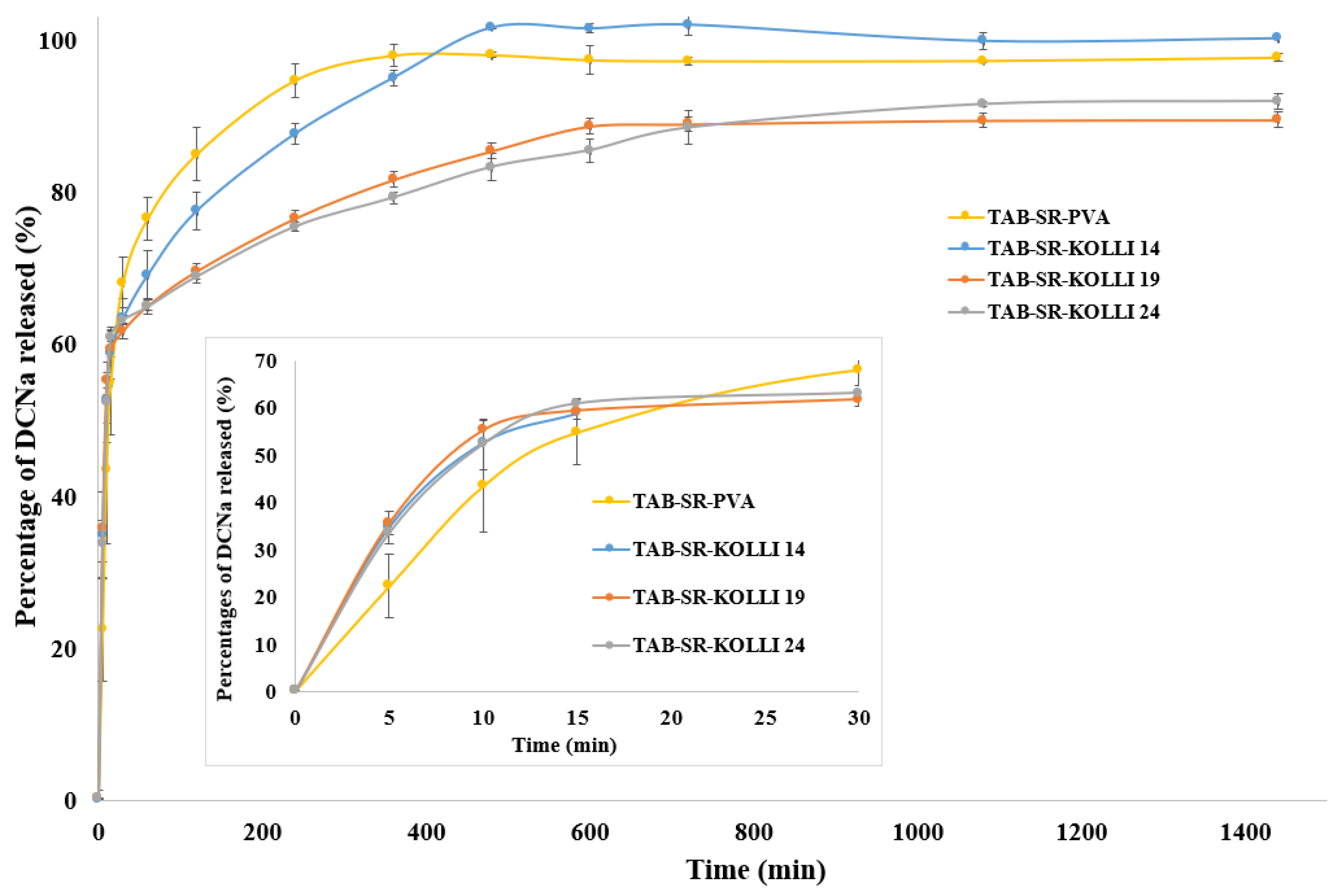
2.7. In Vitro Dissolution Behavior
3. Materials and Methods
3.1. Materials
3.2. Filament Preparation by HME
3.3. Mechanical Characterization of the Hot-Melt-Extruded Filaments
3.4. Fabrication of the FDM-3D-Printed Bilayer Tablets and Evaluation of Their Physical Characteristics
3.5. Solid State Evaluations
3.5.1. Thermogravimetric Analysis
3.5.2. Differential Scanning Calorimetry
3.5.3. X-ray Diffraction
3.5.4. Fourier-Transform Infrared Spectroscopy
3.6. Contact Angle Measurements
3.7. Drug Content Analysis
3.8. Dissolution Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laracuente, M.-L.; Yu, M.H.; McHugh, K.J. Zero-order drug delivery: State of the art and future prospects. J. Control. Release 2020, 327, 834–856. [Google Scholar] [CrossRef]
- Qian, H.; Chen, D.; Xu, X.; Li, R.; Yan, G.; Fan, T. FDM 3D-Printed Sustained-Release Gastric-Floating Verapamil Hydrochloride Formulations with Cylinder, Capsule and Hemisphere Shapes, and Low Infill Percentage. Pharmaceutics 2022, 14, 281. [Google Scholar] [CrossRef]
- Shah, V.; Khambhla, E.; Nivsarkar, M.; Trivedi, R.; Patel, R.K. An Integrative QbD Approach for the Development and Optimization of Controlled Release Compressed Coated Formulation of Water-Soluble Drugs. AAPS PharmSciTech 2022, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Yang, G.; Wei, Y.; Lei, Y.; Ding, Y.; Gong, W.; Wang, Y.; Gao, C.; Han, C. A pharmacokinetic and pharmacodynamic evaluation of colchicine sustained-release pellets for preventing gout. J. Drug Deliv. Sci. Technol. 2021, 67, 103051. [Google Scholar] [CrossRef]
- Jeong, H.M.; Weon, K.-Y.; Shin, B.S.; Shin, S. 3D-Printed Gastroretentive Sustained Release Drug Delivery System by Applying Design of Experiment Approach. Molecules 2020, 25, 2330. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shi, H.; Yang, S. 3D printed oral solid dosage form: Modified release and improved solubility. J. Control. Release 2022, 351, 407–431. [Google Scholar] [CrossRef]
- Tan, Y.J.N.; Yong, W.P.; Low, H.R.; Kochhar, J.S.; Khanolkar, J.; Lim, T.S.E.; Sun, Y.; Wong, J.Z.E.; Soh, S. Customizable drug tablets with constant release profiles via 3D printing technology. Int. J. Pharm. 2021, 598, 120370. [Google Scholar] [CrossRef]
- Al-Litani, K.; Ali, T.; Martinez, P.R.; Buanz, A. 3D printed implantable drug delivery devices for women’s health: Formulation challenges and regulatory perspective. Adv. Drug Deliv. Rev. 2023, 198, 114859. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, X.; Han, X.; Hong, X.; Li, X.; Zhang, H.; Li, M.; Wang, Z.; Zheng, A. A Review of 3D Printing Technology in Pharmaceutics: Technology and Applications, Now and Future. Pharmaceutics 2023, 15, 416. [Google Scholar] [CrossRef]
- Ayyoubi, S.; Cerda, J.R.; Fernández-García, R.; Knief, P.; Lalatsa, A.; Healy, A.M.; Serrano, D.R. 3D printed spherical mini-tablets: Geometry versus composition effects in controlling dissolution from personalised solid dosage forms. Int. J. Pharm. 2021, 597, 120336. [Google Scholar] [CrossRef]
- Parhi, R. A review of three-dimensional printing for pharmaceutical applications: Quality control, risk assessment and future perspectives. J. Drug Deliv. Sci. Technol. 2021, 64, 102571. [Google Scholar] [CrossRef]
- Alzahrani, A.; Narala, S.; Youssef, A.A.A.; Nyavanandi, D.; Bandari, S.; Mandati, P.; Almotairy, A.; Almutairi, M.; Repka, M. Fabrication of a shell-core fixed-dose combination tablet using fused deposition modeling 3D printing. Eur. J. Pharm. Biopharm. 2022, 177, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Tabesh, F.; Hussain, C.M. A new trend of using poly(vinyl alcohol) in 3D and 4D printing technologies: Process and applications. Adv. Colloid Interface Sci. 2022, 301, 102605. [Google Scholar] [CrossRef]
- Uboldi, M.; Chiappa, A.; Pertile, M.; Piazza, A.; Tagliabue, S.; Foppoli, A.; Palugan, L.; Gazzaniga, A.; Zema, L.; Melocchi, A. Investigation on the use of fused deposition modeling for the production of IR dosage forms containing Timapiprant. Int. J. Pharm. X 2023, 5, 100152. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Crișan, A.G.; Iurian, S.; Porfire, A.; Rus, L.M.; Bogdan, C.; Casian, T.; Lucacel, R.C.; Turza, A.; Porav, S.; Tomuță, I. QbD guided development of immediate release FDM-3D printed tablets with customizable API doses. Int. J. Pharm. 2021, 613, 121411. [Google Scholar] [CrossRef]
- Macedo, J.; Samaro, A.; Vanhoorne, V.; Vervaet, C.; Pinto, J.F. Processability of poly(vinyl alcohol) Based Filaments With Paracetamol Prepared by Hot-Melt Extrusion for Additive Manufacturing. J. Pharm. Sci. 2020, 109, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, I.; Bettini, R.; Giordano, F. Thermal behaviour of diclofenac, diclofenac sodium and sodium bicarbonate compositions. J. Therm. Anal. Calorim. 2007, 90, 903–907. [Google Scholar] [CrossRef]
- Palekar, S.; Nukala, P.K.; Mishra, S.M.; Kipping, T.; Patel, K. Application of 3D printing technology and quality by design approach for development of age-appropriate pediatric formulation of baclofen. Int. J. Pharm. 2018, 556, 106–116. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Banerjee, S. Exploration of theoretical and practical evaluation on Kollidon®SR matrix mediated amorphous filament extrusion of norfloxacin by melt extrusion. J. Drug Deliv. Sci. Technol. 2021, 67, 102894. [Google Scholar] [CrossRef]
- Restrepo-Uribe, L.; Ioannidis, N.; Escobar, M.d.P.N. Influence of screw configuration and processing parameters on the dissolution of ketoprofen in polymer blends. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Liu, Z.-P.; Yu, D.-G.; Wang, K.; Liu, P.; Chen, X. Colon-specific pulsatile drug release provided by electrospun shellac nanocoating on hydrophilic amorphous composites. Int. J. Nanomed. 2018, 13, 2395–2404. [Google Scholar] [CrossRef]
- Ali, S.F.B.; Mohamed, E.M.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A.; Asadi, A.; Rahman, Z. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int. J. Pharm. 2019, 570, 118651. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M.; Alaei-Beirami, M.; Javadzadeh, Y.; Mohammadi, G.; Hamidi, A.; Andalib, S.; Adibkia, K. Comparison of physicochemical characteristics and drug release of diclofenac sodium–eudragit® RS100 nanoparticles and solid dispersions. Powder Technol. 2012, 219, 211–216. [Google Scholar] [CrossRef]
- Wei, C.; Solanki, N.G.; Vasoya, J.M.; Shah, A.V.; Serajuddin, A.T. Development of 3D Printed Tablets by Fused Deposition Modeling Using Polyvinyl Alcohol as Polymeric Matrix for Rapid Drug Release. J. Pharm. Sci. 2020, 109, 1558–1572. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.L.; Gómez-Gallo, A.; Delgado, V.; Gallardo, V. Study of the stability of Kollidon® SR suspensions for pharmaceutical applications. Colloids Surf. A Physicochem. Eng. Asp. 2009, 338, 107–113. [Google Scholar] [CrossRef]
- Shen, X.; Yu, D.; Zhu, L.; Branford-White, C.; White, K.; Chatterton, N.P. Electrospun diclofenac sodium loaded Eudragit® L 100-55 nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2011, 408, 200–207. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Naik, J.B. Diclofenac Sodium-Loaded Eudragit® Microspheres: Optimization Using Statistical Experimental Design. J. Pharm. Innov. 2013, 8, 276–287. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2016, 34, 427–437. [Google Scholar] [CrossRef]
- Oladeji, S.; Mohylyuk, V.; Jones, D.S.; Andrews, G.P. 3D printing of pharmaceutical oral solid dosage forms by fused deposition: The enhancement of printability using plasticised HPMCAS. Int. J. Pharm. 2022, 616, 121553. [Google Scholar] [CrossRef]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef]
- Nasereddin, J.M.; Wellner, N.; Alhijjaj, M.; Belton, P.; Qi, S. Development of a Simple Mechanical Screening Method for Predicting the Feedability of a Pharmaceutical FDM 3D Printing Filament. Pharm. Res. 2018, 35, 151. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Li, J.; Meda, A.; Osei-Yeboah, F.; Peterson, M.L.; Repka, M.; Zhan, X. Development of a quantitative method to evaluate the printability of filaments for fused deposition modeling 3D printing. Int. J. Pharm. 2020, 588, 119760. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & Healthcare. Uniformity of Mass of Single-Dose Preparations. In European Pharmacopoeia; Council of Europe: Strasbourg, France, 2022; 358p, Available online: https://pheur.edqm.eu/subhome/11-0 (accessed on 13 June 2023).
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Maver, T.; Mastnak, T.; Mihelič, M.; Maver, U.; Finšgar, M. Clindamycin-Based 3D-Printed and Electrospun Coatings for Treatment of Implant-Related Infections. Materials 2021, 14, 1464. [Google Scholar] [CrossRef]
- Nguyen, T.-T.; Hwang, K.-M.; Kim, S.-H.; Park, E.-S. Development of novel bilayer gastroretentive tablets based on hydrophobic polymers. Int. J. Pharm. 2019, 574, 118865. [Google Scholar] [CrossRef]
- Goyanes, A.; Kobayashi, M.; Martínez-Pacheco, R.; Gaisford, S.; Basit, A.W. Fused-filament 3D printing of drug products: Microstructure analysis and drug release characteristics of PVA-based caplets. Int. J. Pharm. 2016, 514, 290–295. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Obeid, S.; Madžarević, M.; Krkobabić, M.; Ibrić, S. Predicting drug release from diazepam FDM printed tablets using deep learning approach: Influence of process parameters and tablet surface/volume ratio. Int. J. Pharm. 2021, 601, 120507. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Samaro, A.; Shaqour, B.; Goudarzi, N.M.; Ghijs, M.; Cardon, L.; Boone, M.N.; Verleije, B.; Beyers, K.; Vanhoorne, V.; Cos, P.; et al. Can filaments, pellets and powder be used as feedstock to produce highly drug-loaded ethylene-vinyl acetate 3D printed tablets using extrusion-based additive manufacturing? Int. J. Pharm. 2021, 607, 120922. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, P.; Zhang, J.; Bandari, S.; Repka, M.A. Development of controlled release oral dosages by density gradient modification via three-dimensional (3D) printing and hot-melt extrusion (HME) technology. J. Drug Deliv. Sci. Technol. 2022, 71, 103355. [Google Scholar] [CrossRef]


| Blend Name | DCNa (%) | PVA (%) | Sorbitol (%) | Aerosil (%) | Kolli SR (%) | KTPGS (%) | Filament |
|---|---|---|---|---|---|---|---|
| PM-SR-PVA | 10 | 80 | 9 | 1 | - | - | Fil-SR-PVA |
| PM-SR-KOLLI 14 | 10 | 75 | - | 1 | 14 | - | Fil-SR-KOLLI 14 |
| PM-SR-KOLLI 19 | 10 | 70 | - | 1 | 19 | - | Fil-SR-KOLLI 19 |
| PM-SR-KOLLI 24 | 10 | 65 | - | 1 | 24 | - | Fil-SR-KOLLI 24 |
| PM-IR-PVA | 50 | 40 | - | 1 | - | 9 | Fil-IR-PVA |
| IR Layer | SR Layer | FDM-3D Printed Tablet |
|---|---|---|
| Fil-IR-PVA | Fil-SR-PVA | Tab-SR-PVA |
| Fil-IR-PVA | Fil-SR-KOLLI 14 | Tab-SR-KOLLI 14 |
| Fil-IR-PVA | Fil-SR-KOLLI 19 | Tab-SR-KOLLI 19 |
| Fil-IR-PVA | Fil-SR-KOLLI 24 | Tab-SR-KOLLI 24 |
| Tablet | Dimensions | AW ± SD (mg), n = 4 | DAW (%) | ||
|---|---|---|---|---|---|
| L (mm) | W (mm) | H (mm) | |||
| TAB-SR-PVA | 18.01 ± 0.20 | 8.13 ± 0.06 | 6.00 ± 0.06 | 740.19 ± 14.1 | +1.62 −2.40 |
| TAB-SR-KOLLI 14 | 17.93 ± 0.12 | 8.08 ± 0.06 | 5.97 ± 0.08 | 731.93 ± 9.06 | +1.74 −1.14 |
| TAB-SR-KOLLI 19 | 18.05 ± 0.12 | 8.08 ± 0.08 | 6.00 ± 0.03 | 695.51 ± 3.38 | +0.46 −0.48 |
| TAB-SR-KOLLI 24 | 18.02 ± 0.07 | 7.97 ± 0.05 | 6.03 ± 0.04 | 702.77 ± 2.29 | +0.19 −0.49 |
| Sample | Drug Loading ± SD (%) | Yield ± SD (%) |
|---|---|---|
| Fil-IR-PVA | 47.5 ± 0.53 | 95.1 ± 1.06 |
| Tab-IR-PVA | 46.8 ± 0.53 | 93.6 ± 0.64 |
| Fil-SR-PVA | 9.58 ± 0.2 | 95.8 ± 2.02 |
| Tab-SR-PVA | 9.46 ± 0.04 | 94.6 ± 0.37 |
| Fil-SR-KOLLI 14 | 9.44 ± 0.05 | 94.4 ± 0.46 |
| Tab-SR-KOLLI 14 | 9.22 ± 0.11 | 92.2 ± 1.08 |
| Fil-SR-KOLLI 19 | 9.37 ± 0.06 | 93.7 ± 0.58 |
| Tab-SR-KOLLI 19 | 9.28 ± 0.19 | 92.8 ± 1.91 |
| Fil-SR-KOLLI 24 | 9.68 ± 0.13 | 96.8 ± 1.27 |
| Tab-SR-KOLLI 24 | 9.43 ± 0.22 | 94.3 ± 2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crișan, A.G.; Porfire, A.; Iurian, S.; Rus, L.M.; Lucăcel Ciceo, R.; Turza, A.; Tomuță, I. Development of a Bilayer Tablet by Fused Deposition Modeling as a Sustained-Release Drug Delivery System. Pharmaceuticals 2023, 16, 1321. https://doi.org/10.3390/ph16091321
Crișan AG, Porfire A, Iurian S, Rus LM, Lucăcel Ciceo R, Turza A, Tomuță I. Development of a Bilayer Tablet by Fused Deposition Modeling as a Sustained-Release Drug Delivery System. Pharmaceuticals. 2023; 16(9):1321. https://doi.org/10.3390/ph16091321
Chicago/Turabian StyleCrișan, Andrea Gabriela, Alina Porfire, Sonia Iurian, Lucia Maria Rus, Raluca Lucăcel Ciceo, Alexandru Turza, and Ioan Tomuță. 2023. "Development of a Bilayer Tablet by Fused Deposition Modeling as a Sustained-Release Drug Delivery System" Pharmaceuticals 16, no. 9: 1321. https://doi.org/10.3390/ph16091321
APA StyleCrișan, A. G., Porfire, A., Iurian, S., Rus, L. M., Lucăcel Ciceo, R., Turza, A., & Tomuță, I. (2023). Development of a Bilayer Tablet by Fused Deposition Modeling as a Sustained-Release Drug Delivery System. Pharmaceuticals, 16(9), 1321. https://doi.org/10.3390/ph16091321









