Application of Adipose-Tissue Derived Products for Burn Wound Healing
Abstract
:1. Introduction
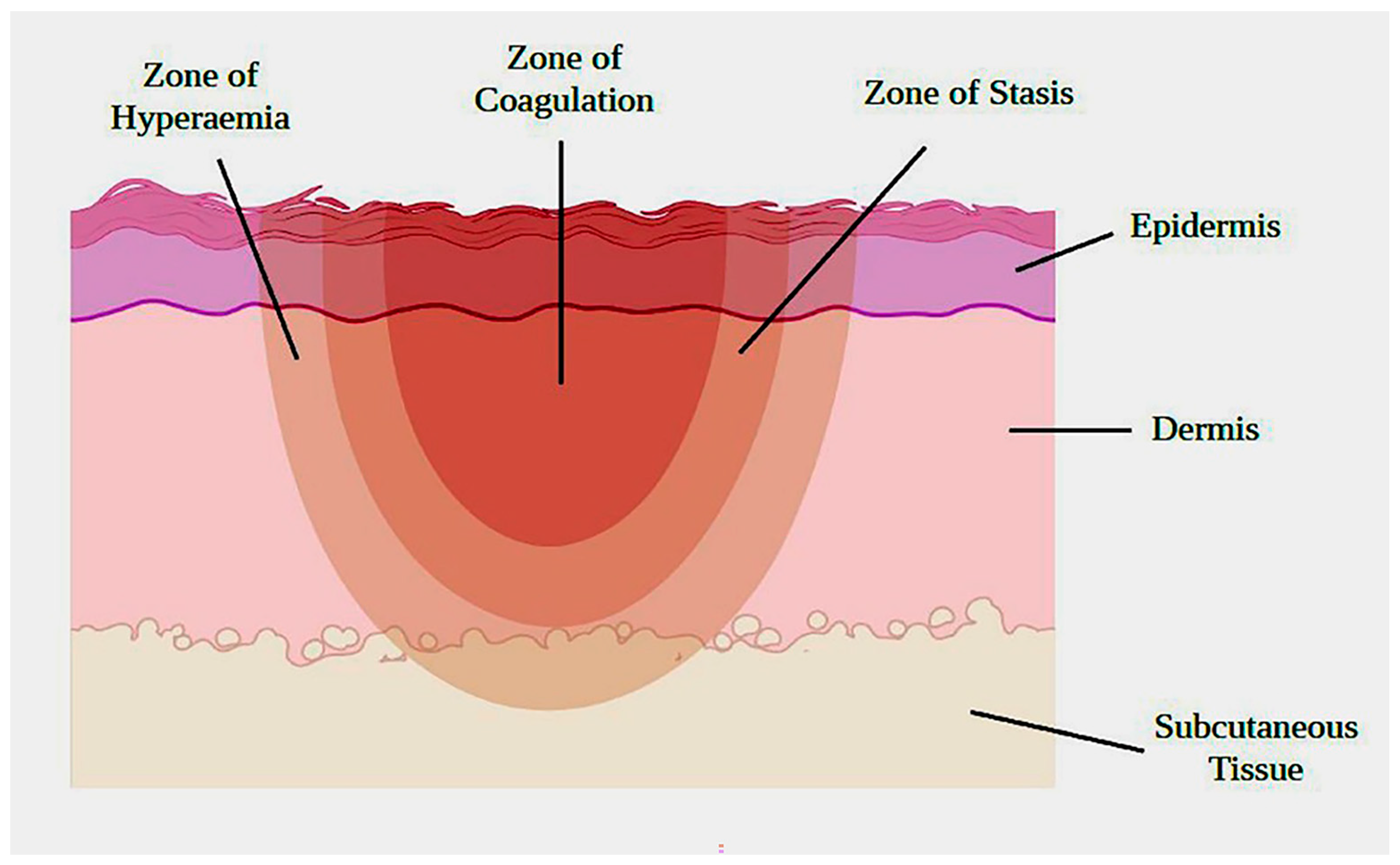
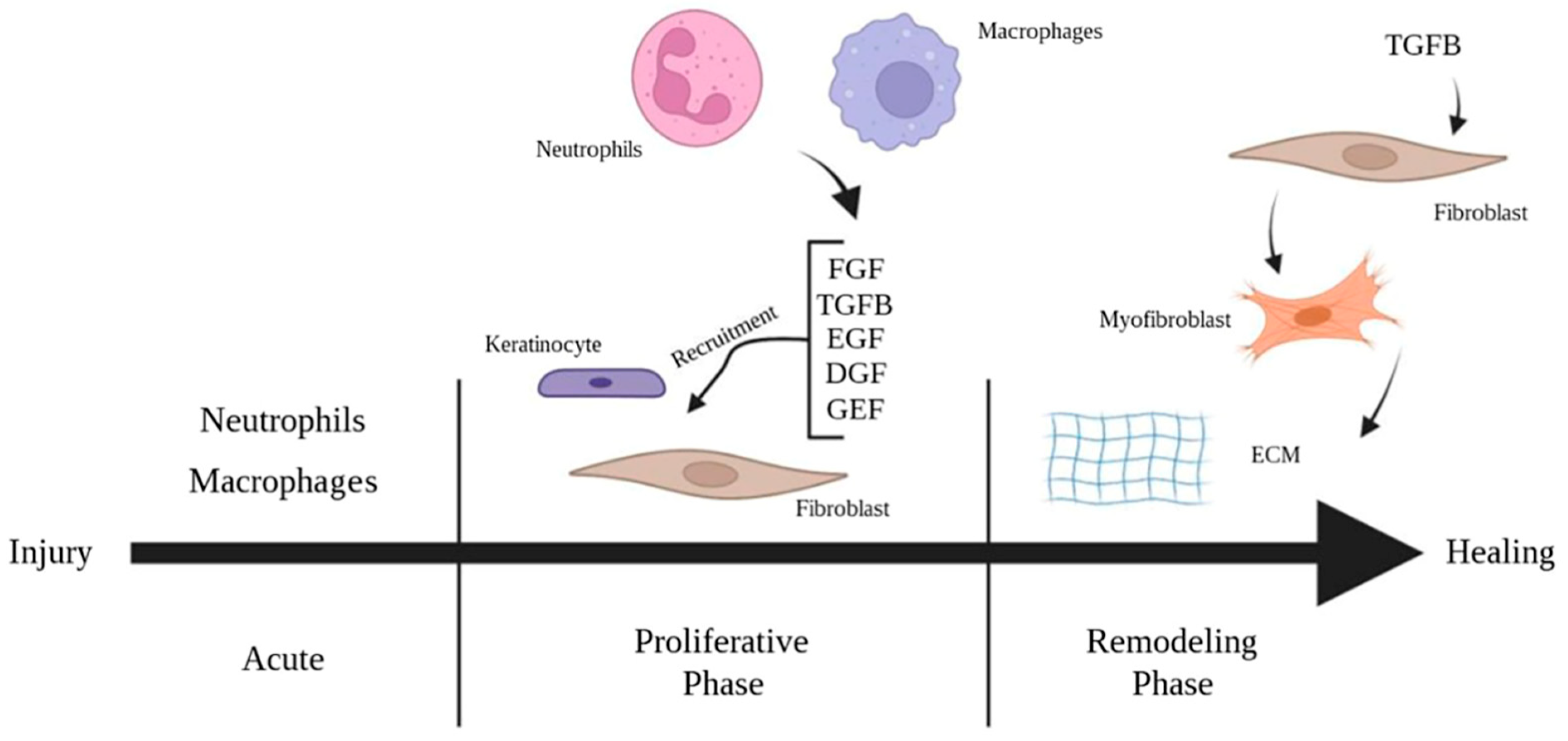
2. The Pathophysiology of Burn Wound Healing
2.1. Current Practice and Limitations
2.2. Regenerative Medicine Approaches for Wound Healing
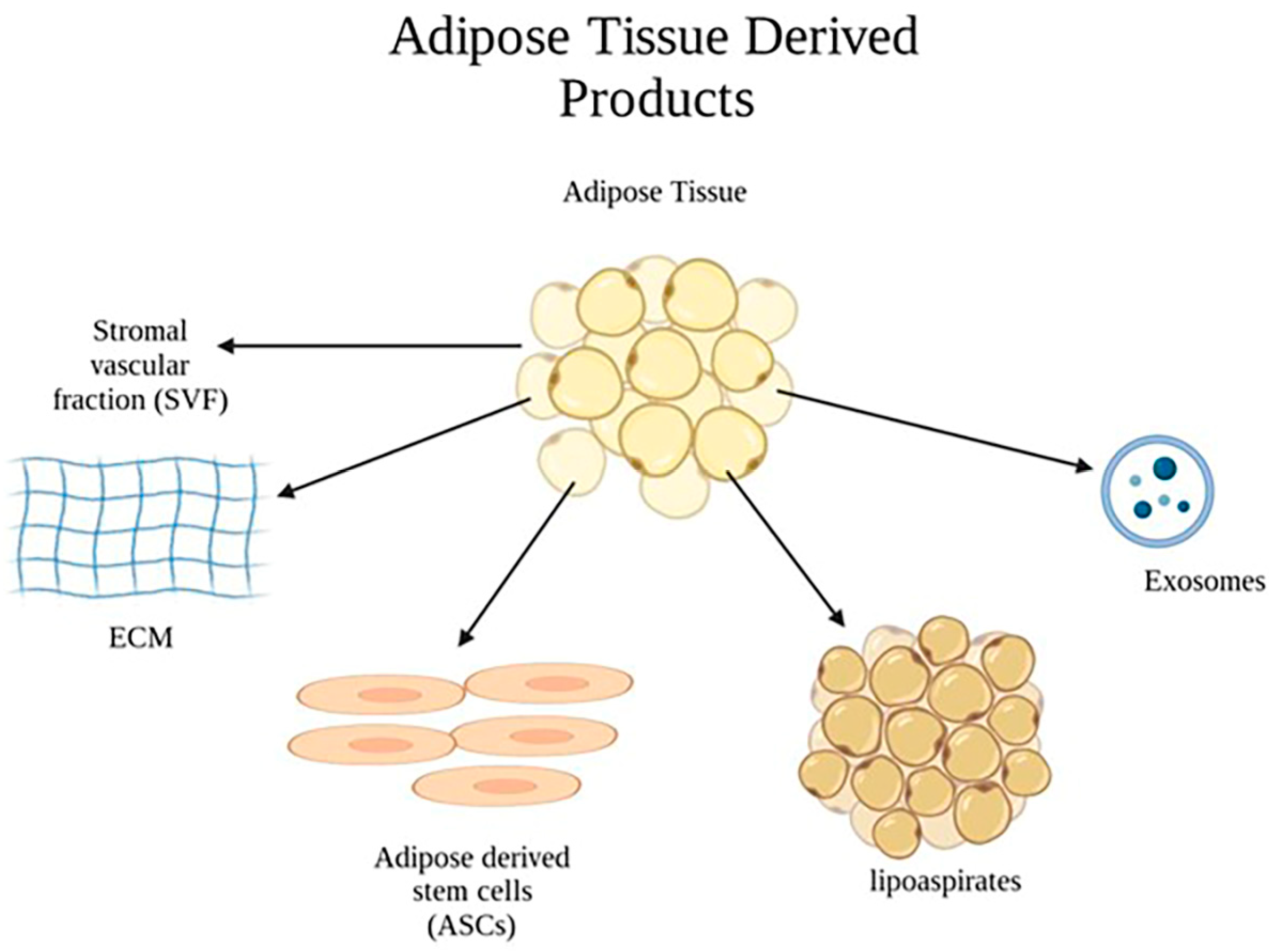
2.3. Application of Adipose-Tissue–Derived Products in Burn Wounds
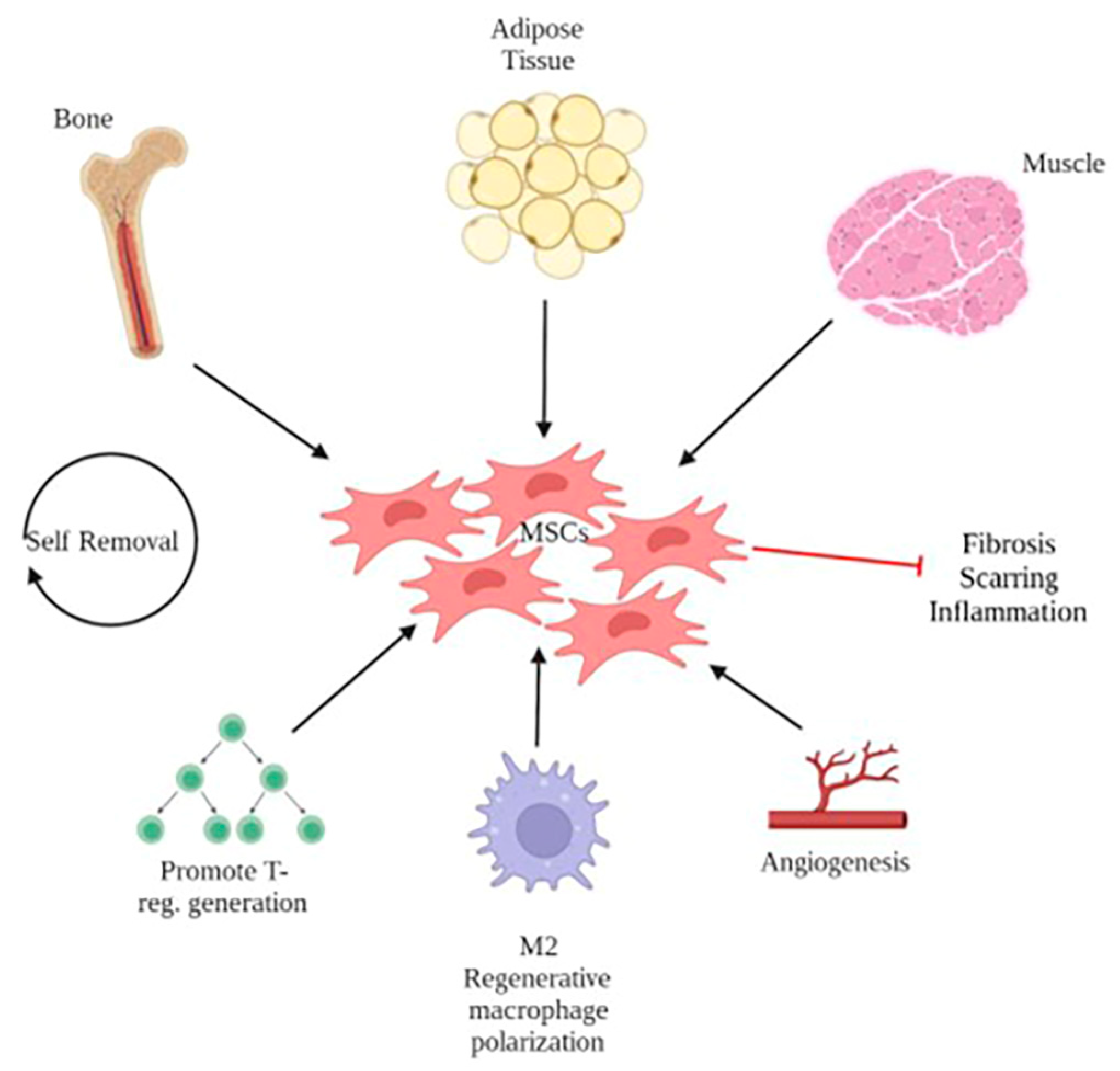
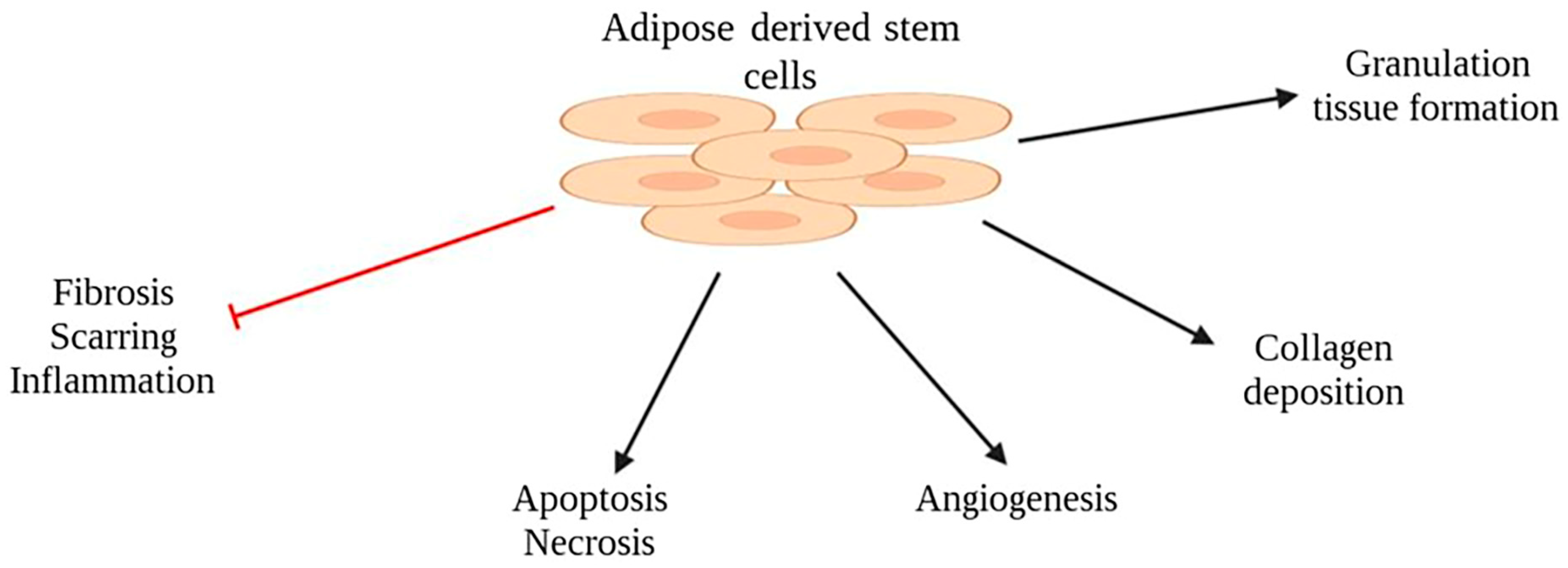
2.4. Adipose-Derived Stem Cells (ASCs)
2.5. Strategies for Delivering ASCs to Enhance Retention at the Wound Site
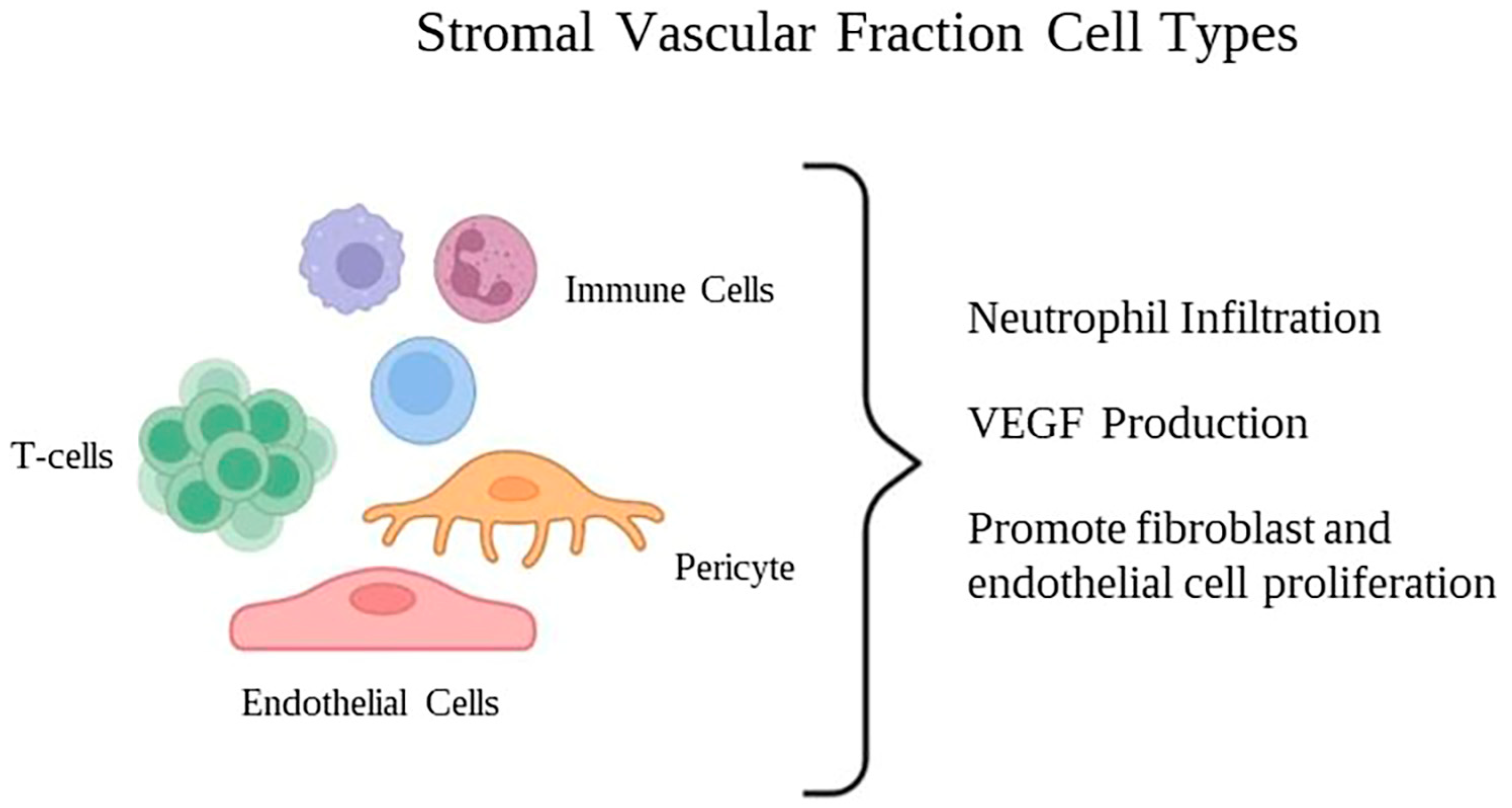
2.6. Stromal Vascular Fraction (SVF)
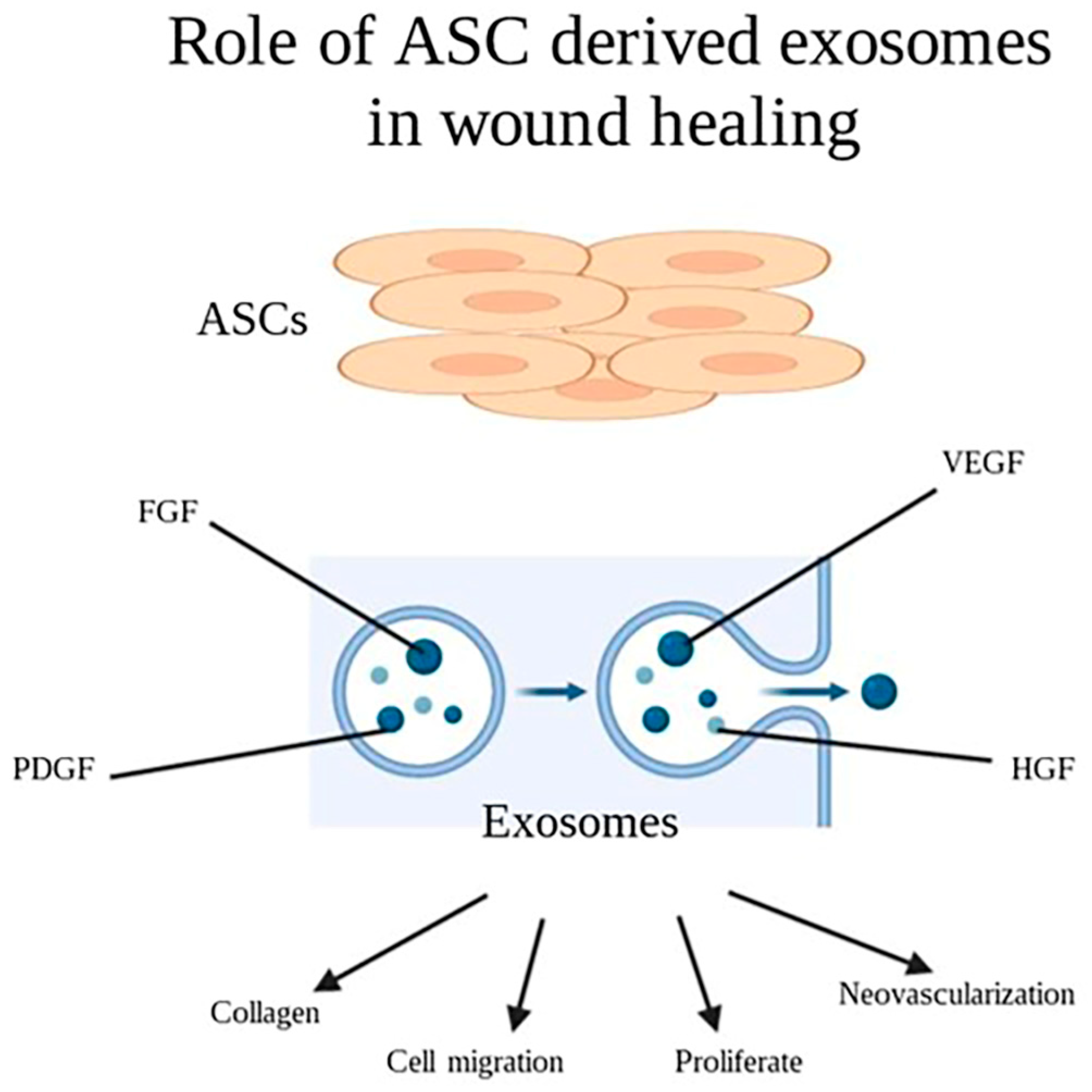
2.7. Cell Derived Exosomes
2.8. From Bench to Bedside
2.9. Limitations and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norton, R.; Kobusingye, O. Injuries. N. Engl. J. Med. 2013, 368, 1723–1730. [Google Scholar] [CrossRef]
- Gerstl, J.V.E.; Ehsan, A.N.; Lassarén, P.; Yearley, A.; Raykar, N.P.; Anderson, G.A.; Smith, T.R.; Sabapathy, R.; Ranganathan, K. The Global Macroeconomic Burden of Burn Injuries. Plast. Reconstr. Surg. 2023. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Burns. 2018. Available online: https://www.who.int/en/news-room/fact-sheets/detail/burns (accessed on 1 March 2023).
- Jeschke, M.G.; van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn injury. Nat. Rev. Dis. Primers 2020, 6, 11. [Google Scholar] [CrossRef]
- Parihar, A.; Parihar, M.S.; Milner, S.; Bhat, S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 2008, 34, 6–17. [Google Scholar] [CrossRef]
- Barrera, J.A.; Trotsyuk, A.A.; Maan, Z.N.; Bonham, C.A.; Larson, M.R.; Mittermiller, P.A.; Henn, D.; Chen, K.; Mays, C.J.; Mittal, S.; et al. Adipose-Derived Stromal Cells Seeded in Pullulan-Collagen Hydrogels Improve Healing in Murine Burns. Tissue Eng. Part A 2021, 27, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Janis, J.E.; Harrison, B. Wound healing: Part I. Basic science. Plast. Reconstr. Surg. 2014, 133, 199e–207e. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.F.; Griffin, M.F.; Parker, J.; Cotterell, A.C.; Wan, D.C.; Longaker, M.T. Beyond the Scar: A Basic Science Review of Wound Remodeling. Adv. Wound Care 2023, 12, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, D.G. Management of burns. N. Engl. J. Med. 2019, 380, 2349–2359. [Google Scholar] [CrossRef] [PubMed]
- Loder, S.; Peterson, J.R.; Agarwal, S.; Eboda, O.; Brownley, C.; DeLaRosa, S.; Ranganathan, K.; Cederna, P.; Wang, S.C.; Levi, B. Wound healing after thermal injury is improved by fat and adipose-derived stem cell isografts. J. Burn Care Res. 2015, 36, 70–76. [Google Scholar] [CrossRef]
- Gabriel, V.A. Transforming growth factor-beta and angiotensin in fibrosis and burn injuries. J. Burn Care Res. 2009, 30, 471–481. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Chapman, M.A. Regenerative Medicine: Charting a New Course in Wound Healing. Adv. Wound Care 2016, 5, 314–328. [Google Scholar] [CrossRef]
- Shi, Y.; Su, J.; Roberts, A.I.; Shou, P.; Rabson, A.B.; Ren, G. How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 2012, 33, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, M.T.; Pirraco, R.P.; Marques, A.P. Stem Cells in Skin Wound Healing: Are We There Yet? Adv. Wound Care 2016, 5, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Shingyochi, Y.; Orbay, H.; Mizuno, H. Adipose-derived stem cells for wound repair and regeneration. Expert Opin. Biol. Ther. 2015, 15, 1285–1292. [Google Scholar] [CrossRef]
- Hocking, A.M.; Gibran, N.S. Mesenchymal stem cells: Paracrine signaling and differentiation during cutaneous wound repair. Exp. Cell Res. 2010, 316, 2213–2219. [Google Scholar] [CrossRef]
- Hu, M.S.; Borrelli, M.R.; Lorenz, H.P.; Longaker, M.T.; Wan, D.C. Mesenchymal Stromal Cells and Cutaneous Wound Healing: A Comprehensive Review of the Background, Role, and Therapeutic Potential. Stem Cells Int. 2018, 2018, 6901983. [Google Scholar] [CrossRef]
- Abdul Kareem, N.; Aijaz, A.; Jeschke, M.G. Stem Cell Therapy for Burns: Story so Far. Biologics 2021, 15, 379–397. [Google Scholar] [CrossRef]
- Li, M.; Luan, F.; Zhao, Y.; Hao, H.; Liu, J.; Dong, L.; Fu, X.; Han, W. Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. Int. Wound J. 2017, 14, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ren, K.K.; Zhang, W.J.; Xiao, L.; Wu, H.Y.; Liu, Q.Y.; Ding, T.; Zhang, X.C.; Nie, W.J.; Ke, Y.; et al. Human amniotic mesenchymal stem cells and their paracrine factors promote wound healing by inhibiting heat stress-induced skin cell apoptosis and enhancing their proliferation through activating PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2019, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Tang, H.; Wu, M.; Liao, Y.; Li, K.; Li, L.; Xu, X. Ozone oil promotes wound healing by increasing the migration of fibroblasts via PI3K/Akt/mTOR signaling pathway. Biosci. Rep. 2017, 37, BSR20170658. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Benoit, D.S. Agonism of Wnt-β-catenin signalling promotes mesenchymal stem cell (MSC) expansion. J. Tissue Eng. Regen. Med. 2015, 9, E13–E26. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Moon, J.H.; Jun, E.K.; Kim, J.; Maeng, I.; Kim, J.S.; Lee, J.H.; Baik, C.S.; Kim, A.; Cho, K.S.; et al. Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev. 2010, 19, 887–902. [Google Scholar] [CrossRef]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef]
- Eschborn, J.; Kruppa, P.; Georgiou, I.; Infanger, M.; Ghods, M. Long-term Results After Autologous Fat Transfer for Treatment of Chronic Lower Extremity Wounds. Int. J. Low. Extrem. Wounds 2023, 22, 524–530. [Google Scholar] [CrossRef]
- Kenny, E.M.; Egro, F.M.; Ejaz, A.; Coleman, S.R.; Greenberger, J.S.; Rubin, J.P. Fat Grafting in Radiation-Induced Soft-Tissue Injury: A Narrative Review of the Clinical Evidence and Implications for Future Studies. Plast. Reconstr. Surg. 2021, 147, 819–838. [Google Scholar] [CrossRef]
- Piccolo, N.S.; Piccolo, M.S.; Piccolo, M.T. Fat grafting for treatment of burns, burn scars, and other difficult wounds. Clin. Plast. Surg. 2015, 42, 263–283. [Google Scholar] [CrossRef]
- Abouzaid, A.M.; El Mokadem, M.E.; Aboubakr, A.K.; Kassem, M.A.; Al Shora, A.K.; Solaiman, A. Effect of autologous fat transfer in acute burn wound management: A randomized controlled study. Burns 2022, 48, 1368–1385. [Google Scholar] [CrossRef]
- Kokai, L.E.; Marra, K.; Rubin, J.P. Adipose stem cells: Biology and clinical applications for tissue repair and regeneration. Transl. Res. 2014, 163, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Zimmerlin, L.; Donnenberg, V.S.; Rubin, J.P.; Donnenberg, A.D. Mesenchymal markers on human adipose stem/progenitor cells. Cytom. A 2013, 83, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Bajada, S.; Mazakova, I.; Richardson, J.B.; Ashammakhi, N. Updates on stem cells and their applications in regenerative medicine. J. Tissue Eng. Regen. Med. 2008, 2, 169–183. [Google Scholar] [CrossRef]
- Chen, Y.W.; Scutaru, T.T.; Ghetu, N.; Carasevici, E.; Lupascu, C.D.; Ferariu, D.; Pieptu, D.; Coman, C.G.; Danciu, M. The Effects of Adipose-Derived Stem Cell-Differentiated Adipocytes on Skin Burn Wound Healing in Rats. J. Burn Care Res. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Chang, Y.W.; Wu, Y.C.; Huang, S.H.; Wang, H.D.; Kuo, Y.R.; Lee, S.S. Autologous and not allogeneic adipose-derived stem cells improve acute burn wound healing. PLoS ONE 2018, 13, e0197744. [Google Scholar] [CrossRef] [PubMed]
- Franck, C.L.; Senegaglia, A.C.; Leite, L.M.B.; de Moura, S.A.B.; Francisco, N.F.; Ribas Filho, J.M. Influence of Adipose Tissue-Derived Stem Cells on the Burn Wound Healing Process. Stem Cells Int. 2019, 2019, 2340725. [Google Scholar] [CrossRef]
- Feng, C.J.; Lin, C.H.; Tsai, C.H.; Yang, I.C.; Ma, H. Adipose-derived stem cells-induced burn wound healing and regeneration of skin appendages in a novel skin island rat model. J. Chin. Med. Assoc. 2019, 82, 635–642. [Google Scholar] [CrossRef]
- Abbas, O.L.; Özatik, O.; Gönen, Z.B.; Öğüt, S.; Özatik, F.Y.; Salkın, H.; Musmul, A. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Dental Pulp as Sources of Cell Therapy for Zone of Stasis Burns. J. Investig. Surg. 2019, 32, 477–490. [Google Scholar] [CrossRef]
- Bliley, J.M.; Argenta, A.; Satish, L.; McLaughlin, M.M.; Dees, A.; Tompkins-Rhoades, C.; Marra, K.G.; Rubin, J.P. Administration of adipose-derived stem cells enhances vascularity, induces collagen deposition, and dermal adipogenesis in burn wounds. Burns 2016, 42, 1212–1222. [Google Scholar] [CrossRef]
- Huang, S.P.; Huang, C.H.; Shyu, J.F.; Lee, H.S.; Chen, S.G.; Chan, J.Y.; Huang, S.M. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J. Biomed. Sci. 2013, 20, 51. [Google Scholar] [CrossRef]
- Riccobono, D.; Agay, D.; Scherthan, H.; Forcheron, F.; Vivier, M.; Ballester, B.; Meineke, V.; Drouet, M. Application of adipocyte-derived stem cells in treatment of cutaneous radiation syndrome. Health Phys. 2012, 103, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Shirado, T.; Mashiko, T.; Feng, J.; Asahi, R.; Kanayama, K.; Mori, M.; Chi, D.; Sunaga, A.; Sarukawa, S.; et al. Therapeutic Effects of Human Adipose-Derived Products on Impaired Wound Healing in Irradiated Tissue. Plast. Reconstr. Surg. 2018, 142, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current Advancements and Strategies in Tissue Engineering for Wound Healing: A Comprehensive Review. Adv. Wound Care 2017, 6, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47–55. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Natesan, S.; Zamora, D.O.; Wrice, N.L.; Baer, D.G.; Christy, R.J. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J. Burn Care Res. 2013, 34, 18–30. [Google Scholar] [CrossRef]
- Lu, T.Y.; Yu, K.F.; Kuo, S.H.; Cheng, N.C.; Chuang, E.Y.; Yu, J.S. Enzyme-Crosslinked Gelatin Hydrogel with Adipose-Derived Stem Cell Spheroid Facilitating Wound Repair in the Murine Burn Model. Polymers 2020, 12, 2997. [Google Scholar] [CrossRef]
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, H.; Yue, Z.; Yu, C.; Jiang, L.; Dong, X.; Yao, M.; Shi, M.; Liang, L.; Wan, Y.; et al. Zwitterionic Polysaccharide-Based Hydrogel Dressing as a Stem Cell Carrier to Accelerate Burn Wound Healing. Adv. Healthc. Mater. 2023, 12, e2202309. [Google Scholar] [CrossRef]
- Motamed, S.; Taghiabadi, E.; Molaei, H.; Sodeifi, N.; Hassanpour, S.E.; Shafieyan, S.; Azargashb, E.; Farajzadeh-Vajari, F.; Aghdami, N.; Bajouri, A. Cell-based skin substitutes accelerate regeneration of extensive burn wounds in rats. Am. J. Surg. 2017, 214, 762–769. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Seifalian, A.M.; Urbanska, A.M.; Omrani, M.D.; Hardy, J.G.; Madjd, Z.; Hashemi, S.M.; Ghanbarian, H.; Brouki Milan, P.; Mozafari, M.; et al. 3D Protein-Based Bilayer Artificial Skin for the Guided Scarless Healing of Third-Degree Burn Wounds in Vivo. Biomacromolecules 2018, 19, 2409–2422. [Google Scholar] [CrossRef] [PubMed]
- Zonari, A.; Martins, T.M.; Paula, A.C.; Boeloni, J.N.; Novikoff, S.; Marques, A.P.; Correlo, V.M.; Reis, R.L.; Goes, A.M. Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomater. 2015, 17, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, T.; Takada, H.; Wu, S.H.; Kanayama, K.; Feng, J.; Tashiro, K.; Asahi, R.; Sunaga, A.; Hoshi, K.; Kurisaki, A.; et al. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J. Tissue Eng. Regen. Med. 2018, 12, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Fang, S.; Li, X.; Zhang, H.; Dai, H.; Fan, H.; Li, Y.; Shen, D.; Tang, W.; et al. Stimulatory effect of engineered three-layer adipose tissue-derived stem cells sheet in atelocollagen matrix on wound healing in a mouse model of radiation-induced skin injury. J. Biomater. Appl. 2019, 34, 498–508. [Google Scholar] [CrossRef]
- Ramakrishnan, V.M.; Boyd, N.L. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part. B Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef]
- National Cancer Institute. Adipose-Derived Stromal Vascular Fraction Cells. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/adipose-derived-stromal-vascular-fraction-cells (accessed on 30 March 2023).
- Atalay, S.; Coruh, A.; Deniz, K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 2014, 40, 1375–1383. [Google Scholar] [CrossRef]
- Foubert, P.; Gonzalez, A.D.; Teodosescu, S.; Berard, F.; Doyle-Eisele, M.; Yekkala, K.; Tenenhaus, M.; Fraser, J.K. Adipose-Derived Regenerative Cell Therapy for Burn Wound Healing: A Comparison of Two Delivery Methods. Adv. Wound Care 2016, 5, 288–298. [Google Scholar] [CrossRef]
- Foubert, P.; Barillas, S.; Gonzalez, A.D.; Alfonso, Z.; Zhao, S.; Hakim, I.; Meschter, C.; Tenenhaus, M.; Fraser, J.K. Uncultured adipose-derived regenerative cells (ADRCs) seeded in collagen scaffold improves dermal regeneration, enhancing early vascularization and structural organization following thermal burns. Burns 2015, 41, 1504–1516. [Google Scholar] [CrossRef]
- Kaita, Y.; Tarui, T.; Yoshino, H.; Matsuda, T.; Yamaguchi, Y.; Nakagawa, T.; Asahi, M.; Ii, M. Sufficient therapeutic effect of cryopreserved frozen adipose-derived regenerative cells on burn wounds. Regen. Ther. 2019, 10, 92–103. [Google Scholar] [CrossRef]
- Dai, W.; Dong, Y.; Han, T.; Wang, J.; Gao, B.; Guo, H.; Xu, F.; Li, J.; Ma, Y. Microenvironmental cue-regulated exosomes as therapeutic strategies for improving chronic wound healing. NPG Asia Mater. 2022, 14, 75. [Google Scholar] [CrossRef]
- Hong, P.; Yang, H.; Wu, Y.; Li, K.; Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: A comprehensive review. Stem Cell Res. Ther. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.H.; Nguyen, T.D.; Nguyen, H.P.; Nguyen, X.H.; Do, P.T.X.; Dang, V.D.; Dam, P.T.M.; Bui, H.T.H.; Trinh, M.Q.; Vu, D.M.; et al. Differential Wound Healing Capacity of Mesenchymal Stem Cell-Derived Exosomes Originated From Bone Marrow, Adipose Tissue and Umbilical Cord Under Serum- and Xeno-Free Condition. Front. Mol. Biosci. 2020, 7, 119. [Google Scholar] [CrossRef]
- Rasulov, M.F.; Vasil’chenkov, A.V.; Onishchenko, N.A.; Krasheninnikov, M.E.; Kravchenko, V.I.; Gorshenin, T.L.; Pidtsan, R.E.; Potapov, I.V. First experience in the use of bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull. Exp. Biol. Med. 2005, 139, 141–144. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, S.; Fu, X. Autologous transplantation of bone marrow-derived mesenchymal stem cells: A promising therapeutic strategy for prevention of skin-graft contraction. Clin. Exp. Dermatol. 2012, 37, 497–500. [Google Scholar] [CrossRef]
- Mansilla, E.; Marín, G.H.; Berges, M.; Scafatti, S.; Rivas, J.; Núñez, A.; Menvielle, M.; Lamonega, R.; Gardiner, C.; Drago, H.; et al. Cadaveric bone marrow mesenchymal stem cells: First experience treating a patient with large severe burns. Burn. Trauma 2015, 3, 17. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Mitsuno, H.; Nonaka, I.; Sen, Y.; Kawanishi, K.; Inada, Y.; Takakura, Y.; Okuchi, K.; Nonomura, A. Wound therapy by marrow mesenchymal cell transplantation. Plast. Reconstr. Surg. 2008, 121, 860–877. [Google Scholar] [CrossRef]
- Abo-Elkheir, W.; Hamza, F.; Elmofty, A.M.; Emam, A.; Abdl-Moktader, M.; Elsherefy, S.; Gabr, H. Role of cord blood and bone marrow mesenchymal stem cells in recent deep burn: A case-control prospective study. Am. J. Stem Cells 2017, 6, 23–35. [Google Scholar]
- Arkoulis, N.; Watson, S.; Weiler-Mithoff, E. Stem cell enriched dermal substitutes for the treatment of late burn contractures in patients with major burns. Burns 2018, 44, 724–726. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Akita, S.; Akino, K.; Hirano, A.; Ohtsuru, A.; Yamashita, S. Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int. 2010, 2010, 532704. [Google Scholar] [CrossRef]
- Iddins, C.J.; Cohen, S.R.; Goans, R.E.; Wanat, R.; Jenkins, M.; Christensen, D.M.; Dainiak, N. Case Report: Industrial X-Ray Injury Treated With Non-Cultured Autologous Adipose-Derived Stromal Vascular Fraction (SVF). Health Phys. 2016, 111, 112–116. [Google Scholar] [CrossRef]
- Frazier, T.P.; Gimble, J.M.; Devay, J.W.; Tucker, H.A.; Chiu, E.S.; Rowan, B.G. Body mass index affects proliferation and osteogenic differentiation of human subcutaneous adipose tissue-derived stem cells. BMC Cell Biol. 2013, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Irons, R.F.; Cahill, K.W.; Rattigan, D.A.; Marcotte, J.H.; Fromer, M.W.; Chang, S.; Zhang, P.; Behling, E.M.; Behling, K.C.; Caputo, F.J. Acceleration of diabetic wound healing with adipose-derived stem cells, endothelial-differentiated stem cells, and topical conditioned medium therapy in a swine model. J. Vasc. Surg. 2018, 68, 115s–125s. [Google Scholar] [CrossRef] [PubMed]
- Bura, A.; Planat-Benard, V.; Bourin, P.; Silvestre, J.S.; Gross, F.; Grolleau, J.L.; Saint-Lebese, B.; Peyrafitte, J.A.; Fleury, S.; Gadelorge, M.; et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy 2014, 16, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Moraci, M.; Armenia, E.; Orabona, C.; Sergio, R.; De Sena, G.; Capuozzo, V.; Barbarisi, M.; Rosso, F.; Giordano, G.; et al. Therapy with autologous adipose-derived regenerative cells for the care of chronic ulcer of lower limbs in patients with peripheral arterial disease. J. Surg. Res. 2013, 185, 36–44. [Google Scholar] [CrossRef]
- Berkowitz, A.L.; Miller, M.B.; Mir, S.A.; Cagney, D.; Chavakula, V.; Guleria, I.; Aizer, A.; Ligon, K.L.; Chi, J.H. Glioproliferative Lesion of the Spinal Cord as a Complication of “Stem-Cell Tourism”. N. Engl. J. Med. 2016, 375, 196–198. [Google Scholar] [CrossRef]
- Dehdashtian, A.; Bratley, J.V.; Svientek, S.R.; Kung, T.A.; Awan, T.M.; Cederna, P.S.; Kemp, S.W. Autologous fat grafting for nerve regeneration and neuropathic pain: Current state from bench-to-bedside. Regen. Med. 2020, 15, 2209–2228. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use. 2020. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed on 15 March 2023).
| Condition or Disease | Intervention/ Treatment | Phase | Sponsor: | Location | First Posted/ Last Update | Recruitment Status | Results | |
|---|---|---|---|---|---|---|---|---|
| NCT02394873 | Deep Second-degree burn | ALLO-ASC-DFU (a hydrogel containing allogenic ASCs) | Phase 1 | Antero gen Co., Ltd. | Seoul, Korea | March 2015/ December 2015 | Completed | Not available |
| NCT03183622 | Deep Second-degree burn | ALLO-ASC-DFU (a hydrogel containing allogenic ASCs) | Phase 1 | Antero gen Co., Ltd. | Seoul, Korea | June 2017/ January 2018 | Completed | Not available |
| NCT02619851 | Deep Second-degree burn | ALLO-ASC-DFU (a hydrogel containing allogenic ASCs) | Phase 2 | Antero gen Co., Ltd. | Seoul, Korea | December 2015/ July 2021 | Unknown | Not available |
| NCT03183648 | Deep Second-degree burn | ALLO-ASC-DFU (a hydrogel containing allogenic ASCs) | Phase 2 | Antero gen Co., Ltd. | Seoul, Korea | June 2017/ July 2021 | Unknown | Not available |
| NCT03113747 | Second- or Third-degree Burns | Allogeneic ASCs | Phase 1 Phase 2 | A A Partners, LLC | Kyiv, Ukraine | April 2017/ April 2017 | Unknown | Not available |
| NCT03686449 | Full-Thickness | ASC-Keratinocyte Suspension | - | - | Cairo, Egypt | September 2018/May 2020 | Unknown | Not available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malekzadeh, H.; Tirmizi, Z.; Arellano, J.A.; Egro, F.M.; Ejaz, A. Application of Adipose-Tissue Derived Products for Burn Wound Healing. Pharmaceuticals 2023, 16, 1302. https://doi.org/10.3390/ph16091302
Malekzadeh H, Tirmizi Z, Arellano JA, Egro FM, Ejaz A. Application of Adipose-Tissue Derived Products for Burn Wound Healing. Pharmaceuticals. 2023; 16(9):1302. https://doi.org/10.3390/ph16091302
Chicago/Turabian StyleMalekzadeh, Hamid, Zayaan Tirmizi, José A. Arellano, Francesco M. Egro, and Asim Ejaz. 2023. "Application of Adipose-Tissue Derived Products for Burn Wound Healing" Pharmaceuticals 16, no. 9: 1302. https://doi.org/10.3390/ph16091302
APA StyleMalekzadeh, H., Tirmizi, Z., Arellano, J. A., Egro, F. M., & Ejaz, A. (2023). Application of Adipose-Tissue Derived Products for Burn Wound Healing. Pharmaceuticals, 16(9), 1302. https://doi.org/10.3390/ph16091302





