The Development of Magnolol-Loaded Intravenous Emulsion with Low Hepatotoxic Potential
Abstract
:1. Introduction
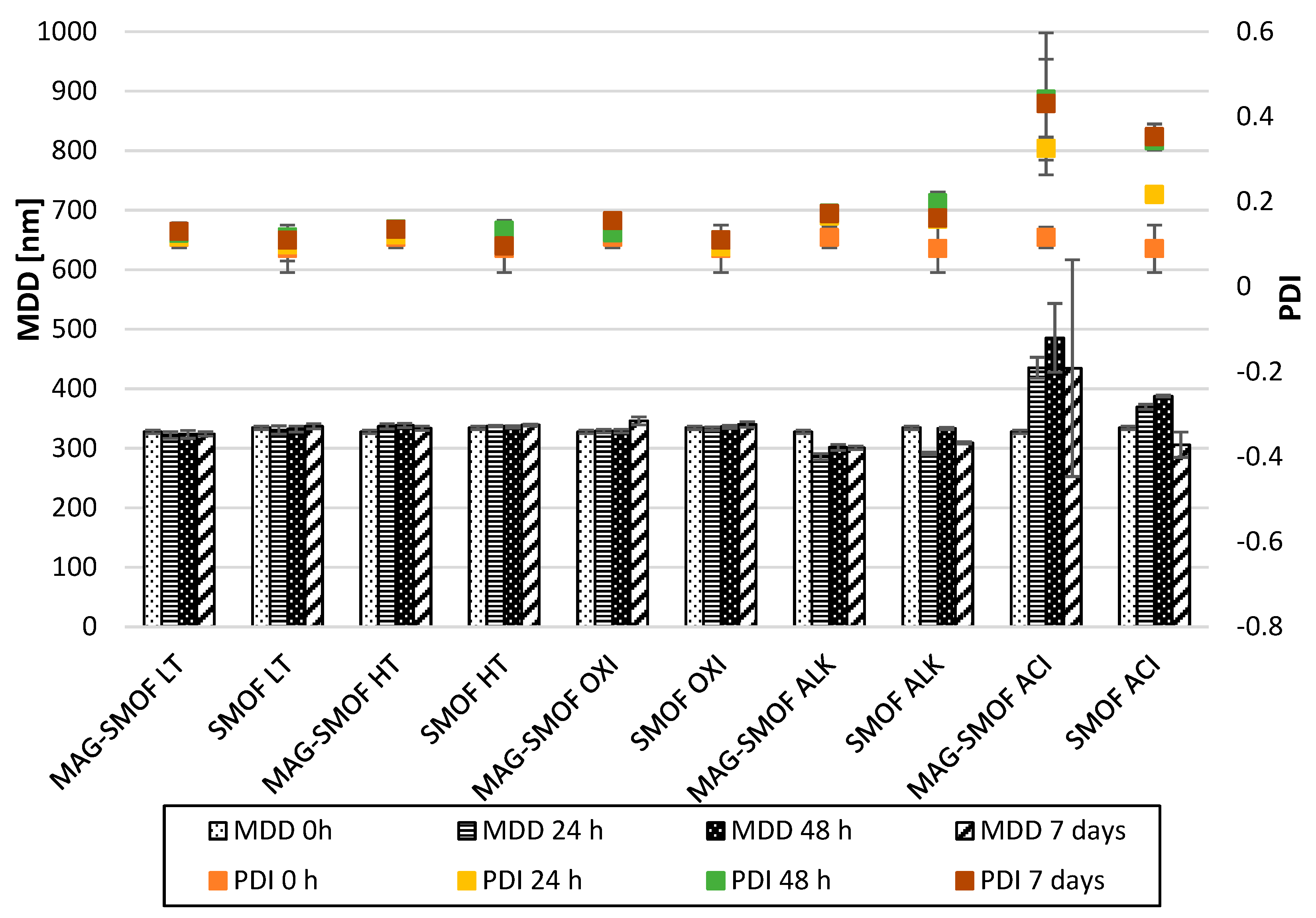
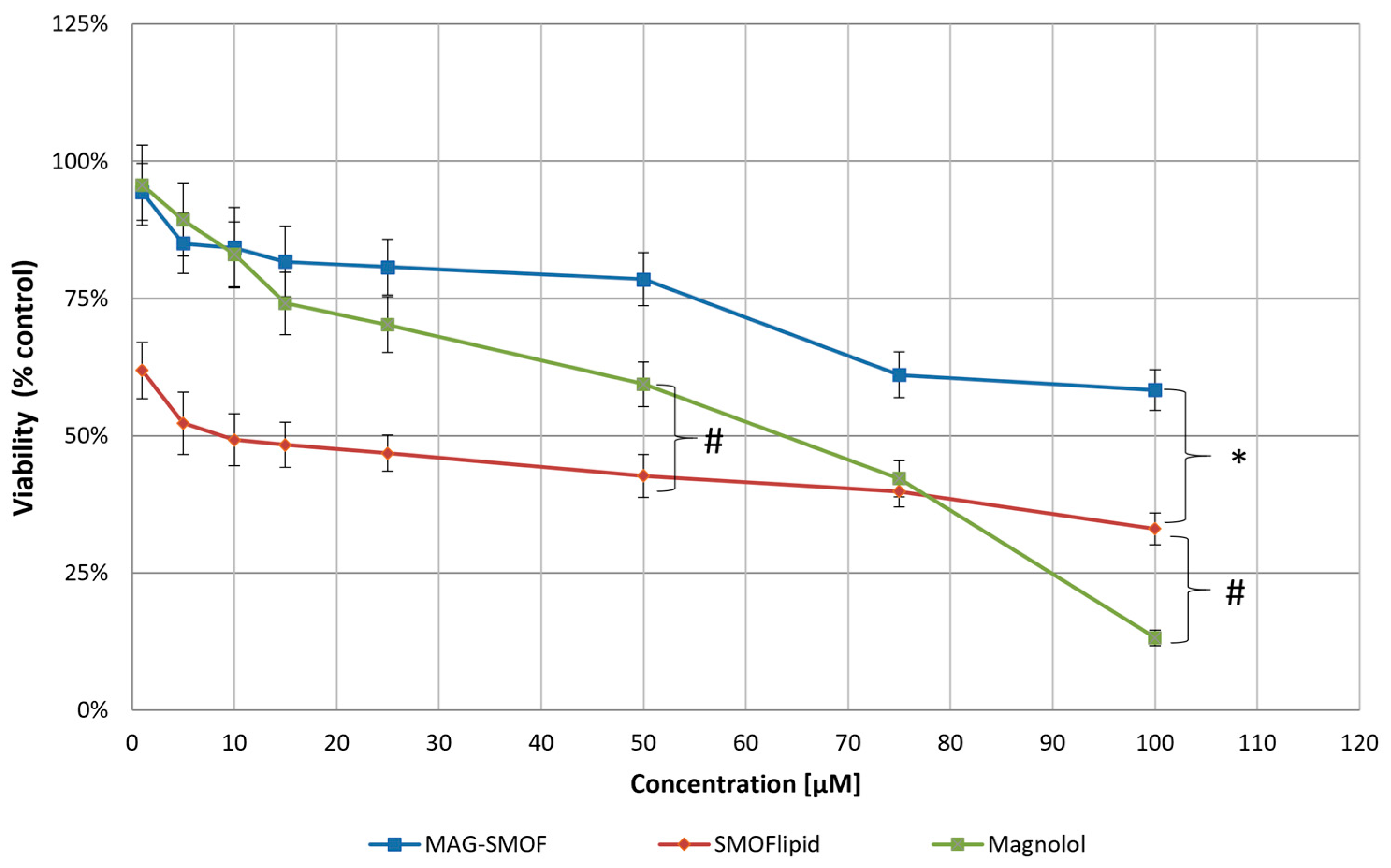
2. Results
3. Discussion
4. Limitations
5. Materials and Methods
5.1. Materials
5.2. Methods
5.2.1. Selection of PN Emulsions
5.2.2. Optimization of the Preparation Process of Magnolol-Loaded PN Emulsion
5.2.3. Determination of Physicochemical Parameters
Determination of pH
Determination of Osmolarity
Determination of Droplet Size
Evaluation of Zeta Potential
Determination of Magnolol Concentration in MAG-SMOF Using Spectrophotometry UV-Vis
Determination of Magnolol Concentration in MAG-SMOF Using HPLC-FLD Method
5.2.4. Calculation of Entrapment Efficiency and Drug Loading of Magnolol in MAG-SMOF
5.2.5. Mid-Term Stability Tests
5.2.6. Short-Term Stress Tests of Optimized MAG-SMOF Formulation
5.2.7. Injectability Test
5.2.8. In Vitro Cytotoxicity Studies
5.2.9. Hemolysis Assay
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, A.; Heyland, D.K.; Ortiz Reyes, L.A.; Laaf, E.; Wendt, S.; Elke, G.; Stoppe, C. Combination of enteral and parenteral nutrition in the acute phase of critical illness: An updated systematic review and meta-analysis. J. Parenter. Enteral Nutr. 2022, 46, 395–410. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. Edinb. Scotl. 2021, 40, 4745–4761. [Google Scholar] [CrossRef]
- Stawny, M.; Olijarczyk, R.; Jaroszkiewicz, E.; Jelińska, A. Pharmaceutical point of view on parenteral nutrition. Sci. World J. 2013, 2013, 415310. [Google Scholar] [CrossRef] [PubMed]
- Sasdelli, A.S.; Agostini, F.; Pazzeschi, C.; Guidetti, M.; Lal, S.; Pironi, L. Assessment of Intestinal Failure Associated Liver Disease according to different diagnostic criteria. Clin. Nutr. Edinb. Scotl. 2019, 38, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Lauriti, G.; Zani, A.; Aufieri, R.; Cananzi, M.; Chiesa, P.L.; Eaton, S.; Pierro, A. Incidence, prevention, and treatment of parenteral nutrition-associated cholestasis and intestinal failure-associated liver disease in infants and children: A systematic review. J. Parenter. Enteral Nutr. 2014, 38, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.D.; Henry, E.; Wiedmeier, S.E.; Burnett, J.; Lambert, D.K. Identifying patients, on the first day of life, at high-risk of developing parenteral nutrition-associated liver disease. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2007, 27, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Gunnar, R.; Mutanen, A.; Merras-Salmio, L.; Pakarinen, M.P. Histopathological liver steatosis linked with high parenteral glucose and amino acid supply in infants with short bowel syndrome. J. Parenter. Enteral Nutr. 2023, 47, 41–50. [Google Scholar] [CrossRef]
- Lim, J.; Oschman, A.; Curiel, K. Current status of lipid emulsions in the prevention of intestinal failure-associated liver disease. Curr. Opin. Organ Transplant. 2019, 24, 188–192. [Google Scholar] [CrossRef]
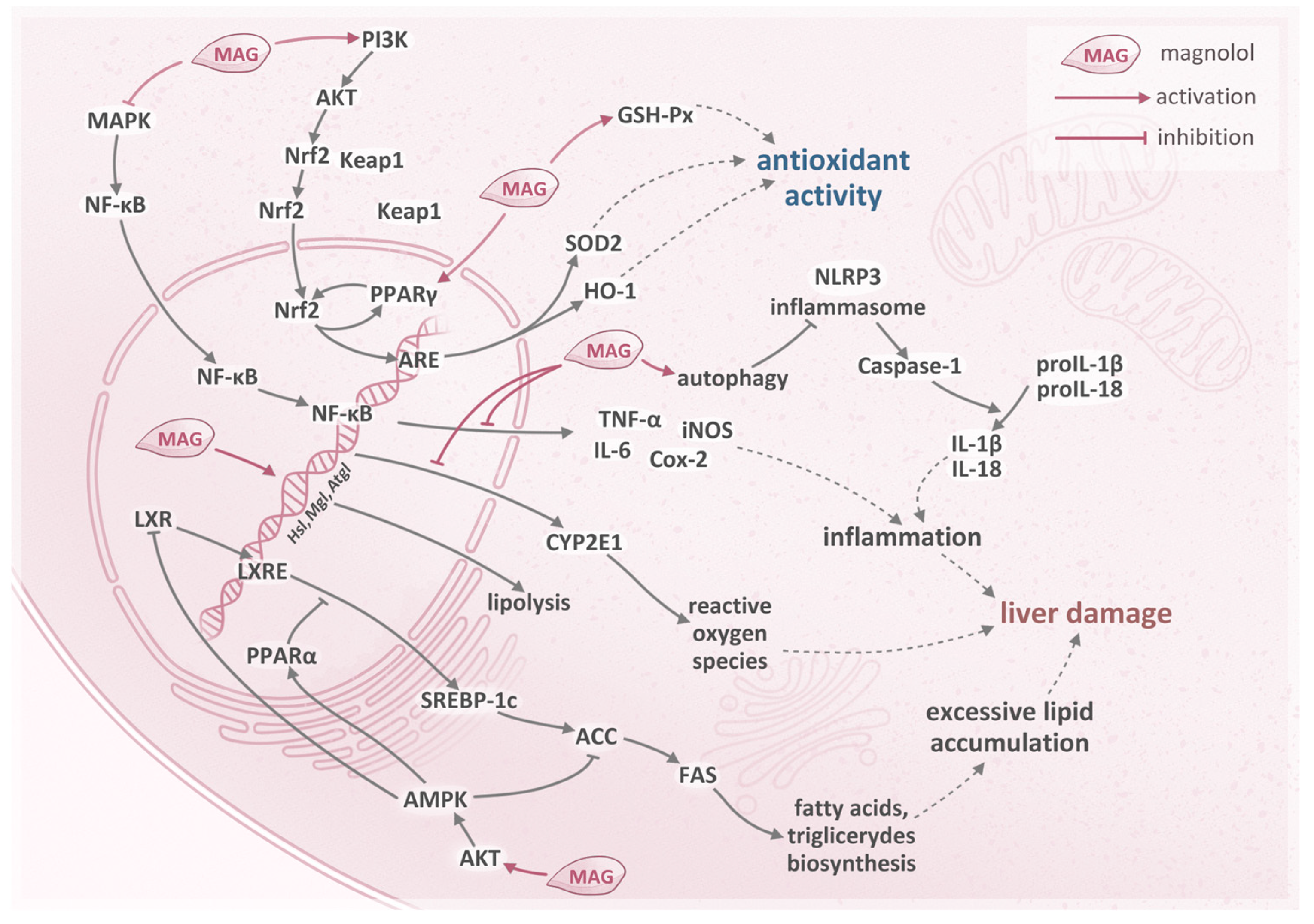
- Liu, X.; Wang, Y.; Wu, D.; Li, S.; Wang, C.; Han, Z.; Wang, J.; Wang, K.; Yang, Z.; Wei, Z. Magnolol Prevents Acute Alcoholic Liver Damage by Activating PI3K/Nrf2/PPARγ and Inhibiting NLRP3 Signaling Pathway. Front. Pharmacol. 2019, 10, 1459. [Google Scholar] [CrossRef]
- Kuo, N.-C.; Huang, S.-Y.; Yang, C.-Y.; Shen, H.-H.; Lee, Y.-M. Involvement of HO-1 and Autophagy in the Protective Effect of Magnolol in Hepatic Steatosis-Induced NLRP3 Inflammasome Activation In Vivo and In Vitro. Antioxidants 2020, 9, 924. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jung, J.Y.; Jang, E.J.; Jegal, K.H.; Moon, S.Y.; Ku, S.K.; Kang, S.H.; Cho, I.J.; Park, S.J.; Lee, J.R.; et al. Combination of honokiol and magnolol inhibits hepatic steatosis through AMPK-SREBP-1 c pathway. Exp. Biol. Med. 2015, 240, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Feng, H.; Han, L.; Wu, L.; Lv, H.; Shen, B.; Li, Z.; Zhang, Q.; Liu, G. Magnolol Alleviates Inflammatory Responses and Lipid Accumulation by AMP-Activated Protein Kinase-Dependent Peroxisome Proliferator-Activated Receptor α Activation. Front. Immunol. 2018, 9, 147. [Google Scholar] [CrossRef]
- Driscoll, D.F. Lipid injectable emulsions: Pharmacopeial and safety issues. Pharm. Res. 2006, 23, 1959–1969. [Google Scholar] [CrossRef]
- Gostyńska, A.; Czerniel, J.; Kuźmińska, J.; Brzozowski, J.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V.; Stawny, M. Honokiol-Loaded Nanoemulsion for Glioblastoma Treatment: Statistical Optimization, Physicochemical Characterization, and an In Vitro Toxicity Assay. Pharmaceutics 2023, 15, 448. [Google Scholar] [CrossRef] [PubMed]
- Weigt, H.U.; Georgieff, M.; Beyer, C.; Föhr, K.J. Activation of neuronal N-methyl-D-aspartate receptor channels by lipid emulsions. Anesth. Analg. 2002, 94, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G.B.; Svensson, L.; Kinnander, N.J.; Zander, M.; Bergström, U.K. Stability of vitamins in soybean oil fat emulsion under conditions simulating intravenous feeding of neonates and children. J. Parenter. Enteral Nutr. 1994, 18, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Hao, L.; Wray, A.E.; Ross, A.C. Lipid Emulsion Administered Intravenously or Orally Attenuates Triglyceride Accumulation and Expression of Inflammatory Markers in the Liver of Nonobese Mice Fed Parenteral Nutrition Formula123. J. Nutr. 2013, 143, 253–259. [Google Scholar] [CrossRef]
- Kazi, M.; Alanazi, Y.; Kumar, A.; Shahba, A.A.-W.; Rizwan Ahamad, S.; Alghamdi, K.M. Oral Bioactive Self-Nanoemulsifying Drug Delivery Systems of Remdesivir and Baricitinib: A Paradigmatic Case of Drug Repositioning for Cancer Management. Molecules 2023, 28, 2237. [Google Scholar] [CrossRef]
- Mundada, V.; Patel, M.; Sawant, K. Submicron Emulsions and Their Applications in Oral Delivery. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 265–308. [Google Scholar] [CrossRef]
- Meirinho, S.; Rodrigues, M.; Ferreira, C.L.; Oliveira, R.C.; Fortuna, A.; Santos, A.O.; Falcão, A.; Alves, G. Intranasal delivery of lipid-based nanosystems as a promising approach for brain targeting of the new-generation antiepileptic drug perampanel. Int. J. Pharm. 2022, 622, 121853. [Google Scholar] [CrossRef]
- Shi, Y.; Porter, W.; Merdan, T.; Li, L.C. Recent advances in intravenous delivery of poorly water-soluble compounds. Expert Opin. Drug Deliv. 2009, 6, 1261–1282. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, R.; Lv, H.-F.; Ying, M.-F.; Jiang, Z. Clinical effects of multi-oil versus pure soybean oil-based lipid emulsions for preterm infants: An observational study. Asia Pac. J. Clin. Nutr. 2023, 32, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kirk, C.; Haigh, L.; Thompson, N.P.; Pearce, M.; Jones, D.E.; Mathers, J.C. The effects of different parenteral nutrition lipid formulations on clinical and laboratory endpoints in patients receiving home parenteral nutrition: A systematic review. Clin. Nutr. Edinb. Scotl. 2022, 41, 80–90. [Google Scholar] [CrossRef]
- de Souza, A.; Yukuyama, M.N.; Barbosa, E.J.; Monteiro, L.M.; Faloppa, A.C.B.; Calixto, L.A.; de Barros Araújo, G.L.; Fotaki, N.; Löbenberg, R.; Bou-Chacra, N.A. A new medium-throughput screening design approach for the development of hydroxymethylnitrofurazone (NFOH) nanostructured lipid carrier for treating leishmaniasis. Colloids Surf. B Biointerfaces 2020, 193, 111097. [Google Scholar] [CrossRef]
- Nemichand, S.K.; Laxman, S.D. Solubility Enhancement of Nebivolol by Micro Emulsion Technique. J. Young Pharm. 2016, 8, 356–367. [Google Scholar] [CrossRef]
- Dubois, V.; Breton, S.; Linder, M.; Fanni, J.; Parmentier, M. Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur. J. Lipid Sci. Technol. 2007, 109, 710–732. [Google Scholar] [CrossRef]
- Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. Clinical Nutrition of Critically Ill Patients in the Context of the Latest ESPEN Guidelines. Med. Kaunas Lith. 2019, 55, 770. [Google Scholar] [CrossRef]
- Yu, L.J.; Anez-Bustillos, L.; Mitchell, P.D.; Ko, V.H.; Secor, J.D.; Hurley, A.P.; Dao, D.T.; Fligor, S.C.; Cho, B.S.; Tsikis, S.T.; et al. Incidence and development of cholestasis in surgical neonates receiving an intravenous mixed-oil lipid emulsion. J. Parenter. Enter. Nutr. 2023, 47, 30–40. [Google Scholar] [CrossRef]
- Daniel, S.; Svoboda, L.; Chen, J. Liver Function in Pediatric Recipients: A Comparison of Intralipid and Smoflipid. J. Pediatr. Pharmacol. Ther. 2021, 26, 258–264. [Google Scholar] [CrossRef]
- Usach, I.; Alaimo, A.; Fernández, J.; Ambrosini, A.; Mocini, S.; Ochiuz, L.; Peris, J.-E. Magnolol and Honokiol: Two Natural Compounds with Similar Chemical Structure but Different Physicochemical and Stability Properties. Pharmaceutics 2021, 13, 224. [Google Scholar] [CrossRef]
- Said Suliman, A.; Tom, R.; Palmer, K.; Tolaymat, I.; Younes, H.M.; Arafat, B.; Elhissi, A.M.A.; Najlah, M. Development, characterization and stability evaluation of ciprofloxacin-loaded parenteral nutrition nanoemulsions. Pharm. Dev. Technol. 2020, 25, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Haidar, I.; Harding, I.H.; Bowater, I.C.; Eldridge, D.S.; Charman, W.N. The role of lecithin degradation on the pH dependent stability of halofantrine encapsulated fat nano-emulsions. Int. J. Pharm. 2017, 528, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Schmidt, S.; Buttle, I.; Akkar, A.; Schmitt, J.; Brömer, S. SolEmuls®—Novel technology for the formulation of i.v. emulsions with poorly soluble drugs. Int. J. Pharm. 2004, 269, 293–302. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeial Convention. The United States Pharmacopeia and National Formulary, 38th ed.; United States Pharmacopeial Convention: Rockville, MD, USA, 2015. [Google Scholar]
- Steger, P.J.K.; Mühlebach, S.F. Lipid peroxidation of IV lipid emulsions in TPN bags: The influence of tocopherols. Nutrition 1998, 14, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.Y.; Benita, S. Design and characterization of a submicronized o/w emulsion of diazepam for parenteral use. Int. J. Pharm. 1989, 54, 103–112. [Google Scholar] [CrossRef]
- Amin, K.; Dannenfelser, R.-M. In vitro hemolysis: Guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef]
- Hoensch, H.P.; Oertel, R. The value of flavonoids for the human nutrition: Short review and perspectives. Clin. Nutr. Exp. 2015, 3, 8–14. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Dettlaff, K.; Jelińska, A.; Ogrodowczyk, M. Stability studies of parenteral nutrition with a high dose of vitamin C. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2020, 26, 1894–1902. [Google Scholar] [CrossRef]
- Stawny, M.; Gostyńska, A.; Olijarczyk, R.; Jelińska, A.; Ogrodowczyk, M. Stability of high-dose thiamine in parenteral nutrition for treatment of patients with Wernicke’s encephalopathy. Clin. Nutr. Edinb. Scotl. 2020, 39, 2929–2932. [Google Scholar] [CrossRef]
- Garib, R.; Garla, P.; Torrinhas, R.S.; Moretti, A.I.S.; Machado, M.C.C.; Waitzberg, D.L. Effect of Previous High Glutamine Infusion on Inflammatory Mediators and Mortality in an Acute Pancreatitis Model. Mediators Inflamm. 2016, 2016, 4261419. [Google Scholar] [CrossRef]
- Hippalgaonkar, K.; Majumdar, S.; Kansara, V. Injectable lipid emulsions-advancements, opportunities and challenges. AAPS PharmSciTech 2010, 11, 1526–1540. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowczyk, M.; Kaczmarek, A.; Gostyńska, A.; Jelińska, A. The role of pharmaceutical analysis in ensuring the safety of drug radiation sterilization. Acta Pol. Pharm.-Drug Res. 2023, 80, 219–233. [Google Scholar] [CrossRef]
- Gostyńska, A.; Starkowska, J.; Sobierajska, P.; Jelińska, A.; Stawny, M. All-in-One Pediatric Parenteral Nutrition Admixtures with an Extended Shelf Life-Insight in Correlations between Composition and Physicochemical Parameters. Pharmaceutics 2021, 13, 1017. [Google Scholar] [CrossRef] [PubMed]
- Czerniel, J.; Gostyńska, A.; Jańczak, J.; Stawny, M. A critical review of the novelties in the development of intravenous nanoemulsions. Eur. J. Pharm. Biopharm. 2023, 191, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Jumaa, M.; Müller, B.W. Lipid emulsions as a novel system to reduce the hemolytic activity of lytic agents: Mechanism of the protective effect. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2000, 9, 285–290. [Google Scholar] [CrossRef]
- Balasubramaniam, P.; Malathi, A. Comparative study of hemoglobin estimated by Drabkin’s and Sahli’s methods. J. Postgrad. Med. 1992, 38, 8–9. [Google Scholar]




| Independent Variables | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Shaking speed (rpm) (A) | 200 | 300 | 400 |
| Time of shaking (min) (B) | 15 | 105 | 195 |
| Magnolol concentration (mg/mL) (C) | 1 | 2 | 3 |
| Formulation Code | A Shaking Speed (rpm) | B Shaking Time (min) | C Magnolol Concentration (mg/mL) |
|---|---|---|---|
| F1 | 200 | 15 | 2 |
| F2 | 400 | 15 | 2 |
| F3 | 200 | 195 | 2 |
| F4 | 400 | 195 | 2 |
| F5 | 200 | 105 | 1 |
| F6 | 400 | 105 | 1 |
| F7 | 200 | 105 | 3 |
| F8 | 400 | 105 | 3 |
| F9 | 300 | 15 | 1 |
| F10 | 300 | 195 | 1 |
| F11 | 300 | 15 | 3 |
| F12 | 300 | 195 | 3 |
| F13 | 300 | 105 | 2 |
| F14 | 300 | 105 | 2 |
| F15 | 300 | 105 | 2 |
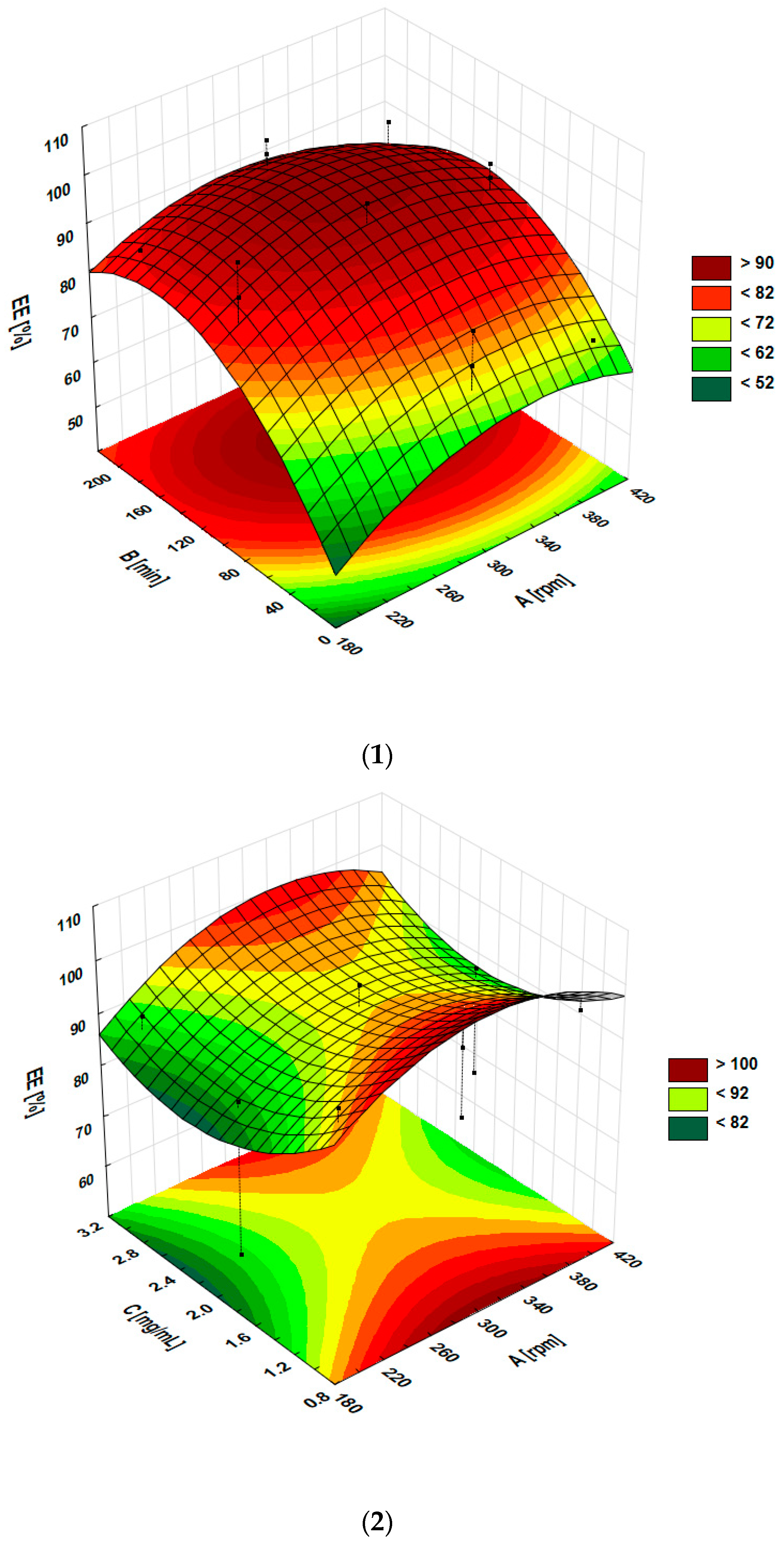
| Formulation Code | EE% ± SD (%) | DL% ± SD (%) |
|---|---|---|
| F1 | 56.39 ± 4.56 | 0.58 ± 0.04 |
| F2 | 70.97 ± 2.67 | 0.71 ± 0.02 |
| F3 | 85.84 ± 3.05 | 0.85 ± 0.02 |
| F4 | 91.24 ± 4.96 | 0.93 ± 0.04 |
| F5 | 97.14 ± 1.78 | 0.49 ± 0.01 |
| F6 | 94.97 ± 2.21 | 0.45 ± 0.01 |
| F7 | 89.88 ± 3.43 | 1.34 ± 0.04 |
| F8 | 91.89 ± 1.67 | 1.36 ± 0.02 |
| F9 | 85.04 ± 6.75 | 0.44 ± 0.03 |
| F10 | 97.93 ± 6.80 | 0.47 ± 0.03 |
| F11 | 77.78 ± 1.66 | 1.19 ± 0.02 |
| F12 | 94.98 ± 1.33 | 1.42 ± 0.02 |
| F13 | 97.77 ± 2.21 | 0.98 ± 0.02 |
| F14 | 90.78 ± 3.34 | 0.89 ± 0.03 |
| F15 | 92.14 ± 2.47 | 0.95 ± 0.02 |
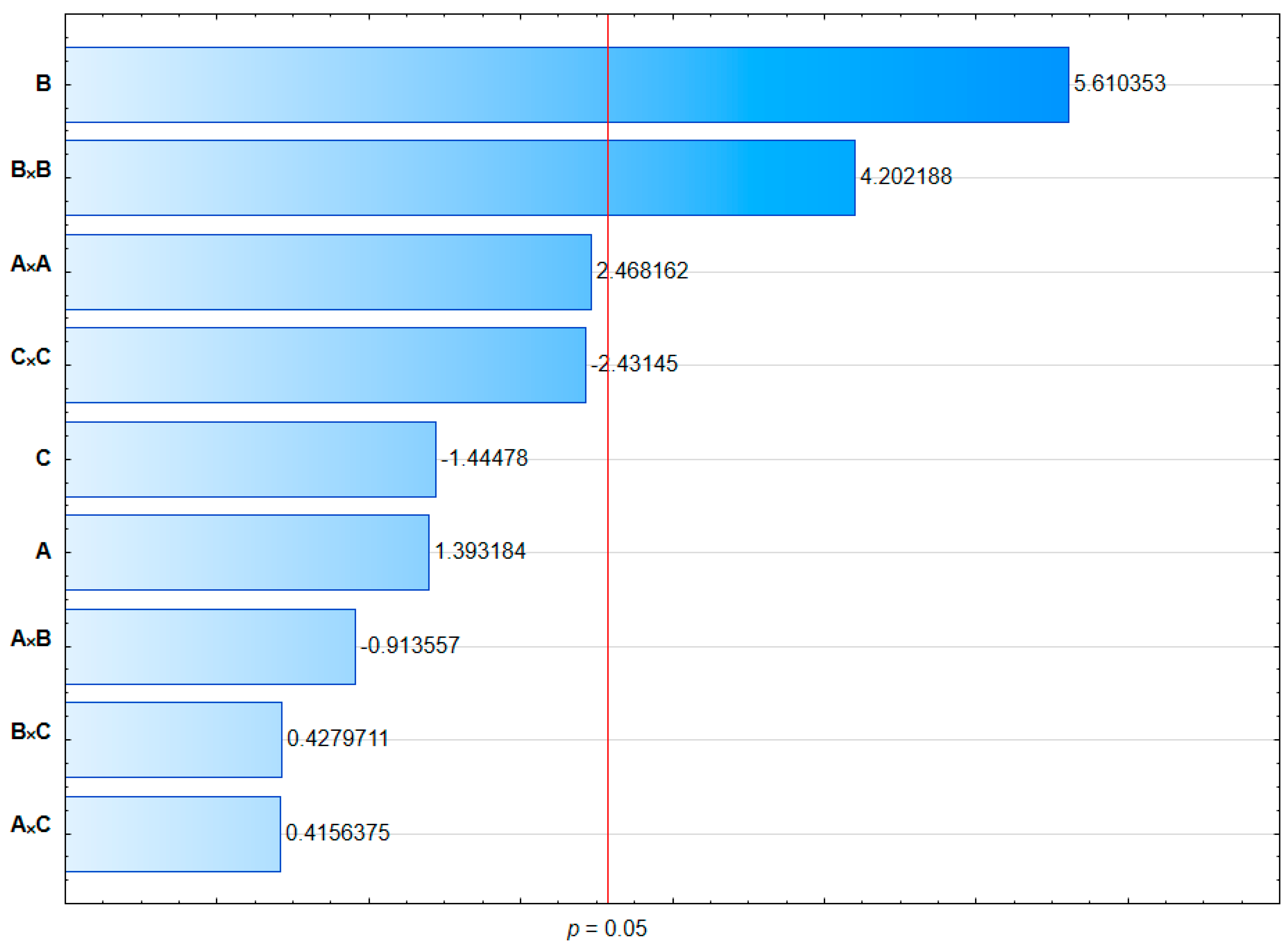
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | p-Values |
|---|---|---|---|---|---|---|
| Model | 1707.83 | 9 | 189.76 | 7.51 | 0.0195 | <0.05 |
| A | 49.10 | 1 | 49.10 | 1.94 | 0.2221 | >0.05 |
| B | 796.20 | 1 | 796.20 | 31.51 | 0.0025 | <0.05 |
| C | 52.79 | 1 | 52.79 | 2.09 | 0.2080 | >0.05 |
| A × B | 21.07 | 1 | 21.07 | 0.8338 | 0.4031 | >0.05 |
| A × C | 4.37 | 1 | 4.37 | 0.1729 | 0.6948 | >0.05 |
| B × C | 4.64 | 1 | 4.64 | 0.1838 | 0.6860 | >0.05 |
| A × A | 153.99 | 1 | 153.99 | 6.09 | 0.0566 | >0.05 |
| B × B | 446.40 | 1 | 446.40 | 17.67 | 0.0085 | <0.05 |
| C × C | 149.57 | 1 | 149.57 | 5.92 | 0.0592 | >0.05 |
| Residual | 126.34 | 5 | 25.27 | |||
| Lack of fit | 98.87 | 3 | 32.96 | 2.40 | 0.3077 | >0.05 |
| Pure error | 27.47 | 2 | 13.73 | |||
| Cor. total | 1834.17 | 14 | ||||
| Regression equation | EE% = 32.75214 + 0.418125 A + 0.448469 B − 32.41917 C − 0.000255 A × B + 0.010450 A × C + 0.011972 B × C − 0.000646 A × A − 0.001357 B × B + 6.36458 C × C | |||||
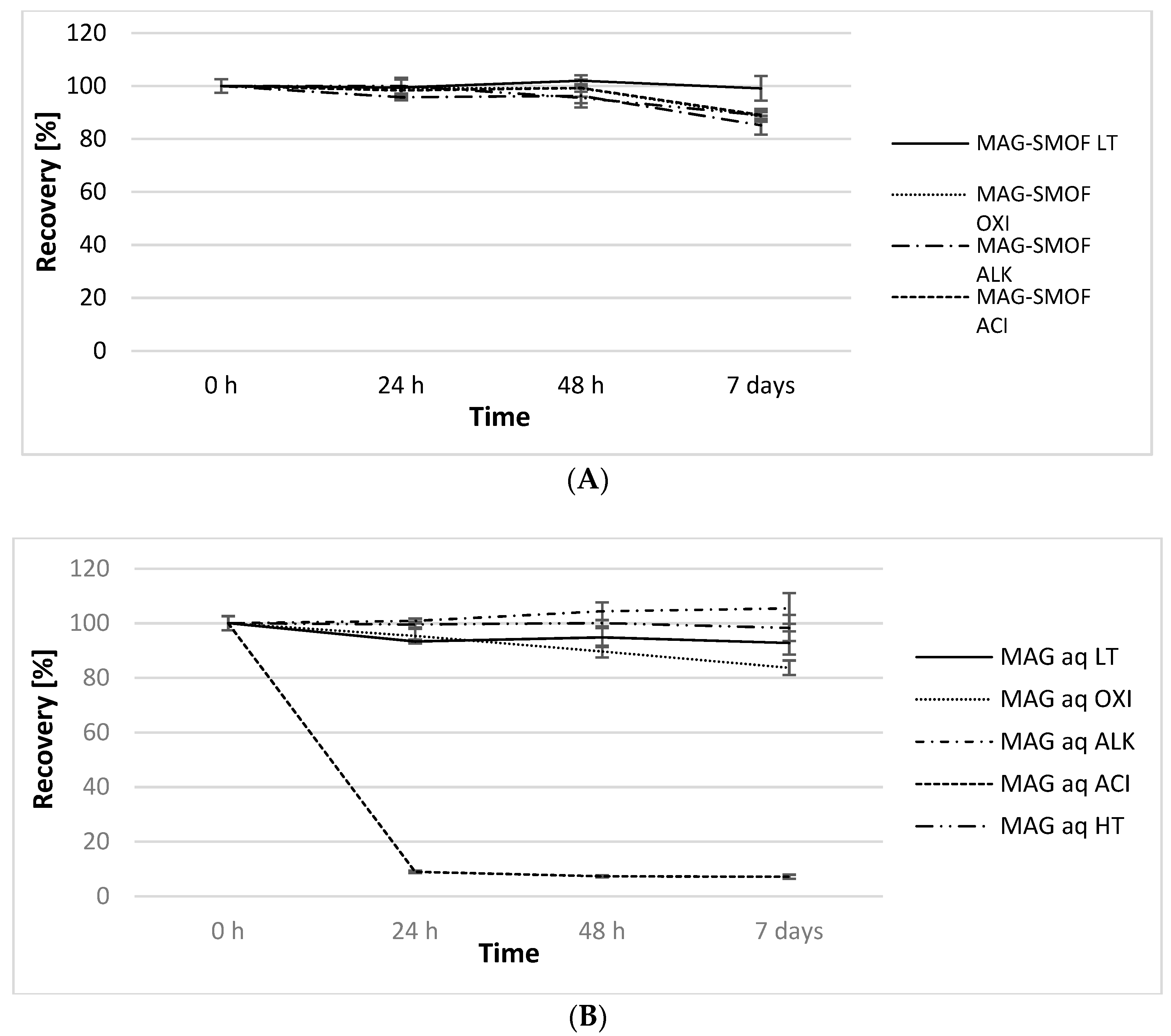
| Sample | Conditions | pH (t = 0 h → t = 7 day) | OSM (mOsm/kg) (t = 0 h → t = 7 day) | ZP (mV) (t = 0 h → t = 7 day) |
|---|---|---|---|---|
| MAG-SMOF LT | 4 ± 2 °C | 7.19 → 7.18 | 401 → 394 | −29.0 → −28.6 |
| MAG-SMOF HT | 80 ± 1 °C | 7.17 → 3.56 | 394 → 411 | −30.6 → −34.3 |
| MAG-SMOF OXI | 30% H2O2 | 4.50 → 4.64 | - | −26.7 → −26.5 |
| MAG-SMOF ALK | 0.5 M NaOH | 12.66 → 12.64 | 645 → 632 | −69.3 → −59.3 |
| MAG-SMOF ACI | 0.5 M HCl | 0.92 → 1.00 | 756 → 767 | −8.4 → −18.4 |
| Sample Name | Infusion Rate (mL/h) | Needle Size (G) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 27 | 26 | 25 | 23 | 22 | 21 | 20 | 18 | ||
| MAG-SMOF | 25 | - | + | + | + | + | + | + | + |
| 50 | - | - | - | - | + | + | + | + | |
| 75 | - | - | - | - | + | + | + | + | |
| 100 | - | - | - | - | - | + | + | + | |
| 200 | - | - | - | - | - | + | + | + | |
| 800 | - | - | - | - | - | + | + | + | |
| SMOF | 25 | - | + | + | + | + | + | + | + |
| 50 | - | - | - | - | + | + | + | + | |
| 75 | - | - | - | - | + | + | + | + | |
| 100 | - | - | - | - | - | + | + | + | |
| 200 | - | - | - | - | - | + | + | + | |
| 800 | - | - | - | - | - | + | + | + | |
| Water for injection | 25 | + | + | + | + | + | + | + | + |
| 50 | + | + | + | + | + | + | + | + | |
| 75 | + | + | + | + | + | + | + | + | |
| 100 | + | + | + | + | + | + | + | + | |
| 200 | - | - | + | + | + | + | + | + | |
| 800 | - | - | - | + | + | + | + | + | |
| Product Name | Soybean Oil | MCT | Olive Oil | Fish Oil |
|---|---|---|---|---|
| Intralipid | 100% | - | - | - |
| Lipofundin 20% MCT/LCT | 50% | 50% | - | - |
| Lipidem | 40% | 50% | - | 10% |
| SMOFlipid | 30% | 30% | 25% | 15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostyńska, A.; Czerniel, J.; Kuźmińska, J.; Żółnowska, I.; Brzozowski, J.; Krajka-Kuźniak, V.; Stawny, M. The Development of Magnolol-Loaded Intravenous Emulsion with Low Hepatotoxic Potential. Pharmaceuticals 2023, 16, 1262. https://doi.org/10.3390/ph16091262
Gostyńska A, Czerniel J, Kuźmińska J, Żółnowska I, Brzozowski J, Krajka-Kuźniak V, Stawny M. The Development of Magnolol-Loaded Intravenous Emulsion with Low Hepatotoxic Potential. Pharmaceuticals. 2023; 16(9):1262. https://doi.org/10.3390/ph16091262
Chicago/Turabian StyleGostyńska, Aleksandra, Joanna Czerniel, Joanna Kuźmińska, Izabela Żółnowska, Jakub Brzozowski, Violetta Krajka-Kuźniak, and Maciej Stawny. 2023. "The Development of Magnolol-Loaded Intravenous Emulsion with Low Hepatotoxic Potential" Pharmaceuticals 16, no. 9: 1262. https://doi.org/10.3390/ph16091262
APA StyleGostyńska, A., Czerniel, J., Kuźmińska, J., Żółnowska, I., Brzozowski, J., Krajka-Kuźniak, V., & Stawny, M. (2023). The Development of Magnolol-Loaded Intravenous Emulsion with Low Hepatotoxic Potential. Pharmaceuticals, 16(9), 1262. https://doi.org/10.3390/ph16091262








