Selenium in Prostate Cancer: Prevention, Progression, and Treatment
Abstract
1. Introduction
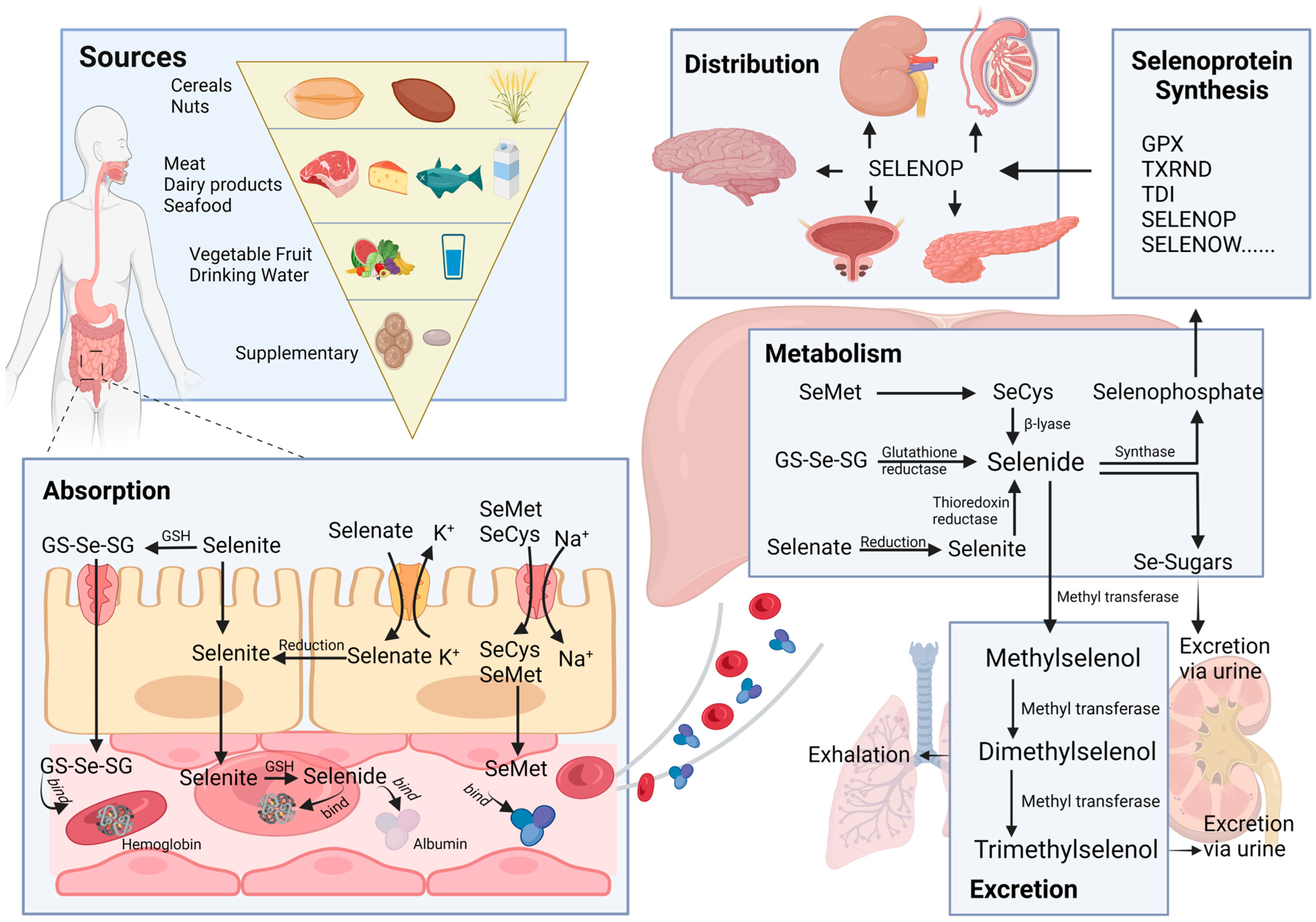
2. Absorption, Distribution, Metabolism, and Excretion (ADME) of Selenium
2.1. Sources
2.2. Absorption
2.3. Distribution
2.4. Metabolism
2.5. Excretion
3. Evidence from Epidemiological Studies
3.1. Observational Studies of Selenium and Prostate Cancer
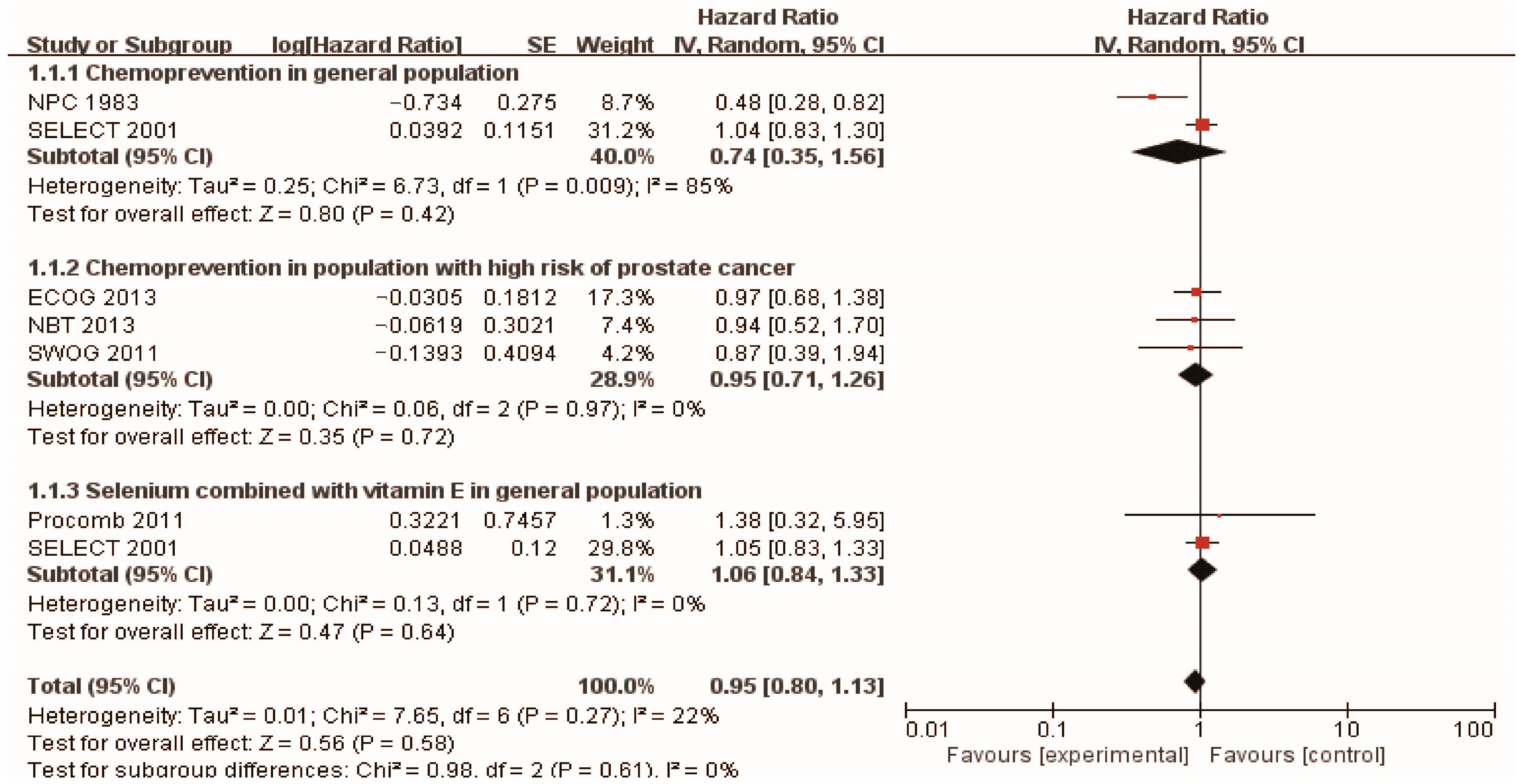
3.2. Chemoprevention of Selenium and Prostate Cancer
| Study | Type of Study | Initiation Year | Participants | Forms of Selenium Supplementation | Follow-Up | Preventive Effects for Prostate Cancer | HR/RR (95%CI) | References |
|---|---|---|---|---|---|---|---|---|
| Selenium supplementation in general population | ||||||||
| NPC | RCT | 1983 | 927 men (457 selenium/470 placebo) | 200 mg/day high selenium yeast | 13 years | Positive | HR 0.48 (0.28, 0.80) | [43] |
| SELECT | RCT | 2001 | 848 men (432 selenium/416 placebo) | 200 µg/day selenized yeast/L-selenomethionine | Median follow-up 5.46 years | NS | HR 1.04 (0.83, 1.30) | [44] |
| Selenium supplementation in population with high risk of prostate cancer | ||||||||
| SWOG | RCT | 2011 | 423 men with HGPIN (212 selenium and 211 placebo) | 200 µg/d selenomethionine | 3 years | NS | RR 0.97 (0.68–1.39) | [45] |
| NBT | RCT | 2013 | 699 men with high risk of prostate cancer (467 selenium and 232 placebo) | 200 µg/day selenium (N =234), or 400 µg/day selenium (N = 233) | 5 years | NS | HR (selenium 200 µg/day) 0.94 (0.52, 1.7) HR (selenium 400 µg/day) 0.90 (0.48, 1.7) | [46] |
| ECOG 5597 | RCT | 2013 | 1561 men with a history of non–small-cell lung cancer (1040 selenium/521 placebo) | selenized yeast 200 µg/day | 4 years | NS | RR 0.87 (0.39–1.95) | [47] |
| Selenium supplementation combined with vitamin E in general population | ||||||||
| SELECT | RCT | 2001 | 853 men (437 selenium + vitamin E/ 416 placebo) | 200 mg/d L-selenomethionine and 400 IU/d vitamin E | Median follow-up 5.46 years | NS | HR 1.05 (0.83, 1.31) | [44] |
| Procomb | RCT | 2011 | 209 men with lower urinary tract symptoms (134 Lycopene + selenium and 75 placebo) | 50 mg/d selenium and 5 mg/d lycopene | 2 years | NS | HR 1.38 (0.32–5.90) | [52] |
4. Selenium and Prostate Cancer Therapy
5. Selenium-Related Protein in Prostate Cancer
5.1. Selenium-Containing Proteins
5.1.1. Classification of Selenium-Containing Proteins
| Selenoproteins | Functions | References |
|---|---|---|
| GPX1 | Metabolize hydrogen peroxide and some organic hydroperoxides | [74] |
| GPX2 | Antioxidant activity in gastroin testinal tissues | [74] |
| GPX3 | Reduce H2O2, fatty acid hydroperoxides, and phospholipid hydroperoxides in the plasma and thyrocytes | [74] |
| GPX4 | Reduce phospholipid- and cholesterol-hydroperoxides by using GSH | [74] |
| GPX6 | Reduce olfactory organs H2O2 | [74] |
| TXNRD1 | Antioxidant activity and regenerate reduction of thioredoxin | [68] |
| TXNRD2 | Regenerates reduced thioredoxin in mitochondria | [68] |
| TXNRD3 | Redox regulation | [68] |
| DIO1 | Production of T3 in thyroid and peripheral tissues | [69] |
| DIO2 | Production of T3 in peripheral tissues | [69] |
| DIO3 | Inactivates thyroid hormone | [69] |
| SELENOK | Antioxidant activity | [75] |
| SELENOR | Antioxidant activity and protein repair | [76] |
| SELENOW | Antioxidant role | [77] |
| SELENOP | Secreted into plasma for selenium transport to tissues | [70] |
| SELENOF | Correcting misglycosylated/misfolded glycoproteins | [71] |
| SELENON | Its mutation leads to early-onset myopathies | [78] |
| SELENOM | Antioxidant activity | [71] |
| SELENOS | Deletes the misfolded proteins in endoplasmic reticulum and responds to endoplasmic reticulum stress | [79] |
| SELENOI | Involved in phospholipid biosynthesis | [80] |
| SELENOT | Oxidoreductase involved in redox regulation and cell anchorage | [81] |
| SELENOO | Unknown | [65] |
| SELENOV | Unknown | [65] |
| SELENOH | Redox sensing and transcriptional regulation of glutathione | [82] |
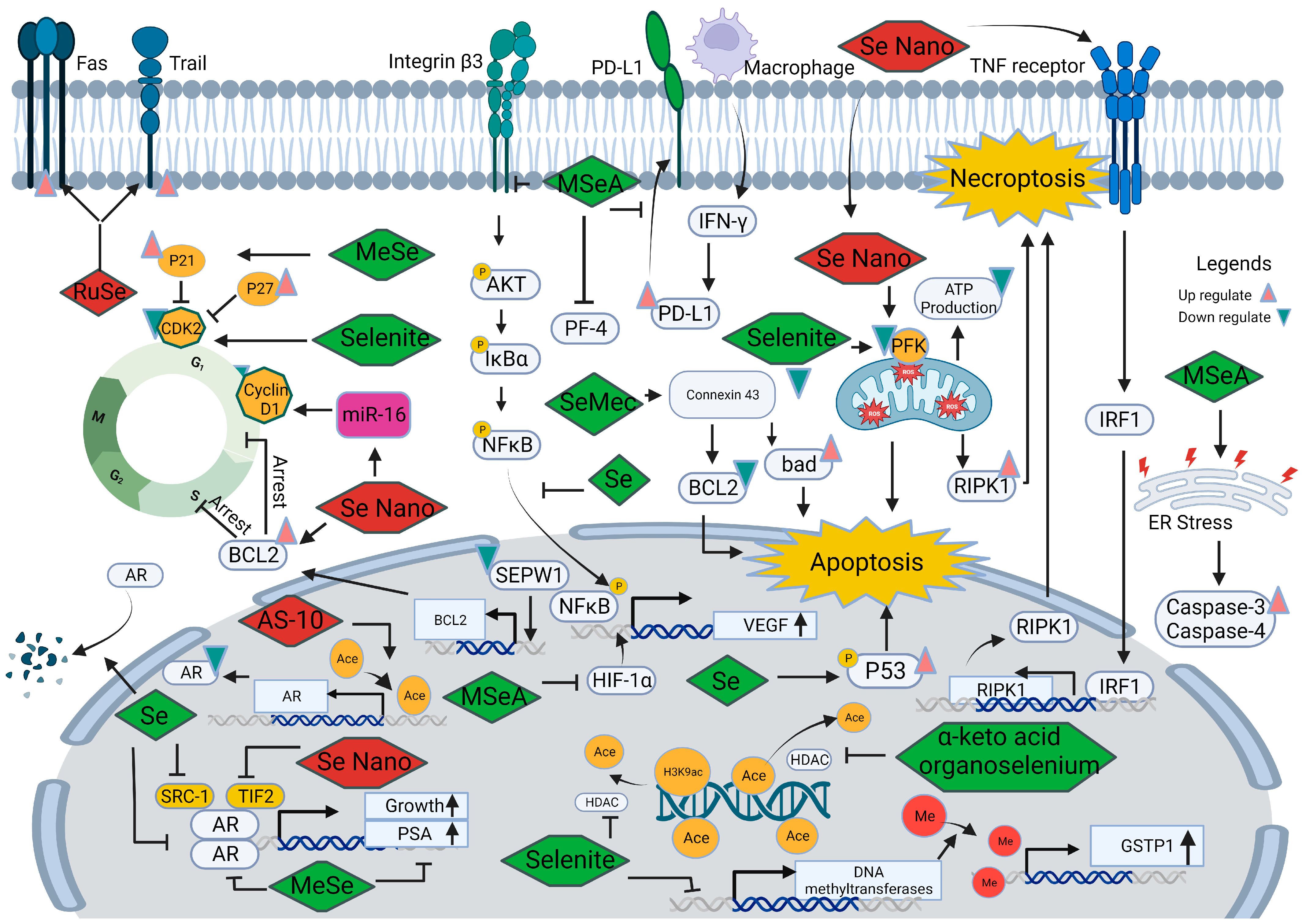
5.1.2. Anti-Tumor Role of Selenium-Containing Proteins
5.2. Selenium in the Development and Progression of Prostate Cancer
5.2.1. Selenium and Androgen Receptor
5.2.2. Selenium and Cell Cycle
5.2.3. Selenium and Angiogenesis
5.2.4. Selenium and Cell Death
5.2.5. Selenium and Epigenetic Modifications
5.2.6. Other Mechanisms
6. Perspectives and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, K.B.; Chan, J.M.; Ryan, C.J.; Kenfield, S.A. Diet and lifestyle considerations for patients with prostate cancer. Urol. Oncol. 2020, 38, 105–117. [Google Scholar]
- Cano-Ibáñez, N.; Barrios-Rodríguez, R.; Lozano-Lorca, M.; Vázquez-Alonso, F.; Arrabal-Martín, M.; Triviño-Juárez, J.M.; Salcedo-Bellido, I.; Jiménez-Moleón, J.J.; Olmedo-Requena, R. Dietary Diversity and Prostate Cancer in a Spanish Adult Population: CAPLIFE Study. Nutrients 2020, 12, 1694. [Google Scholar]
- Ornish, D.; Weidner, G.; Fair, W.R.; Marlin, R.; Pettengill, E.B.; Raisin, C.J.; Dunn-Emke, S.; Crutchfield, L.; Jacobs, F.N.; Barnard, R.J.; et al. Intensive lifestyle changes may affect the progression of prostate cancer. J. Urol. 2005, 174, 1065–1069; discussion 1069–1070. [Google Scholar] [CrossRef]
- Hamilton, R.J. 5-Alpha Reductase Inhibitor Use and Prostate Cancer Prevention: A Victim of the Times. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1259–1260. [Google Scholar] [CrossRef]
- Kumar, N.B.; Hogue, S.; Pow-Sang, J.; Poch, M.; Manley, B.J.; Li, R.; Dhillon, J.; Yu, A.; Byrd, D.A. Effects of Green Tea Catechins on Prostate Cancer Chemoprevention: The Role of the Gut Microbiome. Cancers 2022, 14, 3988. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.; Sultana, H.; Sultana, T.; Al Mamun, A.; Amin, M.N.; Hossain, A.; Ali, C.; Aktar, S.; Sultana, A.; Rahim, Z.B.; et al. Chemotherapeutic Activities of Dietary Phytoestrogens against Prostate Cancer: From Observational to Clinical Studies. Curr. Pharm. Des. 2022, 28, 1561–1580. [Google Scholar] [PubMed]
- Konecki, T.; Juszczak, A.; Cichocki, M. Can Diet Prevent Urological Cancers? An Update on Carotenoids as Chemopreventive Agents. Nutrients 2022, 14, 1367. [Google Scholar] [CrossRef]
- Stanisławska, I.J.; Figat, R.; Kiss, A.K.; Bobrowska-Korczak, B. Essential Elements and Isoflavonoids in the Prevention of Prostate Cancer. Nutrients 2022, 14, 1225. [Google Scholar] [CrossRef]
- Özten, N.; Schlicht, M.; Diamond, A.M.; Bosland, M.C. L-selenomethionine does not protect against testosterone plus 17β-estradiol-induced oxidative stress and preneoplastic lesions in the prostate of NBL rats. Nutr. Cancer 2014, 66, 825–834. [Google Scholar] [CrossRef][Green Version]
- Daragó, A.; Klimczak, M.; Stragierowicz, J.; Stasikowska-Kanicka, O.; Kilanowicz, A. The Effect of Zinc, Selenium, and Their Combined Supplementation on Androgen Receptor Protein Expression in the Prostate Lobes and Serum Steroid Hormone Concentrations of Wistar Rats. Nutrients 2020, 12, 153. [Google Scholar]
- Jiang, C.; Wang, Z.; Ganther, H.; Lu, J. Caspases as key regulators of methyl selenium-induced apoptosis (anoikis) of DU145 prostate cancer cells. Cancer Res. 2000, 61, 3062–3070. [Google Scholar]
- An, Y.; Zhao, J. Functionalized Selenium Nanotherapeutics Synergizes with Zoledronic Acid to Suppress Prostate Cancer Cell Growth Through Induction of Mitochondria-Mediated Apoptosis and Cell Cycle S Phase Arrest. Front. Oncol. 2021, 11, 685784. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, Q.; Zhang, C.; Wang, P.; Xu, X.; Ran, L.; Zhang, L.; Tian, G.; Zhang, G. Amorphous ferric oxide-coating selenium core-shell nanoparticles: A self-preservation Pt(IV) platform for multi-modal cancer therapies through hydrogen peroxide depletion-mediated anti-angiogenesis, apoptosis and ferroptosis. Nanoscale 2022, 14, 11600–11611. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Yang, M.; Chan, J.; Sun, T.; Mucci, L.A.; Penney, K.L.; Lee, G.S.; Kantoff, P.W. Association of genetic variations of selenoprotein genes, plasma selenium levels, and prostate cancer aggressiveness at diagnosis. Prostate 2016, 76, 691–699. [Google Scholar]
- Chan, J.M.; Darke, A.K.; Penney, K.L.; Tangen, C.M.; Goodman, P.J.; Lee, G.M.; Sun, T.; Peisch, S.; Tinianow, A.M.; Rae, J.M.; et al. Selenium- or Vitamin E-Related Gene Variants, Interaction with Supplementation, and Risk of High-Grade Prostate Cancer in SELECT. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1050–1058. [Google Scholar]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid Redox Signal 2011, 14, 1337–1383. [Google Scholar]
- Wolf, W.R.; Goldschmidt, R.J. Updated estimates of the selenomethionine content of NIST wheat reference materials by GC–IDMS. Anal. Bioanal. Chem. 2006, 387, 2449–2452. [Google Scholar]
- Kieliszek, M. Selenium-Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef]
- Wolffram, S. Mechanisms of intestinal absorption of selenium. Med. Klin. 1995, 90 (Suppl. 1), 1–5. [Google Scholar]
- Ha, H.Y.; Alfulaij, N.; Berry, M.J.; Seale, L.A. From selenium absorption to selenoprotein degradation. Biol. Trace Element Res. 2019, 192, 26–37. [Google Scholar]
- Kato, T.; Read, R.; Rozga, J.; Burk, R.F. Evidence for intestinal release of absorbed selenium in a form with high hepatic extraction. Am. J. Physiol. 1992, 262, G854–G858. [Google Scholar] [CrossRef]
- Gammelgaard, B.; Rasmussen, L.H.; Gabel-Jensen, C.; Steffansen, B. Estimating intestinal absorption of inorganic and organic selenium compounds by in vitro flux and biotransformation studies in Caco-2 cells and ICP-MS detection. Biol. Trace Elem. Res. 2012, 145, 248–256. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Ogra, Y.; Ishiwata, K.; Takayama, H.; Aimi, N.; Suzuki, K.T. Selenosugars are key and urinary metabolites for selenium excretion within the required to low-toxic range. Proc. Natl. Acad. Sci. USA 2002, 99, 15932–15936. [Google Scholar] [CrossRef]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484S–1491S. [Google Scholar] [PubMed]
- Olson, G.E.; Winfrey, V.P.; NagDas, S.K.; Hill, K.E.; Burk, R.F. Apolipoprotein E receptor-2 (ApoER2) mediates selenium uptake from selenoprotein P by the mouse testis. J. Biol. Chem. 2007, 282, 12290–12297. [Google Scholar] [CrossRef]
- Burk, R.F.; Hill, K.E.; Olson, G.E.; Weeber, E.J.; Motley, A.K.; Winfrey, V.P.; Austin, L.M. Deletion of apolipoprotein E receptor-2 in mice lowers brain selenium and causes severe neurological dysfunction and death when a low-selenium diet is fed. J. Neurosci. 2007, 27, 6207–6211. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.E.; Winfrey, V.P.; Hill, K.E.; Burk, R.F. Megalin mediates selenoprotein P uptake by kidney proximal tubule epithelial cells. J. Biol. Chem. 2008, 283, 6854–6860. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, M.; Szczyglowska, M.; Konieczka, P.; Namiesnik, J. Methods of Selenium Supplementation: Bioavailability and Determination of Selenium Compounds. Crit. Rev. Food Sci. Nutr. 2016, 56, 36–55. [Google Scholar]
- McConnell, K.P.; Portman, O.W. Excretion of dimethyl selenide by the rat. J. Biol. Chem. 1952, 195, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Robberecht, H.; Van den Berghe, D. Elimination of selenium compounds by mice through formation of different volatile selenides. Experientia 1983, 39, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Gammelgaard, B.; Stürup, S.; Christensen, M.V. Human urinary excretion metabolism of (82)Se-enriched selenite selenate determined by, LC-ICP-MS. Metallomics 2012, 4, 149–155. [Google Scholar] [CrossRef]
- Moncayo, R.; Kroiss, A.; Oberwinkler, M.; Karakolcu, F.; Starzinger, M.; Kapelari, K.; Talasz, H.; Moncayo, H. The role of selenium, vitamin C, and zinc in benign thyroid diseases and of selenium in malignant thyroid diseases: Low selenium levels are found in subacute and silent thyroiditis and in papillary and follicular carcinoma. BMC Endocr. Disord. 2008, 8, 2. [Google Scholar]
- Kluza, M.; Paszek, S.; Kluza, K.; Januszek, S.; Potocka, N.; Skrzypa, M.; Zuchowska, A.; Wróbel, A.; Baszuk, P.; Marciniak, W.; et al. An Assessment of Serum Selenium Concentration in Women with Ovarian Cancer. Nutrients 2023, 15, 850. [Google Scholar]
- Demircan, K.; Bengtsson, Y.; Sun, Q.; Brange, A.; Vallon-Christersson, J.; Rijntjes, E.; Malmberg, M.; Saal, L.H.; Rydén, L.; Borg, Å.; et al. Serum selenium, selenoprotein P and glutathione peroxidase 3 as predictors of mortality and recurrence following breast cancer diagnosis: A multicentre cohort study. Redox Biol. 2021, 47, 102145. [Google Scholar] [PubMed]
- Takata, Y.; Xiang, Y.B.; Burk, R.F.; Li, H.; Hill, K.E.; Cai, H.; Gao, J.; Zheng, W.; Shu, X.O.; Cai, Q. Plasma selenoprotein P concentration and lung cancer risk: Results from a case-control study nested within the Shanghai Men’s Health Study. Carcinogenesis 2018, 39, 1352–1358. [Google Scholar] [CrossRef]
- Cai, X.; Wang, C.; Yu, W.; Fan, W.; Wang, S.; Shen, N.; Wu, P.; Li, X.; Wang, F. Selenium Exposure and Cancer Risk: An Updated Meta-analysis and Meta-regression. Sci. Rep. 2016, 6, 19213. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Deo, P.; Fenech, M. Plasma Micronutrient Profile of Prostate Cancer Cases Is Altered Relative to Healthy Controls-Results of a Pilot Study in South Australia. Cancers 2022, 15, 77. [Google Scholar] [CrossRef]
- Outzen, M.; Tjønneland, A.; Larsen, E.H.; Friis, S.; Larsen, S.B.; Christensen, J.; Overvad, K.; Olsen, A. Selenium status and risk of prostate cancer in a Danish population. Br. J. Nutr. 2016, 115, 1669–1677. [Google Scholar] [CrossRef]
- Gerstenberger, J.P.; Bauer, S.R.; Van Blarigan, E.L.; Sosa, E.; Song, X.; Witte, J.S.; Carroll, P.R.; Chan, J.M. Selenoprotein and antioxidant genes and the risk of high-grade prostate cancer and prostate cancer recurrence. Prostate 2015, 75, 60–69. [Google Scholar] [CrossRef]
- Duffield-Lillico, A.J.; Dalkin, B.L.; Reid, M.E.; Turnbull, B.W.; Slate, E.H.; Jacobs, E.T.; Marshall, J.R.; Clark, L.C. Nutritional Prevention of Cancer Study Group Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: An analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003, 91, 608–612. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Marshall, J.R.; Tangen, C.M.; Sakr, W.A.; Wood, D.P., Jr.; Berry, D.L.; Klein, E.A.; Lippman, S.M.; Parnes, H.L.; Alberts, D.S.; Jarrard, D.F.; et al. Phase III trial of selenium to prevent prostate cancer in men with high-grade prostatic intraepithelial neoplasia: SWOG S9917. Cancer Prev. Res. 2011, 4, 1761–1769. [Google Scholar] [CrossRef]
- Algotar, A.M.; Stratton, M.S.; Ahmann, F.R.; Ranger-Moore, J.; Nagle, R.B.; Thomp son, P.A.; Slate, E.; Hsu, C.H.; Dalkin, B.L.; Sindhwani, P.; et al. Phase 3 clinical trial investigating the effect of selenium supplementation in men at high-risk for prostate cancer. Prostate 2013, 73, 328–335. [Google Scholar] [CrossRef]
- Karp, D.D.; Lee, S.J.; Keller, S.M.; Wright, G.S.; Aisner, S.; Belinsky, S.A.; Johnson, D.H.; Johnston, M.R.; Goodman, G.; Clamon, G.; et al. Randomized, double-blind, placebo-controlled, phase III chemoprevention trial of selenium supplementation in patients with resected stage I non-small-cell lung cancer: ECOG 5597. J. Clin. Oncol. 2013, 31, 4179–4187. [Google Scholar] [CrossRef]
- Adamczyk, P.; Wolski, Z.; Butkiewicz, R.; Nussbeutel, J.; Drewa, T. Significance of atypical small acinar proliferation and extensive high-grade prostatic intraepithelial neoplasm in clinical practice. Cent. Eur. J. Urol. 2014, 67, 136–141. [Google Scholar] [CrossRef]
- Cui, K.; Li, X.; Du, Y.; Tang, X.; Arai, S.; Geng, Y.; Xi, Y.; Xu, H.; Zhou, Y.; Ma, W.; et al. Chemoprevention of prostate cancer in men with high-grade prostatic intraepithelial neoplasia (HGPIN): A systematic review and adjusted indirect treatment comparison. Oncotarget 2017, 8, 36674–36684. [Google Scholar] [CrossRef]
- Horvath, P.M.; Ip, C. Synergistic effect of vitamin E and selenium in the chemoprevention of mammary carcinogenesis in rats. Cancer Res. 1983, 43, 5335–5341. [Google Scholar]
- Gontero, P.; Marra, G.; Soria, F.; Oderda, M.; Zitella, A.; Baratta, F.; Chiorino, G.; Gregnanin, I.; Daniele, L.; Cattel, L.; et al. A randomized double-blind placebo controlled phase I-II study on clinical and molecular effects of dietary supplements in men with precancerous prostatic lesions. Chemoprevention or “chemopromotion”? Prostate 2015, 75, 1177–1186. [Google Scholar] [CrossRef]
- Morgia, G.; Voce, S.; Palmieri, F.; Gentile, M.; Iapicca, G.; Giannantoni, A.; Blefari, F.; Carini, M.; Vespasiani, G.; Santelli, G.; et al. Association between selenium and lycopene supplementation and incidence of prostate cancer: Results from the post-hoc analysis of the procomb trial. Phytomedicine 2017, 34, 1–5. [Google Scholar] [CrossRef]
- Waters, D.J.; Chiang, E.C. Five threads: How U-shaped thinking weaves together dogs, men, selenium, and prostate cancer risk. Free Radic. Biol. Med. 2018, 127, 36–45. [Google Scholar] [CrossRef]
- Gopalakrishna, R.; Gundimeda, U.; Zhou, S.; Bui, H.; Holmgren, A. Redox regulation of protein kinase C by selenometabolites and selenoprotein thioredoxin reductase limits cancer prevention by selenium. Free Radic. Biol. Med. 2018, 127, 55–61. [Google Scholar] [CrossRef]
- Liu, X.; Gao, R.; Dong, Y.; Gao, L.; Zhao, Y.; Zhao, L.; Zhao, X.; Zhang, H. Survivin gene silencing sensitizes prostate cancer cells to selenium growth inhibition. BMC Cancer 2010, 10, 418. [Google Scholar] [CrossRef]
- Stratton, M.S.; Algotar, A.M.; Ranger-Moore, J.; Stratton, S.P.; Slate, E.H.; Hsu, C.H.; Thompson, P.A.; Clark, L.C.; Ahmann, F.R. Oral selenium supplementation has no effect on prostate-specific antigen velocity in men undergoing active surveillance for localized prostate cancer. Cancer Prev. Res. 2010, 3, 1035–1043. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Van Blarigan, E.L.; DuPre, N.; Stampfer, M.J.; Giovannucci, E.L.; Chan, J.M. Selenium supplementation and prostate cancer mortality. J. Natl. Cancer Inst. 2014, 107, 360. [Google Scholar] [CrossRef]
- Tian, J.; Ning, S.; Knox, S.J. Sodium selenite radiosensitizes hormone-refractory prostate cancer xenograft tumors but not intestinal crypt cells in vivo. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 230–236. [Google Scholar] [CrossRef]
- Dhillon, V.S.; Deo, P.; Fenech, M. Effect of Selenium and Lycopene on Radiation Sensitivity in Prostate Cancer Patients Relative to Controls. Cancers 2023, 15, 979. [Google Scholar] [CrossRef]
- Husbeck, B.; Peehl, D.M.; Knox, S.J. Redox modulation of human prostate carcinoma cells by selenite increases radiation-induced cell killing. Free Radic. Biol. Med. 2005, 38, 50–57. [Google Scholar] [CrossRef]
- Muecke, R.; Micke, O.; Schomburg, L.; Buentzel, J.; Kisters, K.; Adamietz, I.A.; AKTE. Selenium in Radiation Oncology-15 Years of Experiences in Germany. Nutrients 2018, 10, 483. [Google Scholar] [CrossRef]
- Freitas, M.; Alves, V.; Sarmento-Ribeiro, A.B.; Mota-Pinto, A. Combined effect of sodium selenite and docetaxel on PC3 metastatic prostate cancer cell line. Biochem. Biophys. Res. Commun. 2011, 408, 713–719. [Google Scholar] [CrossRef]
- Tabassum, A.; Bristow, R.G.; Venkateswaran, V. Ingestion of selenium and other antioxidants during prostate cancer radiotherapy: A good thing? Cancer Treat. Rev. 2010, 36, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Beuth, J.; Van Leendert, R.; Pempelfort, K.; Schneider, B.; Grund, C.; Engelmann, U. Complementary medicine down-regulates side-effects of hormone therapy in prostate cancer patients. In Vivo 2014, 28, 979–982. [Google Scholar] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochem. Biokhimiia 2022, 87 (Suppl. 1), S168–S177. [Google Scholar] [CrossRef]
- Diamond, A.M. The subcellular location of selenoproteins and the impact on their function. Nutrients 2015, 7, 3938–3948. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef]
- Bondareva, A.A.; Capecchi, M.R.; Iverson, S.V.; Li, Y.; Lopez, N.I.; Lucas, O.; Merrill, G.F.; Prigge, J.R.; Siders, A.M.; Wakamiya, M.; et al. Effects of thioredoxin reductase-1 deletion on embryogenesis and transcriptome. Free Radic. Biol. Med. 2007, 43, 911–923. [Google Scholar] [CrossRef]
- Bunevicius, A.; Laws, E.R.; Saudargiene, A.; Tamasauskas, A.; Iervasi, G.; Deltuva, V.; Smith, T.R.; Bunevicius, R. Common genetic variations of deiodinase genes and prognosis of brain tumor patients. Endocrine 2019, 66, 563–572. [Google Scholar] [CrossRef]
- Richardson, D.R. More roles for selenoprotein P: Local selenium storage and recycling protein in the brain. Biochem. J. 2005, 386 Pt 2, e5–e7. [Google Scholar] [CrossRef]
- Ren, B.; Liu, M.; Ni, J.; Tian, J. Role of Selenoprotein F in Protein Folding and Secretion: Potential Involvement in Human Disease. Nutrients 2018, 10, 1619. [Google Scholar] [CrossRef] [PubMed]
- Ansong, E.; Yang, W.; Diamond, A.M. Molecular cross-talk between members of distinct families of selenium containing proteins. Mol. Nutr. Food Res. 2014, 58, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ansong, E.; Ying, Q.; Ekoue, D.N.; Deaton, R.; Hall, A.R.; Kajdacsy-Balla, A.; Yang, W.; Gann, P.H.; Diamond, A.M. Evidence that Selenium Binding Protein 1 is a Tumor Suppressor in Prostate Cancer. PLoS ONE 2015, 10, e0127295. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, H.; Zhang, Q.; Tang, J.; Li, K.; Xia, X.J.; Wang, K.N.; Li, K.; Lei, X.G. Prolonged dietary selenium deficiency or excess does not globally affect selenoprotein gene expression and/or protein production in various tissues of pigs. J. Nutr. 2012, 142, 1410–1416. [Google Scholar] [CrossRef]
- Davis, C.D.; Tsuji, P.A.; Milner, J.A. Selenoproteins and cancer prevention. Annu. Rev. Nutr. 2012, 32, 73–95. [Google Scholar] [CrossRef]
- Yao, H.D.; Wu, Q.; Zhang, Z.W.; Li, S.; Wang, X.L.; Lei, X.G.; Xu, S.W. Selenoprotein W serves as an antioxidant in chicken myoblasts. Biochim. et Biophys. Acta 2013, 1830, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Rederstorff, M.; Krol, A.; Lescure, A. Understanding the importance of selenium and selenoproteins in muscle function. Cell. Mol. Life Sci. 2006, 63, 52–59. [Google Scholar] [CrossRef]
- Turanov, A.A.; Shchedrina, V.A.; Everley, R.A.; Lobanov, A.V.; Yim, S.H.; Marino, S.M.; Gygi, S.P.; Hatfield, D.L.; Gladyshev, V.N. Selenoprotein S is involved in maintenance and transport of multiprotein complexes. Biochem. J. 2014, 462, 555–565. [Google Scholar] [CrossRef]
- Horibata, Y.; Hirabayashi, Y. Identification and characterization of human ethanolaminephosphotransferase1. J. Lipid Res. 2007, 48, 503–508. [Google Scholar] [CrossRef]
- Boukhzar, L.; Hamieh, A.; Cartier, D.; Tanguy, Y.; Alsharif, I.; Castex, M.; Arabo, A.; El Hajji, S.; Bonnet, J.J.; Errami, M.; et al. Selenoprotein T Exerts an Essential Oxidoreductase Activity That Protects Dopaminergic Neurons in Mouse Models of Parkinson’s Disease. Antioxid. Redox Signal. 2016, 24, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.L.; Mendelev, N.; Kumari, S.; Andy Li, P. Overexpression of human selenoprotein H in neuronal cells enhances mitochondrial biogenesis and function through activation of protein kinase A, protein kinase B, and cyclic adenosine monophosphate response element-binding protein pathway. Int. J. Biochem. Cell Biol. 2013, 45, 604–611. [Google Scholar] [CrossRef]
- Ghuge, S.A.; Kadam, U.S.; Hong, J.C. Selenoprotein: Potential Player in Redox Regulation in Chlamydomonas reinhardtii. Antioxidants 2022, 11, 1630. [Google Scholar] [CrossRef] [PubMed]
- Arsova-Sarafinovska, Z.; Eken, A.; Matevska, N.; Erdem, O.; Sayal, A.; Savaser, A.; Banev, S.; Petrovski, D.; Dzikova, S.; Georgiev, V.; et al. Increased oxidative/nitrosative stress and decreased antioxidant enzyme activities in prostate cancer. Clin. Biochem. 2009, 42, 1228–1235. [Google Scholar] [CrossRef]
- Sajjaboontawee, N.; Supasitthumrong, T.; Tunvirachaisakul, C.; Nantachai, K.; Snabboon, T.; Reiche, E.M.V.; Simão, A.N.C.; Maes, M. Lower thiol, glutathione, and glutathione peroxidase levels in prostate cancer: A meta-analysis study. Aging Male 2020, 23, 1533–1544. [Google Scholar] [CrossRef]
- Kuznetsova, Y.P.; Goltyaev, M.V.; Gorbacheva, O.S.; Novoselov, S.V.; Varlamova, E.G.; Fesenko, E.E. Influence of Sodium Selenite on the mRNA Expression of the Mammalian Selenocysteine-Containing Protein Genes in Testicle and Prostate Cancer Cells. Doklady. Biochem. Biophys. 2018, 480, 131–134. [Google Scholar] [CrossRef]
- Elhodaky, M.; Hong, L.K.; Kadkol, S.; Diamond, A.M. Selenium-binding protein 1 alters energy metabolism in prostate cancer cells. Prostate 2020, 80, 962–976. [Google Scholar] [CrossRef]
- Fang, W.; Goldberg, M.L.; Pohl, N.M.; Bi, X.; Tong, C.; Xiong, B.; Koh, T.J.; Diamond, A.M.; Yang, W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis 2010, 31, 1360–1366. [Google Scholar] [CrossRef]
- Jerome-Morais, A.; Wright, M.E.; Liu, R.; Yang, W.; Jackson, M.I.; Combs, G.F., Jr.; Diamond, A.M. Inverse association between glutathione peroxidase activity and both selenium-binding protein 1 levels and Gleason score in human prostate tissue. Prostate 2012, 72, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moreno, O.; Boque, N.; Redrado, M.; Milagro, F.; Campion, J.; Endermann, T.; Takahashi, K.; Saito, Y.; Catena, R.; Schomburg, L.; et al. Selenoprotein-P is down-regulated in prostate cancer, which results in lack of protection against oxidative damage. Prostate 2011, 71, 824–834. [Google Scholar] [CrossRef]
- Luchman, H.A.; Villemaire, M.L.; Bismar, T.A.; Carlson, B.A.; Jirik, F.R. Prostate epithelium-specific deletion of the selenocysteine tRNA gene Trsp leads to early onset intraepithelial neoplasia. Am. J. Pathol. 2014, 184, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.; Kim, C.Y.; Lee, J.M.; Ryu, B.; Kim, J.; Bang, J.; Ahn, N.; Park, J.H. Loss of glutathione peroxidase 3 induces ROS and contributes to prostatic hyperplasia in Nkx3.1 knockout mice. Andrology 2020, 8, 1486–1493. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.N.; Han, J.; Abdelkader, T.S.; Kim, T.H.; Lee, J.M.; Song, J.; Kim, K.S.; Park, J.H.; Park, J.H. High animal fat intake enhances prostate cancer progression and reduces glutathione peroxidase 3 expression in early stages of TRAMP mice. Prostate 2014, 74, 1266–1277. [Google Scholar] [CrossRef]
- Xu, Y.; Fang, F.; Zhang, J.; Josson, S.; St Clair, W.H.; St Clair, D.K. miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes. PLoS ONE 2010, 5, e14356. [Google Scholar] [CrossRef]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A. THE MAIN CYTOTOXIC EFFECTS OF METHYLSELENINIC ACID ON VARIOUS CANCER CELLS. Int. J. Mol. Sci. 2021, 22, 6614. [Google Scholar] [CrossRef] [PubMed]
- Romero-Ramirez, L.; Cao, H.; Nelson, D.; Hammond, E.; Lee, A.H.; Yoshida, H.; Mori, K.; Glimcher, L.H.; Denko, N.C.; Giaccia, A.J.; et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004, 64, 5943–5947. [Google Scholar] [CrossRef]
- Goltyaev, M.V.; Mal’tseva, V.N.; Varlamova, E.G. Expression of ER-resident selenoproteins and activation of cancer cells apoptosis mechanisms under ER-stress conditions caused by methylseleninic acid. Gene 2020, 755, 144884. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Goltyaev, M.V.; Mal’tseva, V.N.; Turovsky, E.A.; Sarimov, R.M.; Simakin, A.V.; Gudkov, S.V. Mechanisms of the Cytotoxic Effect of Selenium Nanoparticles in Different Human Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 7798. [Google Scholar] [CrossRef]
- Hamieh, A.; Cartier, D.; Abid, H.; Calas, A.; Burel, C.; Bucharles, C.; Jehan, C.; Grumolato, L.; Landry, M.; Lerouge, P.; et al. Selenoprotein T is a novel OST subunit that regulates UPR signaling and hormone secretion. EMBO Rep. 2017, 18, 1935–1946. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, W.; Chen, L.L.; Huang, J.Q.; Lei, X.G. Selenoprotein V protects against endoplasmic reticulum stress and oxidative injury induced by pro-oxidants. Free Radic. Biol. Med. 2020, 160, 670–679. [Google Scholar] [CrossRef]
- Méplan, C.; Rohrmann, S.; Steinbrecher, A.; Schomburg, L.; Jansen, E.; Linseisen, J.; Hesketh, J. Polymorphisms in thioredoxin reductase and selenoprotein K genes and selenium status modulate risk of prostate cancer. PLoS ONE 2012, 7, e48709. [Google Scholar] [CrossRef]
- Cooper, M.L.; Adami, H.O.; Grönberg, H.; Wiklund, F.; Green, F.R.; Rayman, M.P. Interaction between single nucleotide polymorphisms in selenoprotein P and mitochondrial superoxide dismutase determines prostate cancer risk. Cancer Res. 2008, 68, 10171–10177. [Google Scholar] [CrossRef]
- Geybels, M.S.; van den Brandt, P.A.; Schouten, L.J.; van Schooten, F.J.; van Breda, S.G.; Rayman, M.P.; Green, F.R.; Verhage, B.A. Selenoprotein gene variants, toenail selenium levels, and risk for advanced prostate cancer. J. Natl. Cancer Inst. 2014, 106, dju003. [Google Scholar] [CrossRef]
- Penney, K.L.; Li, H.; Mucci, L.A.; Loda, M.; Sesso, H.D.; Stampfer, M.J.; Ma, J. Selenoprotein P genetic variants and mrna expression, circulating selenium, and prostate cancer risk and survival. Prostate 2013, 73, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Geybels, M.S.; Hutter, C.M.; Kwon, E.M.; Ostrander, E.A.; Fu, R.; Feng, Z.; Stanford, J.L.; Peters, U. Variation in selenoenzyme genes and prostate cancer risk and survival. Prostate 2013, 73, 734–742. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen Signaling in Prostate Cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Anderson, L.B.; Witthuhn, B.; Xu, Y.; Lü, J. Proteomic profiling of potential molecular targets of methyl-selenium compounds in the transgenic adenocarcinoma of mouse prostate model. Cancer Prev. Res. 2010, 3, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Yeon Chun, J.; Nadiminty, N.; Trump, D.L.; Ip, C.; Dong, Y.; Gao, A.C. Monomethylated selenium inhibits growth of LNCaP human prostate cancer xenograft accompanied by a decrease in the expression of androgen receptor and prostate-specific antigen (PSA). Prostate 2006, 66, 1070–1075. [Google Scholar] [CrossRef]
- Chun, J.Y.; Nadiminty, N.; Lee, S.O.; Onate, S.A.; Lou, W.; Gao, A.C. Mechanisms of selenium down-regulation of androgen receptor signaling in prostate cancer. Mol. Cancer Ther. 2006, 5, 913–918. [Google Scholar] [CrossRef]
- Kong, L.; Yuan, Q.; Zhu, H.; Li, Y.; Guo, Q.; Wang, Q.; Bi, X.; Gao, X. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials 2011, 32, 6515–6522. [Google Scholar] [CrossRef]
- Karelia, D.N.; Kim, S.; Plano, D.; Sharma, A.K.; Jiang, C.; Lu, J. Seleno-aspirin compound AS-10 promotes histone acetylation ahead of suppressing androgen receptor transcription, G1 arrest, and apoptosis of prostate cancer cells. Prostate 2023, 83, 16–29. [Google Scholar] [CrossRef]
- Amanatullah, D.F.; Reutens, A.T.; Zafonte, B.T.; Fu, M.; Mani, S.; Pestell, R.G. Cell-cycle dysregulation and the molecular mechanisms of prostate cancer. Front. Biosci. 2000, 5, D372–D390. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Wang, T.T.; Alkan, Z.; Richter, B.D.; Dawson, K. Selenoprotein W modulates control of cell cycle entry. Biol. Trace Elem. Res. 2009, 131, 229–244. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, H.J.; Chai, Y.; Hu, H.; Wang, L.; Zhang, Y.; Jiang, C.; Lü, J. Persistent p21Cip1 induction mediates G(1) cell cycle arrest by methylseleninic acid in DU145 prostate cancer cells. Curr. Cancer Drug Targets 2010, 10, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Tang, J.; Wang, D.; Zuo, H.; Zhang, Q.; Liu, Y.; Xiong, H. Selenium nanoparticles (SeNPs) have potent antitumor activity against prostate cancer cells through the upregulation of miR-16. World J. Surg. Oncol. 2020, 18, 81. [Google Scholar] [CrossRef]
- Zhao, R.; Xiang, N.; Domann, F.E.; Zhong, W. Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr. Cancer 2009, 61, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef] [PubMed]
- Melegh, Z.; Oltean, S. Targeting Angiogenesis in Prostate Cancer. Int. J. Mol. Sci. 2019, 20, 2676. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, N.M.; Najdovska, M.; Costello, A.J. Inorganic selenium retards progression of experimental hormone refractory prostate cancer. J. Urol. 2004, 171, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, H.; Li, G.; Lee, H.J.; Jiang, C.; Kim, S.H.; Lü, J. Methylseleninic acid inhibits microvascular endothelial G1 cell cycle progression and decreases tumor microvessel density. Int. J. Cancer 2008, 122, 15–24. [Google Scholar] [CrossRef]
- Cai, Z.; Dong, L.; Song, C.; Zhang, Y.; Zhu, C.; Zhang, Y.; Ling, Q.; Hoffmann, P.R.; Li, J.; Huang, Z.; et al. Methylseleninic Acid Provided at Nutritional Selenium Levels Inhibits Angiogenesis by Down-regulating Integrin β3 Signaling. Sci. Rep. 2017, 7, 9445. [Google Scholar] [CrossRef]
- Sinha, I.; Null, K.; Wolter, W.; Suckow, M.A.; King, T.; Pinto, J.T.; Sinha, R. Methylseleninic acid downregulates hypoxia-inducible factor-1α in invasive prostate cancer. Int. J. Cancer 2012, 130, 1430–1439. [Google Scholar] [CrossRef] [PubMed]
- Cervi, D.; Pak, B.; Venier, N.A.; Sugar, L.M.; Nam, R.K.; Fleshner, N.E.; Klotz, L.H.; Venkateswaran, V. Micronutrients attenuate progression of prostate cancer by elevating the endogenous inhibitor of angiogenesis, platelet factor-4. BMC Cancer 2010, 10, 258. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Chen, X.; Gong, H.; Qiu, P.; Xiao, X.; Dang, S.; Hong, A.; Ma, Y. Delivery of a TNF-α-derived peptide by nanoparticles enhances its antitumor activity by inducing cell-cycle arrest caspase-dependent apoptosis. FASEB J. 2018, 32, fj201800377R. [Google Scholar] [CrossRef]
- Sarveswaran, S.; Liroff, J.; Zhou, Z.; Nikitin, A.Y.; Ghosh, J. Selenite triggers rapid transcriptional activation of p53, and p53-mediated apoptosis in prostate cancer cells: Implication for the treatment of early-stage prostate cancer. Int. J. Oncol. 2010, 36, 1419–1428. [Google Scholar]
- Lu, Z.; Qi, L.; Li, G.X.; Bo, X.J.; Liu, G.D.; Wang, J.M. Se-methylselenocysteine suppresses the growth of prostate cancer cell DU145 through connexin 43-induced apoptosis. J. Cancer Res. Ther. 2015, 11, 840–845. [Google Scholar] [PubMed]
- Xiang, N.; Zhao, R.; Zhong, W. Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells. Cancer Chemother. Pharmacol. 2009, 63, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yan, M.; Liu, X.; Yin, S.; Lu, S.; Fan, L.; Hu, H. Inorganic Selenium Induces Nonapoptotic Programmed Cell Death in PC-3 Prostate Cancer Cells Associated with Inhibition of Glycolysis. J. Agric. Food Chem. 2019, 67, 10637–10645. [Google Scholar] [CrossRef]
- Sonkusre, P. Specificity of Biogenic Selenium Nanoparticles for Prostate Cancer Therapy with Reduced Risk of Toxicity: An in vitro and in vivo Study. Front. Oncol. 2020, 9, 1541. [Google Scholar] [CrossRef]
- Sonkusre, P.; Cameotra, S.S. Biogenic selenium nanoparticles induce necroptosis in PC-3 cancer cells through TNF activation. J. Nanobiotechnol. 2017, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nature reviews. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar]
- Ghoochani, A.; Hsu, E.C.; Aslan, M.; Rice, M.A.; Nguyen, H.M.; Brooks, J.D.; Corey, E.; Paulmurugan, R.; Stoyanova, T. Ferroptosis Inducers Are a Novel Therapeutic Approach for Advanced Prostate Cancer. Cancer Res. 2021, 81, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Exquisite sensitivity of adrenocortical carcinomas to induction of ferroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 22269–22274. [CrossRef]
- Rebsch, C.M.; Penna, F.J.; Copeland, P.R. Selenoprotein expression is regulated at multiple levels in prostate cells. Cell Res. 2006, 16, 940–948. [Google Scholar] [CrossRef]
- Johnstone, S.E.; Gladyshev, V.N.; Aryee, M.J.; Bernstein, B.E. Epigenetic clocks, aging, and cancer. Science 2022, 378, 1276–1277. [Google Scholar] [CrossRef]
- Jabłońska, E.; Reszka, E. Selenium and Epigenetics in Cancer: Focus on DNA Methylation. Adv. Cancer Res. 2017, 136, 193–234. [Google Scholar] [PubMed]
- Xiang, N.; Zhao, R.; Song, G.; Zhong, W. Selenite reactivates silenced genes by modifying DNA methylation and histones in prostate cancer cells. Carcinogenesis 2008, 29, 2175–2181. [Google Scholar] [CrossRef]
- Ramachandran, K.; Navarro, L.; Gordian, E.; Das, P.M.; Singal, R. Methylation-mediated silencing of genes is not altered by selenium treatment of prostate cancer cells. Anticancer Res. 2007, 27, 921–925. [Google Scholar]
- Lee, J.I.; Nian, H.; Cooper, A.J.; Sinha, R.; Dai, J.; Bisson, W.H.; Dashwood, R.H.; Pinto, J.T. Alpha-keto acid metabolites of naturally occurring organoselenium compounds as inhibitors of histone deacetylase in human prostate cancer cells. Cancer Prev. Res. 2009, 2, 683–693. [Google Scholar] [CrossRef]
- Kok, D.E.; Kiemeney, L.A.; Verhaegh, G.W.; Schalken, J.A.; van Lin, E.N.; Sedelaar, J.P.; Witjes, J.A.; Hulsbergen-van de Kaa, C.A.; van ‘t Veer, P.; Kampman, E.; et al. A short-term intervention with selenium affects expression of genes implicated in the epithelial-to-mesenchymal transition in the prostate. Oncotarget 2017, 8, 10565–10579. [Google Scholar] [CrossRef] [PubMed]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Zeng, D.; Liu, C.; Zhang, Q.; Wang, X.; Chen, T. Selenium-containing ruthenium complex synergizes with natural killer cells to enhance immunotherapy against prostate cancer via activating TRAIL/FasL signaling. Biomaterials 2019, 219, 119377. [Google Scholar] [CrossRef]
- Hu, W.; Ma, Y.; Zhao, C.; Yin, S.; Hu, H. Methylseleninic acid overcomes programmed death-ligand 1-mediated resistance of prostate cancer and lung cancer. Mol. Carcinog. 2021, 60, 746–757. [Google Scholar] [CrossRef] [PubMed]
| Gene | SNP ID | Risk of Prostate Cancer | Response to Selenium Supplementation | References |
|---|---|---|---|---|
| SELENOP | rs13168440 | NS | NA | [104] |
| TT decreases the risk of prostate cancer, compared to allele CC | NA | [105] | ||
| rs230813 | NS | NA | [104] | |
| rs230819 | NS | NA | [104] | |
| rs3877899 | NS | NA | [104] | |
| AA decreases the risk of distant prostate cancer, compared to allele GG | NA | [106] | ||
| rs7579 | AG and AA increase the risk of advanced (Stage III, IV) prostate cancer, compared to allele GG | NA | [104] | |
| NS | NA | [15] | ||
| rs3797310 | TT increases the risk of distant prostate cancer, compared to allele CC | NA | [106] | |
| rs3877899 | NS | NA | [15] | |
| rs11959466 | NS | NA | [105] | |
| rs12517112 | NS | NA | [105] | |
| rs230820 | NS | NA | [105] | |
| SELENO15 | rs561104 | NS | NA | [106] |
| rs540049 | NS | NA | [15] | |
| rs5859 | NS | NA | [15] | |
| SELENOK | rs9880056 | NS | NA | [102] |
| GPX1 | rs17650792 | AG and GG decrease the risk of high-grade prostate cancer, compared to allele AA | NA | [16] |
| GG and AG increase the risk of high-grade (Stage III, IV) prostate cancer, compared to allele AA | NA | [104] | ||
| rs1800668 | TT and CT decrease the risk of high-grade (Stage III, IV) prostate cancer, compared to allele CC | NA | [104] | |
| rs3448 | NS | NA | [104] | |
| TT decreases the risk of overall (local and distant) prostate cancer, compared to allele CC | NA | [106] | ||
| rs1050450 | NS | NA | [15] | |
| GPX2 | rs4902346 | GG increases the risk of Gleason 7–10 prostate cancer, compared to allele AA | NA | [106] |
| GPX3 | rs8177447 | NS | NA | [106] |
| GPX4 | rs2075710 | TT increases the risk of local prostate cancer, compared to allele CC | NA | [106] |
| rs713041 | NS | NA | [15] | |
| TXNRD1 | rs7310505 | NS | NA | [102] |
| TXNRD2 | rs3804047 | NA | Selenium supplementation increases the risk of high-grade prostate cancer in candidates with allele AA, AG | [16] |
| Selenium supplementation decreases the risk of high-grade prostate cancer in candidates with allele GG | [16] | |||
| rs8141691 | AG and AA increase the risk of high-grade prostate cancer, compared to allele GG. | Selenium supplementation increases the risk of high-grade prostate cancer in candidates with allele AA, GG | [16] | |
| Selenium supplementation decreases the risk of high-grade prostate cancer in candidates with allele AA | [16] | |||
| rs9605030 | NS | NA | [102] | |
| rs9605031 | NS | NA | [102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Chen, B.; Tang, B.; Wei, Q. Selenium in Prostate Cancer: Prevention, Progression, and Treatment. Pharmaceuticals 2023, 16, 1250. https://doi.org/10.3390/ph16091250
Jiang J, Chen B, Tang B, Wei Q. Selenium in Prostate Cancer: Prevention, Progression, and Treatment. Pharmaceuticals. 2023; 16(9):1250. https://doi.org/10.3390/ph16091250
Chicago/Turabian StyleJiang, Jinjiang, Bo Chen, Bo Tang, and Qiang Wei. 2023. "Selenium in Prostate Cancer: Prevention, Progression, and Treatment" Pharmaceuticals 16, no. 9: 1250. https://doi.org/10.3390/ph16091250
APA StyleJiang, J., Chen, B., Tang, B., & Wei, Q. (2023). Selenium in Prostate Cancer: Prevention, Progression, and Treatment. Pharmaceuticals, 16(9), 1250. https://doi.org/10.3390/ph16091250








