Selective Androgen Receptor Modulators Combined with Treadmill Exercise Have No Bone Benefit in Healthy Adult Rats
Abstract
1. Introduction
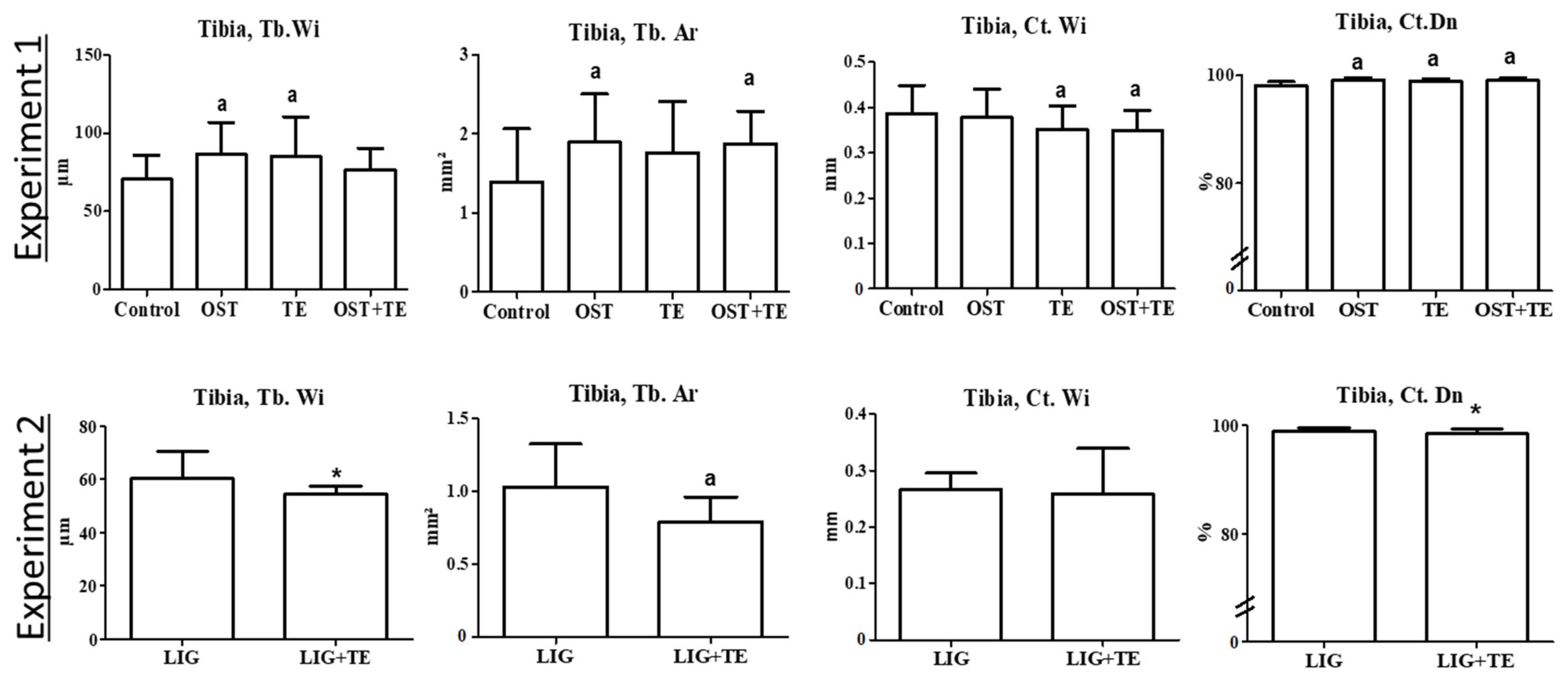
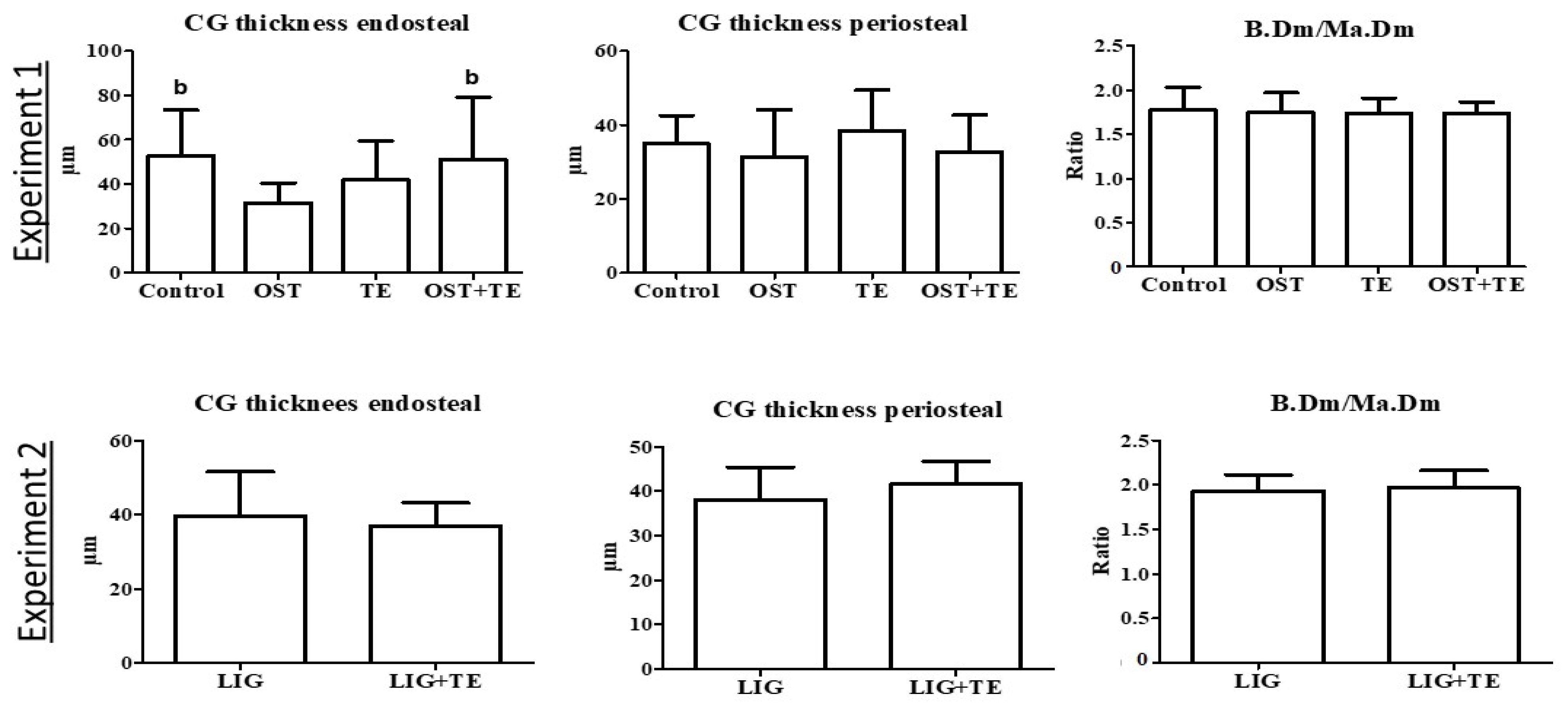
2. Results
3. Discussion
4. Materials and Methods
4.1. General Procedures
4.2. Serum Analyses
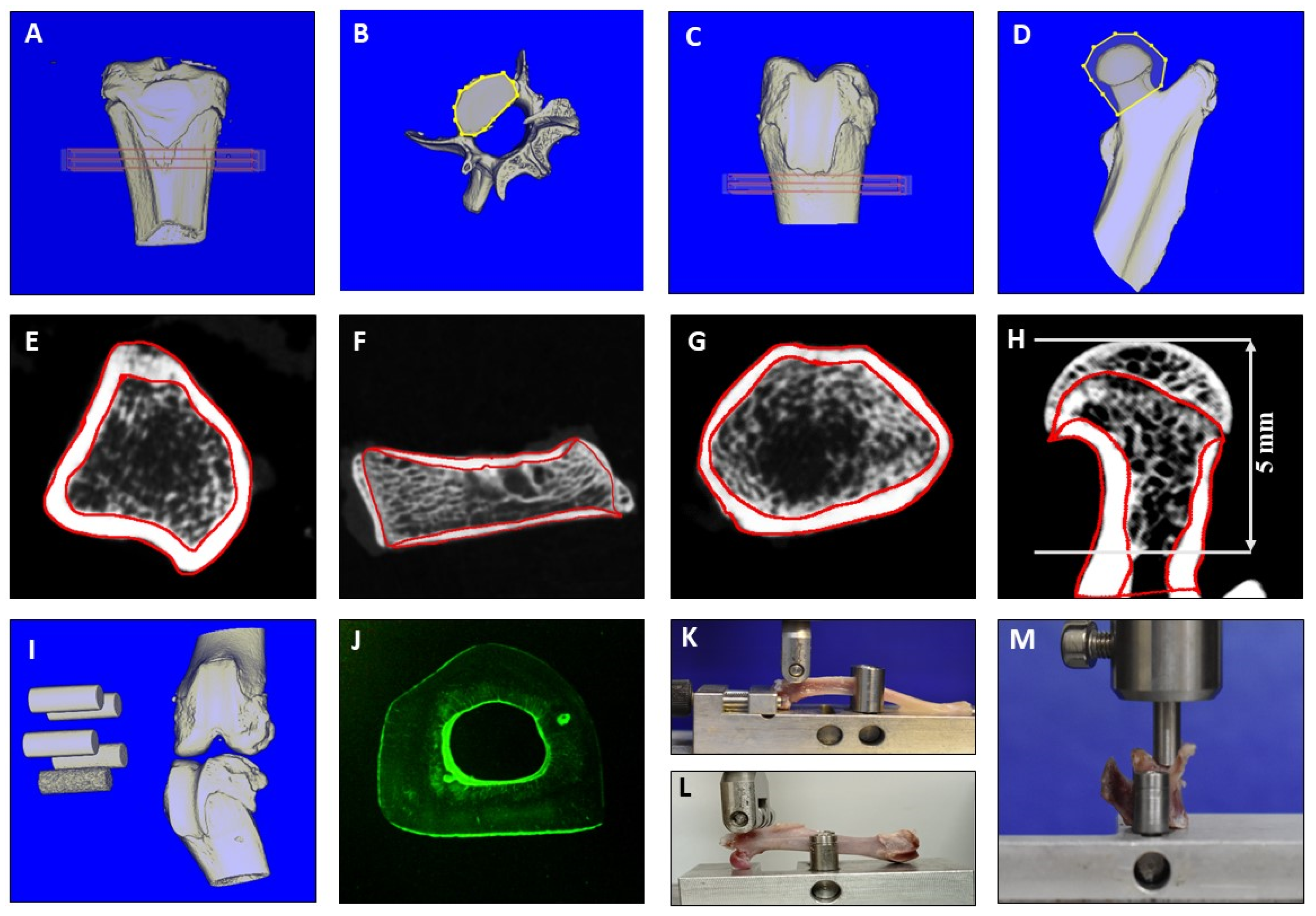
4.3. Biomechanical Analysis
4.4. Micro-CT Analyses
4.5. Histological Analysis
4.6. Ashing Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, L.; Elliott-Sale, K.J.; Sale, C. Exercise and bone health across the lifespan. Biogerontology 2017, 18, 931–946. [Google Scholar] [CrossRef] [PubMed]
- Demontiero, O.; Vidal, C.; Duque, G. Aging and bone loss: New insights for the clinician. Ther. Adv. Musculoskelet. Dis. 2012, 4, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Lenrow, D.A.; Holmes, J.H.; Dlewati, A.; Santanna, J.; Rosen, C.J.; et al. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J. Clin. Endocrinol. Metab. 1999, 84, 2647–2653. [Google Scholar] [CrossRef] [PubMed]
- Gruenwald, D.A.; Matsumoto, A.M. Testosterone supplementation therapy for older men: Potential benefits and risks. J. Am. Geriat. Soc. 2003, 51, 101–115. [Google Scholar] [CrossRef]
- Dalton, J.T. The long and winding road for selective androgen receptor modulators. Br. J. Clin. Pharmacol. 2017, 83, 2131–2133. [Google Scholar] [CrossRef]
- Narayanan, R.; Coss, C.C.; Dalton, J.T. Development of selective androgen receptor modulators (SARMs). Mol. Cell. Endocrinol. 2018, 465, 134–142. [Google Scholar] [CrossRef]
- Zilbermint, M.F.; Dobs, A.S. Nonsteroidal selective androgen receptor modulator Ostarine in cancer cachexia. Future Oncol. 2009, 5, 1211–1220. [Google Scholar] [CrossRef]
- Basaria, S.; Collins, L.; Dillon, E.L.; Orwoll, K.; Storer, T.W.; Miciek, R.; Ulloor, J.; Zhang, A.; Eder, R.; Zientek, H.; et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 87–95. [Google Scholar] [CrossRef]
- Lambert, C.P. Should the FDA’s criteria for the clinical efficacy of cachexia drugs be changed? Is Ostarine safe and effective? J. Cachexia Sarcopenia Muscle 2021, 12, 531–532. [Google Scholar] [CrossRef]
- Crawford, J.; Prado, C.M.; Johnston, M.A.; Gralla, R.J.; Taylor, R.P.; Hancock, M.L.; Dalton, J.T. Study design and rationale for the phase 3 clinical development program ofenobosarm, a selective androgen receptor modulator, for the prevention and treatment of muscle wasting in cancer patients (POWER trials). Curr. Oncol. Rep. 2016, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Garber, K. No longer going to waste. Nat. Biotechnol. 2016, 34, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, A.R.; Lipshultz, L.I.; Hotaling, J.M.; Pastuszak, A.W. Selective androgen receptor modulators: The future of androgen therapy? Transl. Androl. Urol. 2020, 9, S135–S148. [Google Scholar] [CrossRef] [PubMed]
- Cardaci, T.D.; Machek, S.B.; Wilburn, D.T.; Heileson, J.L.; Harris, D.R.; Cintineo, H.P.; Willoughby, D.S. LGD-4033 and MK-677 use impacts body composition, circulating biomarkers, and skeletal muscle androgenic hormone and receptor content: A case report. Exp Physiol. 2022, 107, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Naafs, M.A. Selective androgen receptor modulators (SARMs): A mini-review. Open Access J. Reprod. Syst. Sex. Disord. 2018, 1, 1–7. [Google Scholar] [CrossRef]
- Komrakova, M.; Büchler, G.; Böker, K.O.; Lehmann, W.; Schilling, A.F.; Roch, P.J.; Taudien, S.; Hoffmann, D.B.; Sehmisch, S. A combined treatment with selective androgen and estrogen receptor modulators prevents bone loss in orchiectomized rats. J. Endocrinol. Investig. 2022, 45, 2299–2311. [Google Scholar] [CrossRef]
- Chen, J.-F.; Lin, P.-W.; Tsai, Y.-R.; Yang, Y.-C.; Kang, H.-Y. Androgens and androgen receptor actions on bone health and disease: From androgen deficiency to androgen therapy. Cells 2019, 8, 1318. [Google Scholar] [CrossRef]
- Burmeister, M.A.; Fincher, T.K.; Graham, W.H. Recreational use of selective androgen receptor modulators. US Pharm. 2020, 45, 15–18. [Google Scholar]
- Yeh, J.K.; Aloia, J.F.; Tierney, J.M.; Sprintz, S. Effect of treadmill exercise on vertebral and tibial bone mineral content and bone mineral density in the aged adult rat: Determined by dual energy X-ray absorptiometry. Calcif. Tissue Int. 1993, 52, 234–238. [Google Scholar] [CrossRef]
- Oxlund, H.; Andersen, N.B.; Ortoft, G.; Orskov, H.; Andreassen, T.T. Growth hormone and mild exercise in combination markedly enhance cortical bone formation and strength in old rats. Endocrinology 1998, 139, 1899–1904. [Google Scholar] [CrossRef]
- Liu, S.Y.; Li, Z.; Xu, S.Y.; Xu, L.; Yang, M.; Ni, G.X. Intensity dependent effect of treadmill running on differentiation of rat bone marrow stromal cells. Mol. Med. Rep. 2018, 17, 7746–7756. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Shimamura, C.; Takeda, T.; Abe, H.; Ichimura, S.; Sato, Y.; Toyama, Y. Effects of treadmill exercise on bone mass, bone metabolism, and calciotropic hormones in young growing rats. J. Bone Miner. Metab. 2004, 22, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhong, P.; Ning, P.; Tan, L.; Huang, X.; Peng, T.; Yin, L.; Luo, F.; Qu, M.; Zhou, J. Treadmill training mitigates bone deterioration via inhibiting NLRP3/Caspase1/IL-1β signaling in aged rats. BMC Musculoskelet. Disord. 2022, 23, 1089. [Google Scholar] [CrossRef] [PubMed]
- Stuermer, E.K.; Komrakova, M.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Utesch, C.; Mangal, O.; Zimmer, S.; et al. Musculoskeletal response to whole body vibration during fracture healing in intact and ovariectomized rats. Calcif. Tissue Int. 2010, 87, 168–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, J.; Bao, Q.; Chen, S.; Liu, H.; Feng, J.; Qin, H.; Li, A.; Liu, D.; Shen, Y.; Zhao, Y.; et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/β-catenin signaling in mice. J. Orthop. Res. 2017, 35, 812–819. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Cariati, I.; Bonanni, R.; Onorato, F.; Mastrogregori, A.; Rossi, D.; Iundusi, R.; Gasbarra, E.; Tancredi, V.; Tarantino, U. Role of physical activity in bone-muscle crosstalk: Biological aspects and clinical implications. J. Funct. Morphol. Kinesiol. 2021, 21, 55. [Google Scholar] [CrossRef]
- Xu, X.; Liu, S.; Liu, H.; Ru, K.; Jia, Y.; Wu, Z.; Liang, S.; Khan, Z.; Chen, Z.; Qian, A.; et al. Piezo channels: Awesome mechanosensitive structures in cellular mechanotransduction and their role in bone. Int. J. Mol. Sci. 2021, 22, 6429. [Google Scholar] [CrossRef]
- Müller-Reiter, M.S. The Effect of the Selective Androgen Receptor Modulator Ligandrol on the Bone Tissue of the Tibia in an Osteoporotic Rat Model. Doctoral Thesis, Georg-August-University of Goettingen, Goettigen, Germany, 2021. [Google Scholar] [CrossRef]
- Barbara, M.; Dhingra, S.; Mindikoglu, A.L. Ligandrol (LGD-4033)-induced liver injury. ACG Case Rep. J. 2020, 7, e00370. [Google Scholar] [CrossRef]
- Emmanuelle, N.E.; Marie-Cécile, V.; Florence, T.; Jean-François, A.; Françoise, L.; Coralie, F.; Alexia, V. Critical role of estrogens on bone homeostasis in both male and female: From physiology to medical implications. Int. J. Mol. Sci. 2020, 22, 1568. [Google Scholar] [CrossRef]
- Dalton, J.T.; Barnet, K.G.; Bohl, C.E. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: Results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2011, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Carroll, M.D.; Kit, B.K.; Lacher, D.A. Total and high-density lipoprotein cholesterol in adults: National Health and Nutrition Examination Survey, 2009–2010. NCHS Data Brief 2012, 92, 1–8. [Google Scholar]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kroschel, L.-K. Retrospective Analysis of Historical Effects in Control Groups in Animal Experiments in Osteoporosis Research. Doctoral Thesis, Georg-August-Univerisity of Goettingen, Goettingen, Germany, 2018. [Google Scholar] [CrossRef]
- Roch, P.J.; Henkies, D.; Carstens, J.C.; Krischek, C.; Lehmann, W.; Komrakova, M.; Sehmisch, S. Ostarine and ligandrol improve muscle tissue in an ovariectomized rat model. Front. Endocrinol. 2018, 11, 556581. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Amabile, M.I.; Giorgi, A.; Monti, M.; D’Andrea, V.; Muscaritoli, M. Investigational drugs for the treatment of cancer cachexia: A focus on phase I and phase II clinical trials. Expert Opin. Investig. Drugs 2019, 28, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.I.; Noakes, T.D. Dissociation of changes in VO2 max, muscle QO2, and performance with training in rats. J. Appl. Physiol. 1989, 66, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, K.N.; Boyadjiev, N.P. Effects of nandrolone decanoate on VO2max, running economy, and endurance in rats. Med. Sci. Sports Exerc. 2004, 36, 1336–1341. [Google Scholar] [CrossRef]
- Tezval, M.; Stuermer, E.K.; Sehmisch, S.; Rack, T.; Stary, A.; Stebener, M.; Konietschke, F.; Stuermer, K.M. Improvement of trochanteric bone quality in an osteoporosis model after short-term treatment with parathyroid hormone: A new mechanical test for trochanteric region of rat femur. Osteoporos. Int. 2010, 21, 251–261. [Google Scholar] [CrossRef][Green Version]
- Stürmer, E.K.; Seidlová-Wuttke, D.; Sehmisch, S.; Rack, T.; Wille, J.; Frosch, K.H.; Wuttke, W.; Stürmer, K.M. Standardized bending and breaking test for the normal and osteoporotic metaphyseal tibias of the rat: Effect of estradiol, testosterone, and raloxifene. J. Bone Miner. Res. 2006, 21, 89–96. [Google Scholar] [CrossRef]
- Albers, J.; Markus, M.A.; Alves, F.; Dullin, C. X-ray based virtual histology allows guided sectioning of heavy ion stained murine lungs for histological analysis. Sci. Rep. 2018, 8, 7712. [Google Scholar] [CrossRef]
- Saul, D.; Ninkovic, M.; Komrakova, M.; Wolff, L.; Simka, P.; Gasimov, T.; Menger, B.; Hoffmann, D.B.; Rohde, V.; Sehmisch, S. Effect of zileuton on osteoporotic bone and its healing, expression of bone, and brain genes in rats. J. Appl. Physiol. 2018, 124, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using microcomputed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR histomorphometry nomenclature committee. J. Bone Miner. Res. 1987, 2, 595–610. [Google Scholar] [CrossRef] [PubMed]
- CEN. European Committee for Standardization. Determination of Calcium and Magnesium. EN ISO 7980. 2002. Available online: https://www.iso.org/standard/14972.html (accessed on 31 August 2023).
- CEN. European Committee for Standardization. Determination of Orthophosphate. EN ISO 6878. 2004. Available online: https://standards.iteh.ai/catalog/standards/cen/b7cc71c5-a6c5-4314-9547-d2054be03b72/en-iso-15681-2-2004 (accessed on 31 August 2023).
| Parameters | Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|---|
| Control n = 10 | OST n = 9 | TE n = 9 | OST + TE n = 10 | LIG n = 10 | LIG + TE n = 10 | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Initial body weight (g) | 229 ± 21 | 228 ± 26 | 223 ± 14 | 230 ± 21 | 234 ± 18 | 233 ± 16 |
| End body weight (g) | 422 ± 43 | 419 ± 29 | 413 ± 38 | 442 ± 45 | 391 ± 23 | 377 ± 53 |
| Serum analyses (mmol/L) | ||||||
| Cholesterol | 1.5 ± 0.2 | 1.9 a ± 0.2 | 1.7 ± 0.3 | 1.7 ± 0.2 | 2.0 ± 0.3 | 1.9 ± 0.5 |
| Glucose | 6.4 ± 2.0 | 5.0 ± 1.1 | 6.6 ± 1.4 | 5.2 ± 1.8 | 3.9 ± 12 | 3.3 ± 8 |
| HDL | 1.3 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.2 | 1.5 a ± 0.1 | 1.5 ± 0.2 | 1.3 ± 0.3 |
| LDL | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.2 ± 0.1 | 0.5 * ± 0.2 |
| Triglyceride | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.5 ± 0.3 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.3 |
| Biomechanical analyses Femur | ||||||
| Stiffness (N/mm) | 308 ± 49 | 289 ± 51 | 244 ± 48 | 246 ± 52 | 222 ± 39 | 241 ± 80 |
| Fmax (N) | 169 ± 18 | 169 ± 20 | 157 ± 28 | 148 ± 24 | 139 ± 19 | 142 ± 19 |
| Tibia | ||||||
| Stiffness (N/mm) | 106 ± 28 | 114 ± 41 | 104 ± 41 | 97 ± 33 | 128 ± 47 | 99 ± 18 |
| Fmax (N) | 56 ± 8 | 61 ± 11 | 59 ± 15 | 54 ± 5 | 70 ± 16 | 69 ± 22 |
| L3 | ||||||
| Stiffness (N/mm) | 281 ± 54 | 279 ± 31 | 277 ± 45 | 275 ± 61 | 258 ± 46 | 294 ± 81 |
| Fmax (N) | 209 ± 46 | 199 ± 31 | 194 ± 51 | 198 ± 23 | 211 ± 37 | 186 ± 63 |
| Ashing analysis, femur | ||||||
| Mineral content (%) | 44 ± 2 | 42 ± 2 | 42 ± 2 | 42 ± 1 | 41 ± 2 | 41 ± 3 |
| Mg+ (%) | 0.77 ± 0.03 | 0.75 ± 0.02 | 0.77 ± 0.03 | 0.75 ± 0.01 | 0.76 ± 0.02 | 0.77 ± 0.03 |
| Ca2+/PO43− | 1.66 ± 0.03 | 1.68 ± 0.06 | 1.73 ± 0.09 | 1.71 ± 0.06 | 1.35 ± 0.02 | 1.37 ±0.05 |
| Parameters | Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|---|
| Control n = 10 | OST n = 9 | TE n = 9 | OST + TE n = 10 | LIG n = 10 | LIG + TE n = 10 | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Femur distal | ||||||
| Total BMD (g/cm3) | 0.54 ± 0.05 | 0.58 ± 0.06 | 0.55 ± 0.07 | 0.52 ± 0.06 | 0.58 ± 0.05 | 0.50 * ± 0.07 |
| BV/TV (%) | 64 ± 8 | 70 ± 11 | 68 ± 8 | 65 ± 9 | 54 ± 8 | 44 * ± 9 |
| Ct. BMD (g/cm3) | 1.12 ± 0.04 | 1.13 ± 0.05 | 1.11 ± 0.03 | 1.11 ± 0.04 | 1.14 ± 0.02 | 1.13 ± 0.02 |
| Tb. BMD (g/cm3) | 0.57 ± 0.01 | 0.56 ± 0.02 | 0.56 ± 0.02 | 0.55 ± 0.01 | 0.65 ± 0.01 | 0.64 ± 0.01 |
| Femur proximal | ||||||
| Total BMD (g/cm3) | 0.75 ± 0.03 | 0.70 ± 0.06 | 0.71 ± 0.04 | 0.72 ± 0.05 | 0.71 ± 0.05 | 0.67 ± 0.07 |
| BV/TV (%) | 73 ± 4 | 69 ± 7 | 58 ± 6 | 70 ± 6 | 70 ± 6 | 62 ± 11 |
| Ct. BMD (g/cm3) | 1.10 ± 0.03 | 1.12 ± 0.06 | 1.13 ± 0.05 | 1.12 ± 0.04 | 1.09 ± 0.02 | 1.09 ± 0.04 |
| Tb. BMD (g/cm3) | 0.77 ± 0.01 | 0.76 ± 0.01 | 0.75 ± 0.02 | 0.76 ± 0.02 | 0.76 ± 0.02 | 0.75 ± 0.02 |
| Tibia proximal | ||||||
| Total BMD (g/cm3) | 0.36 ± 0.04 | 0.39 ± 0.04 | 0.38 ± 0.05 | 0.40 ± 0.04 | 0.46 ± 0.04 | 0.43 ± 0.07 |
| BV/TV (%) | 44 ± 7 | 50 ± 9 | 50 ± 5 | 51 ± 6 | 46 ± 6 | 42 ± 7 |
| Ct. BMD (g/cm3) | 1.03 ± 0.03 | 1.00 ± 0.04 | 1.00 ± 0.06 | 1.01 ± 0.07 | 1.12 ± 0.03 | 1.12 ± 0.05 |
| Tb. BMD (g/cm3) | 0.47 ± 0.01 | 0.46 ± 0.01 | 0.46 ± 0.02 | 0.46 ± 0.01 | 0.56 ± 0.01 | 0.57 ± 0.01 |
| L3 corpus vertebrae | ||||||
| Total BMD (g/cm3) | 0.59 ± 0.01 | 0.57 ± 0.03 | 0.57 ± 0.03 | 0.58 ± 0.03 | 0.62 ± 0.04 | 0.57 ± 0.08 |
| BV/TV (%) | 74 ± 5 | 72 ± 5 | 73 ± 3 | 74 ± 2 | 68 ± 5 | 61 ± 12 |
| Ct. BMD (g/cm3) | 1.01 ± 0.01 | 1.02 ± 0.02 | 1.01 ± 0.01 | 1.02 ± 0.02 | 1.06 ± 0.02 | 1.05 ± 0.02 |
| Tb. BMD (g/cm3) | 0.60 ± 0.01 | 0.59 ± 0.01 | 0.59 ± 0.02 | 0.59 ± 0.01 | 0.65 ± 0.01 | 0.65 ± 0.02 |
| Parameters | Experiment 1 | Experiment 2 | ||||
|---|---|---|---|---|---|---|
| Control n = 10 | OST n = 9 | TE n = 9 | OST + TE n = 10 | LIG n = 10 | LIG + TE n = 10 | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Femur distal | ||||||
| N.Nd (n/mm2) | 9.5 ± 2.4 | 9.5 ± 2.3 | 10.7 ± 1.3 | 8.9 c ± 1.8 | 9.1 ± 1.8 | 8.3 ± 1.7 |
| Tb.Wi (µm) | 87 ± 23 | 98 ± 24 | 94 ± 22 | 87 ± 18 | 87 ± 18 | 69 * ± 9 |
| Tb.Dn (%) | 21 ± 7 | 24 ± 8 | 24 ± 4 | 23 ± 6 | 22 ± 5 | 16 ± 4 |
| Ct.Dn (%) | 98 ± 1 | 98 ± 1 | 97 ad ± 1 | 98 ± 1 | 98 ± 1 | 98 ± 1 |
| Ct.Wi (mm) | 0.33 ± 0.04 | 0.34 ± 0.05 | 0.31 b ± 0.05 | 0.30 ab ± 0.05 | 0.30 ± 0.05 | 0.26 * ± 0.07 |
| Femur proximal | ||||||
| N.Nd (n/mm2) | 4.2 ± 2.0 | 4.4 ± 1.1 | 4.8 ± 1.2 | 5.6 a ± 1.8 | 4.0 ± 1.2 | 3.8 ± 1.2 |
| Tb.Wi (µm) | 78 ± 13 | 82 ± 8 | 87 ± 17 | 95 ab ± 14 | 76 ± 10 | 78 ± 11 |
| Tb.Dn (%) | 27 ± 9 | 29 ± 4 | 31 ± 7 | 34 a ± 7 | 26 ± 4 | 26 ± 5 |
| Ct.Dn (%) | 96 ± 2 | 96 ± 1 | 97 ± 1 | 97 ± 1 | 97 ± 1 | 98 ± 1 |
| L3 | ||||||
| N.Nd (n/mm2) | 8.5 ± 2.0 | 9.0 ± 1.6 | 7.9 ± 1.5 | 9.6 c ± 2.1 | 9.6 ± 2.1 | 8.0 * ± 1.5 |
| Tb.Wi (µm) | 131 ± 31 | 122 ± 27 | 124 ± 39 | 108 ± 23 | 87 ± 17 | 85 ± 23 |
| Tb.Dn (%) | 30 ± 6 | 30 ± 5 | 30 ± 5 | 28 ± 5 | 24 ± 3 | 22 ± 8 |
| Ct.Dn (%) | 94 ± 2 | 93 ± 3 | 94 ± 2 | 94 ± 3 | 94 ± 3 | 92 * ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komrakova, M.; Schilling, A.F.; Lehmann, W.; Vasilev, V.; Georgieva, K.; Gerginska, F.; Boyadjiev, N.; Delchev, S. Selective Androgen Receptor Modulators Combined with Treadmill Exercise Have No Bone Benefit in Healthy Adult Rats. Pharmaceuticals 2023, 16, 1249. https://doi.org/10.3390/ph16091249
Komrakova M, Schilling AF, Lehmann W, Vasilev V, Georgieva K, Gerginska F, Boyadjiev N, Delchev S. Selective Androgen Receptor Modulators Combined with Treadmill Exercise Have No Bone Benefit in Healthy Adult Rats. Pharmaceuticals. 2023; 16(9):1249. https://doi.org/10.3390/ph16091249
Chicago/Turabian StyleKomrakova, Marina, Arndt Friedrich Schilling, Wolfgang Lehmann, Veselin Vasilev, Katerina Georgieva, Fanka Gerginska, Nikolay Boyadjiev, and Slavi Delchev. 2023. "Selective Androgen Receptor Modulators Combined with Treadmill Exercise Have No Bone Benefit in Healthy Adult Rats" Pharmaceuticals 16, no. 9: 1249. https://doi.org/10.3390/ph16091249
APA StyleKomrakova, M., Schilling, A. F., Lehmann, W., Vasilev, V., Georgieva, K., Gerginska, F., Boyadjiev, N., & Delchev, S. (2023). Selective Androgen Receptor Modulators Combined with Treadmill Exercise Have No Bone Benefit in Healthy Adult Rats. Pharmaceuticals, 16(9), 1249. https://doi.org/10.3390/ph16091249







