In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster
Abstract
1. Introduction
2. Results
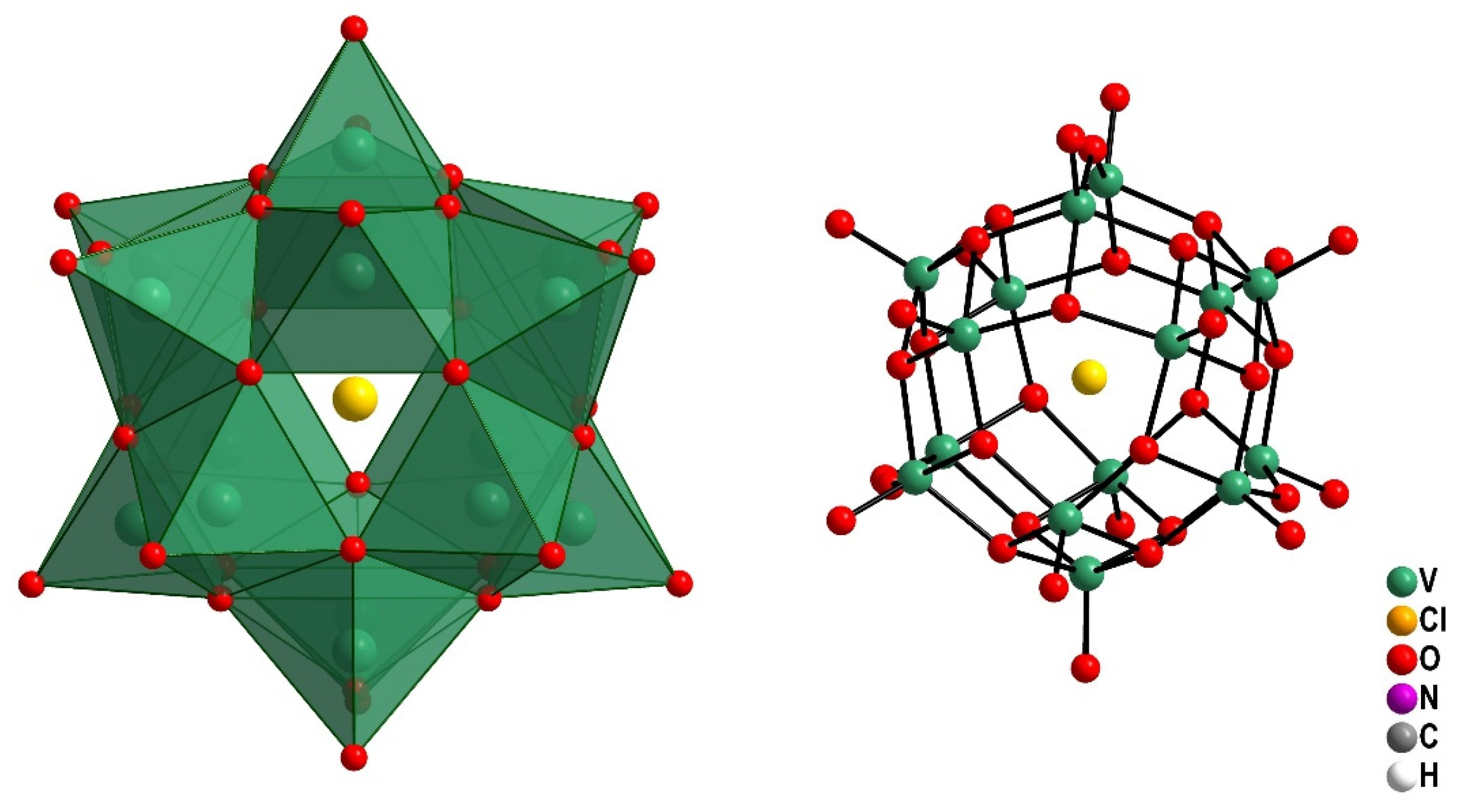
2.1. Preparation and Characterization of the Pentadecavanadate V15
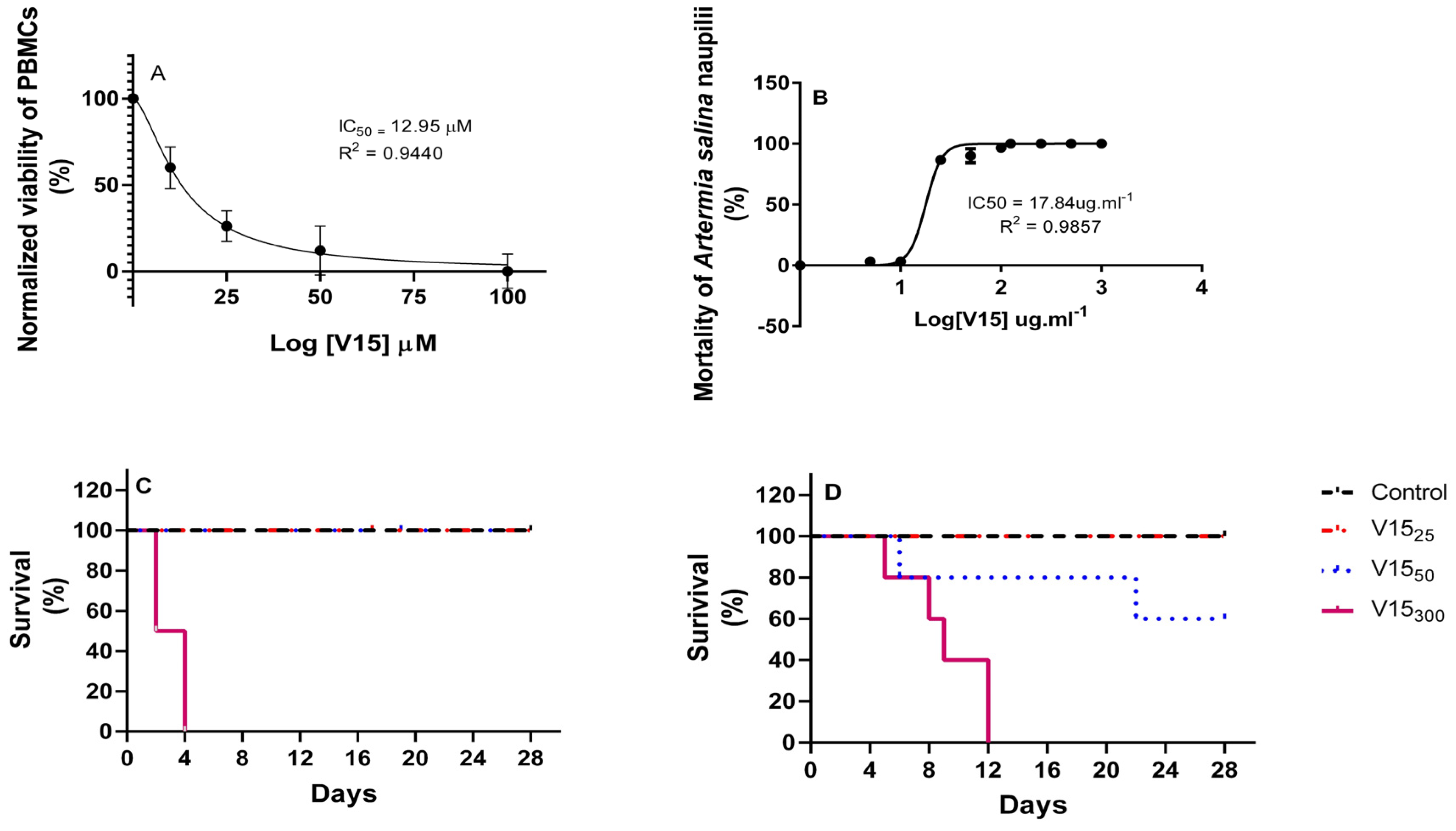
2.2. In Vitro Toxicity
2.3. Acute Oral Toxicity of V15
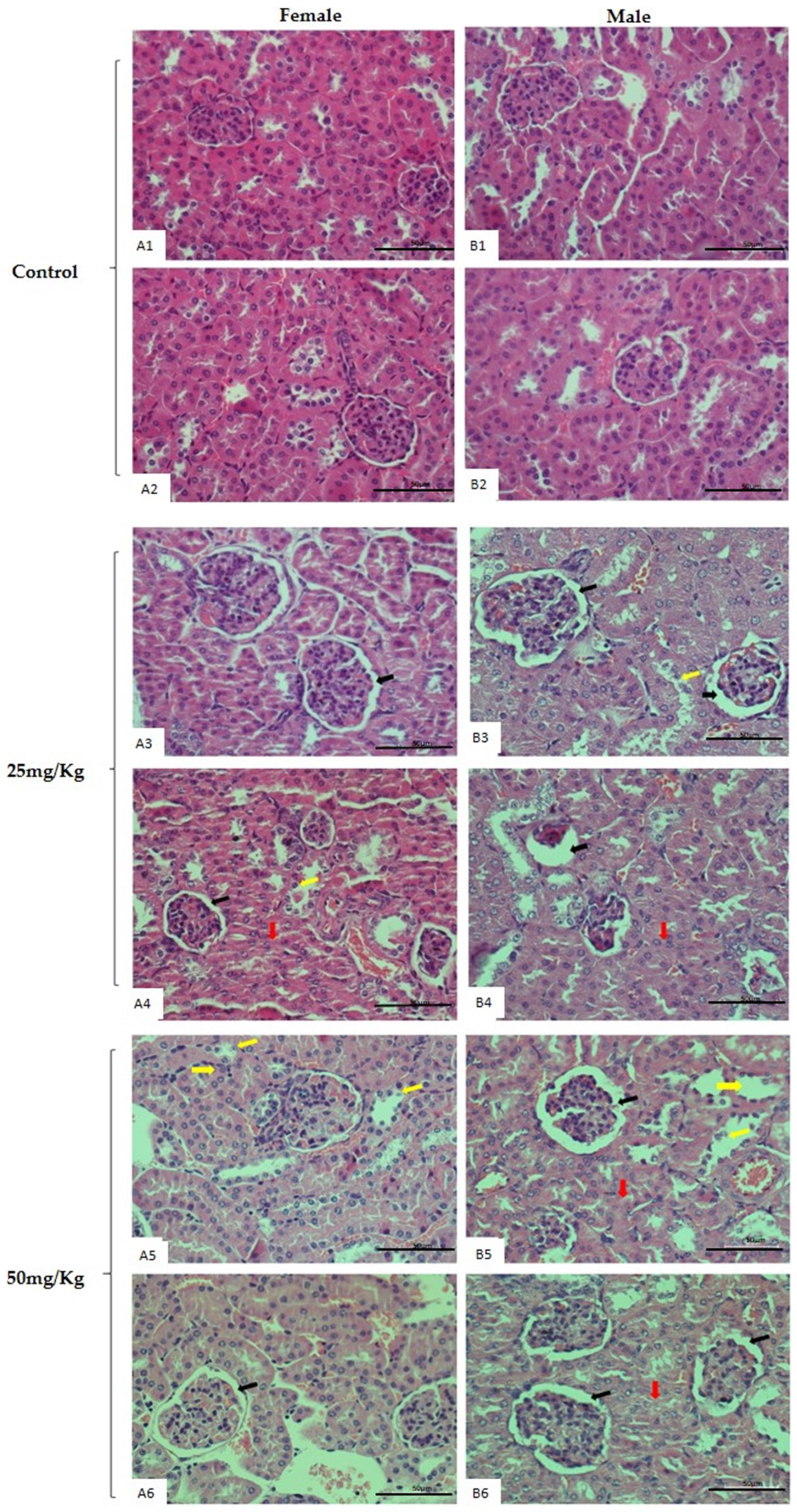
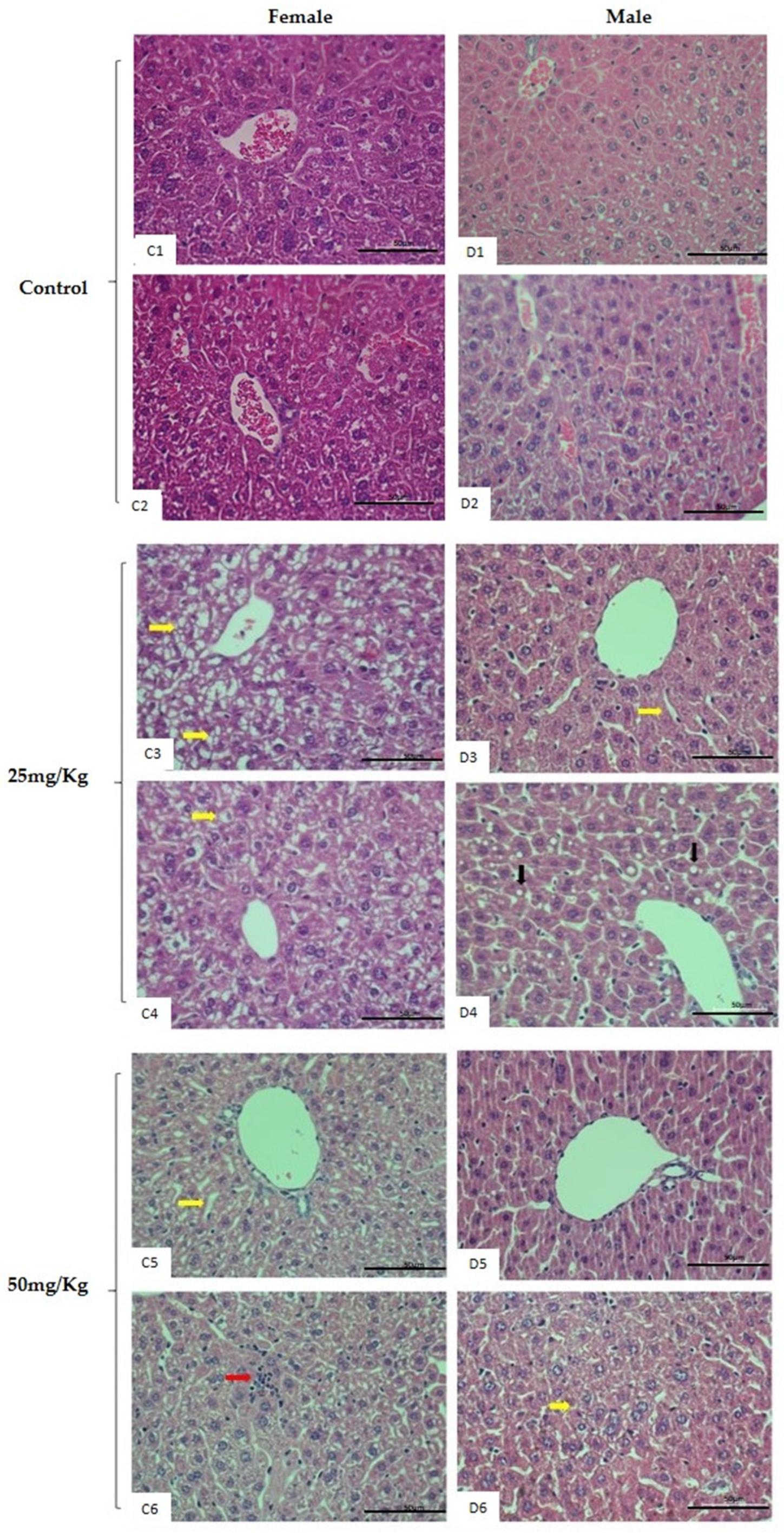
2.4. Repeated-Dose 28-Day Oral Toxicity Study in Rodents
2.5. Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Chemicals and General Methods
4.2.1. Synthesis of V15-(Me4N)6[V15O36(Cl)]
4.2.2. Characterization of V15 in the Solid and Solution States
4.3. Peripheral Blood Mononuclear Cells (PBMC) and Cytotoxicity Assay by 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) of V15
4.4. Toxicity against Larvae of Artemia Salina Leach
4.5. Oral Acute Toxicity
4.6. Toxicity Administered by the Repeated 28-Day Oral Toxicity Dose
4.7. Biochemical and Hematological Analysis
4.8. Oxidative Stress Evaluation in the Liver
4.9. Organ Mass and Histopathological Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALB | Albumin |
| ALT | Alanine aminotransferase |
| ANOVA | One-way analysis of variance |
| AST | Aspartate aminotransferase |
| BMOV | Bis(maltolato)oxidovanadium(IV) |
| BUN | Blood urea nitrogen |
| b.w. | Body weight |
| CAT | Catalase |
| CEUA | Ethics Committee on the Use of Animals |
| CRE | Creatinine |
| CHO | Chinese Hamster Ovary Cells |
| DMSO | Dimethyl sulfoxide |
| DNA | Deoxyribonucleic acid |
| EDL | Extensor digitorum longus |
| EPR | Eletronic paragmagnetic ressonance |
| FBS | Fetal bovine serum |
| GSH | Glutathione reduced |
| GSSG | Glutathione oxidized |
| GLB | Globulin |
| GPx | Glutathione peroxidase |
| Hb | Hemoglobin |
| HCT | Hematocrit |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| i.p | Intraperitoneal administration |
| LC50 | Lethal concentration median dose |
| LD50 | Median lethal dose |
| LPO | Lipid peroxidation |
| LYM | Lymphocytes |
| MCH | Mean corpuscular hemoglobin |
| MCHC | Mean corpuscular hemoglobin concentration |
| MDA | Malondialdehyde |
| MVP | Mixed-valence polyoxovanadates |
| MTT | 3-(4,5- dimethyl-2-thiazolyl)-2,5-diphenyl-2H-Tetrazolium bromide |
| NMR | Nuclear magnetic ressonance |
| o.g. | Oral gavage |
| OECD | Organization for Economic Cooperation and Development |
| PBMC | Peripheral blood mononuclear cells |
| POM | Polyoxometalate |
| POV | Polyoxovanadate |
| RBC | Red blood cells |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| Rpm | Rotation per minute |
| SD | Standard deviation |
| SEM | Standard error of the mean |
| SOD | Superoxide dismutase |
| STZ | Streptozotocin |
| TC | Total cholesterol |
| TP | Total protein |
| TBAR | Thiobarbituric acid |
| UA | Uric acid |
| WBC | White blood cells |
References
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Speciation atlas of polyoxometalates in aqueous solutions. Sci. Adv. 2023, 9, eadi0814. [Google Scholar] [CrossRef]
- Wang, X.; Wei, S.; Zhao, C.; Li, X.; Jin, J.; Shi, X.; Su, Z.; Li, J.; Wang, J. Promising application of polyoxometalates in the treatment of cancer, infectious diseases and Alzheimer’s disease. J. Biol. Inorg. Chem. 2022, 27, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Carvalho, E.; Eriksson, J.W.; Crans, D.C.; Aureliano, M. Effects of decavanadate and insulin enhancing vanadium compounds on glucose uptake in isolated rat adipocytes. J. Inorg. Biochem. 2009, 103, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Treviño, S.; González-Vergara, E. Metformin-decavanadate treatment ameliorates hyperglycemia and redox balance of the liver and muscle in a rat model of alloxan-induced diabetes. New J. Chem. 2019, 43, 17850–17862. [Google Scholar] [CrossRef]
- Colović, M.B.; Lacković, M.; Lalatović, J.; Mougharbel, A.S.; Kortz, U.; Krstić, D.Z. Polyoxometalates in biomedicine: Update and overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Samart, N.; Saeger, J.; Haller, K.J.; Aureliano, M.; Crans, D.C. Interaction of decavanadate with interfaces and biological model membrane systems: Characterization of soft oxometalate systems. J. Mol. Eng. Mater. 2014, 2, 1440007. [Google Scholar] [CrossRef]
- Althumairy, D.; Pozeal, K.; Barisas, B.G.; Nunes, G.G.; Roess, D.A.; Crans, D.C. Polyoxometalates function as indirect activators of a G protein-coupled receptor. Metallomics 2020, 12, 1044–1061. [Google Scholar] [CrossRef]
- Zhai, F.; Wang, X.; Li, D.; Zhang, H.; Li, R.; Song, L. Synthesis and biological evaluation of decavanadate Na4Co(H2O)6V10O28·18H2O. Biomed. Pharmacother. 2009, 63, 51–55. [Google Scholar] [CrossRef]
- Ferretti, V.A.; León, I.E. An Overview of Vanadium and Cell Signaling in Potential Cancer Treatments. Inorganics 2022, 10, 47. [Google Scholar] [CrossRef]
- Kostenkova, K.; Althumairy, D.; Rajan, A.; Kortz, U.; Barisas, B.G.; Roess, D.A.; Crans, D.C. Polyoxidovanadates [MoVIVV9O28]5− and [H2PtIVVV9O28]5− interact with CHO cell plasma membrane lipids causing aggregation and activation of a G protein-coupled receptor. Front. Chem. Biol. 2023, 2, 1126975. [Google Scholar] [CrossRef]
- Samart, N.; Althumairy, D.; Zhang, D.; Roess, D.A.; Crans, D.C. Initiation of a novel mode of membrane signaling: Vanadium facilitated signal transduction. Coord. Chem. Rev. 2020, 416, 213–286. [Google Scholar] [CrossRef]
- Bulgheroni, A.; Kinsner-Ovaskainen, A.; Hoffmann, S.; Hartung, T.; Prieto, P. Estimation of acute oral toxicity using the No Observed Adverse Effect Level (NOAEL) from the 28 days repeated dose toxicity studies in rats. Regul. Toxicol. Pharmacol. 2009, 53, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Xu, K.; Zhao, C.; Song, X.; Li, J.; Li, L.; Nie, W.; Bao, H.; Wang, J.; Niu, F.; et al. Genotoxicity and acute and subchronic toxicity studies of a bioactive polyoxometalate in Wistar rats. BMC Pharmacol. Toxicol. 2017, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.A.; Murakami, H.; Gaebler, D.J.; Silva, W.E.; Belian, M.F.; Lira, E.C.; Crans, D.C. Acute Toxicity Evaluation of Non-Innocent Oxidovanadium(V) Schiff Base Complex. Inorganics 2021, 9, 42. [Google Scholar] [CrossRef]
- Cohen, M.D.; Sisco, M.; Prophete, C.; Yoshida, K.; Chen, L.C.; Zelikoff, J.T.; Smee, J.; Holder, A.A.; Stonehuerner, J.; Crans, D.C.; et al. Effects of metal compounds with distinct physicochemical properties on iron homeostasis and antibacterial activity in the lungs: Chromium and vanadium. Inhal. Toxicol. 2010, 22, 169–178. [Google Scholar] [CrossRef]
- Cohen, M.D.; Sisco, M.; Prophete, C.; Chen, L.C.; Zelikoff, J.T.; Ghio, A.J.; Stonehuerner, J.D.; Smee, J.J.; Holder, A.A.; Crans, D.C. Pulmonary immunotoxic potentials of metals are governed by select physicochemical properties: Vanadium agents. J. Immunotoxicol. 2007, 4, 49–60. [Google Scholar] [CrossRef][Green Version]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Decavanadate induces mitochondrial membrane depolarization and inhibits oxygen consumption. J. Inorg. Biochem. 2007, 101, 789–796. [Google Scholar] [CrossRef]
- Soares, S.S.; Martins, H.; Duarte, R.O.; Moura, J.J.; Coucelo, J.; Gutiérrez-Merino, C.; Aureliano, M. Vanadium distribution, lipid peroxidation and oxidative stress markers upon decavanadate in vivo administration. J. Inorg. Biochem. 2007, 101, 80–88. [Google Scholar] [CrossRef]
- Soares, S.S.; Gutiérrez-Merino, C.; Aureliano, M. Mitochondria as a target for decavanadate toxicity in Sparus aurata heart. Aquat. Toxicol. 2007, 83, 1–9. [Google Scholar] [CrossRef]
- Samart, N.; Arhouma, Z.; Kumar, S.; Murakami, H.A.; Crick, D.C.; Crans, D.C. Decavanadate inhibits microbacterial growth more potently than other oxovanadates. Front. Chem. 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Arhouma, Z.; Murakami, H.A.; Koehn, J.T.; Li, X.; Roess, D.A.; Crick, D.C.; Crans, D.C. Exploring growth of Mycobacterium smegmatis treated with anticarcinogenic vanadium compounds. Inorganics 2022, 10, 50. [Google Scholar] [CrossRef]
- Kostenkova, K.; Arhouma, Z.; Postal, K.; Rajan, A.; Kortz, U.; Nunes, G.G.; Crick, D.C.; Crans, D.C. PtIV- or MoVI- substituted decavanadate inhibits the growth of Mycobacterium smegmatis. J. Inorg. Biochem. 2021, 217, 111356. [Google Scholar] [CrossRef] [PubMed]
- Kioseoglou, E.; Petanidis, S.; Gabriel, C.; Salifoglou, A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord. Chem. Rev. 2015, 301–302, 87–105. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, B.; Qi, Y.; Guo, S.; Tian, R.; Sun, J.; Zhao, M. The Anti-Proliferation Activity and Mechanism of Action of K12[V18O42(H2O)]∙6H2O on Breast Cancer Cell Lines. Molecules 2017, 22, 1535. [Google Scholar] [CrossRef] [PubMed]
- Roubatis, L.; Anastasiadis, N.C.; Paratriantafyllopoulou, C.; Moushi, E.; Tasiopoulos, A.J.; Karkabounas, S.C.; Veltsistas, P.G.; Perlepes, S.P.; Evangelou, A.M. A missing oxidation-state level in the family of polyoxo(azide)octadecavanadate(IV/V) clusters: Synthesis, structure and antitumoural properties of [VIV11VV7O44(N3)]10− in a sodium containing-3D architecture. Inorg. Chem. Commun. 2016, 69, 85–88. [Google Scholar] [CrossRef]
- Carvalho, F.; Aureliano, M. Polyoxometalates Impact as Anticancer Agents. Int. J. Mol. Sci. 2023, 24, 5043. [Google Scholar] [CrossRef]
- Nunes, G.G.; Bonatto, A.C.; Albuquerque, C.G.; Barison, A.; Ribeiro, R.R.; Back, D.F.; Andrade, A.V.C.; de Sá, E.L.; Pedrosa, F.D.O.; Soares, J.F.; et al. Synthesis, characterization and chemoprotective activity of polyoxovanadates against DNA alkylation. J. Inorg. Biochem. 2012, 108, 36–46. [Google Scholar] [CrossRef]
- Postal, K.; Maluf, D.L.; Valdameri, A.; Rudiger, A.L.; Hughes, D.L.; de Sá, E.L.; Ribeiro, R.R.; de Souza, E.M.; Soares, J.F.; Nunes, G.G. Chemoprotective activity of mixed valence polyoxovanadates against diethylsulphate in E. coli cultures: Insights from solution speciation studies. RSC Adv. 2016, 6, 114955. [Google Scholar] [CrossRef]
- Arhouma, Z.A. Antibacterial Growth Effects and Speciations of Several Vanadium Salts and Complexes. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2022. [Google Scholar]
- Kita, D.H.; de Andrade, G.A.; Missina, J.M.; Postal, K.; Boell, V.K.; Santana, F.S.; Zattoni, I.F.; Zanzarini, I.D.S.; Moure, V.R.; Rego, F.G.M.; et al. Polyoxovanadates as new P-glycoprotein inhibitors: Insights into the mechanism of inhibition. FEBS Lett. 2022, 596, 381–399. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, G.; Aureliano, M. Polyoxovanadates Contribution to Pharmacological, Antimicrobial and Toxicological Actions of Vanadium. Med. Sci. Forum 2022, 11, 8. [Google Scholar] [CrossRef]
- Doucette, K.A.; Hassell, K.N.; Crans, D.C. Selective Speciation Improves Efficacy and Lowers Toxicity of Platinum Anticancer and Vanadium Antidiabetic Drugs. J. Inorg. Biochem. 2016, 165, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Llobet, J.M.; Domingo, J.L. Acute Toxicity of Vanadium Compounds in Rats and Mice. Toxicol. Lett. 1984, 23, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Gomez, M.; Sanchez, D.J.; Llobet, J.M.; Keen, C.L. Toxicology of Vanadium Compounds in Diabetic Rats: The Action of Chelating Agents on Vanadium Accumulation. Mol. Cell. Biochem. 1995, 153, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.S.; Li, J.B.; Liu, Y.Y.; Wu, H.; Cao, A.; Wang, H. Cytotoxicity and genotoxicity of low-dose vanadium dioxide nanoparticles to lung cells following long-term exposure. Toxicology 2021, 459, 152859. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Trujillo, A.M.; Pharazyn, P.S.; Cohen, M.D. How environment affects drug activity: Localization, compartmentalization and reactions of a vanadium insulin-enhancing compound, dipicolinatooxovanadium(V). Coord. Chem. Rev. 2011, 19–20, 2178–2192. [Google Scholar] [CrossRef]
- Aureliano, M.; de Sousa-Coelho, A.L.; Dolan, C.C.; Roess, D.A.; Crans, D.C. Biological Consequences of Vanadium Effects on Formation of Reactive Oxygen Species and Lipid Peroxidation. Int. J. Mol. Sci. 2023, 24, 5382. [Google Scholar] [CrossRef]
- Scibior, A.; Pietrzyk, L.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacological mechanisms and multi-application with a summary of further research trends. J. Trace Elem. Med. Bio. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Muller, A.; Krickemeyer, E.; Penk, M.; Walberg, H.J.; Bogge, H. Spherical Mixed-Valence [V15O36]5⊖, an Example from an Unusual Cluster Family. Chem. Int. Ed. 1987, 26, 1045–1046. [Google Scholar] [CrossRef]
- Rhaiem, A.B.; Hlel, F.; Guidara, K.; Gargouri, M. Vibrational study of [(CH3)4N]2Cu0.5Zn0.5Cl4. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2007, 66, 1107–1109. [Google Scholar] [CrossRef] [PubMed]
- Mabbs, F.E. Some aspects of the electron paramagnetic resonance spectroscopy of d-transition metal compounds. Chem. Soc. Rev. 1993, 22, 313–324. [Google Scholar] [CrossRef]
- Postal, K.; Santana, F.S.; Hughes, D.L.; Rüdiger, A.L.; Ribeiro, R.R.; de Sá, E.L.; de Souza, E.M.; Soares, J.F.; Nunes, G.G. Stability in solution and chemoprotection by octadecavanadates(IV/V) in E. coli cultures. J. Inorg. Biochem. 2021, 219, 1114–1138. [Google Scholar] [CrossRef]
- OECD. Test No. 423: Acute Oral Toxicity—Acute Toxic Class Method. In OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2002. [Google Scholar]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalates in medicine. Chem. Rev. 1998, 98, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. Polyoxometalates as potential next-generation metallodrugs in the combat against cancer. Angew. Chem. Int. Ed. 2019, 58, 2980–2999. [Google Scholar] [CrossRef] [PubMed]
- Bijelic, A.; Aureliano, M.; Rompel, A. The antibacterial activity of polyoxometalates: Structures, antibiotic effects and future perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xing, C.; Zhan, S.; Zhao, M.; Li, M.; Liu, H. A polyoxometalate-modified magnetic nanocomposite: A promising antibacterial material for water treatment. J. Mater. Chem. B 2019, 7, 1933–1944. [Google Scholar] [CrossRef]
- Kiss, T.; Jakusch, T.; Hollender, D.; Dörnyei, A.; Enyedy, E.A.; Pessoa, J.C.; Sakurai, H.; Sanz-Medel, A. Biospeciation of antidiabetic VO(IV) complexes. Coord. Chem. Rev. 2008, 252, 1153–1162. [Google Scholar] [CrossRef]
- Pessoa, J.C.; Santos, M.F.A.; Correia, I.; Sanna, D.; Sciortino, G.; Garribba, E. Binding of vanadium ions and complexes to proteins and enzymes in aqueous solution. Coord. Chem. Rev. 2021, 449, 214192. [Google Scholar] [CrossRef]
- Rehder, D. The potentiality of vanadium in medicinal applications. Future Med. Chem. 2012, 4, 1823–1837. [Google Scholar] [CrossRef]
- Corona-Motolinia, N.D.; Martínez-Valencia, B.; Noriega, L.; Sánchez-Gaytán, B.L.; Melendez, F.J.; García-García, A.; Choquesillo-Lazarte, D.; Rodríguez-Diéguez, A.; Castro, M.E.; González-Vergara, E. Tris(2-Pyridylmethylamine)V(O)2 Complexes as Counter Ions of Diprotonated Decavanadate Anion: Potential Antineoplastic Activity. Front. Chem. 2022, 10, 830511. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Noriega, L.; Meléndez-Bustamante, F.J.; Castro, M.E.; Sánchez-Gaytán, B.L.; Choquesillo-Lazarte, D.; González-Vergara, E.; Rodríguez-Diéguez, A. 2-Aminopyrimidinium Decavanadate: Experimental and Theoretical Characterization, Molecular Docking, and Potential Antineoplastic Activity. Inorganics 2021, 9, 67. [Google Scholar] [CrossRef]
- Olaolorun, F.A.; Obasa, A.A.; Balogun, H.A.; Alnda, O.O.; Olopade, J.O. Lactational vitamin E protects against the histotoxic effects of systematically administers vanadium in neonatal rats. Niger. J. Physiol. Sci. 2014, 29, 125–129. Available online: https://pubmed.ncbi.nlm.nih.gov/26196578/ (accessed on 14 June 2023). [PubMed]
- Adebiyi, O.E.; Obisesan, A.D.; Olayemie, F.O.; Olopase, J.O. Protective effect of ethanolic extract of Grewia carpinifolia leaves on vanadium induced toxicity. Alex. J. Vet. Sci. 2015, 47, 1–6. [Google Scholar] [CrossRef]
- Beusen, J.M.; Neven, B. Toxicity of vanadium to different freshwater organisms. Bull. Environ. Contam. Toxicol. 1987, 39, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Borges, G.; Medonça, P.; Joaquim, N.; Coucelo, J.; Aureliano, M. Acute effects of vanadate oligomers on heart, kidney, and liver histology in the Lusitanian toadsifh (Halobatrachus didactylys). Arch. Environ. Contam. Toxicol. 2003, 45, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Callander, R.; Duffy, P.; Jacobesen, M.; Knight, R.; Boobis, A. Target organ profiles in toxicity studies supporting human dosing: Does severity progress with longer duration of exposure? Regul. Toxicol. Pharmacol. 2015, 73, 737–746. [Google Scholar] [CrossRef]
- Lipnick, R.L.; Cotruvo, J.A.; Hill, R.N.; Bruce, R.D.; Stitzel, K.A.; Walker, A.P.; Chu, I.; Goddard, M.; Segal, L.; Springer, J.A.; et al. Comparison of the Up-and-down, Conventional LD50, and Fixed-Dose Acute Toxicity Procedures. Food Chem. Toxicol. 1995, 33, 223–231. [Google Scholar] [CrossRef]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.; McLaughilin, J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica 1982, 45, 31–34. [Google Scholar] [CrossRef]
- Sarah, Q.S.; Anny, F.; Misbahuddin, M. Brine shrimp lethality assay. Bangladesh J. Pharmacol. 2017, 12, 5. [Google Scholar] [CrossRef]
- David, J.P.; Silva, E.F.; Moura, D.L.; Guedes, M.L.; Assunção, R.J.; David, J.M. Lignanas e triterpenos do extrato citotóxico de Eriope blanchetii. Quim. Nova 2001, 24, 730–733. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Jiang, S.; Zhang, M.; Lu, J.; Huang, L.; Zhang, T.; Gong, K.; Yan, S.; Yang, Z.; et al. Vanadate-induced antiproliferative and apoptotic response in esophageal squamous carcinoma cell line EC109. J. Toxicol. Environ. Health 2016, 79, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Dávalos, A.; Gonzalez-Villava, A.; Rodriguéz-Lara, V.; Montaño, L.F.; Fortoul, T.I.; López-Marure, R. Vanadium pentoxide induces activation and death of endothelial cells. J. Appl. Toxicol. 2012, 32, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Rodrígues-Mercado, J.J.; Roldán-Reyes, E.; Altamirano-Lozano, M.A. Genotoxic effects of vanadium(IV) in human peripheral blood cells. Toxicol. Lett. 2003, 144, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, V.P.; Kar, A.B. Antitesticular effect of metallic and rate earth salts. J. Reprod. Fertil. 1964, 7, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lara, V.; Morales-Rivero, A.; Rivera-Cambas, A.M.; Fortoul, T.I. Vanadium inhalation induces actin changes in mice testicular cells. Toxicol. Ind. Health 2016, 32, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K. Anti-Diabetic and Toxic Effects of Vanadium Compounds. Mol. Cell. Biochem. 2000, 206, 177–182. [Google Scholar] [CrossRef]
- Wilk, A.; Szypulska-Koziarska, D.; Wiszniewska, B. The toxicity of vanadium on gastrointestinal, urinary and reproductive system, and its influence on fertility and fetuses malformations. Adv. Hyg. Exp. Med. 2017, 71, 850–859. [Google Scholar] [CrossRef]
- Domingo, J.L. Vanadium: A review of the reproductive and developmental toxicity. Reprod. Toxicol. 1996, 10, 175–182. [Google Scholar] [CrossRef]
- Shrivastava, S.; Joshi, D.; Bhadauria, M.; Shukla, S.; Mathur, R. Cotherapy of tiron and selenium Against vanadium induced toxic effects in lactating rats. Iran. J. Reprod. Med. 2011, 9, 229–238. Available online: https://pubmed.ncbi.nlm.nih.gov/26396569/ (accessed on 14 June 2023).
- Sánchez-González, C.; Rivas-García, L.; López-Chaves, C.; Rodríguez-Nogales, A.; ALgieri, F.; Gálvez, J.; Gómez-Aracena, J.; Vera-Ramírez, L.; Montes-Bayon, M.; Sanz-Medel, L.; et al. Exposure to bis(maltolato)oxovanadium(IV) increases levels of hepcidin mRNA and impairs the homeostasis of iron but not that of manganese. J. Food Chem. Toxicol. 2014, 73, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; McNeill, J.H. One-year treatment of non-diabetic and streptozotocin-diabetic rats with vanadyl sulphate did not alter blood pressure or haematological indices. Pharmacol. Toxicol. 1994, 74, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Villalva, A.; Fortoul, T.I.; Avila-Costa, M.R.; Piñon-Zarate, G.; Rodriguez-Lara, V.; Martinéz-Levi, G.; Rojas-Lemus, M.; Bizarro-Nevarez, P.; Díaz-Bech, P.; Mussali-Galante, P.; et al. Thrombocytosis induced in mice after subacute and subchronic V2O5 inhalation. Toxicol. Ind. Health 2006, 22, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Sanna, D.; Garribba, E. Pharmacologically active vanadium species: Distribution in biological media and interaction with molecular targets. Curr. Med. Chem. 2021, 28, 7339–7384. [Google Scholar] [CrossRef]
- Soares, S.S.; Martins, H.; Aureliano, M. Vanadium distribution following decavanadate administration. Arch. Environ. Contam. Toxicol. 2006, 50, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Espionsa-Zurutuza, M.; González-Villalva, A.; Albarrán-Alonso, J.C.; Colín-Barenque, L.; Bizarro-Nevares, P.; Rojas-Lemus, M.; López-Valdez, N.; Fortoul, T.I. Oxidative stress as a mechanism involved in kidney damage after subchronic exposure to vanadium inhalation and oral sweetened beverages in a mouse model. Int. J. Toxicol. 2018, 37, 45–52. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Saha, R.; Saha, B. Toxicity of inorganic vanadium compounds. Res. Chem. Intermed. 2015, 41, 4873–4897. [Google Scholar] [CrossRef]
- Donaldson, J.; Hemming, R.; Labella, F. Vanadium exposure enhances lipid peroxidation in the kidney of rats and mice. Can. J. Physiol. Pharmacol. 1984, 63, 1961–1999. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Bermudez-Peña, C.; Trenzado, C.E.; Goenaga-Infante, H.; Montes-Bayon, M.; Sanz-Medel, A.; Llopis, J. Change in the antioxidant defense in selenium concentration in tissues of vanadium exposed rats. Metallomics 2012, 4, 814–819. [Google Scholar] [CrossRef]
- Koubaa, F.G.; Chaâbane, M.; Turki, M.; Ayadi, F.M.; El Feki, A. Anti-oxidant and hepatoprotective effects of Salvia officinalis essential oil against vanadium-induced oxidative stress and histological changes in the rat liver. Environ. Sci. Pollut. Res. Int. 2021, 28, 11001–11015. [Google Scholar] [CrossRef]
- Oster, M.H.; Llobet, J.M.; Domingo, J.L.; Bruce German, J.; Keen, C.L. Vanadium treatment of diabetic Sprague-Dawley rats results in tissue vanadium accumulation and pro-oxidant effects. Toxicology 1993, 83, 115–130. [Google Scholar] [CrossRef]
- Gândara, R.M.C.; Soares, S.S.; Martins, H.; Gutiérrez-Merino, C.; Aureliano, M. Vanadate oligomers: In vivo effects in hepatic vanadium accumulation and stress markers. J. Inorg. Biochem. 2005, 99, 238–1244. [Google Scholar] [CrossRef] [PubMed]
- Nnama, A.U.; Ekeh, F.N.; Aguzie, I.F.; Udegbuman, S.L.; Nwani, C.D. Vanadium pentoxide induces hematological, oxidative stress and histological chages in Oryctolagus cuniculus. J. Hazard. Mater. Adv. 2022, 5, 100048. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, L.L.; Liu, T.Y. Evaluation of gender-related differences in various oxidative stress in enzymes in mice. Chin. J. Physiol. 2011, 54, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J. Cell. Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Oinam, S.; Basu, M.; Chatterjee, M. Vanadium chemoprevention of 7,12-dimethylbenz(a)anthracene-induced rat mammary carcinogenesis: Probable involvement of representative hepatic phase I and II xenobiotic metabolizing enzymes. Breast Cancer Res. Treat. 2000, 63, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.H.; McNeill, J.H. Effect of vanadyl sulfate feeding on susceptibility to peroxidative change in diabetic rats. Res. Commun. Chem. Pathol. Pharmacol. 1993, 80, 187–200. Available online: https://pubmed.ncbi.nlm.nih.gov/8100638 (accessed on 14 June 2023).
- Crans, D.C.; Zhang, B.; Gaidamauskas, E.; Keramidas, A.D.; Willsky, G.R.; Roberts, C.R. Is vanadate reduced by thiols under biological conditions?: Changing the redox potential of V(V)/V(IV) by complexation in aqueous solution. Inorg. Chem. 2010, 49, 4245–4256. [Google Scholar] [CrossRef]
- Kang, D.H.; Nakagawa, T.; Feng, L.; Watanabe, S.; Han, L.; Mazzali, M.; Truong, L.; Harris, R.; Johnson, R.J. A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 2002, 13, 2888–2897. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Liu, J.; Cui, H.; Liu, X.; Peng, X.; Deng, H.; Zuo, Z.; Cui, W.; Deng, Y.; Wang, K. Dietary high vanadium causes oxidative damage-induced renal and hepatic toxicity in broilers. Biol. Trace Elem. Res. 2012, 145, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shi, Y.; Wu, Q.; Ma, W. Epigallocatechin-3-gallate alleviates vanadium-induced reduction of antioxidant capacity via keap1-Nrf2-sMaf pathway in the liver, kidney, and ovary of laying hens. Biol. Trace Elem. Res. 2021, 199, 2707–2716. [Google Scholar] [CrossRef] [PubMed]
- Cano-Gutiérrez, G.; Acevedo-Nava, S.; Santamaría, A.; Altamirano-Lozano, M.; Cano-Rodríguez, M.C.; Fortoul, T.I. Hepatic megalocytosis due to vanadium inhalation: Participation of oxidative stress. Toxicol. Ind. Health 2012, 28, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Fortoul, T.; Rodriguez-Lara, V.; González-Villalva, A.; Rojas-Lemus, M.; Cano-Gutiérrez, G.; Ustarroz-Cano, M.; Colín-Barenque, L.; Bizarro-Nevares, P.; García-Pealez, I.; Montaño, L.; et al. Inhalation of vanadium pentoxide and its effects in a mouse model. Inorg. Chim. Acta 2014, 420, 9–15. [Google Scholar] [CrossRef]
- Xiong, Z.; Xing, C.; Xu, T.; Yang, Y.; Liu, G.; Hu, G.; Cao, H.; Zhang, C.; Guo, X.; Yang, F. Vanadium Induces Oxidative Stress and Mitochondrial Quality Control Disorder in the Heart of Ducks. Front. Vet. Sci. 2021, 26, 756534. [Google Scholar] [CrossRef]
- Wang, J.P.; Cui, R.Y.; Zhang, K.Y.; Ding, X.M.; Luo, Y.H.; Bai, S.P.; Zeng, Q.F.; Xuan, Y.; Su, Z.W. High-fat diet increased renal and hepatic oxidative stress induced by vanadium of Wistar rat. Biol. Trace Elem. Res. 2016, 170, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Barbosa, H.M.; Do Nascimento, J.N.; Araújo, T.A.S.; Duarte, F.S.; Albuquerque, U.P.; Vieira, J.R.C.; De Santana, E.R.B.; Yara, R.; Lima, C.S.A.; Gomes, D.A.; et al. Acute Toxicity and Cytotoxicity Effect of Ethanolic Extract of Spondias Tuberose Arruda Bark: Hematological, Biochemical and Histopathological Evaluation. An. Acad. Bras. Cienc. 2016, 88, 1993–2004. [Google Scholar] [CrossRef]
- OECD. Test No 407: Repeated dose 28-day oral toxicity study in rodent. In OECD Guidelines for the Testing of Chemicals, Section 4, Health Effects; OECD Publishing: Paris, France, 2008. [Google Scholar]
- Jiang, P.; Ni, Z.; Wang, B.; Ma, B.; Duan, H.; Li, X.; Ma, X.; Wei, Q.; Ji, X.; Liu, Q.; et al. Acute toxicity, twenty-eight days repeated dose toxicity and genotoxicity of vanadyl trehalose in Kunming mice. Regul. Toxicol. Pharmacol. 2017, 85, 86–97. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Hissin, P.J.; Hilf, R. A Fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 10, 3170–3175. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]

| Parameters | Control | V15–50 | V15–300 | V15–2000 |
|---|---|---|---|---|
| Total body weight (g) | 470.5 ± 11.7 a | 484.3 ± 15.8 a | 463.0 ± 14.8 a | 503.0 ± 20.0 a |
| Total food intake (g) | 96.5 ± 3.8 a | 89.7 ± 4.1 a | 102.7 ± 4.2 a | 103.3 ± 1.9 a |
| Total fluid intake (mL) | 194.0 ± 9.9 a | 189.3 ± 3.7 a | 234.0 ± 4.5 b | 235.0 ± 9.5 b |
| Organ | Control | V15–50 | V15–300 | V15–2000 |
|---|---|---|---|---|
| Heart | 0.56 ± 0.13 a | 0.69 ± 0.26 a | 0.54 ± 0.06 a | 0.57 ± 0.02 a |
| Lung | 0.54 ± 0.01 a | 0.58 ± 0.07 a | 0.72 ± 0.05 b | 0.68 ± 0.07 b |
| Liver | 5.93 ± 0.19 a | 5.15 ± 0.15 a | 5.94 ± 0.18 a | 6.70 ± 0.29 b |
| Kidney | 1.46 ± 0.04 a | 1.29 ± 0.03 a | 1.38 ± 0.036 a | 1.38 ± 0.04 a |
| Spleen | 0.54 ± 0.02 a | 0.46 ± 0.04 a | 0.64 ± 0.03 a | 0.66 ± 0.04 a |
| Tibial anterior | 0.13 ± 0.01 a | 0.15 ± 0.02 a | 0.12 ± 0.01 a | 0.13 ± 0.01 a |
| Soleus | 0.024 ± 0.002 a | 0.021 ± 0.001 a | 0.016 ± 0.002 a | 0.018 ± 0.001 a |
| Parameters | Control | V15–50 | V15–300 | V15–2000 |
|---|---|---|---|---|
| AST (mmol/L) | 58.5 ± 1.1 a | 87.0 ± 3.1 b | 117.9 ± 15.4 c | 139.7 ± 19.4 c |
| ALT (mmol/L) | 66.1 ± 5.3 a | 59.9 ± 0.6 a | 141.6 ± 8.9 b | 165.5 ± 14.4 b |
| Total protein (mg/dL) | 45.6 ± 4.2 a | 41.0 ± 1.1 a | 49.5 ± 2.5 a | 48.0 ± 3.6 a |
| Albumin (μmol/L) | 314.0 ± 29.0 a | 357.4 ± 4.8 a | 236.8 ± 11.3 b | 218.7 ± 5.4 c |
| BUN (mg/dL) | 11.2 ± 0.6 a | 11.2 ± 0.6 a | 11.2 ± 0.7 a | 10.5 ± 0.4 a |
| Male | |||
|---|---|---|---|
| Parameters | Control | V15–25 | V15–50 |
| Body weight (g) | 1071.80 ± 21.8 a | 1.08000 ± 12.1 a | 1.07000 ± 29.2 a |
| Food intake (g) | 177.0 ± 6.6 a | 170.8 ± 2.8 a | 155.6 ± 5.4 b |
| Fluid intake (mL) | 316.0 ± 5.89 a | 327.5 ± 14.2 a | 308.40 ± 10.5 a |
| Female | |||
| Parameters | Control | V15–25 | V15–50 |
| Body weight (g) | 941.20 ± 28.7 a | 928.80 ± 15.4 a | 912.70 ± 15.5 a |
| Food intake (g) | 146.8 ± 6.9 a | 154.2 ± 5.7 a | 136.3 ± 5.4 b |
| Fluid intake (mL) | 303.6 ± 9.0 a | 303.2 ± 18.1 a | 322.7 ± 3.5 a |
| Male Mice | |||
|---|---|---|---|
| Organ (mg/100 g of b.w.) | Control | V15–25 | V15–50 |
| Brain | 1.13 ± 0.03 a | 1.10 ± 0.06 a | 1.05 ± 0.03 a |
| Heart | 0.41 ± 0.01 a | 0.51 ± 0.11 a | 0.45 ± 0.01 a |
| Lung | 0.62 ± 0.02 a | 0.63 ± 0.05 a | 0.52 ± 0.06 a |
| Liver | 4.69 ± 0.24 a | 5.41 ± 0.10 a | 4.99 ± 0.66 a |
| Kidney | 1.41 ± 0.05 a | 1.44 ± 0.08 a | 1.22 ± 0.08 b |
| Spleen | 0.35 ± 0.04 a | 0.42 ± 0.07 a | 0.39 ± 0.02 a |
| Tibial anterior | 0.15 ± 0.01 a | 0.14 ± 0.01 a | 0.13 ± 0.01 a |
| Gastrocnemius | 0.36 ± 0.01 a | 0.36 ± 0.02 a | 0.34 ± 0.02 a |
| Teste | 0.74 ± 0.08 a | 0.64 ± 0.08 b | 0.60 ± 0.01 b |
| Female mice | |||
| Organ (mg/100 g of b.w.) | Control | V15–25 | V15–50 |
| Brain | 1.24 ± 0.09 a | 1.23 ± 0.04 a | 1.25 ± 0.03 a |
| Heart | 0.48 ± 0.04 a | 0.41 ± 0.01 a | 0.39 ± 0.02 a |
| Lung | 0.67 ± 0.09 a | 0.64 ± 0.05 a | 0.70 ± 0.05 a |
| Liver | 5.03 ± 0.22 a | 5.30 ± 0.23 a | 5.78 ± 0.05 a |
| Kidney | 1.34 ± 0.06 a | 1.16 ± 0.04 a | 1.14 ± 0.05 a |
| Spleen | 0.47 ± 0.04 a | 0.48 ± 0.04 a | 0.50 ± 0.05 a |
| Tibial anterior | 0.24 ± 0.06 a | 0.14 ± 0.01 b | 0.13 ± 0.01 b |
| Gastrocnemius | 0.25 ± 0.01 a | 0.28 ± 0.01 a | 0.31 ± 0.03 a |
| Ovaries and uterus | 1.32 ± 0.07 a | 0.94 ± 0.04 b | 0.92 ± 0.07 b |
| Male Mice | |||
|---|---|---|---|
| Parameters | Control | V15–25 | V15–50 |
| RBC (×103/μ) | 6.80 ± 0.50 a | 6.90 ± 0.50 a | 5.50 ± 0.70 a |
| Hemoglobin (%) | 15.20 ± 1.30 a | 12.50 ± 0.50 b | 13.30 ± 0.60 b |
| MCV (μm3) | 58.50 ± 2.70 a | 46.70 ± 0.60 b | 47.70 ± 0.40 b |
| MCH (pg) | 20.60 ± 0.70 a | 18.70 ± 0.70 a | 17.40 ± 3.20 b |
| MCHC (g/dL) | 35.50 ± 1.80 a | 38.60 ± 0.10 a | 37.40 ± 6.80 a |
| HCT (%) | 43.40 ± 4.70 a | 32.20 ± 1.90 b | 26.40 ± 3.20 c |
| Platelet count (×103/μ) | 531.00 ± 86.94 a | 778.00 ± 23.35 b | 942.75 ± 17.33 c |
| WBC (×103/μ) | 14.00 ± 0.70 a | 13.30 ± 0.10 a | 14.50 ± 0.40 a |
| Monocytes (%) | 6.90 ± 1.00 a | 7.70 ± 0.30 a | 7.60 ± 2.50 a |
| Lymphocyte (%) | 89.50 ± 2.10 a | 89.00 ± 0.30 a | 87.40 ± 4.00 a |
| Female mice | |||
| Parameters | Control | V15–25 | V15–50 |
| RBC (×103/μ) | 14.80 ± 0.50 a | 13.90 ± 0.20 a | 15.2 ± 0.20 a |
| Hemoglobin (%) | 13.80 ± 0.60 a | 11.20 ± 0.80 b | 11.40 ± 0.70 b |
| MCV (μm3) | 56.80 ± 5.40 a | 47.30 ± 0.80 b | 42.40 ± 0.40 c |
| MCH (pg) | 20.90 ± 1.10 a | 15.10 ± 0.40 b | 15.30 ± 7.00 c |
| MCHC (g/dL) | 37.30 ± 1.60 a | 35.30 ± 2.40 a | 33.50 ± 17.50 a |
| HCT (%) | 37.30 ± 2.30 a | 31.80 ± 0.40 a | 11.4 ± 5.80 c |
| Platelet count (×103/μ) | 438.82 ± 13.82 a | 499.00 ± 14.47 b | 911.66 ± 16.23 b |
| WBC (×103/μ) | 6.70 ± 0.60 a | 7.40 ± 0.60 a | 3.60 ± 0.90 b |
| Monocytes (%) | 3.50 ±0.60 a | 4.90 ± 0.60 a | 6.10 ± 1.30 b |
| Lymphocyte (%) | 88.30 ± 4.70 a | 91.1 ± 0.60 a | 90.40 ± 2.40 a |
| Male Mice | |||
|---|---|---|---|
| Parameters | Control | V15–25 | V15–50 |
| ALT (mmol/L) | 39.90 ± 1.80 a | 103.70 ± 5.40 b | 122.50 ± 8.30 c |
| AST (mmol/L) | 74.80 ± 2.40 a | 81.90 ± 2.90 a | 145.40 ± 8.30 b |
| Total protein (g/L) | 46.60 ± 5.60 a | 46.00 ± 11.90 a | 51.60 ± 2.10 a |
| BUN (mg/dL) | 12.00 ± 0.20 a | 13.40 ± 0.90 a | 11.60 ± 0.01 a |
| Creatinine (µmol/L) | 29.80 ± 2.40 a | 23.86 ± 2.60 a | 71.90 ± 20.50 b |
| Uric acid (µmol/L) | 122.31 ± 15.30 a | 185.30 ± 17.30 b | 211.80 ± 32.70 b |
| Total cholesterol (mmol/L) | 1.60 ± 0.18 a | 1.58 ± 0.41 a | 1.80 ± 0.19 a |
| Triglycerides (mmol/L) | 1.62 ± 0.11 a | 1.84 ± 0.13 a | 1.30 ± 0.10 b |
| Female mice | |||
| Parameters | Control | V15–25 | V15–50 |
| ALT (mmol/L) | 35.40 ± 2.70 a | 55.40 ± 3.10 b | 76.30 ± 12.10 c |
| AST (mmol/L) | 60.30 ± 3.10 a | 73.20 ± 4.70 a | 76.30 ± 12.10 b |
| Total protein (g/L) | 41.80 ± 5.40 a | 47.60 ± 6.00 a | 50.70 ± 1.50 a |
| BUN (mg/dL) | 11.49 ± 1.10 a | 13.41 ± 0.28 a | 11.74 ± 0.22 a |
| Creatinine (µmol/L) | 37.53 ± 6.44 a | 36.60 ± 3.60 a | 34.50 ± 1.80 a |
| Uric acid (µmol/L) | 114.92 ± 19.72 a | 170.60 ± 42.0 b | 204.10 ± 68.1 b |
| Total cholesterol (mmol/L) | 2.03 ± 0.14 a | 1.46 ± 0.17 b | 1.83 ± 0.06 a |
| Triglycerides (mmol/L) | 1.50 ± 0.14 a | 1.21 ± 0.12 b | 1.40 ± 0.07 a |
| Male Mice | |||
|---|---|---|---|
| Parameters | Control | V15–25 | V15–50 |
| GSH (nmol/mg ptn) | 10.2 ± 0.3 a | 10.4 ± 0.4 a | 10.1 ± 0.6 a |
| GSSG (nmol/mg ptn) | 7.4 ± 0.4 a | 7.4 ± 0.4 a | 7.3 ± 0.6 a |
| GSH/GSSG (nmol/mg ptn) | 1.4 ± 0.1 a | 1.4 ± 0.1 a | 1.4 ± 0.05 a |
| MDA (U/mg ptn) | 1.3 ± 0.1 a | 5.3 ± 0.3 b | 4.8 ± 0.5 b |
| SOD (U/mg ptn) | 19.9 ± 3.0 a | 14.0 ± 2.2 b | 15.7 ± 1.3 b |
| CAT (U/mg ptn) | 0.023 ± 0.005 a | 0.027 ± 0.010 a | 0.028 ± 0.010 a |
| Female mice | |||
| Parameters | Control | V15–25 | V15–50 |
| GSH (nmol/mg ptn) | 6.6 ± 0.2 a | 7.2 ± 0.2 a | 6.9 ± 0.2 a |
| GSSG (nmol/mg ptn) | 2.4 ± 0.4 a | 5.0 ± 0.3 b | 4.2 ± 0.1 b |
| GSH/GSSG (nmol/mg ptn) | 2.8 ± 0.5 a | 1.5 ± 0.1 b | 1.6 ± 0.1 c |
| MDA (U/mg ptn) | 0.9 ± 0.1 a | 1.0 ± 0.2 a | 1.3 ± 0.1 b |
| SOD (U/mg ptn) | 16.6 ± 3.6 a | 33.7 ± 3.8 b | 24.3 ± 1.9 c |
| CAT (U/mg ptn) | 0.011 ± 0.003 a | 0.029 ± 0.008 b | 0.026 ± 0.004 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbosa, M.d.M.; Lima, L.M.A.d.; Alves, W.A.d.S.; Lima, E.K.B.d.; Silva, L.A.d.; Silva, T.D.d.; Postal, K.; Ramadan, M.; Kostenkova, K.; Gomes, D.A.; et al. In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster. Pharmaceuticals 2023, 16, 1232. https://doi.org/10.3390/ph16091232
Barbosa MdM, Lima LMAd, Alves WAdS, Lima EKBd, Silva LAd, Silva TDd, Postal K, Ramadan M, Kostenkova K, Gomes DA, et al. In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster. Pharmaceuticals. 2023; 16(9):1232. https://doi.org/10.3390/ph16091232
Chicago/Turabian StyleBarbosa, Mariana de M., Lidiane M. A. de Lima, Widarlane A. da S. Alves, Eucilene K. B. de Lima, Luzia A. da Silva, Thiago D. da Silva, Kahoana Postal, Mohammad Ramadan, Kateryna Kostenkova, Dayane A. Gomes, and et al. 2023. "In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster" Pharmaceuticals 16, no. 9: 1232. https://doi.org/10.3390/ph16091232
APA StyleBarbosa, M. d. M., Lima, L. M. A. d., Alves, W. A. d. S., Lima, E. K. B. d., Silva, L. A. d., Silva, T. D. d., Postal, K., Ramadan, M., Kostenkova, K., Gomes, D. A., Nunes, G. G., Pereira, M. C., Silva, W. E. d., Belian, M. F., Crans, D. C., & Lira, E. C. (2023). In Vitro, Oral Acute, and Repeated 28-Day Oral Dose Toxicity of a Mixed-Valence Polyoxovanadate Cluster. Pharmaceuticals, 16(9), 1232. https://doi.org/10.3390/ph16091232







